Abstract
Angiogenesis is considered a hallmark pathophysiological process in tumor development. Aberrant vasculature resulting from tumor angiogenesis plays a critical role in the development of resistance to breast cancer treatments, via exacerbation of tumor hypoxia, decreased effective drug concentrations within tumors, and immune-related mechanisms. Antiangiogenic therapy can counteract these breast cancer resistance factors by promoting tumor vascular normalization. The combination of antiangiogenic therapy with chemotherapy, targeted therapy, or immunotherapy has emerged as a promising approach for overcoming drug resistance in breast cancer. This review examines the mechanisms associated with angiogenesis and the interactions among tumor angiogenesis, the hypoxic tumor microenvironment, drug distribution, and immune mechanisms in breast cancer. Furthermore, this review provides a comprehensive summary of specific antiangiogenic drugs, and relevant studies assessing the reversal of drug resistance in breast cancer. The potential mechanisms underlying these interventions are discussed, and prospects for the clinical application of antiangiogenic therapy to overcome breast cancer treatment resistance are highlighted.
Keywords: Angiogenesis, breast cancer, chemotherapy, drug resistance, vascular normalization, immunologic therapy, tumor microenvironment (TME)
Introduction
Breast cancer is currently the most prevalent type of cancer among women1, and its incidence is increasing every year2. Breast cancer treatments include primarily endocrine therapy, surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy, which function by mobilizing the autoimmune system. With advancements in therapeutic drugs and the development of auxiliary detection methods, the 5-year survival rate of patients with breast cancer has increased. Nevertheless, drug resistance in breast cancer decreases the patient survival rate; consequently, breast cancer remains the leading cause of cancer death among women worldwide3. Investigating the mechanisms underlying drug resistance in breast cancer and developing novel treatment strategies will be crucial to reverse drug resistance during breast cancer treatment.
Drug resistance in breast cancer is associated with factors including apoptosis, ferroptosis, drug efflux systems, and tumor angiogenesis. Angiogenesis is considered a marker of tumor growth. Normal blood vessels serve as conduits for the delivery of oxygen and nutrients. Tumor angiogenesis, the formation of morphologically abnormal blood vessels from the existing capillaries or postcapillary venules, results in the development of an immature vascular network. These newly formed vessels have extremely thin walls and lack smooth muscle components, thus hindering proper oxygen delivery and drug transport during tumor treatment. When the diameter of the tumor is > 0.5 mm, the oxygen obtained by tumor cells via simple diffusion is insufficient to support cell growth. Therefore, tumor cells rely on angiogenesis to obtain more oxygen and maintain growth. Tumor angiogenesis leads to disordered internal tumor vasculature and aggravation of hypoxia, which in turn directly promotes the transcription of drug-resistance genes such as Multi-drug Resistance Protein1 (MDR1), Multi-drug Resistant Associate Protein1 (MRP1), and Breast Cancer Resistance Protein (BRCP)4,5. Concurrently, tumor angiogenesis affects drug distribution, and alters the immune system both inside and outside tumors6. Vascular endothelial growth factor (VEGF) is highly expressed in tumor angiogenesis and binds VEGF receptor (VEGFR), thereby preventing antigen presentation and inhibiting T-cell activation. Simultaneously, angiogenesis-related factors induce macrophage polarization toward the M2 phenotype, thereby leading to the development of an immunosuppressive tumor microenvironment (TME). These factors directly or indirectly contribute to the occurrence of tumor drug resistance. Notably, tumor angiogenesis plays a crucial role in the occurrence of drug resistance in breast cancer. Anti-tumor angiogenesis increases the normalization of blood vessels, inhibits tumor hypoxia and the immunosuppressive TME, increases the effective drug concentrations in tumors, and enhances drug efficacy. Therefore, anti-angiogenesis therapy can be used to reverse drug resistance in breast cancer7.
Tumor vascular normalization, a novel angiogenesis-specific concept for the treatment of tumors, was first proposed by R. K. Jain in 1974. Targeting VEGF or hypoxia-inducible factor (HIF-1) can help normalize the tumor blood vessels, promote drug delivery, improve tumor hypoxia and the immunosuppressive microenvironment8, and ultimately prevent or reverse drug resistance in breast cancer. This review describes the mechanisms underlying angiogenesis and drug resistance in breast cancer, and explores the possibility of reversing drug resistance via vascular normalization. The efficacy of a single anti-angiogenesis targeted agent on the inhibition of tumor growth is short-lived9. Investigating the relationship between angiogenesis and drug resistance in breast cancer, and the combination of anti-angiogenic and vascular normalization, may provide novel ideas for reversing drug resistance in breast cancer.
Mechanism of angiogenesis
Tumor angiogenesis involves the activation of transcription factors, such as HIF-1α, within the hypoxic tumor environment. This activation leads to the proliferation and migration of endothelial cells, as mediated by growth factors such as VEGF and Platelet-derived Growth Factor (PDGF), thus resulting in the formation of a complicated and disordered tumor vasculature.
Classical growth factors
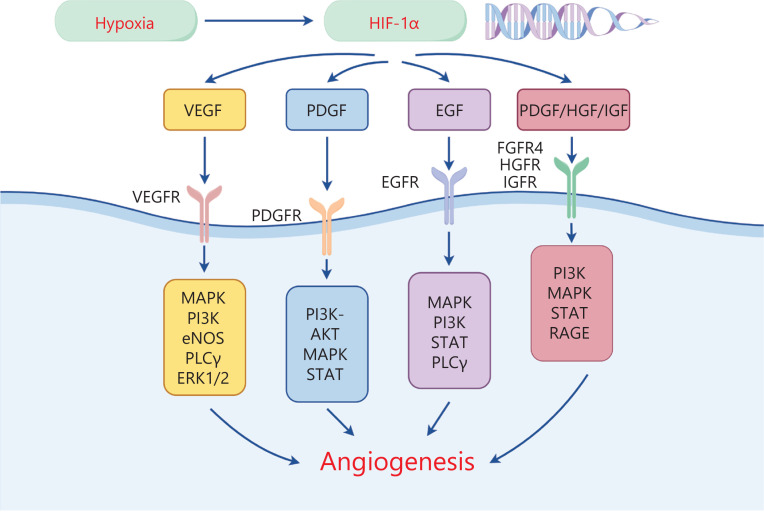
VEGF and VEGFR
The discovery of VEGF has greatly advanced understanding of tumor angiogenesis and enabled its potential targeting in cancer therapy. The binding of VEGF-A to VEGFR2 is crucial for angiogenesis. Notably, VEGF-A binding leads to the tyrosine phosphorylation of VEGFR2, which in turn further activates downstream signaling pathways including the MAPK and PI3K pathways. Furthermore, pathways such as PLCγ10, ERK1/2, and PI3K11 stimulate endothelial cell division. The activation of endothelial nitric oxide synthase (eNOS)12 increases vascular permeability13 (Figure 1). A recent study has revealed that VEGF inhibition effectively inhibits tumor angiogenesis, promotes vascular normalization, and inhibits tumor growth14. Nevertheless, the normalization of blood vessels by targeting VEGF anti-tumor angiogenesis therapy is short-lived. Therefore, investigating the appropriate dose and time window of drugs to promote and maintain tumor vascular normalization may become a primary focus of future research on anti-tumor angiogenesis15.
Figure 1.
The hypoxia-inducible factor HIF promotes the transcription of angiogenesis-related factors. The VEGF/VEGFR signaling axis promotes angiogenesis via MAPK, PI3K, PLCγ, ERK1/2, and eNOS. The PDGF/PDGFR signaling axis promotes angiogenesis via MAPK, PI3K-AKT, and STAT. The EGF/EGFR signaling axis promotes angiogenesis via MAPK, PI3K, PLCγ, and STAT. Other signaling axes (PDGF/HGF/IGF) promote angiogenesis through MAPK, PI3K, STAT, and RAGE.
