Abstract
Gastric cancer (GC) is a major cause of cancer-related mortality worldwide. GC is determined by multiple (epi)genetic and environmental factors; can occur at distinct anatomic positions of the stomach; and displays high heterogeneity, with different cellular origins and diverse histological and molecular features. This heterogeneity has hindered efforts to fully understand the pathology of GC and develop efficient therapeutics. In the past decade, great progress has been made in the study of GC, particularly in molecular subtyping, investigation of the immune microenvironment, and defining the evolutionary path and dynamics. Preclinical mouse models, particularly immunocompetent models that mimic the cellular and molecular features of human GC, in combination with organoid culture and clinical studies, have provided powerful tools for elucidating the molecular and cellular mechanisms underlying GC pathology and immune evasion, and the development of novel therapeutic strategies. Herein, we first briefly introduce current progress and challenges in GC study and subsequently summarize immunocompetent GC mouse models, emphasizing the potential application of genetically engineered mouse models in antitumor immunity and immunotherapy studies.
Keywords: Gastric cancer, heterogeneity, mouse model, GEMM, immunocompetent
Introduction
Gastric cancer (GC) is a major cause of cancer-related mortality worldwide1,2. To date, surgery and radio-chemotherapy remain the major clinical treatments for GC. However, these treatments are frequently challenged by patients presenting in advanced or metastatic disease stages, because of a lack of early diagnosis markers3. Recently, immunotherapies such as anti-PD1/PD-L14,5 and targeted therapy (anti-Her2/Claudin18.2)6,7 have entered clinical trials and have been used as tumor treatments. However, many or even most patients with GC do not respond well to these treatments, thus emphasizing the highly heterogeneous nature of GC and the urgent need for in-depth understanding of GC pathology. Heterogeneity remains a major barrier to GC management8.
The first aspect of GC heterogeneity is the ever-refined subtyping. Traditional Lauren classification of GC includes intestinal-type, diffuse-type, and mixed-type GC9. Recent studies have comprehensively characterized the molecular features of gastric adenocarcinoma. In a milestone in this regard, The Cancer Genome Atlas project, at the molecular level, has revealed 295 primary GCs and defined 4 GC subtypes including Epstein-Barr virus-positive, microsatellite instability, chromosomal instability and genomic stability10. Single cell RNA sequencing (scRNA-seq) has been applied to decipher the cellular heterogeneity of the tumor microenvironment in primary and metastatic lesions of patients with GC11–23. ScRNA-seq primary and peritoneal carcinomatosis cells from patients with GC has demonstrated that the diversity in tumor cell lineage/state compositions is a key contributor to intratumoral heterogeneity11,12. These studies have identified a group of genes associated with differentiation and prognosis, and showing high diversity within and between tumors. Some subgroups show different degrees of differentiation, consistently with the histopathological features of the Lauren subtype. Two newly identified subgroups show unique transcriptional profiles: one expressing master cell markers and Wnt/β-catenin pathway signature genes, and the other expressing immune-related signature genes associated with Epstein-Barr virus infection. Despite progress in understanding of the molecular and cellular mechanisms of GC pathology, the underlying clonal evolution dynamics and cellular malignant transformation of human GC remain to be fully elucidated.
The second aspect of GC heterogeneity includes cell origin and genetic mutations. The homeostasis of the gastric epithelia, which is directly exposed to food intake and gastric acid, can be easily disrupted. As a countermeasure, gastric epithelial cells are continually renewed to maintain gastric gland structure and function. The gastric gland is composed primarily of pit cells, neck cells, parietal cells, isthmus stem cells, reserve stem cells, chief cells, and a small number of endocrine cells and tuft cells24. Gastric adenocarcinomas can originate from both stem cells and terminal differentiated cells located at the cardia, corpus, and antrum of the stomach. Furthermore, genetic mutations such as inactivation of tumor suppressors, including RNF4325, TP5326,27, and ROHA10,28, as well as activation of oncogenic Kras29 and YAP30, synergistically initiate and drive the tumor evolution of GC. Among them, the Hippo signaling pathway has been extensively investigated as a major driving force of both gastric tumorigenesis and acquired drug resistance31,32. Targeting recovery of Hippo activity, such as with SHAP33 and SAIP-1/234 peptide agonists or chemical agonists35, is a promising strategy to curb GC. However, the specific cellular origins and genetic drivers of human GC remain to be clarified, thus hindering the development of precision medicine and targeted therapies.
The third aspect of GC heterogeneity involves the dynamic remodeling of the immune microenvironment associated with disease progression and treatment. A comprehensive single-cell atlas characterizing the microenvironment across various stages of GC progression, from precancerous lesions to metastatic tumors, has identified 6 ecotypes associated with the phenotypic progression and outcomes of GC14. In particular, IgA+ plasma cells accumulate in precancerous lesions, whereas immunosuppressive myeloid and SDC2+ cancer-associated fibroblasts dominate late-stage GC14. In addition, scRNA-seq of GC biopsy samples has revealed elevated plasma cell proportions in diffuse-type GC, in agreement with the upregulation of KLF2 expression in epithelial cells mediating plasma cell recruitment15. Using paired pre- and on-treatment samples during standard frontline chemotherapy, Kim et al.16 have identified chemotherapy-induced NK cell infiltration, macrophage repolarization, and increased antigen presentation among responders. In contrast, the non-responders showed increased LAG3 expression and decreased dendritic cell abundance, thus suggesting remodeling of the tumor microenvironment during chemotherapy response and resistance. However, how the immune microenvironment regulates GC remains largely unknown. For example, how do the unique regional immune properties of the stomach regulate human GC initiation? How does the heterogeneity of the immune microenvironment determine the sensitivity or response to immuno-therapy?
A new dimension of GC heterogeneity, nerve-cancer crosstalk, is increasingly being implicated in gastric tumorigenesis. Although the stomach is innervated predominantly by the autonomic, non-autonomic, and enteric nervous systems, to maintain epithelial homeostasis and hormone secretion36, elevated infiltration of other neuronal fibers derived from vagal nerves, sympathetic nerves, and choline-acetyltransferase positive stromal neurons is frequently observed in GC tissues, and neural density positively correlates with GC progression and poor survival prognosis37–39. Indeed, sole surgical vagotomy or myenteric denervation has been found to efficiently decrease the incidence of gastric tumors, enhance chemotherapy effects, and prolong overall survival in both mouse and rat models, as well as in human patients with GC37,40, thus adding a new layer of complexity underlying GC carcinogenosis36–38,41. Exploring how nerves, or even emotions, regulate the initiation, progression, and response to targeted and/or immunotherapy of human GC should prove interesting.
Beyond intrinsic factors, environmental factors, such as microorganisms, have been well established to participate in GC initiation and development2. For example, gastric tissue injury and chronic inflammation triggered by Helicobacter pylori infection initiate sequential histopathologic progression of gastritis to gastric atrophy, intestinal metaplasia, dysplasia, and finally gastric adenocarcinoma42. Recently, several oral pathogens, including Streptococcus anginosus43, Candida albicans44, and intracellular bacteria45, have been identified to colonize the stomach and promote gastric tumorigenesis. Nevertheless, how these novel microbe-host interactions and genetic mutations synergistically drive GC tumor evolution awaits further investigation. In addition, how microbiota determine the sensitivity or response to targeted treatment and immunotherapy for GC remains poorly understood.
To address GC heterogeneity and uncover its pathological nature, multiple model systems can be applied, including cell lines, organoids, animal models, and clinical samples. In the past decade, organoids have been developed as powerful tools for both mechanistic study of tumorigenesis and drug screening. Comparisons between patient-derived organoids and single cells from primary tumors have highlighted inter- and intralineage similarities and differences15, thus suggesting that heterogeneity may decrease or disappear during the continuous passage of organoids. Moreover, current organoid models do not adequately mimic the complex microenvironment in vivo, where immune cells interact with tumor cells. Furthermore, tumorigenesis is increasingly understood to be regulated by crosstalk between the stomach and other organs, e.g., the liver and brain. Therefore, various GC animal models must be developed to meet research needs, including mechanistic study and target intervention, genealogical tracing of different GC subtypes, and assessment of chemotherapy and immunotherapy.
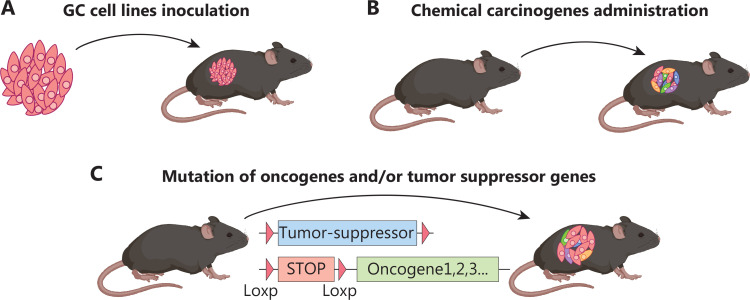
Multiple immunocompetent GC mouse models have been developed, including GC cell line transplantation, chemical carcinogen administration, and genetic engineered models (Figure 1). These models enable study of the cellular origins, clonal evolution, relapse, host-microbe interaction, tumor immunity, and neuronal/emotional control of tumor immunity during various stages of GC, including initiation, progression, invasion, and metastasis, with or without therapeutic treatment. Herein, we review the roles of well-established and emerging GC mouse models, particularly genetically engineered mouse models (GEMMs), in deciphering the heterogeneity in human GC; we further compare their pathological features, applications, and limitations.
Figure 1.
Immunocompetent mouse models of GC. (A) Allograft GC models generated by subcutaneous and orthotopic transplantation of mouse gastric cell lines, such as MFC and YTN16, show favorable replicability and stability, but also induce an unnaturally hyperinflammatory response. (B) Chemical carcinogen-induced GC mouse models, such as those using MNU and MNNG, exhibit high mutational burden and immunogenicity. (C) Genetically engineered mouse models of GC generated through genetic manipulation within stomach specific cells have uncovered genotype-phenotype relationships during GC initiation and progression.