PDGF and PDGF receptor (PDGFR)
PDGF is a growth factor that is secreted by platelets and their stromal cells and is involved in the regulation of angiogenesis. This growth factor comprises the following 4 subunits: PDGF-A, PDGF-B, PDGF-C, and PDGF-D16. PDGFR is divided primarily into PDGFR-α and PDGF-β. Studies have focused on the binding between PDGF-B and PDGFR-β, and subsequent promotion of angiogenesis. PDGFR-β is significantly expressed in breast cancer cells17,18. Phosphorylated PDGFR regulates cell proliferation and migration via PI3K-AKT19 and other signaling pathways, thereby participating in tumor angiogenesis (Figure 1). Wang et al. have reported that decreased levels of PDGF-B promote vascular normalization of breast cancer cells, increase cytotoxic drug delivery, and inhibit tumor growth17. In a mouse model of triple-negative breast cancer with lung metastasis formation, PDGFR-β blockade has been found to decrease cancer cell growth and migration, and consequently prevent lung metastasis20. Nevertheless, the specific mechanism of the PDGF/PDGFRs axis in the angiogenesis of breast cancer cells remains unclear. Further basic experiments are warranted to elucidate the relevant mechanisms.
Epidermal growth factor (EGF) and EGF receptor (EGFR)
EGF is a mediator with crucial roles in the proliferation, survival, differentiation, and migration of vascular endothelial cells, through EGFR binding. EGFR is activated by phosphorylation and is involved in regulation of tumor angiogenesis via its downstream signaling pathways (MAPK, PI3K/AKT/PKB, STAT, and PLCγ/PKC)21 (Figure 1). HER2, a subtype of EGFR, is a frequent target in breast cancer treatment. EGFR-mediated downregulation of the JAK-1/STAT-3 signaling pathway has been found to inhibit angiogenesis, significantly decrease the volume of breast cancer tissues, and inhibit tumor growth21. EGFR and VEGFR frequently share downstream signaling pathways. Furthermore, the expression of EGFR leads to an increase in VEGFR levels and consequently plays a crucial role in promoting angiogenesis22. Therefore, targeting EGFR to downregulate VEGFR expression is advantageous in cancer treatment. Nevertheless, many patients who undergo targeted EGFR therapy exhibit progression after 1 year23. Therefore, mechanisms underlying resistance and strategies to reverse resistance via targeted EGFR therapy must be further explored.
Other factors
Beyond the VEGF/VEGFR, EGF/EGFR, and PDGF/PDGFR axes, many other growth factors play indispensable roles in angiogenesis, such as fibroblast growth factor (FGF)/FGFR4, hepatocyte growth factor (HGF)/c-Met, insulin-like growth factor (IGF)/IGFR, and transforming growth factor (TGF-β) (Figure 1). Angiogenesis-related FGFR4 is significantly upregulated in breast cancer, and promotes vascular endothelial cell proliferation and breast cancer angiogenesis via the PI3K/AKT signaling pathway24. HGF activates c-Met through phosphorylation of the tyrosine residues Y1234 and Y1235. Adaptor proteins bind several substrates, and downstream MAPK and STAT signaling pathways are consequently activated, thereby promoting angiogenesis in breast cancer25. Muoio et al. have reported that elevated IGF expression in patients with obesity and diabetes promotes breast cancer angiogenesis via the S7A1/RAGE downstream signaling pathway26 (Figure 1). Notably, TGF-β increases angiogenesis in breast cancer by modulating endothelial–mesenchymal transition, potentially through TGF-β-induced expression of Snail and Slug27. Various growth factors play important roles in the angiogenesis of breast cancer. Therefore, anti-angiogenic therapies against these vascular factors can be beneficial. Further studies are warranted to validate the possibility of targeting single or multiple growth factors to increase the anti-angiogenic efficacy of breast cancer treatment.
Hypoxia-induced mechanisms
Oxygen is a crucial factor required for tumor growth, and the increasing oxygen demand during tumor development contributes to the formation of a hypoxic microenvironment. Under hypoxic conditions, tumors activate HIF-1α, which in turn stimulates tumor angiogenesis. Paradoxically, the resultant abnormal vasculature exacerbates hypoxic conditions and creates a vicious cycle, wherein the progressively abnormal TME continues to foster angiogenesis and hypoxia in tumors.
HIF-induced angiogenesis
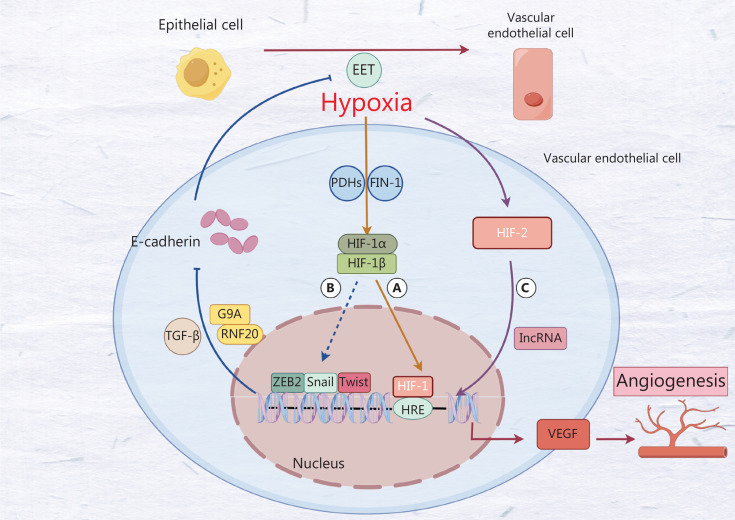
Under hypoxia, the activity of FIN-1 and PHDs, acting as oxidase-related groups, is inhibited, thus leading to decreased HIF-1α hydroxylation and proteasome degradation. Consequently, the cytoplasmic level of HIF-1α increases, and HIF-1α forms a heterodimer with HIF-1β. This heterodimer translocates to the nucleus, where HIF-1 binds the activating protein CREB (P300) and the hypoxia reactive element (HRE), and subsequently initiates a series of hypoxia-induced cascades, including angiogenesis, through increased transcription of target genes, such as VEGF28. Recent studies have elucidated the role of HIF-1 in breast cancer angiogenesis through the SNHG1/miR-199a-3p/TFAM axis29. Beyond HIF-1, the hypoxia-induced HIF-2-dependent pathway promotes angiogenesis in breast cancer by upregulating levels of the lncRNA RAB1B-AS231 through HIF-2. This event in turn increases the transcription of angiogenesis-related factor VEGFA. Knockdown of HIF-2 has been shown to eliminate the increase in RAB1B-AS231 expression, thereby decreasing angiogenesis in breast cancer30 (Figure 2C). Given the current limitations of targeted VEGF treatment regimens, targeting HIF, a key regulator of angiogenesis in breast cancer, may emerge as a promising new therapeutic approach.
Figure 2.
A. Hypoxia inhibits the activity of FIH-1 and PHD, thereby promoting the expression of HIF-1. Subsequently, HIF-1 stimulates the expression of VEGF, which in turn facilitates angiogenesis. B. HIF-1 induces endothelial to mesenchymal transition by promoting the activity of the transcription factors Twist, Snail, and ZEB2, thereby stimulating angiogenesis. However, its specific mechanism is unclear. C. Hypoxia promotes HIF-2 expression through a lncRNA that also promotes angiogenesis.