Non-genetically engineered GC mouse models
Cell line-derived GC graft model
Cell lines derived from patients with GC and mice provide powerful tools to explore the nature of tumor progression, and responsiveness to targeted therapy and immunotherapy. In addition to the multiple human GC cell lines available for xenograft study in immunocompromised mice, several mouse GC cell lines (e.g., MFC, MGCC3I, NCC-S1/3, YTN16, and M12), which can be transplanted into immunocompetent mice, have been generated to investigate the molecular and cellular mechanisms governing gastric tumorigenesis and related immune response.
The widely used mouse GC cell line MFC was generated from forestomach carcinoma developed in N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) treated mice on a 615 inbred background. MFC cells form tumors after orthotopic or subcutaneous implantation in mice, and are prone to spontaneous metastasis to the lungs46. MFC cells have been applied in investigating the molecular mechanisms through which tumor cells promote immune evasion47,48 and impede responses to anti-PD-1 immunotherapy48–50. For example, through SLC6A6-mediated competitive uptake of taurine, gastric cancer cells induce CD8+ T cell exhaustion by increasing ER stress and ATF4 mediated upregulation of immune checkpoint genes, thus resulting in immune evasion and tumor progression47. MGCC3I, another forestomach carcinoma-derived mouse GC cell line, forms poorly differentiated gastric carcinoma after orthotopic transplantation into the stomach serosa, and liver metastasis after intrasplenic injection into the syngeneic ICR mice51. The NCC-S1 and NCC-S3 cell lines were derived from primary GC developed in Smad4fl/fl; Trp53fl/fl; Cdh1fl/+; Villincre and Trp53fl/fl; Cdh1fl/fl; Pdx1cre mice, respectively52. Metastatic NCC-S1M and NCC-S3M subclones were then isolated from lung metastatic foci. Activation of the Wnt/β-catenin signaling pathway is required for the metastatic phenotype52.
Notably, 2 mouse GC cell lines (YTN16 and M12) transplantable into mice with a C57BL/6 background have been developed53,54. YTN16 cells were subcloned and established from N-methyl-N-nitrosourea (MNU) treated p53 heterozygous knockout mice. YTN16 cells form orthotopic tumors and metastasis foci in lymph nodes, the peritoneum, and lungs53. YTN16 cells have been used to develop novel mouse models of lymphatic and peritoneal metastasis55–57, gastric tumor immunogenicity58, neoantigen identification59, and response to immune checkpoint inhibitors in GC60–62. M12 cells have also been derived from gastric carcinoma in p53 knockout mice on a C57BL/6 background treated with a zinc-deficient diet and MNU administered in the drinking water. M12 cells show tumorigenic and metastatic properties in C57BL/6 syngeneic mice54. Serine/threonine-protein kinase 24 (STK24) is essential for immune regulation during the tumor progression of M12 cells in vivo. Knockdown of STK24 promotes myeloid derived suppressor cell (MDSC) expansion and tumor growth in C57BL/6 mice54.
Chemical carcinogen-induced GC mouse models
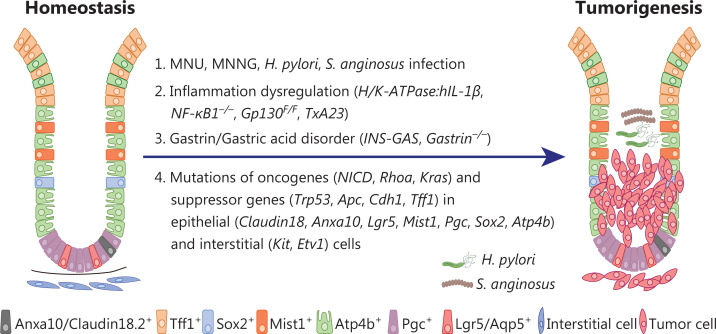
MNU, one of the best-characterized chemical carcinogens, can be supplied in the drinking water to induce GC in mice63. By introducing alkyl radicals into DNA, MNU causes DNA mutation and dysfunction, thereby promoting gastric tumorigenesis64. MNU-induced primary GCs are usually localized in the antrum, and involve well to poorly differentiated adenocarcinoma63,65. The tumorigenic efficacy of MNU varies in mice with different genetic backgrounds; male mice on a BALB/c background are relatively susceptible to MNU-induced tumors65. In addition, MNU-induced GC is significantly enhanced in combination with other GC risk factors, such as a high-salt diet66, H. pylori infection67, and Streptococcus anginosus infection43. Notably, genetic alterations also significantly influence MNU-dependent tumorigenesis. For example, p53 knockout mice are relatively sensitive to MNU-induced carcinogenesis68,69.
MNNG is another chemical carcinogen particularly widely used in combination with Helicobacter infection to induce GC in mice70. MNNG is supplied in the drinking water in 3 cycles at 2 week intervals to induce GC in mice. MNNG-induced primary GC varies across model organisms, including squamous cell carcinoma in the forestomach in mice46,70 and adenocarcinomas in the glandular stomach in Mongolian gerbils71. Similarly, environmental GC risk factors, including a high-salt diet72, calcium-deficient diet71, or catechol73, promote the incidence and progression of GC induced by MNNG administration. Moreover, this model has been extensively used to investigate gastric tumorigenesis and targeted therapy against GC32,33,74–76.
Genetically engineered GC mouse models
Inflammation-induced GC
IL-1β transgenic mice
Gastrointestinal cancers are frequently associated with chronic inflammation. For example, chronic inflammation triggered by H. pylori infection or tissue injury in the stomach can initiate sequential histopathologic progression of gastritis to gastric atrophy, intestinal metaplasia, dysplasia, and finally gastric adenocarcinoma. Interleukin-1 polymorphisms have been associated with increased risks of both hypochlorhydria induced by H. pylori infection and gastric carcinogenesis77. H/K-ATPase:hIL-1β transgenic mice expressing secretory human IL-1β specifically in parietal cells have been generated to explore the pathogenic role of hIL-1β during gastric tumorigenesis. These mice spontaneously develop chronic gastritis, hyperplasia, and high-grade dysplasia/adenocarcinoma without invasion into the submucosa or metastasis to distant organs78. In a setting of H. felis Infection, IL-1β has been found to accelerate the development of gastric inflammation and carcinoma, thereby indicating a causative effect of IL-1β in inflammation-associated GC79. This model has been used to explore mechanisms of tumor resistance to immune checkpoint blockade of GC. Overexpression of IL-1β in the stomach results in recruitment of MDSCs through the IL-1RI/NF-κB signaling pathway. MDSCs exert an immunosuppressive function through upregulation of PDL1, and anti-PD-1 treatment does not block GC progression in these IL1β transgenic mice78.
NF-κB1-deficient mice
Deficiency in NF-κB1, even loss of a single allele, can lead to spontaneous intestinal-type gastric adenocarcinoma in mice. Interestingly, such gastric adenocarcinoma is not accelerated by H. pylori infection and a high salt diet in these NF-κB1-deficient mice80, thus indicating a GC pathology independent of commensal microorganisms. This model has been used to study inflammation associated malignancy in GC. Deficiency in NF-κB1 results in increased expression of a variety of inflammatory cytokines, including tumor necrosis factor (TNF), interleukin-6 (IL-6), IL-22, and IL-11, thereby driving aberrant activation of signal transducer and activator of transcription 1 (STAT1). Further genetic depletion of TNF or STAT1 in NF-κB1-deficient mice has been found to prevent invasive GC development81. In agreement with these observations, genetic analysis has identified a significant association between the Nfκb1 locus and gastric tumor susceptibility in a collaborative cross-mouse population82.
Gp130F/F transgenic mice
Excessive secretion of IL-6 cytokine family members, including IL-6, IL-11, IL-27, IL-31, oncostatin M, leukemia inhibitory factor, ciliary neurotrophic factor, ardiotrophin-like cytokine factor 1, and cardiotrophin 1, promote GC through persistent activation of the JAK-STAT1/3 and/or SHP2-Ras-ERK signaling pathways through the IL-6 cytokine family of receptors83. To investigate the role of dysregulated activation of STAT3 in regulating gastrointestinal epithelial cell homeostasis, Tebbutt et al.84 have generated gp130F/F mice by using a phenylalanine knock-in substitution of the IL-6 receptor β-chain Gp130 at the cytoplasmic tyrosine 757 residue, thus preventing its binding to the suppressor of cytokine signaling 3 and enhancing activation of STAT3. Gp130F/F mice spontaneously develop gastric adenoma at the antrum by 4–6 weeks of age, accompanied by splenomegaly and extra-gastric pathologies in the liver and lung84,85. Further knockout of STAT3 in Gp130F/F mice alleviates gastric adenoma progression, thereby highlighting an essential role of STAT3 hyperactivation in GC pathology85.
Among IL-6 cytokine family members, IL-11 is a major cytokine promoting gastrointestinal tumorigenesis. Pharmacological inhibition of IL-11 signaling through mIL-11 Mutein administration or genetic depletion of IL-11 ligand–binding receptor subunit in Gp130F/F mice has been found to inhibit GC development86,87. Beyond the IL-6 cytokine family, STAT3 activation directly increases Toll-like receptor (TLR) 2 expression, thereby promoting gastric tumor cell survival and proliferation. Accordingly, genetic or therapeutic targeting of TLR2 has been found to alleviate gastric tumorigenesis88. Moreover, IL-6/IL-11-gp130-dependent mTORC1 activation has been implicated in promoting inflammation-associated gastrointestinal tumorigenesis, which is druggable through treatment with the mTORC1-specific inhibitor RAD00189. Finally, Gp130F/F transgenic mice have also been used to study the interaction of tumor cells with immune cells involved in GC progression. For example, mast cells have been found to be activated by GC cell-derived alarmin IL-33 and to recruit macrophages via secreting attracting chemokines, such as CSF2, CCL3, and IL-6; moreover, deletion of macrophages has been found to suppress gastric tumorigenesis90.