Epithelial–endothelial transition (EET)
Epithelial–mesenchymal transition (EMT) is a prevalent phenomenon. Research has revealed that the same key factors are involved in both EET and EMT. Consequently, EET is generally considered a phenotype of EMT31. Under hypoxia, breast cancer cells show a decrease in tight junction proteins (such as E-cadherin and occludin) and an increase in vimentin and VE-cadherin. Tumor epithelial cell transition to endothelial cell phenotypes subsequently promotes the proliferation of tumor vascular endothelial cells and angiogenesis32 (Figure 2A) Numerous EET-associated signaling pathways are directly or indirectly regulated by hypoxia. Transcription factors such as Twist, Snail, and ZEB2, which are directly regulated by hypoxia, play major roles in this process and share a frequent promoter: the HRE. Under hypoxia, binding of HIF to HRE promotes the transcription of Twist, Snail, and ZEB233–35 (Figure 2B). Twist proteins downregulate the expression of E-cadherin in breast cancer, thereby promoting EET. This process may be involved in EET progression via activation of Wnt/β-catenin signaling by the JPX/miR-33a-5p/Twist1 axis36. However, this signaling pathway was discovered and validated by Pan et al. in the EET in lung cancer, and further research is warranted to confirm its presence in breast cancer37. The Snail gene has been found to interact with RNF20 (E120 ubiquitin-protein ligase with monoubiquitinated H3BK2) and G9a (methyltransferase of H3K9me2) in breast cancer, and to subsequently inhibit the expression of E-cadherin. The Snail gene also promotes tumor EET and induces tumor angiogenesis38,39. Consequently, hypoxia plays a crucial role in tumor angiogenesis, and reversing the tumor hypoxic microenvironment may aid in the treatment of anti-angiogenic and tumor vascular normalization.
Tumor angiogenesis and drug resistance
The abnormal new vasculature formed through tumor angiogenesis impedes oxygen delivery and increases the hypoxic microenvironment of breast tumors. This condition has been found to directly or indirectly induce drug resistance in breast cancer through various mechanisms5. The abnormal vasculature disrupts the delivery and distribution of drugs, thereby preventing effective drug concentrations from being achieved within tumors and leading to drug resistance. Tumor angiogenesis further alters the composition of immune cells in the microenvironment, by converting immune cells into tumor-related immunosuppressive cells, and promoting breast cancer progression and drug resistance7,40.
Hypoxia-induced resistance
Immediate effects
Hypoxia directly induces the transcription of drug resistance-associated genes, namely MDR1, MRP1, and BRCP, which are members of the same ABC transporter family. The expression of ABC transporters increases under hypoxic conditions, thus facilitating drug efflux, decreasing effective drug concentrations, and fostering drug resistance in breast cancer. Expression of the multidrug resistance gene (MDR1) frequently contributes to drug resistance in various cancers. Che et al. have demonstrated that HIF-1α directly binds the MDR1 promoter and promotes MDR1 expression, as confirmed with ChIP assays41. In breast cancer cells, AGR2 expression decreases the degradation of HIF-1α and increases MDR1 transcription, thus decreasing epirubicin uptake and promoting doxorubicin (DOX) resistance in breast cancer cells42. MDR1 is regulated through hypoxia-induced Notch1 signaling and contributes to the development of tumor resistance43. Under hypoxic conditions, increased HIF-1α activity promotes the BRCP expression41. DOX has been found to induce the production of reactive oxygen species, thereby synergistically upregulating the expression of MDR1 and BRCP in conjunction with the high activity of HIF-1α under hypoxia. This process limits the uptake of DOX and promotes the emergence of drug resistance44.
Indirect effects
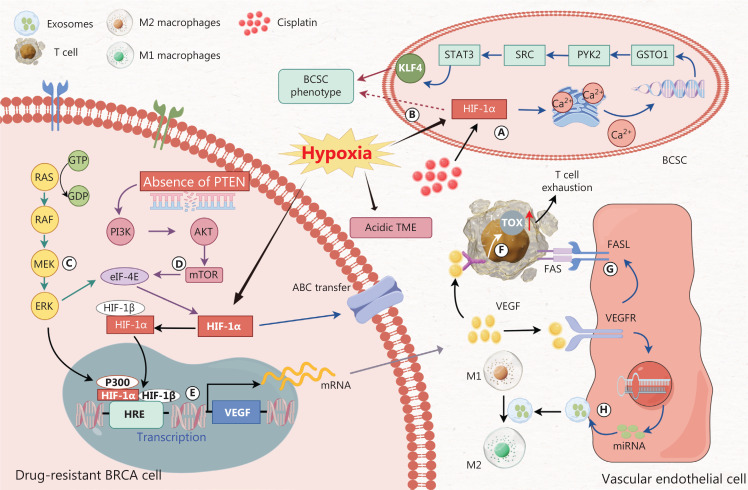
The hypoxia-induced acidic microenvironment of tumors can affect drug efficacy45, and promote the phenotypic expression of breast cancer stem cells (CSCs) and the acquisition of MDR in breast cancer. In a hypoxic environment, HIF-1α is essential for promoting the expression of the target gene VEGF, which in turn stimulates angiogenesis, exacerbates hypoxia, and supports the development of drug resistance in breast cancer. Under hypoxia, enhanced anaerobic metabolism leads to the accumulation of acidic substances, such as lactic acid, and an increase in the microenvironment pH. The pH value of the TME is typically 10 to 30 times higher than that of normal tissues45. The efficacy of DOX against breast cancer cells is limited by the low pH TME induced by hypoxia simulation. Simultaneously, the degree of pH decrease precisely corresponds to the extent of tumor growth delay46. Breast CSCs exhibit unlimited proliferation and diverse differentiation potential, and consequently promote tumor immune evasion. Cancer stem cells usually overexpress ABC transporters, which contribute to dysregulation of the signal transduction network and play essential roles in tumor MDR47. HIF is involved in the expression of CSC in breast cancer. Through FACS, Brooks et al. have observed that HIF is highly active under hypoxic conditions and promotes the transcription of the target gene ITGA6, which, in conjunction with other integrins, is enriched in breast stem cells and promotes the acquisition of MDR phenotypes in breast cancer48. HIF participates in the expression of glutathione S-transferase omega 1 (GSTO1), thereby promoting endoplasmic reticulum release of Ca2+; inducing the recruitment of CSC through the PYK2 → SRC → STAT3 signal transduction induced by cisplatin; and contributing to the occurrence of drug resistance in breast cancer49 (Figure 3A). HIF-1α activity increases under hypoxia. HIF-1α, which promotes transcription under hypoxia, directly promotes the expression of cancer stem cell phenotypes in liver cancer. However, whether HIF-1α directly promotes the expression of breast cancer stem cell phenotypes has not been validated (Figure 3B). The RAS/RAF/MEK/ERK kinase cascade enhances the translation of HIF-1α by phosphorylating eukaryotic translation initiation factor 4E (eIF-4E). Furthermore, this kinase cascade enhances the transcriptional activity of HIF-1α by binding the TAD sequence of HIF-1α through the transcriptional co-activators p300/CBP (Figure 3C). In breast cancer cells, loss of the tumor suppressor gene PTEN is frequently observed. The loss of PTEN promotes activation of the PI3K/AKT pathway, and its downstream mTOR protein activates eIF-4E and consequently promotes the translation of HIF-1α protein50,51 (Figure 3D). HIF-1α forms a heterodimer with HIF-1β in the cytoplasm, translocates into the nucleus, and binds specific HREs on DNA, where it acts as a transcription factor for the downstream target gene VEGF (Figure 3E). Subsequently, the proliferation of endothelial cells increases, angiogenesis in breast cancer is improved, and hypoxia is further exacerbated. Thus, the development of drug resistance in breast cancer occurs through various direct and indirect mechanisms.
Figure 3.