Transgenic mice with aberrant inflammation induced by T cells
Deregulated T cell activation mediates gastritis and promotes gastric hyperplasia and adenocarcinomas. For example, T cell-specific deletion of the tumor suppressor liver kinase B1 (LKB1) results in excessive production of proinflammatory cytokines and chemokines such as IL-6, IL-11 and CXCL2, which is accompanied by increased STAT3 activation and infiltration of inflammatory monocytes and neutrophils. The related inflammation promotes development of gastrointestinal polyposis, a cancer predisposition syndrome91. In addition, autoimmune gastritis mediated by self-reactive CD4+ T cells has been found to promote GC development. In a T cell receptor transgenic mouse model of autoimmune gastritis, the T cell receptor targets a peptide from the H+/K+ ATPase proton pump, which is highly expressed on parietal cells in the stomach. Transgenic mice display chronic gastritis with intensive CD4+ T cell infiltration, and elevated IFNγ and IL1-17 production, which is followed by initiation and progression of GC from oxyntic atrophy, mucinous hyperplasia to spasmolytic polypeptide-expressing metaplasia, and intraepithelial neoplasia92. Collectively, these mouse models illustrate a causal link between gastric inflammation and GC development.
Gastrin/gastric acid disorder-induced GC
INS-GAS mice
Gastrin, produced by antrum G cells, is crucial for gastric acid secretion and parietal cell differentiation. A transgenic mouse model termed INS-GAS expressing human gastrin specifically in β islet cells under control of the insulin promoter was originally generated to investigate the potential role of gastrin in regulating islet differentiation93,94. INS-GAS mice show a twofold elevation of serum amidated gastrin and gastrointestinal mucosal hyperplasia94. These mice have been further used to examine the role of hypergastrinemia in GC pathology and have shown elevated maximal gastric acid secretion and parietal cell number within 4 months old, but progressive sustained loss of parietal and hypochlorhydria95. Eventually, INS-GAS mice develop metaplasia, dysplasia, and invasive GC at 20 months of age (Figure 2).
Figure 2.
Mouse GC models induced by chemical carcinogen administration and/or genetic engineering in immunocompetent mice. Modeling of gastric tumorigenesis by genetic manipulation within distinct cell types has revealed the high heterogeneity in cell origin during human GC initiation. Infection with microorganisms such as H. pylori and S. anginosus accelerates GC tumorigenesis.
Notably, INS-GAS mice exhibit accelerated progression to gastric carcinoma in the presence of H. felis infection95. Moreover, this tumor progression is influenced by sex, genetic background, and commensal flora. Female INS-GAS mice are more resistant than male INS-GAS mice to H. felis infection-induced GC96–98. Ovary derived estradiol may contribute to the protective role of this sexual dimorphism, because ovariectomized female mice develop more severe gastritis and gastrointestinal neoplasia than intact female mice97. In addition, INS-GAS mice on an FVB/N background have been found to be susceptible to H. felis infection-induced GC, whereas those on a C57BL/6 background develop only metaplasia and dysplasia99. In addition, H. felis-infected INS-GAS mice raised in germ-free conditions develop mitigatory gastritis and delayed intraepithelial neoplasia, in contrast to those raised in specific-pathogen-free conditions100. Moreover, gastric colonization with restrict altered Schaedler’s flora in male germ-free INS-GAS mice is sufficient to promote gastric inflammation and dysplasia to a similar extent as diverse intestinal microbiota in the presence of H. pylori infection101. In the setting H. felis infection, INS-GAS mouse models have served as an important tool for validation of anti-inflammatory strategies for GC treatment. The combination of the nonsteroidal anti-inflammatory drug sulindac and antibiotic mediated H. pylori eradication has been found to alleviate the production of pro-inflammatory cytokines in the stomach, as well as the progression from H. pylori-associated severe dysplasia to GC102. In addition, the combination of the gastrin receptor antagonist YF476 and the histamine H2-receptor antagonist loxtidin has been found to completely suppress gastric acid secretion and progression to neoplasia103.
Gastrin−/− mice
Gastrin-deficient mice (gastrin−/−) have been generated to investigate the role of gastrin in regulating the development and function of the gastrointestinal tract104,105. Gastrin−/− mice show impaired gastric acid secretion, accompanied by marked abnormalities in gastric gland architecture, with diminished numbers of parietal and enterochromaffin-like cells, and enhanced numbers of mucous neck cells104,105. The loss of parietal cells in gastrin−/− mice has been attributed to bacterial overgrowth and chronic gastritis, and the parietal cell number has been found to normalize after antibiotic treatment106. The chronic inflammation resulting from gastric acid secretion disorder in gastrin−/− mice promotes intestinal metaplasia of the gastric epithelium, which eventually develops into polyps by the age of 12-month107–109 (Figure 2).
GCs induced by mutation of oncogenes and/or tumor suppressor genes
GCs induced by genetic mutations in gastric epithelial cells
Genetically engineered mice with genetic mutations in pan-epithelial cells
Claudin18.2 is a tight junction membrane protein specifically expressed in the gastric epithelium. Claudin18.2 knockout mice (CLDN18KM) exhibit preneoplastic lesions at 7 weeks and eventually develop high-grade intraepithelial neoplasia at 2 years of age110. However, CLDN18KM mice are resistant to H. pylori colonization and are not suitable to investigate pro-tumor of H. pylori infection. Of note, the yes-associated protein 1 (YAP1) signaling pathway has been found to be up-regulated and to contribute to the proliferation of metaplastic cells in CLDN18KM mice110. Given that Wnt/β-catenin signaling, receptor tyrosine kinase, and Trp53 pathways are commonly perturbed pathways in GC, Fatehullah et al.111 have developed a claudin18.2-IRES-CreERT2 allele to selectively drive conditional knock in of KrasG12D and deletion of Apc and Trp53 (Cldn18-ATK) in the gastric epithelium. Cldn18-ATK mice develop high grade CIN GC, which metastasizes to the liver, lymph nodes, and diaphragm111. This model has been used to evaluate the roles of Lgr5+ stem cells in GC initiation and distant metastasis111.
Expression of Anxa10, a member of the annexin family of calcium-dependent phospholipid-binding proteins, is restricted to the gastric epithelium. Mutations in oncogenes and/or tumor suppressor genes specifically in Anxa10+ cells result in mouse GCs with various subtypes possibly mimicking human GC. Knock in of KrasG12D and Trp53R172H, and deletion of Smad4 in Anxa10+ cells leads to intestinal-type GC, which is prone to metastasis to the liver and the lungs. Knock in of KrasG12D and deletion of Cdh1 and Smad4 in Anxa10+ cells result in poorly differentiated signet ring cell carcinoma, and metastasis to the lung and peritoneum; moreover, knock in of KrasG12D and deletion of Cdh1 and Apc in Anxa10+ cells lead to serrated adenomatous GC112. Tumor organoids have been derived from these models to test responses to conventional chemotherapeutics and targeted therapeutics. Intestinal-type CIN organoids are relatively sensitive to docetaxel treatment but resistant to trametinib treatment targeting the EGF receptor (EGFR). In addition, overexpression of peroxisome proliferator-activated receptor delta (Ppard1/2) in Villin+ cells promotes gastric inflammation and tumorigenesis113.
Genetically engineered mice with genetic mutations in stem cells
With their capabilities of self-renewal, proliferation, and differentiation into various types of functional cells, stem cells play important roles in tissue homeostasis and injury repair24,114. Moreover, stem cells have been found to be the cellular origins of gastric tumorigenesis and metastasis24,114. Lgr5 marks homeostatic stem cells in multiple tissues including the gastrointestinal tract. In the human and mouse stomach, Lgr5 is expressed in a subpopulation of chief cells located at the base of the corpus gland. Lgr5-expressing chief cells drive epithelial renewal after injury and are the cells of origin of GC115. Knock in of KrasG12D or Trp53 deletion in Lgr5-expressing chief cells promotes metaplastic lesions in the corpus69,115. In Cldn18-ATK mice, Lgr5+ cells function as cancer stem cells in gastric tumorigenesis and distal metastasis111.
Recently, Aqp5 has been identified as a new pyloric-specific marker of Lgr5-expressing stem cells. Hyperactivation of the WNT/β-catenin, PI3K, and KRAS signaling pathways by deletion of Apc and Pten and knock in of KrasG12D in Aqp5+ stem cells cooperatively drives invasive gastric tumorigenesis116. In addition, pepsinogen C (PGC) is a predominant marker secreted by gastric chief cells, and successive activation of KrasG12D and depletion of Apc and Trp53 in Pgc+ chief cells have been found to result in progressive development of metaplasia, dysplasia, and invasive and metastatic gastric carcinoma117. Recently, knock in of KrasG12D alone in zymogen-secreting chief cells has been reported to lead to the development of precancerous metaplasia and high-grade dysplasia. Metabolic rewiring from glycolysis to fatty acid metabolism occurs during the progression from metaplasia to dysplasia118. Stearoyl-coenzyme A desaturase dependent production of monounsaturated fatty acids fuels dysplastic cells118.
Mist1 expression marks the stem cells located in the isthmus of the gastric corpus24. Knock in of KrasG12D and Apc deletion in Mist1-expressing stem cells give rise to intestinal-type metaplasia and cancer. In addition, depletion of Cdh1 in Mist1+ stem cells may cause diffuse-type GC. Importantly, Cxcl12+ endothelial cells recruit Cxcr4+ innate lymphoid cells (ILCs), which form a peri-vascular inflammatory niche supporting diffuse-type GC development from Mist1+ cells through Wnt5a produced by ILCs119. ILC-derived Wnt5a mediates RhoA activation and promotes tumor cell survival. Concordantly, RHOA gain of function through knock in RHOAY42C combined with Cdh1 deletion in Mist1+ cells induces metastatic diffuse-type GC120. Mechanistically, Cdh1 loss and RHOAY42C mutation induce cytoskeletal rearrangements and focal adhesion kinase activation, which in turn further promote the activation of YAP/TAZ, PI3K/AKT, and WNT/β-catenin signaling120.
The transcription factor Sox2 marks adult stem cells in multiple epithelial tissues, including the glandular stomach, anus, cervix, testes, and lens121. Sox2 is highly expressed in the basal progenitor cells of the stratified epithelium in the esophagus and forestomach121, and drives gastric specification and regionalization by maintaining chromatin accessibility of forestomach lineage-specific genes122. Overexpression of Sox2 in basal progenitor cells results in the development of invasive squamous cancer in the forestomach and is involved in inflammation-mediated Stat3 activation123. However, deletion of Apc in Sox2+ cells leads to gastric adenoma formation in the corpus, and loss of Sox2 enhances gastric tumorigenesis, thus suggesting that Sox2 may also act as a tumor suppressor by restraining Wnt/β-catenin signaling and intestinal genes124. Moreover, activation of KRAS in Sox2+ cells also leads to precancerous lesions in gastric tissues, accompanied by accumulation of Sox9+ cells in the stomach. A combined Cre-loxp and Flipase-Frt system to specifically activate Kras and deplete Trp53 in Sox2+Sox9+ cells has been found to result in the development of aggressive GC, in which SOX9 promotes the transformation of SOX2+ stem cells through biased symmetric cell division125. In addition, SOX9 in epithelial tumor cells promotes M2 macrophage polarization and CD8+ T cell functional inhibition through paracrine secretion of LIF, thus driving the progression and metastasis of gastric adenocarcinoma.
Genetically engineered mice with genetic mutations in terminally differentiated cells
Tff1−/− mice
Trefoil factor 1 (TFF1) is a tumor suppressor gene that belongs to the trefoil factor family and is expressed predominantly in gastric pit cells. TFF1 transcription is positively regulated by the gastrin hormone126, and decreased abundance of TFF1 resulting from epigenetic silencing is involved in gastric carcinogenesis127,128. TFF1 knockout mice (Tff1−/−) have been generated by Lefebvre et al.129 to investigate the roles of this factor in gastrointestinal homeostasis and tumorigenesis. Tff1−/− mice develop severe hyperplasia and dysplasia, marked by elongated pits and enlarged nuclei. Moreover, 30% of Tff1−/− mice develop invasive pyloric adenoma, but no metastatic dissemination to the lung or liver, at the age of 5 months129. In line with the role of dysregulated inflammation in promoting GC, knockout of TFF1in mice and progressive loss of TFF1in human gastric tissues have been associated with activation of NF-κB-mediated inflammation and progression to gastric tumorigenesis130. This model has been used to investigate the role of prostanoid metabolism in GC progression. Expression of cyclooxygenase-2 (Cox-2) is elevated in pyloric adenoma in Tff1−/− mice, and is involved in the conversion of arachidonic acid to prostanoid precursors. Moreover, inhibition of Cox-2 through genetic deletion or treatment with the selective inhibitor celecoxib decreases adenoma size and ulceration in Tff1−/− mice131,132.
Mutations in parietal cells
Parietal cells, marked by Atp4b expression, account for one-third of all gastric epithelial cells. Parietal cells secrete gastric acid in response to gastrin stimulation, thereby maintaining the acidic environment of the stomach and inhibiting the invasion of pathogenic microorganisms. Manipulation of oncogenes and/or tumor suppressor genes in parietal cells also contributes to gastric tumorigenesis. For example, activation of Notch signaling by knock in of Notch1 intracellular domain (NICD1) in Atp4b+ parietal cells induces dedifferentiation into multipotential progenitors that populate the gastric epithelium. Sustained Notch activation within parietal cells eventually induces adenomas characterized by focal Wnt/β-catenin signaling activation133. In addition, loss of AT-rich interaction domain 1A (Arid1a), a key subunit of the chromatin remodeling BAF complex in Notch-signaling-activated parietal cells, further accelerates GC progression in a dose-dependent manner134. Mechanistically, homozygous depletion of Arid1a leads to a competitive disadvantage through activation of the p53 pathway and thus promotes gastric tumorigenesis134.
Multiple studies have shown that the Cdh1 gene, encoding E-cadherin, displays hypermutation in diffuse-type GC10,135. Deletion of Cdh1 alone in Atp4b+ parietal cells leads to mucosal hyperplasia and spasmolytic polypeptide-expressing metaplasia136. Synergistic depletion of Cdh1 and Trp53 in parietal cells results in the development of invasive diffuse-type GC, thus leading to a high frequency of lymphatic metastases and tumorigenic activity in immunodeficient mice137. In addition to specific deficiency in Cdh1 and Trp53, knock in of oncogenic KrasG12D in parietal cells accelerates intestinal and diffuse-type gastric tumorigenesis, and lymphatic and hematogenous metastasis in the lymph nodes, liver, and lungs138. In this regard, KRAS activation promotes epithelial-to-mesenchymal transition and the generation of cancer stem cells, and consequently metastasis to the lungs139.
Mutations in neuroendocrine cells
Neuroendocrine cells in Neurogenin 3-expressing progenitor cells in the gastric epithelium play an essential role in maintaining gastrointestinal homeostasis and have been proposed as a potential cellular origin of gastric neuroendocrine neoplasms140–142. Neuroendocrine neoplasms are characterized by the expression of neuroendocrine markers and are divided into subclasses of well-differentiated neuroendocrine tumors, aggressive poorly differentiated neuroendocrine carcinoma, and mixed neuroendocrine/non-neuroendocrine neoplasia143. A missense mutation (p.R703C) in the human ATP4a gene has been identified in aggressive familial gastric neuroendocrine tumors. Mice with knock in of human ATP4aR703C develop severe metaplasia and dysplasia in the stomach144. Recently, by characterizing the genomic landscapes and transcriptional subtyping of human gastric neuroendocrine carcinoma (G-NEC), Griger et al.145 have identified MYC as a critical driver of G-NEC. The Cγ1-cre allele was used to drive overexpression of MYC in the gastric neuroendocrine compartment. MYC-driven mouse G-NECs develop aggressive malignancies and distal metastatic foci in the lungs and liver145. The G-NEC cell line and organoid resources derived from this GC model were generated to perform genome-scale CRISPR and pharmacologic screens.
Gastrointestinal stromal tumors (GISTs)
GISTs are among the most common human sarcomas in human gastrointestinal tracts. GIST originates from the interstitial cells of Cajal (ICC) which depends on high expression of KIT for lineage commitment. GIST is characterized primarily by activating mutations in KIT or PDGFRA receptor tyrosine kinase146,147. Multiple mouse models of GIST have been established through knock in of KIT mutations. For example, knock in of KitV558∆ or KitK641E results in the development of human GIST-like tumors marked by ICC hyperplasia within the myenteric plexus of the GI tract148,149. Imatinib (Gleevec), a multitargeted tyrosine kinase inhibitor targeting KIT/PDGFR, is the standard first-line therapy for advanced GIST. However, patients with GIST frequently develop imatinib resistance resulting from second-site mutations of KIT. Further knock in of KitT669I or KitK653A in KitV558∆ mice promotes GIST development and induces resistance to imatinib150,151.
The ETS family member ETV1 is another lineage survival factor of ICC. Activating mutation of KIT stabilizes the ETV1 protein through constitutive activation of the KIT-MAPK signaling pathway, and augments ETV1 transcriptional output, thus promoting GIST152. In the KitV558∆ mouse model, ETV1 ablation inhibits GIST initiation and progression153. Moreover, the Forkhead family member FOXF1 directly controls the transcription of KIT and ETV1, and is required for tumor growth and maintenance via regulating the GIST lineage-specific transcriptome154. Combining knock in of BrafV600E with Trp53 deletion in ETV1+ ICC or smooth muscle cells drives ICC hyperplasia and multifocal GIST-like tumor formation in the mouse gastrointestinal tract155,156.
Conclusions and perspectives
Although gastric anatomy differs between mice and humans, many mouse models have been established to study GC pathology and related immune responses. In combination with gastric disorders, such as H. pylori infection, sophisticated GC mouse models—including cell line-derived graft tumors, treatment with chemical carcinogens, and genetic engineering—have laid an important foundation for exploring GC pathogenesis and antitumor immunity (Figure 1). Mutation of oncogenes and tumor suppressor genes in gastric epithelial and interstitial cells induces different types of GC, thus contributing to the high heterogeneity in GC (Table 1). Therefore, illustrating the pathogenesis and molecular features of GC induced by specific genetic mutations in specific types of gastric cells will not only uncover cell signaling networks crucial for GC development, but also promote accurate diagnosis and efficient treatment of GC.
Table 1.