A. Cisplatin-induced HIF-1α facilitates the release of calcium ions from the endoplasmic reticulum. The released calcium ions in turn promote the expression of glutathione S-transferase omega 1 (GSTO1). GSTO1 induces the recruitment of cancer stem cells (CSCs) through the PYK2 → SRC → STAT3 signal transduction pathway. STAT3 activity promotes the BCSC phenotype by upregulating the expression of the pluripotency factor KLF4. B. Hypoxia-induced HIF-1α directly promotes the cancer stem cell phenotype in liver cancer. However, whether HIF-1α directly promotes the breast cancer stem cell phenotype remains to be validated. C. The RAS/RAF/MEK/ERK kinase cascade enhances the translation of HIF-1α by phosphorylating the eukaryotic translation initiation factor 4E (eIF-4E). Furthermore, this cascade pathway facilitates the interaction between transcriptional co-activators p300/CBP and HIF-1α, thereby augmenting the binding capacity of HIF-1α to HRE. D. Loss of PTEN promotes activation of the PI3K/AKT/mTOR pathway, thereby activating eIF-4E, which in turn promotes HIF-1α translation. E. HIF-1α forms a heterodimer with HIF-1β in the cytoplasm, translocates into the nucleus, and acts as a transcription factor binding specific HREs on DNA, thus promoting transcription of the downstream target gene VEGF. F. VEGF binding to VEGFR on the surfaces of T cells promotes TOX expression and leads to T cell exhaustion. G. VEGF binds VEGFR and promotes the expression of FASL ligands in epithelial cells, thereby facilitating T cell apoptosis. H. VEGF/VEGFR signaling promotes the transcription of miRNAs (miR-142-5p, miR-183-5p, and miR-222-3p) and facilitates their extracellular transport via exosomes, thereby promoting the polarization of M2 macrophages.
Drug concentration, efficacy, and resistance
Drug concentration
The drugs used to treat tumors typically must reach effective concentrations within tumors to exert anti-tumor effects. Drug concentrations are affected by the abnormal and immature tumor vasculature system, as well as by the activation of drug efflux transporters under the hypoxic tumor environment52.
MDR1, also known as permeability glycoprotein (P-gp), is a transmembrane transport protein that recognizes and controls drug efflux from tumor tissues and eventually promotes drug resistance in breast cancer by altering drug distribution in tumors52. HIF-1α is highly expressed after tumor angiogenesis, and subsequently binds P-gp, and upregulates P-gp gene expression. This upregulation then promotes drug efflux. Consequently, achieving effective drug concentrations in the tumor becomes difficult and ultimately leads to drug resistance in breast cancer. HIF-1α inhibition decreases P-gp levels in tumors and partially reverses MDR caused by MDR1 (P-gp)41. A recent study has described the use of novel nanomolecular materials to directly downregulate P-gp gene expression and enhance the sensitivity of breast cancer cells to chemotherapy52.
Drug efficacy
Antineoplastic drugs typically require certain conditions to exert optimal therapeutic effects. For example, compared to cells with an inactive cell cycle, chemotherapy drugs are usually more effective against active tumor cells. Tumor cells typically undergo active DNA replication, and chemotherapy drugs act on the cell cycle53. Abnormal tumor vascular systems often affect pH levels and create a hypoxic environment, which in turn affects the efficacy of antineoplastic drugs.
Tumor angiogenesis often results in a low-pH and hypoxic TME, which eventually limits drug efficacy. The low-pH TME has been reported to promote the resistance of breast cancer cells to DOX46. In addition, the hypoxic microenvironment caused by impaired oxygen delivery due to abnormal vascular systems affects drug efficacy. In breast cancer treatment, chemotherapeutic drugs, such as docetaxel, are among the most frequently used therapeutic agents. Typically, these drugs exert therapeutic effects by interfering with DNA synthesis, inducing apoptosis, and inhibiting cell division in breast cancer cells. Although chemotherapeutic drugs are more effective against actively proliferating cancer cells than proliferating inactive cancer cells, decreased aerobic oxidation in cancer cells under hypoxia decreases cell activity and proliferation, and consequently limits the effectiveness of chemotherapeutic drugs53. However, no specific measures are currently available to prevent or treat drug resistance caused by angiogenesis in breast cancer. Therefore, investigating the mechanisms of action of various drugs and devising strategies to prevent drug resistance will be imperative to achieve more precise and effective targeting of breast cancer cells.
Tumor immunity and resistance
The formation of abnormal vascular systems due to tumor angiogenesis leads to decreased blood perfusion, which in turn hinders the immune cell infiltration that effectively kills tumor cells. Tumor angiogenesis is associated with intrinsic mechanisms such as the MAPK/PI3K and WNT/β-catenin pathways, which contribute to drug resistance development54. In contrast, angiogenic factors promote changes in extracellular factors and contribute to immunosuppressive TME formation, such as peripheral dendritic cell (DC) maturation, inhibition of T-cell activation, and polarization of macrophages toward the M2-like tumor-associated phenotype55. Angiogenesis induces an immunosuppressive TME that promotes the resistance of breast cancer to immunotherapeutic drugs56.
Tumor-associated internal resistance
The oncogenic signaling of the MAPK pathway associated with breast cancer is linked to the c-myc pathway through the VEGF promoter, which promotes VEGF transcription; subsequently, secreted proteins inhibit the recruitment and infiltration of specific T-cells in tumors57. In addition, the loss of PTEN, a tumor suppressor gene, plays a major role in the invasion and progression of breast cancer. PTEN loss and VEGF overexpression are closely correlated in breast cancer. PTEN loss promotes VEGF expression by inducing HIF-1α and activating the PI3K-AKT pathway58,59. PTEN loss in breast cancer is associated with resistance to chemotherapy58 and immune checkpoint-associated immunotherapy, which may be associated with the activation of the PI3K-AKT pathway, thereby affecting T-cell infiltration in tumors60. Both miR526b and miR655 regulate PTEN expression and upregulate VEGF expression, and hence promote angiogenesis in breast cancer. Altered PTEN pathway expression has been found to enhance the efficacy of anti-PD-1 and anti-CTLA-4 antibodies in mouse models60 and to improve the chemoresistance of breast cancer cells to DOX61. Moreover, miR526b and miR655 may serve as targets for improving drug resistance in breast cancer via the PTEN/PI3K pathway. The WNT pathway, which is often involved in tumorigenesis signaling, is crucial for tumor angiogenesis. This pathway, under stabilization by β-catenin, is closely associated with immune suppression in tumors62. Moreover, this pathway has been associated with increased β-catenin levels, and decreased DC chemokine CCL103 and mature CD4 DC levels in tumors. The Wnt/β-catenin pathway, targeting suppresses stemness and angiogenesis, improves MDR in breast cancer62. Compared with mice with Wnt/β-catenin pathway activation, mice lacking β-catenin exhibit markedly enhanced immunotherapeutic efficacy54. Conventionally, breast cancer has not been considered an immunogenic tumor. However, several recent studies have shown that breast cancer cells express immune inhibitory ligands, such as PD-1, which suppress CD8 T-cell activity and lead to immune resistance in breast cancer63. In addition, tumor-associated intrinsic pathways such as MAPK/PI3K, WNT/β-catenin, and PD-1 are closely associated with tumor angiogenesis, and interactions among these pathways affect DC maturation, and T-cell recruitment and activation, and subsequently promote drug resistance in breast cancer. Targeting the key factor VEGFR2 promotes tumor vascular normalization, thus increasing immune cell infiltration and activation. Furthermore, low-dose anti-angiogenic therapy promotes osteopontin (OPN) secretion by CD8+ T-cells. Subsequently, OPN induces tumor cells to produce TGF-β, which in turn upregulates PD-1 expression on immune cells and ultimately increases the sensitivity of breast cancer to PD-1 immunotherapy. The combination of anti-angiogenic therapy and anti-PD-1 immunotherapy has been found to effectively overcome drug resistance in breast cancer64.
Tumor-associated external resistance
Beyond abnormal vascularization due to tumor angiogenesis, hypoxia, and low pH induce an immunosuppressive TME65. VEGF highly expressed on the endothelium of peripheral tumor blood vessels binds VEGFR1, and subsequently inhibits DC maturation and antigen presentation, and impedes T-cell activation and infiltration66. High VEGF expression promotes the accumulation of peripheral myeloid-derived suppressor cells (MDSCs), which are closely associated with cancer angiogenesis and immunosuppression67. MDSCs regulate breast CSCs via the CXCL2-CXCR2 pathway, thereby inducing resistance to docetaxel68. Furthermore, MDSC targeting and inhibition via the SDF1α/CXCR4 axis enhance the anti-tumor activity of chimeric antigen receptor (CAR)-T-cell immunotherapy in breast cancer69. High VEGF expression during angiogenesis in breast cancer decreases CD8 T-cell levels via the HMG-bOX (TOX) pathway, thereby increasing tumor-cell tolerance to immunotherapy70 (Figure 3F). The combination of VEGF, interleukin-10, and prostaglandin E2 promotes the expression of the death ligand FasL in endothelial cells. FasL is an ectopic expression product in mouse solid tumors and is undetectable in normal vascular systems. FasL promotes the tumor-specific exclusion of cytotoxic T-cells, facilitates the formation of an immunosuppressive TME, and leads to the development of drug resistance71 (Figure 3G). In addition, angiopoietin 2 and placental growth factor, which are associated with tumor angiogenesis, induce macrophage polarization toward the M2 phenotype, thereby leading to the development of an immunosuppressive TME72. Moreover, vascular endothelial cells in the breast cancer microenvironment release several miRNAs (miR-142-5p, miR-183-5p, and miR-222-3p) that are transported through extracellular vesicles, and subsequently regulate macrophage remodeling and promote macrophage polarization toward the M2 phenotype73 (Figure 3H). Anti-angiogenesis treatment to promote tumor vessel normalization has been found to reverse the tumor immunosuppressive microenvironment and improve cancer treatment efficacy74. Thus, the combination of anti-angiogenesis therapy and immunotherapy may enhance the efficacy of immunotherapy against breast cancer. However, several challenges exist in the application of such combination treatment strategies. Future studies should focus on the appropriate dosages of anti-angiogenic drugs and their potential toxicity after combined treatment, to overcome persisting challenges.
Drugs to reverse drug resistance in breast cancer
Breast cancer treatments include primarily chemotherapy, targeted therapy, and immunotherapy. Because angiogenesis plays a crucial role in the development of drug resistance in breast cancer, anti-angiogenic therapy has been found to improve the TME and drug perfusion, thereby partially reversing drug resistance. Studies to date have investigated various anti-angiogenesis methods, including the use of nanoparticle drugs, to promote tumor vessel normalization and reverse drug resistance75.
Reversal of chemotherapy drug resistance
Eribulin mesylate, a synthetic analog of halichondrin B, directly inhibits cancer cells by binding microtubule proteins. This analog has been widely applied in locally advanced or metastatic breast cancer. However, eribulin directly acts on angiogenesis-associated pathways, including VEGF (Vegfa, Vegfr1, Vegfr2, and Vegfr3), Notch (Dll4, Jag1, and Notch4), Eph (Efnb2, Epha2, and EphB1), and WNT (Wnt5a, Wnt11, and BPM4), thus resulting in vascular remodeling. Increased vascular perfusion and enhanced drug delivery after eribulin treatment markedly increase capecitabine’s efficacy, thereby preventing resistance of breast cancer cells to capecitabine76 (Table 1). Gambogic acid (GA), a naturally occurring dry resin found in Garcinia hanburyi, selectively targets the HIF-1α/VEGF pathway and inhibits tumor angiogenesis77. Furthermore, GA inhibits P-gp gene expression, thereby preventing drug efflux. GA alters drug distribution, increases perfusion, enhances intratumoral drug concentration, and reverses resistance to DOX in drug-resistant breast cancer cells via the combined action of these 2 mechanisms, and ultimately increases sensitivity to the drug78 (Table 1). Recently, novel nanomedicines have been investigated to provide additional avenues for reversing drug resistance in breast cancer by promoting tumor vascular normalization via anti-angiogenesis. The nanodrug AuNP-Qu-5 synthesized from quercetin (Qu) and gold nanoparticles (AuNPs), enhances tumor vascular normalization by downregulating VEGFR2 expression, improving blood flow, and increasing drug penetration79 (Table 1). A nanodrug comprising low-molecular-weight heparin and QU has been reported to target basic fibroblast growth factor and VEGF, inhibit drug efflux protein expression (P-gp, MRP1, and BCRP), and prevent drug resistance in breast cancer80 (Table 1). Xihuang pills act on PI3K/Akt/mTOR signaling, a key pathway in blood vessel formation, thus providing a possibility for reversing resistance to chemotherapy81. In addition, preliminary studies have elucidated the mechanism of reversing resistance to paclitaxel in breast cancer via anti-angiogenesis. CircBACH217 reverses the resistance of breast cancer to paclitaxel by inhibiting G1BP7 expression82. However, basic experiments remain necessary to verify the feasibility of nanodrug therapies.
Table 1.
Drugs for reversing drug resistance
| Drugs | Targets | Effect/mechanism | ||
|---|---|---|---|---|
| Reversal of chemotherapy drug resistance | ||||
| Eribulin | VEGF, Notch, Eph, and WNT | Eribulin increases the efficacy of capecitabine | ||
| Gambogic acid (GA) | HIF-1α, VEGF, and P-gp | GA enhances intratumoral doxorubicin concentration | ||
| Au NPs-Qu-5 | VEGFR2 | Au NPs-Qu-5 increases drug penetration | ||
| LMWH and QU | Bfgf, VEGF, P-gp, MRP1, and BCRP | LMWH and QU inhibit drug efflux | ||
| Reversal of targeted drug resistance | ||||
| Nintedanib | VEGFR, PDGF, FGFR, FLT3, and SRC family | Nintedanib increases the efficacy of single-target VEGF drugs | ||
| Matrine | HIF, BRCA CSC | Matrine inhibits resistance of breast cancer to anti-angiogenic therapy | ||
| Lapatinib | EGFR/HER-2 | Lapatinib reverses resistance to trastuzumab | ||
| Gold nanoparticles and pulsed femtosecond lasers | HER-2 | Gold nanoparticles with anti-HER2 antibody functionality reverse resistance to trastuzumab | ||
Reversal of targeted drug resistance
Targeted therapies such as VEGF inhibitor treatment, have faced challenges in achieving ideal therapeutic effects in breast cancer treatment83. Nintedanib, a multi-target receptor tyrosine kinase inhibitor, targets VEGFR, PDGF, FGFR, FMS-associated tyrosine kinase 3, and SRC family kinases, thus increasing the efficacy of single-target VEGF drugs84 (Table 1). Matrine and bortezomib selectively target and inhibit HIF, alleviate the hypoxic environment, and prevent hypoxia-induced resistance to anti-angiogenic therapy in breast cancer. The matrine and trastuzumab combination targets breast CSCs and alleviates the resistance of breast cancer cells to anti-angiogenic therapy85 (Table 1). High FGF2 expression in breast cancer cells promotes resistance to targeted VEGF and PDGF drugs via a PDGFRβ-dependent mechanism. Moreover, combination therapy targeting VEGF and PDGF reverses the resistance of high FGF2-expressing breast cancer cells to single-targeted drugs14. Nevertheless, patients with HER-2-positive breast cancer can develop resistance to targeted HER-2 therapy, such as trastuzumab, after several years86. A phase III clinical trial examining lapatinib, a dual EGFR/HER-2 tyrosine kinase inhibitor that inhibits the EGFR/HER-2 signaling pathway, has shown that lapatinib reverses resistance to trastuzumab in patients with breast cancer with trastuzumab resistance86 (Table 1). In addition, Nunes et al. have developed AuNPs with anti-HER2 antibody functionality, used in combination with pulsed femtosecond lasers, to reverse the resistance of breast cancer cells to trastuzumab. The combination of the AuNPs and photothermal therapy markedly decreases microvascular density in the tumor area, thereby inhibiting the growth of trastuzumab-resistant breast cancer cells via anti-angiogenesis, and ultimately reversing resistance to trastuzumab87 (Table 1).