Genetically engineered mouse models of gastric carcer
| GEMM | Cell type | Time/lesion | Location | Subtype | Metastasis | References |
|---|---|---|---|---|---|---|
| H/K-ATPase:hIL-1β | Parietal cell | 12 months | Corpus | Intestinal | N | Tu et al.79 |
| NF-κB1−/− | Epithelial and hematopoietic cell | 18 months | Corpus + antrum | Intestinal | ND | O’Reilly et al.80 |
| Gp130F/F | ND | 6 months | Corpus + antrum | Intestinal | Y | Tebbutt et al.85 |
| TxA23 | CD4+ T cell | 12 months | Corpus | Intestinal | ND | Nguyen et al.92 |
| INS-GAS | β islet cell | 20 months | Corpus | Intestinal | N | Wang et al.94 |
| Gastrin−/− | G cell | 12 months | Antrum | Intestinal | N | Zavros et al.106 |
| Claudin18KM | Pan-epithelial cell | 5 months | Corpus | Intestinal | ND | Hagen et al.110 |
| Claudin18creERT2; Apcfl/+; KrasG12D; Trp53fl/fl | Pan-epithelial cell | 4 months | Corpus | Intestinal | Y | Fatehullah et al.111 |
| Anxa10creERT2; KrasG12D; Trp53R172H; Smad4fl/fl | Pan-epithelial cell | 2 months | Corpus | Intestinal | Y | Seidlitz et al.112 |
| Anxa10creERT2; KrasG12D; Cdh1fl/fl; Smad4fl/fl | Pan-epithelial cell | 2 months | Corpus | Diffuse | Y | Seidlitz et al.112 |
| Ppard1/2 transgenic | Pan-epithelial cell | 7 months | Corpus | Intestinal | Y | Zuo et al.113 |
| Lgr5creERT2; KrasG12D | Stem cell | 4 months | Corpus | ND | ND | Leushacke et al.115 |
| Lgr5creERT2; Trp53fl/fl + MNU | Stem cell | 12 months | Antrum | ND | ND | Sethi et al.69 |
| Aqp5creERT2; Apcfl/fl; KrasG12D; Pten fl/fl | Stem cell | 1 month | Antrum | Intestinal | ND | Tan et al.116 |
| PgccreERT2; Apcfl/+; KrasG12D; Trp53fl/fl | Chief cell | ND | Corpus + antrum | ND | Y | Douchi et al.117 |
| Gif-rtTA; TetOCre; KrasG12D | Chief cell | 3 months | Corpus | Intestinal | ND | Won et al.118 |
| Mist1creERT2; Apcfl/fl; KrasG12D | Stem cell | 4 months | Corpus | Intestinal | ND | Hayakawa et al.119 |
| Mist1creERT2; Cdh1fl/fl + H. felis | Stem cell | 12 months | Corpus | Diffuse | ND | Hayakawa et al.119 |
| Mist1creERT2; Cdh1fl/fl; RhoaY42C | Stem cell | ND | ND | Diffuse | Y | Zhang et al.120 |
| Krt5creERT2; Rosa26sox2/sox2 | Stem cell | 3 months | Fore-stomach | Squamous cancer | ND | Liu et al.123 |
| Sox2creERT2; Apcfl/fl | Stem cell | 12 months | Corpus | Intestinal | ND | Sarkar et al.124 |
| Sox2creERT2; Sox9Flp; KrasG12D; Trp53R172H | Stem cell | 5 months | Corpus | Intestinal | Y | Chen et al.125 |
| Tff1−/− | Pit cell | 5 months | Antrum | Intestinal | ND | Lefebvre et al.129 |
| Atp4bcre; Rosa26NICD | Parietal cell | 4.5 months | Corpus | Intestinal | ND | Kim et al.133 |
| Atp4bcre; Rosa26NICD; Arid1afl/fl | Parietal cell | 6 months | Corpus | Intestinal | ND | Loe et al.134 |
| Atp4bcre; Cdh1fl/fl | Parietal cell | 3 months | Corpus | ND | ND | Mimata et al.136 |
| Atp4bcre; Cdh1fl/fl; Trp53fl/fl | Parietal cell | 12 months | Corpus | Diffuse | Y | Shimada et al.137 |
| Atp4bcre; Cdh1fl/fl; Trp53fl/fl; KrasG12D | Parietal cell | 2 months | Corpus | Intestinal and diffuse | Y | Till et al.138 |
| Cγ1cre; Rosa26MYC | Neuroendocrine cell | 3 months | Corpus | Neuroendocrine cancer | Y | Griger et al.145 |
| KitV558∆; KitT669I or KitV558∆; KitK653A | ICC | 9 months | Interstitial | GIST | ND | Sommer et al.148 |
| Etv1creERT2; BrafV600E; Trp53fl/fl | ICC | 1.5 months | Interstitial | GIST | ND | Ran et al.155 |
Y, Yes; N, No; ND, Not determined.
Mouse GC cell lines provide powerful tools to explore the molecular mechanisms regulating immune responses. The most widely used mouse GC cell line is MFC, derived from squamous carcinoma in the forestomach from mice on a 615 background. However, mice with conditional knockout of certain immune system genes (e.g., Cd4cre in T cells and Cd19cre in B cells) are usually bred on a C57BL/6 background, which is not suitable for MFC cell tumorigenicity. In this regard, the development of mouse gastric adenocarcinoma cell lines, particularly those with a C57BL/6 background, has become an urgent need for tumor immunity research in GC. In addition, graft tumor models usually induce an unnaturally hyperinflammatory state after tumor cell transplantation157. Without high spatial and temporal specificity, chemical carcinogens such as MNU induce a high mutational burden and immunogenicity, but also dampen the immune system, including T cells. In contrast, GEMMs of GC provide multiple advantages for studying tumor cell initiation, progression, and antitumor immunity (Figure 2). The advantages of GEMMs include the following: (1) Modeling gastric tumorigenesis through genetic manipulation within distinct cell types aids in understanding the high heterogeneity arising from different cell origins and the genotype-phenotype relationships during human GC progression. (2) In combination with the lineage tracing strategy, the gastric local spontaneity of GEMM helps reveal the clonal evolution of tumor cells within a complete immune system. (3) GEMMs of GC can replicate the effects of the gastric immune microenvironment and the crosstalk between the stomach and other organs, such as the brain and liver, in GC initiation and metastasis. However, the extent to which currently available genetically engineered GC mouse models reflect the pathological nature of human GC is questionable. Systemic comparative studies defining the similarity between human and mouse GC subtypes at single cell-resolution are lacking. Moreover, use of GEMMs is usually time- and resource-consuming, because of the need to intercross multiple germline strains. Recently, Leibold et al.158 have developed somatic mouse models of GC by introducing various oncogenic lesions into the murine gastric epithelium through an electroporation-based approach. This strategy accelerates the development of GC mouse models, although the cellular origin of GC is in suspense and the injury is unavoidable during surgery158.
In addition, patient-derived xenografts (PDXs) and human gastric cell line-derived xenografts in immunocompromised mice are crucial tools for GC research159,160. Originating from human gastric tumor tissue, PDXs retain the genetic and phenotypic characteristics of tumors in the presence of stroma and immune cells, thus favorably modeling the natural tumor microenvironment. These models reflect the heterogeneity in patients’ cancers as well as the biological characteristics and mutational landscape of cancer cells161,162. Additionally, humanized mouse models are an important GC research tool providing a humanized immune microenvironment for PDX and gastric cell line-derived xenograft growth, which may serve as a platform for the evaluation of drugs modulating the anti-tumor immune response159.
Given the heterogeneity in GC, no single GC model can answer all GC-associated scientific questions. Taking advantage of GEMM in GC research, further endeavors may focus on clarifying the cellular origins and clonal evolution of tumor cells during GC initiation and metastasis, through intercrossing with lineage tracing germline strains; the molecular and cellular mechanisms driving GC invasion, metastasis and metastatic organ tropism, by using cell lines and organoid resources derived from GEMMs; the heterogeneity in the immune microenvironment of primary GC located in the distinct anatomy of the stomach; the roles of stomach-brain and stomach-liver organ communication in regulating GC progression; the mechanisms mediating T cell exhaustion, formation of tertiary lymphoid structures, and resistance to immunotherapy; and the effects and mechanisms of emotions, biological rhythm, nerves, and microorganism infections in regulating GC tumorigenesis, immune evasion, and responses to targeted treatments and immunotherapy.
Funding Statement
This work was supported by the National Key R&D Program of China (Grant No. 2020YFA0803200 and 2023YFC2505903), National Natural Science Foundation of China (Grant Nos. 82003014, 31930026, 81972876, 82150112, 92168116, 81725014, 81822035, and 82222052), China Postdoctoral Science Foundation (Grant No. 2020M671231), and Fundamental Research Funds for the Central Universities (Grant No. 22120240327).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Weihong Zhang, Shi Jiao, Liwei An, Zhaocai Zhou.
Collected the data: Weihong Zhang, Yan Meng, Liwei An.
Contributed data or analysis tools: Shilong Wang, Hui Zhang.
Performed the analysis: Weihong Zhang, Yan Meng, Liwei An.