Reversal of immunotherapy drug resistance
Immunotherapy has emerged as a treatment method in recent years. Antibody-drug conjugates (ADCs) have been found to be effective in combined immunotherapy and targeted therapy. This class of drugs is formed by linking a specific antibody to a toxic payload and works by binding target antigens on the surfaces of breast cancer cells through their specific antibodies. After the drugs enter the cells through endocytosis, the toxic payload is released inside the cells through the action of lysosomes and subsequently exerts cell-killing effects88. The combination of specific antibodies and targeted toxins partially reverses resistance to conventional targeted drugs89. In the EMILIA trial (NCT00829166), ADC class drugs, compared with lapatinib plus capecitabine, in populations with low HER-2 expression extended the progression-free survival by more than 3 months and the overall survival by 4 months. However, patients with breast cancer exposed to ADC therapy continue to develop resistance after a certain period of time, primarily because ADC drugs can maintain progression-free survival in patients with advanced breast cancer patients for only several months to several years. Breast cancer cells acquire resistance through pathways such as downregulation of HER-2 expression and upregulation of ABC transporter protein expression90. Currently, many strategies are being explored to reverse drug resistance to ADC drugs. For example, the newly developed drug DS-8201a has higher membrane penetration and a greater drug payload than the traditional ADC drug T-DM191. Moreover, anti-angiogenic therapy can increase the effective concentrations of ADC drugs within tumors by inhibiting the expression of ABC transport proteins. However, the dose toxicity effects of ADC drugs, such as lung injury, remain challenges that must be addressed.
Conclusions
Tumor angiogenesis, a major pathological process in breast cancer, is induced by a hypoxic TME and various angiogenic factors. An abnormal tumor vascular system further exacerbates tumor hypoxia and an immunosuppressive TME, affects drug distribution, and promotes drug resistance in breast cancer. Drug resistance is a major cause of poor prognosis in many patients with breast cancer. The diverse mechanisms of angiogenesis in tumor treatment act as a double-edged sword by providing various therapeutic targets for targeting angiogenesis but also enabling tumors to escape from the action of single-target drugs, such as VEGF. This escape may be attributable to the activation of alternative growth factor-associated signaling pathways through diverse mechanisms, thus leading to continual promotion of tumor angiogenesis. Nevertheless, inhibiting tumor angiogenesis and promoting tumor vascular normalization are potential new mechanisms for reversing drug resistance in breast cancer. Anti-angiogenic approaches have improved the efficacy of chemotherapy and targeted therapy for breast cancer. Immunotherapy is a newly emerging treatment modality against breast cancer; however, the specific mechanism underlying the resistance of breast cancer cells to immune drugs remains unclear. Nevertheless, anti-angiogenic therapy has been demonstrated to increase the efficacy of immune drugs in tumor treatment92.
This review provided an overview of the correlation between tumor angiogenesis and drug resistance development in breast cancer, as well as methods for reversing drug resistance via anti-angiogenic therapy. However, challenges persist in applying these approaches in clinical practice. First, determining the appropriate dosage of anti-angiogenic drugs to increase the efficacy of drug-resistant treatments against breast cancer is difficult. In addition, whether combination therapy might increase drug toxicity remains uncertain. Hence, more preclinical studies must be performed to validate the rationale and safety of combination therapy74. Anti-angiogenic therapy often has a reversible and transient vascular normalization window, depending on the drug type and dosage. Thus, future studies focusing on reversing drug resistance in breast cancer via anti-angiogenic therapy should examine the use of specific anti-angiogenic drugs and their methods of administration93. Moreover, different breast cancer cell lines may exhibit varying sensitivity to anti-angiogenic therapy. To further investigate tumor angiogenesis markers for breast cancer for evaluation of anti-angiogenic therapy efficacy, promoting vascular normalization may be a necessary prerequisite for reversing drug resistance. Finally, this review summarized the potential mechanisms of resistance to anti-angiogenic therapy in reversing drug resistance in breast cancer immunotherapy. For instance, targeting the SDF1α/CXCR4 axis enhances the efficacy of CAR-T cell immunotherapy. Specific drugs targeting drug resistance reversal via anti-angiogenic therapy in breast cancer immunotherapy are under development69.
Acknowledgements
We acknowledge the State Administration of Traditional Chinese Medicine, Zhejiang Provincial Key Laboratory, and Key Laboratory for the Diagnosis and treatment of Upper Limb Edema and Stasis of Breast Cancer.
Funding Statement
This research was supported by the National Natural Science Foundation of China (Grant No. 81973861), Zhejiang Provincial Ministry Medical and Health Co-construction Major Project (Grant No. 20214355173), Zhejiang Science and Technology Department “Vanguard” “Leading Goose” research (Grant No. 2023C03044), Zhejiang Provincial Health “Leading Talents” Project, and Zhejiang Medical and Health Science and Technology Project (Grant No. 2022KY558).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Drafted the manuscript and designed the figures: Jiancheng Mou.
Substantially contributed to analysis and manuscript preparation: Jiancheng Mou, Lichen Hong.
Helped perform the analysis and engaged in constructive discussions: Hongchao Tang.
Conceived and critically revised the manuscript and figures: Xuli Meng, Qinghui Zheng.
Wrote the paper: Jiancheng Mou, Lichen Hong.