Wrote the paper: Weihong Zhang, Yan Meng, Liwei An, Zhaocai Zhou.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338–49. doi: 10.1038/s41571-023-00747-0. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Li W. Early detection of gastric cancer in China: progress and opportunities. Cancer Biol Med. 2022;19:1622–8. doi: 10.20892/j.issn.2095-3941.2022.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20:155–70. doi: 10.1038/s41575-022-00703-w. [DOI] [PubMed] [Google Scholar]
- 5.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 6.Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30. doi: 10.1038/s41586-021-04161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133–41. doi: 10.1038/s41591-023-02465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao M, He L, Sun D, He S, Yan X, Yang F, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19:1121–38. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Hu S, Min M, Ni Y, Lu Z, Sun X, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70:464–75. doi: 10.1136/gutjnl-2019-320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Dang M, Harada K, Han G, Wang F, Pool Pizzi M, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med. 2021;27:141–51. doi: 10.1038/s41591-020-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Jia Y, Sun G, Yang H, Liu L, Qu X, et al. Single-cell analysis of gastric signet ring cell carcinoma reveals cytological and immune microenvironment features. Nat Commun. 2023;14:2985. doi: 10.1038/s41467-023-38426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Song S, Qin J, Yoshimura K, Peng F, Chu Y, et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell. 2023;41:1407–26.e9. doi: 10.1016/j.ccell.2023.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar V, Ramnarayanan K, Sundar R, Padmanabhan N, Srivastava S, Koiwa M, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov. 2022;12:670–91. doi: 10.1158/2159-8290.CD-21-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim R, An M, Lee H, Mehta A, Heo YJ, Kim KM, et al. Early tumor-immune microenvironmental remodeling and response to first-line fluoropyrimidine and platinum chemotherapy in advanced gastric cancer. Cancer Discov. 2022;12:984–1001. doi: 10.1158/2159-8290.CD-21-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang B, Camps J, Fan B, Jiang H, Ibrahim MM, Hu X, et al. Parallel single-cell and bulk transcriptome analyses reveal key features of the gastric tumor microenvironment. Genome Biol. 2022;23:265. doi: 10.1186/s13059-022-02828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun K, Xu R, Ma F, Yang N, Li Y, Sun X, et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat Commun. 2022;13:4943. doi: 10.1038/s41467-022-32627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022;12:620–38. doi: 10.7150/thno.60540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang XZ, Pang MJ, Li JY, Chen HY, Sun JX, Song YX, et al. Single-cell sequencing of ascites fluid illustrates heterogeneity and therapy-induced evolution during gastric cancer peritoneal metastasis. Nat Commun. 2023;14:822. doi: 10.1038/s41467-023-36310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowicki-Osuch K, Zhuang L, Cheung TS, Black EL, Masqué-Soler N, Devonshire G, et al. Single-cell RNA sequencing unifies developmental programs of esophageal and gastric intestinal metaplasia. Cancer Discov. 2023;13:1346–63. doi: 10.1158/2159-8290.CD-22-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takada H, Sasagawa Y, Yoshimura M, Tanaka K, Iwayama Y, Hayashi T, et al. Single-cell transcriptomics uncovers EGFR signaling-mediated gastric progenitor cell differentiation in stomach homeostasis. Nat Commun. 2023;14:3750. doi: 10.1038/s41467-023-39113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Liu K, Luo Y, Kang M, Wang J, Chen G, et al. Single-cell profiling of tumor immune microenvironment reveals immune irresponsiveness in gastric signet-ring cell carcinoma. Gastroenterology. 2023;165:88–103. doi: 10.1053/j.gastro.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa Y, Nakagawa H, Rustgi AK, Que J, Wang TC. Stem cells and origins of cancer in the upper gastrointestinal tract. Cell Stem Cell. 2021;28:1343–61. doi: 10.1016/j.stem.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noto JM, Peek RM., Jr RNF43: a biomarker with potential ramifications for therapeutic intervention in gastric cancer. Cell Mol Gastroenterol Hepatol. 2021;11:1202–3. doi: 10.1016/j.jcmgh.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An L, Han Y, Jiao S, Zhou Z. Road of no return - loss of TP53 paves a defined evolution path from gastric preneoplasia-to-cancer. Cancer Biol Med. 2024;20:885–90. doi: 10.20892/j.issn.2095-3941.2023.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson K, Przybilla MJ, Kotler E, Khan A, Xu H, Karagyozova K, et al. Deterministic evolution and stringent selection during preneoplasia. Nature. 2023;618:383–93. doi: 10.1038/s41586-023-06102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaefer A, Der CJ. RHOA takes the RHOad less traveled to cancer. Trends Cancer. 2022;8:655–69. doi: 10.1016/j.trecan.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Wong GS, Zhou J, Liu JB, Wu Z, Xu X, Li T, et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med. 2018;24:968–77. doi: 10.1038/s41591-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–8. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 31.Cao Z, An L, Han Y, Jiao S, Zhou Z. The Hippo signaling pathway in gastric cancer. Acta Biochim Biophys Sin (Shanghai) 2023;55:893–903. doi: 10.3724/abbs.2023038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao S, Guan J, Chen M, Wang W, Li C, Wang Y, et al. Targeting IRF3 as a YAP agonist therapy against gastric cancer. J Exp Med. 2018;215:699–718. doi: 10.1084/jem.20171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Fang G, Guo F, Zhang H, Chen X, An L, et al. Selective inhibition of STRN3-containing PP2A phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell. 2020;38:115–28.e9. doi: 10.1016/j.ccell.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 34.An L, Cao Z, Nie P, Zhang H, Tong Z, Chen F, et al. Combinatorial targeting of Hippo-STRIPAK and PARP elicits synthetic lethality in gastrointestinal cancers. J Clin Invest. 2022;132:e155468. doi: 10.1172/JCI155468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Z, Hou Y, Zhao Z, Zhang H, Tian L, Zhang Y, et al. Reactivating hippo by drug compounds to suppress gastric cancer and enhance chemotherapy sensitivity. J Biol Chem. 2024;300:107311. doi: 10.1016/j.jbc.2024.107311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaes N, Idris M, Boesmans W, Alves MM, Melotte V. Nerves in gastrointestinal cancer: from mechanism to modulations. Nat Rev Gastroenterol Hepatol. 2022;19:768–84. doi: 10.1038/s41575-022-00669-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi YH, Yang LZ, Zhou L, Gao L, Hou J, Yan Z, et al. Sympathetic nerve infiltration promotes stomach adenocarcinoma progression via norepinephrine/β2-adrenoceptor/YKL-40 signaling pathway. Heliyon. 2022;8:e12468. doi: 10.1016/j.heliyon.2022.e12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polli-Lopes AC, Zucoloto S, de Queiros Cunha F, da Silva Figueiredo LA, Garcia SB. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003;190:45–50. doi: 10.1016/s0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 2019;10:788. doi: 10.1038/s41419-019-2030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu K, Cheung AHK, Wong CC, Liu W, Zhou Y, Wang F, et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell. 2024;187:882–96.e17. doi: 10.1016/j.cell.2024.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Dohlman AB, Klug J, Mesko M, Gao IH, Lipkin SM, Shen X, et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell. 2022;185:3807–22.e12. doi: 10.1016/j.cell.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973–80. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian SS, Gao J, Wang JX, Liu Y, Dong HY. Establishment of a mouse forestomach carcinoma cell line (MFC) with spontaneous hematogenous metastasis and preliminary study of its biological characteristics. Zhonghua Zhong Liu Za Zhi. 1987;9:261–4. [PubMed] [Google Scholar]
- 47.Cao T, Zhang W, Wang Q, Wang C, Ma W, Zhang C, et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8+ T cells. Cell. 2024;187:2288–304.e27. doi: 10.1016/j.cell.2024.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Shi T, Zhang Y, Wang Y, Song X, Wang H, Zhou X, et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. 2022;10:1506–24. doi: 10.1158/2326-6066.CIR-22-0218. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Wang CM, Wu HX, Wang F, Chai YY, Hu Y, et al. MFSD2A potentiates gastric cancer response to anti-PD-1 immunotherapy by reprogramming the tumor microenvironment to activate T cell response. Cancer Commun (Lond) 2023;43:1097–116. doi: 10.1002/cac2.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X, Jia S, Wang G, Liang M, Guo T, Du H, et al. TRIM28 promotes the escape of gastric cancer cells from immune surveillance by increasing PD-L1 abundance. Signal Transduct Target Ther. 2023;8:246. doi: 10.1038/s41392-023-01450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan YS, Fang JH, Lai MD, Yen MC, Lin PW, Hsu HP, et al. Establishment of an orthotopic transplantable gastric cancer animal model for studying the immunological effects of new cancer therapeutic modules. Mol Carcinog. 2011;50:739–50. doi: 10.1002/mc.20668. [DOI] [PubMed] [Google Scholar]