References
- 1.Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- 2.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524–41. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 4.Doublier S, Belisario DC, Polimeni M, Annaratone L, Riganti C, Allia E, et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12:4. doi: 10.1186/1471-2407-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Zhu X, Zhang K, Yin Y, Chen Y, Zhang T. Interleukin-6 contributes to chemoresistance in MDA-MB-231 cells via targeting HIF-1alpha. J Biochem Mol Toxicol. 2018;32:e22039. doi: 10.1002/jbt.22039. [DOI] [PubMed] [Google Scholar]
- 6.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–6. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Lu Z, Qi C, Yu C, Li Y, Huan W, et al. N(6)-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol Cancer. 2022;21:111. doi: 10.1186/s12943-022-01549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watari K, Shibata T, Fujita H, Shinoda A, Murakami Y, Abe H, et al. NDRG1 activates VEGF-A-induced angiogenesis through PLCgamma1/ERK signaling in mouse vascular endothelial cells. Commun Biol. 2020;3:107. doi: 10.1038/s42003-020-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu M, Chen L, Qi Y, Ci H, Mou S, Yang J, et al. Human umbilical cord mesenchymal stem cell promotes angiogenesis via integrin beta1/ERK1/2/HIF-1alpha/VEGF-A signaling pathway for off-the-shelf breast tissue engineering. Stem Cell Res Ther. 2022;13:99. doi: 10.1186/s13287-022-02770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marisi G, Azzali I, Passardi A, Rebuzzi F, Bartolini G, Urbini M, et al. Prospective validation of VEGF and eNOS polymorphisms as predictors of first-line bevacizumab efficacy in patients with metastatic colorectal cancer. Sci Rep. 2023;13:12921. doi: 10.1038/s41598-023-40220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin H, Zhong C, Nudleman E, Ferrara N. Evidence for pro-angiogenic functions of VEGF-Ax. Cell. 2016;167:275–84.e6. doi: 10.1016/j.cell.2016.08.054. [DOI] [PubMed] [Google Scholar]
- 14.Hosaka K, Yang Y, Seki T, Du Q, Jing X, He X, et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat Commun. 2020;11:3704. doi: 10.1038/s41467-020-17525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen R, Jiang Q, Li P, Wang D, Yu C, Meng T, et al. “Targeted plus controlled” - Composite nano delivery system opens the tumor vascular and microenvironment normalization window for anti-tumor therapy. Int J Pharm. 2023;647:123512. doi: 10.1016/j.ijpharm.2023.123512. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Wu D, Qin X, Mi LZ. PDGF-D prodomain differentially inhibits the biological activities of PDGF-D and PDGF-B. J Mol Biol. 2022;434:167709. doi: 10.1016/j.jmb.2022.167709. [DOI] [PubMed] [Google Scholar]
- 17.Wang JC, Li GY, Wang B, Han SX, Sun X, Jiang YN, et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J Exp Clin Cancer Res. 2019;38:235. doi: 10.1186/s13046-019-1211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Shi YH, Xu QC, Zhu YQ, Liu ZD, Zhao GY, Liu Q, et al. Imatinib facilitates gemcitabine sensitivity by targeting epigenetically activated PDGFC signaling in pancreatic cancer. Mol Ther. 2023;31:503–16. doi: 10.1016/j.ymthe.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camorani S, Hill BS, Collina F, Gargiulo S, Napolitano M, Cantile M, et al. Targeted imaging and inhibition of triple-negative breast cancer metastases by a PDGFRbeta aptamer. Theranostics. 2018;8:5178–99. doi: 10.7150/thno.27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajakumar T, Pugalendhi P. Allyl isothiocyanate inhibits invasion and angiogenesis in breast cancer via EGFR-mediated JAK-1/STAT-3 signaling pathway. Amino Acids. 2023;55:981–92. doi: 10.1007/s00726-023-03285-2. [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Li W, Deng Q, You S, Liu H, Peng S, et al. Neoalbaconol inhibits angiogenesis and tumor growth by suppressing EGFR-mediated VEGF production. Mol Carcinog. 2017;56:1414–26. doi: 10.1002/mc.22602. [DOI] [PubMed] [Google Scholar]
- 23.Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22:101–26. doi: 10.1038/s41573-022-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cejalvo JM, Martinez de Duenas E, Galvan P, García-Recio S, Burgués Gasión O, Paré L, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res. 2017;77:2213–21. doi: 10.1158/0008-5472.CAN-16-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parr C, Ali AY. Boswellia frereana suppresses HGF-mediated breast cancer cell invasion and migration through inhibition of c-Met signalling. J Transl Med. 2018;16:281. doi: 10.1186/s12967-018-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muoio MG, Talia M, Lappano R, Sims AH, Vella V, Cirillo F, et al. Activation of the S100A7/RAGE pathway by IGF-1 contributes to angiogenesis in breast cancer. Cancers (Basel) 2021;13:621. doi: 10.3390/cancers13040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ZX, Chen JX, Zheng ZJ, Cai WJ, Yang XB, Huang YY, et al. TGF-beta1 promotes human breast cancer angiogenesis and malignant behavior by regulating endothelial-mesenchymal transition. Front Oncol. 2022;12:1051148. doi: 10.3389/fonc.2022.1051148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schanza LM, Seles M, Stotz M, Fosselteder J, Hutterer GC, Pichler M, et al. MicroRNAs associated with Von Hippel-Lindau pathway in renal cell carcinoma: a comprehensive review. Int J Mol Sci. 2017;18:2495. doi: 10.3390/ijms18112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo Y, Qu C, Tian Y, Wen Y, Xia S, Ma M. The HIF-1/SNHG1/miR-199a-3p/TFAM axis explains tumor angiogenesis and metastasis under hypoxic conditions in breast cancer. Biofactors. 2021;47:444–60. doi: 10.1002/biof.1702. [DOI] [PubMed] [Google Scholar]
- 30.Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang B, et al. HIF2-induced long noncoding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer Res. 2020;80:964–75. doi: 10.1158/0008-5472.CAN-19-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun B, Zhang D, Zhao N, Zhao X. Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget. 2017;8:30502–10. doi: 10.18632/oncotarget.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L, Xiong W, Zhang L, Wang D, Wang Y, Wu Y, et al. circSETD3 regulates MAPRE1 through miR-615-5p and miR-1538 sponges to promote migration and invasion in nasopharyngeal carcinoma. Oncogene. 2021;40:307–21. doi: 10.1038/s41388-020-01531-5. [DOI] [PubMed] [Google Scholar]
- 33.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 34.Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ. Hypoxia-induced snail expression through transcriptional regulation by HIF-1alpha in pancreatic cancer cells. Dig Dis Sci. 2013;58:3503–15. doi: 10.1007/s10620-013-2841-4. [DOI] [PubMed] [Google Scholar]
- 35.Nakuluri K, Mukhi D, Nishad R, Saleem MA, Mungamuri SK, Menon RK, et al. Hypoxia induces ZEB2 in podocytes: implications in the pathogenesis of proteinuria. J Cell Physiol. 2019;234:6503–18. doi: 10.1002/jcp.27387. [DOI] [PubMed] [Google Scholar]
- 36.Tan R, Wang L, Song J, Li J, He T. Expression and significance of Twist, estrogen receptor, and E-cadherin in human breast cancer cells and tissues. J Cancer Res Ther. 2017;13:707–14. doi: 10.4103/jcrt.JCRT_1396_16. [DOI] [PubMed] [Google Scholar]
- 37.Pan J, Fang S, Tian H, Zhou C, Zhao X, Tian H, et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/beta-catenin signaling. Mol Cancer. 2020;19:9. doi: 10.1186/s12943-020-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Wang Y, Wu X, Kong X, Li J, Dong C. RNF20 is critical for snail-mediated E-Cadherin repression in human breast cancer. Front Oncol. 2020;10:613470. doi: 10.3389/fonc.2020.613470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q, et al. ZEB2 promotes vasculogenic mimicry by TGF-beta1 induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Exp Mol Pathol. 2015;98:352–9. doi: 10.1016/j.yexmp.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Li S, Tang H, Meng X, Zheng Q. Molecular mechanisms of immunotherapy resistance in triple-negative breast cancer. Front Immunol. 2023;14:1153990. doi: 10.3389/fimmu.2023.1153990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, et al. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One. 2014;9:e98882. doi: 10.1371/journal.pone.0098882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Zhu Q, Hu L, Chen H, Wu Z, Li D. Anterior gradient 2 is a binding stabilizer of hypoxia inducible factor-1alpha that enhances CoCl2-induced doxorubicin resistance in breast cancer cells. Cancer Sci. 2015;106:1041–9. doi: 10.1111/cas.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Xu D, Chen P, Xie J. Notch1 signaling modulates hypoxia-induced multidrug resistance in human laryngeal cancer cells. Mol Biol Rep. 2022;49:6235–40. doi: 10.1007/s11033-022-07421-1. [DOI] [PubMed] [Google Scholar]