- 52.Park JW, Park DM, Choi BK, Kwon BS, Seong JK, Green JE, et al. Establishment and characterization of metastatic gastric cancer cell lines from murine gastric adenocarcinoma lacking Smad4, p53, and E-cadherin. Mol Carcinog. 2015;54:1521–7. doi: 10.1002/mc.22226. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto M, Nomura S, Hosoi A, Nagaoka K, Iino T, Yasuda T, et al. Established gastric cancer cell lines transplantable into C57BL/6 mice show fibroblast growth factor receptor 4 promotion of tumor growth. Cancer Sci. 2018;109:1480–92. doi: 10.1111/cas.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu HP, Wang CY, Hsieh PY, Fang JH, Chen YL. Knockdown of serine/threonine-protein kinase 24 promotes tumorigenesis and myeloid-derived suppressor cell expansion in an orthotopic immunocompetent gastric cancer animal model. J Cancer. 2020;11:213–28. doi: 10.7150/jca.35821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banan B, Beckstead JA, Dunavant LE, Sohn Y, Adcock JM, Nomura S, et al. Development of a novel murine model of lymphatic metastasis. Clin Exp Metastasis. 2020;37:247–55. doi: 10.1007/s10585-020-10025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimori D, Kinoshita J, Yamaguchi T, Nakamura Y, Gunjigake K, Ohama T, et al. Established fibrous peritoneal metastasis in an immunocompetent mouse model similar to clinical immune microenvironment of gastric cancer. BMC Cancer. 2020;20:1014. doi: 10.1186/s12885-020-07477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura Y, Kinoshita J, Yamaguchi T, Aoki T, Saito H, Hamabe-Horiike T, et al. Crosstalk between cancer-associated fibroblasts and immune cells in peritoneal metastasis: inhibition in the migration of M2 macrophages and mast cells by Tranilast. Gastric Cancer. 2022;25:515–26. doi: 10.1007/s10120-021-01275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai X, Wong CC, Pan Y, Chen H, Liu W, Zhai J, et al. Loss of YTHDF1 in gastric tumors restores sensitivity to antitumor immunity by recruiting mature dendritic cells. J Immunother Cancer. 2022;10:e003663. doi: 10.1136/jitc-2021-003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagaoka K, Sun C, Kobayashi Y, Kanaseki T, Tokita S, Komatsu T, et al. Identification of neoantigens in two murine gastric cancer cell lines leading to the neoantigen-based immunotherapy. Cancers (Basel) 2021;14:106. doi: 10.3390/cancers14010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagaoka K, Shirai M, Taniguchi K, Hosoi A, Sun C, Kobayashi Y, et al. Deep immunophenotyping at the single-cell level identifies a combination of anti-IL-17 and checkpoint blockade as an effective treatment in a preclinical model of data-guided personalized immunotherapy. J Immunother Cancer. 2020;8:e001358. doi: 10.1136/jitc-2020-001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith JP, Cao H, Chen W, Mahmood K, Phillips T, Sutton L, et al. Gastrin vaccine alone and in combination with an immune checkpoint antibody inhibits growth and metastases of gastric cancer. Front Oncol. 2021;11:788875. doi: 10.3389/fonc.2021.788875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee D, Choi J, Oh HJ, Ham IH, Lee SH, Nomura S, et al. Molecular and immune profiling of syngeneic mouse models predict response to immune checkpoint inhibitors in gastric cancer. Cancer Res Treat. 2023;55:167–78. doi: 10.4143/crt.2022.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Shimizu N, Kobayashi K, et al. N-methyl-N-nitrosourea concentration-dependent, rather than total intake-dependent, induction of adenocarcinomas in the glandular stomach of BALB/c mice. Jpn J Cancer Res. 1998;89:385–91. doi: 10.1111/j.1349-7006.1998.tb00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faustino-Rocha AI, Ferreira R, Oliveira PA, Gama A, Ginja M. N-Methyl-N-nitrosourea as a mammary carcinogenic agent. Tumour Biol. 2015;36:9095–117. doi: 10.1007/s13277-015-3973-2. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto M, Furihata C, Ogiu T, Tsukamoto T, Inada Ki, Hirano K, et al. Independent variation in susceptibilities of six different mouse strains to induction of pepsinogen-altered pyloric glands and gastric tumor intestinalization by N-methyl-N-nitrosourea. Cancer Lett. 2002;179:121–32. doi: 10.1016/s0304-3835(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 66.Leung WK, Wu KC, Wong CY, Cheng AS, Ching AK, Chan AW, et al. Transgenic cyclooxygenase-2 expression and high salt enhanced susceptibility to chemical-induced gastric cancer development in mice. Carcinogenesis. 2008;29:1648–54. doi: 10.1093/carcin/bgn156. [DOI] [PubMed] [Google Scholar]
- 67.Han SU, Kim YB, Joo HJ, Hahm KB, Lee WH, Cho YK, et al. Helicobacter pylori infection promotes gastric carcinogenesis in a mice model. J Gastroenterol Hepatol. 2002;17:253–61. doi: 10.1046/j.1440-1746.2002.02684.x. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto M, Tsukamoto T, Sakai H, Shirai N, Ohgaki H, Furihata C, et al. p53 knockout mice (-/-) are more susceptible than (+/-) or (+/+) mice to N-methyl-N-nitrosourea stomach carcinogenesis. Carcinogenesis. 2000;21:1891–7. doi: 10.1093/carcin/21.10.1891. [DOI] [PubMed] [Google Scholar]
- 69.Sethi NS, Kikuchi O, Duronio GN, Stachler MD, McFarland JM, Ferrer-Luna R, et al. Early TP53 alterations engage environmental exposures to promote gastric premalignancy in an integrative mouse model. Nat Genet. 2020;52:219–30. doi: 10.1038/s41588-019-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danon SJ, Eaton KA. The role of gastric Helicobacter and N-methyl-N’-nitro- N-nitrosoguanidine in carcinogenesis of mice. Helicobacter. 1998;3:260–8. doi: 10.1046/j.1523-5378.1998.08017.x. [DOI] [PubMed] [Google Scholar]
- 71.Tatematsu M, Yamamoto M, Shimizu N, Yoshikawa A, Fukami H, Kaminishi M, et al. Induction of glandular stomach cancers in Helicobacter pylori-sensitive Mongolian gerbils treated with N-methyl-N-nitrosourea and N-methyl-N’-nitro-N-nitrosoguanidine in drinking water. Jpn J Cancer Res. 1998;89:97–104. doi: 10.1111/j.1349-7006.1998.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T. Effects in rats of sodium chloride on experimental gastric cancers induced by N-methyl-N-nitro-N-nitrosoguanidine or 4-nitroquinoline-1-oxide. J Natl Cancer Inst. 1975;55:101–6. doi: 10.1093/jnci/55.1.101. [DOI] [PubMed] [Google Scholar]
- 73.Wada S, Hirose M, Shichino Y, Ozaki K, Hoshiya T, Kato K, et al. Effects of catechol, sodium chloride and ethanol either alone or in combination on gastric carcinogenesis in rats pretreated with N-methyl-N’-nitro-N-nitrosoguanidine. Cancer Lett. 1998;123:127–34. doi: 10.1016/s0304-3835(97)00407-2. [DOI] [PubMed] [Google Scholar]
- 74.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 75.An L, Nie P, Chen M, Tang Y, Zhang H, Guan J, et al. MST4 kinase suppresses gastric tumorigenesis by limiting YAP activation via a non-canonical pathway. J Exp Med. 2020;217:e20191817. doi: 10.1084/jem.20191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nie P, Zhang W, Meng Y, Lin M, Guo F, Zhang H, et al. A YAP/TAZ-CD54 axis is required for CXCR2-CD44- tumor-specific neutrophils to suppress gastric cancer. Protein Cell. 2023;14:513–31. doi: 10.1093/procel/pwac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 78.Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, et al. PD-1 signaling promotes tumor-infiltrating myeloid-derived suppressor cells and gastric tumorigenesis in mice. Gastroenterology. 2021;160:781–96. doi: 10.1053/j.gastro.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Reilly LA, Putoczki TL, Mielke LA, Low JT, Lin A, Preaudet A, et al. Loss of NF-κB1 causes gastric cancer with aberrant inflammation and expression of immune checkpoint regulators in a STAT-1-dependent manner. Immunity. 2018;48:570–83.e8. doi: 10.1016/j.immuni.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Low JT, Christie M, Ernst M, Dumoutier L, Preaudet A, Ni Y, et al. Loss of NFKB1 results in expression of tumor necrosis factor and activation of signal transducer and activator of transcription 1 to promote gastric tumorigenesis in mice. Gastroenterology. 2020;159:1444–58.e15. doi: 10.1053/j.gastro.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 82.Wang P, Wang Y, Langley SA, Zhou YX, Jen KY, Sun Q, et al. Diverse tumour susceptibility in Collaborative Cross mice: identification of a new mouse model for human gastric tumourigenesis. Gut. 2019;68:1942–52. doi: 10.1136/gutjnl-2018-316691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 84.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–97. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–52. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 86.Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–38. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24:257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–78. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767–81. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eissmann MF, Dijkstra C, Jarnicki A, Phesse T, Brunnberg J, Poh AR, et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun. 2019;10:2735. doi: 10.1038/s41467-019-10676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poffenberger MC, Metcalfe-Roach A, Aguilar E, Chen J, Hsu BE, Wong AH, et al. LKB1 deficiency in T cells promotes the development of gastrointestinal polyposis. Science. 2018;361:406–11. doi: 10.1126/science.aan3975. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen TL, Khurana SS, Bellone CJ, Capoccia BJ, Sagartz JE, Kesman RA, Jr, et al. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 2013;73:2117–26. doi: 10.1158/0008-5472.CAN-12-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest. 1993;92:1349–56. doi: 10.1172/JCI116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 96.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–50. [PubMed] [Google Scholar]
- 97.Ohtani M, García A, Rogers AB, Ge Z, Taylor NS, Xu S, et al. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597–604. doi: 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- 98.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–90. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 99.Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, et al. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365–75. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, Feng Y, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54–63. doi: 10.1136/gutjnl-2013-305178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee CW, Rickman B, Rogers AB, Muthupalani S, Takaishi S, Yang P, et al. Combination of sulindac and antimicrobial eradication of Helicobacter pylori prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2009;69:8166–74. doi: 10.1158/0008-5472.CAN-08-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, et al. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–83. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 104.Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, et al. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–25. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 105.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, et al. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–8. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 106.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology. 2002;122:119–33. doi: 10.1053/gast.2002.30298. [DOI] [PubMed] [Google Scholar]
- 107.Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6:936–46. doi: 10.1038/nrc2014. [DOI] [PubMed] [Google Scholar]
- 108.Friis-Hansen L, Rieneck K, Nilsson HO, Wadström T, Rehfeld JF. Gastric inflammation, metaplasia, and tumor development in gastrin-deficient mice. Gastroenterology. 2006;131:246–58. doi: 10.1053/j.gastro.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 109.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 110.Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, et al. Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology. 2018;155:1852–67. doi: 10.1053/j.gastro.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fatehullah A, Terakado Y, Sagiraju S, Tan TL, Sheng T, Tan SH, et al. A tumour-resident Lgr5(+) stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat Cell Biol. 2021;23:1299–313. doi: 10.1038/s41556-021-00793-9. [DOI] [PubMed] [Google Scholar]
- 112.Seidlitz T, Chen YT, Uhlemann H, Schölch S, Kochall S, Merker SR, et al. Mouse models of human gastric cancer subtypes with stomach-specific CreERT2-mediated pathway alterations. Gastroenterology. 2019;157:1599–614.e2. doi: 10.1053/j.gastro.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zuo X, Deguchi Y, Xu W, Liu Y, Li HS, Wei D, et al. PPARD and interferon gamma promote transformation of gastric progenitor cells and tumorigenesis in mice. Gastroenterology. 2019;157:163–78. doi: 10.1053/j.gastro.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin SA, Barker N. Gastrointestinal stem cells in self-renewal and cancer. J Gastroenterol. 2011;46:1039–55. doi: 10.1007/s00535-011-0424-8. [DOI] [PubMed] [Google Scholar]
- 115.Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19:774–86. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 116.Tan SH, Swathi Y, Tan S, Goh J, Seishima R, Murakami K, et al. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature. 2020;578:437–43. doi: 10.1038/s41586-020-1973-x. [DOI] [PubMed] [Google Scholar]
- 117.Douchi D, Yamamura A, Matsuo J, Melissa Lim YH, Nuttonmanit N, Shimura M, et al. Induction of gastric cancer by successive oncogenic activation in the corpus. Gastroenterology. 2021;161:1907–23.e26. doi: 10.1053/j.gastro.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 118.Won Y, Jang B, Lee SH, Reyzer ML, Presentation KS, Kim H, et al. Oncogenic fatty acid metabolism rewires energy supply chain in gastric carcinogenesis. Gastroenterology. 2024;166:772–86.e14. doi: 10.1053/j.gastro.2024.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell Niche. Cancer Cell. 2015;28:800–14. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H, Schaefer A, Wang Y, Hodge RG, Blake DR, Diehl JN, et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 2020;10:288–305. doi: 10.1158/2159-8290.CD-19-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Francis R, Guo H, Streutker C, Ahmed M, Yung T, Dirks PB, et al. Gastrointestinal transcription factors drive lineage-specific developmental programs in organ specification and cancer. Sci Adv. 2019;5:eaax8898. doi: 10.1126/sciadv.aax8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu K, Jiang M, Lu Y, Chen H, Sun J, Wu S, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–15. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sarkar A, Huebner AJ, Sulahian R, Anselmo A, Xu X, Flattery K, et al. Sox2 suppresses gastric tumorigenesis in mice. Cell Rep. 2016;16:1929–41. doi: 10.1016/j.celrep.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 125.Chen Q, Weng K, Lin M, Jiang M, Fang Y, Chung SSW, et al. SOX9 modulates the transformation of gastric stem cells through biased symmetric cell division. Gastroenterology. 2023;164:1119–36.e12. doi: 10.1053/j.gastro.2023.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan ZE, Wang TC, Cui G, Chi AL, Dimaline R. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology. 2003;125:510–21. doi: 10.1016/s0016-5085(03)00908-9. [DOI] [PubMed] [Google Scholar]
- 127.Beckler AD, Roche JK, Harper JC, Petroni G, Frierson HF, Jr, Moskaluk CA, et al. Decreased abundance of trefoil factor 1 transcript in the majority of gastric carcinomas. Cancer. 2003;98:2184–91. doi: 10.1002/cncr.11789. [DOI] [PubMed] [Google Scholar]
- 128.Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, Jin G, et al. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879–91. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 130.Soutto M, Belkhiri A, Piazuelo MB, Schneider BG, Peng D, Jiang A, et al. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J Clin Invest. 2011;121:1753–67. doi: 10.1172/JCI43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saukkonen K, Tomasetto C, Narko K, Rio MC, Ristimäki A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003;63:3032–6. [PubMed] [Google Scholar]
- 132.Thiel A, Narko K, Heinonen M, Hemmes A, Tomasetto C, Rio MC, et al. Inhibition of cyclooxygenase-2 causes regression of gastric adenomas in trefoil factor 1 deficient mice. Int J Cancer. 2012;131:1032–41. doi: 10.1002/ijc.27331. [DOI] [PubMed] [Google Scholar]
- 133.Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–88. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loe AKH, Francis R, Seo J, Du L, Wang Y, Kim JE, et al. Uncovering the dosage-dependent roles of Arid1a in gastric tumorigenesis for combinatorial drug therapy. J Exp Med. 2021;218:e20200219. doi: 10.1084/jem.20200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 136.Mimata A, Fukamachi H, Eishi Y, Yuasa Y. Loss of E-cadherin in mouse gastric epithelial cells induces signet ring-like cells, a possible precursor lesion of diffuse gastric cancer. Cancer Sci. 2011;102:942–50. doi: 10.1111/j.1349-7006.2011.01890.x. [DOI] [PubMed] [Google Scholar]
- 137.Shimada S, Mimata A, Sekine M, Mogushi K, Akiyama Y, Fukamachi H, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut. 2012;61:344–53. doi: 10.1136/gutjnl-2011-300050. [DOI] [PubMed] [Google Scholar]
- 138.Till JE, Yoon C, Kim BJ, Roby K, Addai P, Jonokuchi E, et al. Oncogenic KRAS and p53 loss drive gastric tumorigenesis in mice that can be attenuated by E-cadherin expression. Cancer Res. 2017;77:5349–59. doi: 10.1158/0008-5472.CAN-17-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon C, Till J, Cho SJ, Chang KK, Lin JX, Huang CM, et al. KRAS activation in gastric adenocarcinoma stimulates epithelial-to-mesenchymal transition to cancer stem-like cells and promotes metastasis. Mol Cancer Res. 2019;17:1945–57. doi: 10.1158/1541-7786.MCR-19-0077. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–47. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–54. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 142.Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med. 2017;23:1–10. doi: 10.1038/nm.4341. [DOI] [PubMed] [Google Scholar]
- 143.Rindi G, Wiedenmann B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine. Nat Rev Endocrinol. 2020;16:590–607. doi: 10.1038/s41574-020-0391-3. [DOI] [PubMed] [Google Scholar]
- 144.Calvete O, Varro A, Pritchard DM, Barroso A, Oteo M, Morcillo MÁ, et al. A knockin mouse model for human ATP4aR703C mutation identified in familial gastric neuroendocrine tumors recapitulates the premalignant condition of the human disease and suggests new therapeutic strategies. Dis Model Mech. 2016;9:975–84. doi: 10.1242/dmm.025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Griger J, Widholz SA, Jesinghaus M, de Andrade Krätzig N, Lange S, Engleitner T, et al. An integrated cellular and molecular model of gastric neuroendocrine cancer evolution highlights therapeutic targets. Cancer Cell. 2023;41:1327–44.e10. doi: 10.1016/j.ccell.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 146.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 147.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 148.Sommer G, Agosti V, Ehlers I, Rossi F, Corbacioglu S, Farkas J, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci USA. 2003;100:6706–11. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rubin BP, Antonescu CR, Scott-Browne JP, Comstock ML, Gu Y, Tanas MR, et al. A knock-in mouse model of gastrointestinal stromal tumor harboring kit K641E. Cancer Res. 2005;65:6631–9. doi: 10.1158/0008-5472.CAN-05-0891. [DOI] [PubMed] [Google Scholar]
- 150.Bosbach B, Deshpande S, Rossi F, Shieh JH, Sommer G, de Stanchina E, et al. Imatinib resistance and microcytic erythrocytosis in a KitV558Δ;T669I/+ gatekeeper-mutant mouse model of gastrointestinal stromal tumor. Proc Natl Acad Sci USA. 2012;109:E2276–83. doi: 10.1073/pnas.1115240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang JQ, Bosbach B, Loo JK, Vitiello GA, Zeng S, Seifert AM, et al. The V654A second-site KIT mutation increases tumor oncogenesis and STAT activation in a mouse model of gastrointestinal stromal tumor. Oncogene. 2020;39:7153–65. doi: 10.1038/s41388-020-01489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–53. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ran L, Sirota I, Cao Z, Murphy D, Chen Y, Shukla S, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5:304–15. doi: 10.1158/2159-8290.CD-14-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ran L, Chen Y, Sher J, Wong EWP, Murphy D, Zhang JQ, et al. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov. 2018;8:234–51. doi: 10.1158/2159-8290.CD-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ran L, Murphy D, Sher J, Cao Z, Wang S, Walczak E, et al. ETV1-positive cells give rise to BRAF(V600E)-mutant gastrointestinal stromal tumors. Cancer Res. 2017;77:3758–65. doi: 10.1158/0008-5472.CAN-16-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kondo J, Huh WJ, Franklin JL, Heinrich MC, Rubin BP, Coffey RJ. A smooth muscle-derived, Braf-driven mouse model of gastrointestinal stromal tumor (GIST): evidence for an alternative GIST cell-of-origin. J Pathol. 2020;252:441–50. doi: 10.1002/path.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hill W, Caswell DR, Swanton C. Capturing cancer evolution using genetically engineered mouse models (GEMMs) Trends Cell Biol. 2021;31:1007–18. doi: 10.1016/j.tcb.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 158.Leibold J, Tsanov KM, Amor C, Ho YJ, Sánchez-Rivera FJ, Feucht J, et al. Somatic mouse models of gastric cancer reveal genotype-specific features of metastatic disease. Nat Cancer. 2024;5:315–29. doi: 10.1038/s43018-023-00686-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zeng M, Pi C, Li K, Sheng L, Zuo Y, Yuan J, et al. Patient-derived xenograft: a more standard “Avatar” model in preclinical studies of gastric cancer. Front Oncol. 2022;12:898563. doi: 10.3389/fonc.2022.898563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv Y, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–11. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ajani JA, Xu Y, Huo L, Wang R, Li Y, Wang Y, et al. YAP1 mediates gastric adenocarcinoma peritoneal metastases that are attenuated by YAP1 inhibition. Gut. 2021;70:55–66. doi: 10.1136/gutjnl-2019-319748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin R, Zhang H, Yuan Y, He Q, Zhou J, Li S, et al. Fatty acid oxidation controls CD8+ tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol Res. 2020;8:479–92. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]