- 44.Pinzon-Daza ML, Cuellar-Saenz Y, Nualart F, Ondo-Mendez A, Del Riesgo L, Castillo-Rivera F, et al. Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions. J Cell Biochem. 2017;118:1868–78. doi: 10.1002/jcb.25890. [DOI] [PubMed] [Google Scholar]
- 45.Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int J Mol Sci. 2021;22:5703. doi: 10.3390/ijms22115703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–9. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 47.Duan H, Liu Y, Gao Z, Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. 2021;11:55–70. doi: 10.1016/j.apsb.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks DL, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer. 2016;15:26. doi: 10.1186/s12943-016-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu H, Chen I, Shimoda LA, Park Y, Zhang C, Tran L, et al. Chemotherapy-induced Ca(2+) release stimulates breast cancer stem cell enrichment. Cell Rep. 2021;34:108605. doi: 10.1016/j.celrep.2020.108605. [DOI] [PubMed] [Google Scholar]
- 50.Ba X, Huang Y, Shen P, Huang Y, Wang H, Han L, et al. WTD attenuating rheumatoid arthritis via suppressing angiogenesis and modulating the PI3K/AKT/mTOR/HIF-1alpha pathway. Front Pharmacol. 2021;12:696802. doi: 10.3389/fphar.2021.696802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z, Zhu C, Chen C, Zong Y, Feng H, Liu D, et al. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1alpha/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9:974. doi: 10.1038/s41419-018-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang W, Chen L, Guo X, Cheng C, Luo Y, Wang J, et al. Combating multidrug resistance and metastasis of breast cancer by endoplasmic reticulum stress and cell-nucleus penetration enhanced immunochemotherapy. Theranostics. 2022;12:2987–3006. doi: 10.7150/thno.71693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8:790–7. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- 54.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai R, Li Y, Jian L, Yang Y, Zhao L, Wei M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: mechanisms and clinical treatment strategies. Mol Cancer. 2022;21:177. doi: 10.1186/s12943-022-01645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao X, Qin T, Mao J, Zhang J, Fan S, Lu Y, et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38:256. doi: 10.1186/s13046-019-1260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu L, Loo WT, Louis WC. PTEN and VEGF: possible predictors for sentinel lymph node micro-metastasis in breast cancer. Biomed Pharmacother. 2007;61:558–61. doi: 10.1016/j.biopha.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunter S, Nault B, Ugwuagbo KC, Maiti S, Majumder M. Mir526b and Mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakunrangsit N, Ketchart W. Plumbagin inhibits cancer stem-like cells, angiogenesis and suppresses cell proliferation and invasion by targeting Wnt/beta-catenin pathway in endocrine resistant breast cancer. Pharmacol Res. 2019;150:104517. doi: 10.1016/j.phrs.2019.104517. [DOI] [PubMed] [Google Scholar]
- 63.Vranic S, Cyprian FS, Gatalica Z, Palazzo J. PD-L1 status in breast cancer: current view and perspectives. Semin Cancer Biol. 2021;72:146–54. doi: 10.1016/j.semcancer.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res. 2020;26:1712–24. doi: 10.1158/1078-0432.CCR-19-2179. [DOI] [PubMed] [Google Scholar]
- 65.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–22. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 67.Vetsika EK, Koukos A, Kotsakis A. Myeloid-derived suppressor cells: major figures that shape the immunosuppressive and angiogenic network in cancer. Cells. 2019;8:1647. doi: 10.3390/cells8121647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang R, Dong M, Tu J, Li F, Deng Q, Xu J, et al. PMN-MDSCs modulated by CCL20 from cancer cells promoted breast cancer cell stemness through CXCL2-CXCR2 pathway. Signal Transduct Target Ther. 2023;8:97. doi: 10.1038/s41392-023-01337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun R, Luo H, Su J, Di S, Zhou M, Shi B, et al. Olaparib suppresses MDSC recruitment via SDF1alpha/CXCR4 axis to improve the anti-tumor efficacy of CAR-T cells on breast cancer in mice. Mol Ther. 2021;29:60–74. doi: 10.1016/j.ymthe.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–8. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–15. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44:1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Njock MS, O’Grady T, Nivelles O, Lion M, Jacques S, Cambier M, et al. Endothelial extracellular vesicles promote tumour growth by tumour-associated macrophage reprogramming. J Extracell Vesicles. 2022;11:e12228. doi: 10.1002/jev2.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen W, Shen L, Jiang J, Zhang L, Zhang Z, Pan J, et al. Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment. Biomark Res. 2021;9:59. doi: 10.1186/s40364-021-00312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee M, Devi Rajeswari V. Inhibition of WNT signaling by conjugated microRNA nano-carriers: a new therapeutic approach for treating triple-negative breast cancer a perspective review. Crit Rev Oncol Hematol. 2023;182:103901. doi: 10.1016/j.critrevonc.2022.103901. [DOI] [PubMed] [Google Scholar]
- 76.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105:1334–42. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Chen Y, Lin L, Li H. Gambogic acid as a candidate for cancer therapy: a review. Int J Nanomedicine. 2020;15:10385–99. doi: 10.2147/IJN.S277645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S, Wang L, Chen M, Wang Y. Gambogic acid sensitizes resistant breast cancer cells to doxorubicin through inhibiting P-glycoprotein and suppressing survivin expression. Chem Biol Interact. 2015;235:76–84. doi: 10.1016/j.cbi.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Liang P, Ballou B, Lv X, Si W, Bruchez MP, Huang W, et al. Monotherapy and combination therapy using anti-angiogenic nanoagents to fight cancer. Adv Mater. 2021;33:e2005155. doi: 10.1002/adma.202005155. [DOI] [PubMed] [Google Scholar]
- 80.Tian F, Dahmani FZ, Qiao J, Ni J, Xiong H, Liu T, et al. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018;75:398–412. doi: 10.1016/j.actbio.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, You X, Lin Q, Xiong W, Guo Y, Huang Z, et al. Exploring the pharmacological mechanisms of Xihuang Pills against prostate cancer via integrating network pharmacology and experimental validation in vitro and in vivo. Front Pharmacol. 2021;12:791269. doi: 10.3389/fphar.2021.791269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia W, Chen W, Ni C, Meng X, Wu J, Yang Q, et al. Chemotherapy-induced exosomal circBACH1 promotes breast cancer resistance and stemness via miR-217/G3BP2 signaling pathway. Breast Cancer Res. 2023;25:85. doi: 10.1186/s13058-023-01672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerbel RS. Reappraising antiangiogenic therapy for breast cancer. Breast. 2011;20(Suppl 3):S56–60. doi: 10.1016/S0960-9776(11)70295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakelee HA, Lee JW, Hanna NH, Traynor AM, Carbone DP, Schiller JH. A double-blind randomized discontinuation phase-II study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: eastern cooperative oncology group study E2501. J Thorac Oncol. 2012;7:1574–82. doi: 10.1097/JTO.0b013e31826149ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conley SJ, Baker TL, Burnett JP, Theisen RL, Lazarus D, Peters CG, et al. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res Treat. 2015;150:559–67. doi: 10.1007/s10549-015-3349-8. [DOI] [PubMed] [Google Scholar]
- 86.Bedard PL, de Azambuja E, Cardoso F. Beyond trastuzumab: overcoming resistance to targeted HER-2 therapy in breast cancer. Curr Cancer Drug Targets. 2009;9:148–62. doi: 10.2174/156800909787581024. [DOI] [PubMed] [Google Scholar]
- 87.Nunes T, Pons T, Hou X, Van Do K, Caron B, Rigal M, et al. Pulsed-laser irradiation of multifunctional gold nanoshells to overcome trastuzumab resistance in HER2-overexpressing breast cancer. J Exp Clin Cancer Res. 2019;38:306. doi: 10.1186/s13046-019-1305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang HL, Schwettmann B, McArthur HL, Chan IS. Antibody-drug conjugates in breast cancer: overcoming resistance and boosting immune response. J Clin Invest. 2023;133:e172156. doi: 10.1172/JCI172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12:3528. doi: 10.1038/s41467-021-23793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabaud O, Berger L, Crompot E, Adélaide J, Finetti P, Garnier S, et al. Overcoming resistance to Anti-Nectin-4 antibody-drug conjugate. Mol Cancer Ther. 2022;21:1227–35. doi: 10.1158/1535-7163.MCT-22-0013. [DOI] [PubMed] [Google Scholar]
- 91.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]