Abstract
The objective of this retrospective study was the evaluation of the administration of a haemoglobin (Hb)-based oxygen carrying solution (Oxyglobin®) to cats over a time period of 4 years. Indication, infusion volume/24 h, number of Oxyglobin® infusions/cat, Hb concentration pre- and post-infusion, adverse events, and patient outcome were evaluated. Forty-eight anaemic cats received 65 Oxyglobin® infusions. Prior to administration of Oxyglobin®, Hb concentration ranged from 2 to 7.8 g/dl (median 4.9 g/dl), the volume of Oxyglobin® administered was 4.4–25 ml/kg/24 h (median 9.8 ml/kg/24 h). An increase of Hb was noted after 41 of 49 infusion events. Severe side effects were noted in seven cats with cardiac disease, which developed pulmonary oedema (five), pleural effusion (three), and respiratory distress (one). They received 6.7–19.8 ml/kg/24 h (median 12.3 ml/kg/24 h) of Oxyglobin®. Four of these seven cats received whole blood transfusions on the same day; five cats died and one was euthanased. Overall 24-h survival rate was 77%. Administration of Oxyglobin® efficiently increased the Hb concentration. However, in cats suffering from cardiac disease, there is a high risk of life-threatening circulatory overload at the doses used in this study.
Blood transfusions have become an important component of intensive medical and surgical care in cats. Feline blood transfusions are safe and efficient, however, they are labour-intense as blood typing of donor and recipient and, possibly, cross-matching are needed (Weingart et al 2004, Weinstein et al 2007). Moreover, a clinical examination of the donor, a haematology and clinical chemistry is recommended before each donation. Infections with feline leukaemia virus, feline immunodeficiency virus, Haemoplasma species and Bartonella species have to be ruled out, but absolute safety in regard to transmission of infectious diseases can never be guaranteed (Wardrop et al 2005). The administration of haemoglobin (Hb) solutions such as Oxyglobin® is less time-consuming. Hb solutions can be stored for several years and are, therefore, readily available in emergency situations, and the transmission of infectious diseases is believed to be minimal compared to blood products (Muir and Wellmann 2003).
Oxyglobin® has a lower viscosity than blood, thereby improving tissue perfusion. Average molecular weight is 200 kD, which results in a distinctly higher colloid osmotic pressure in comparison to other colloidal solutions. Therefore, there is the potential risk for circulatory overload (Rentko and Sharpe 2000).
An advantage of Oxyglobin® in comparison to blood products is a storage life of 3 years at room temperature. However, once a bag (containing 125 ml) is opened, its content has to be used up within 24 h due to oxidation of Hb to methaemoglobin and possible bacterial growth (Adamantos et al 2005). In contrast to blood transfusions, special infusion sets for administration are not needed. As the Hb solution contains no red blood cell antigens, there is no need for blood typing or cross-matching.
An advantage of the small molecular size is an even distribution within the vascular system (Muir and Wellmann 2003). In healthy dogs, half-life is 24 h after administration of 15 ml/kg. After 5–9 days, more than 95% is eliminated. Oxyglobin® is metabolised by the reticuloendothelial system, except for the less than 5% that exists as an unstable tetramer and is excreted by the kidney (Callan and Rentko 2003). Comparable data for cats are not available.
Indications for administration of Oxyglobin® are anaemias, especially due to haemolysis or blood loss. Treatment of an anaemia caused by ineffective erythropoiesis is less effective due to the product's short half-life, but can be life-saving until blood products are available. Further indication for Oxyglobin® infusion is the presence of haemorrhagic or hypovolaemic shock (Callan and Rentko 2003). The dosage recommendations in cats range from 10 to 40 ml/kg depending on the literature (Callan and Rentko 2003, Adamantos et al 2005). Slow infusion rates of 0.5–5 ml/kg/h are recommended to prevent circulatory overload. An increased risk for volume overload in patients suffering from cardiac and respiratory diseases, or in patients displaying cerebral oedema or oliguria/anuria due to acute renal failure has been reported (Gibson et al 2002, Callan and Rentko 2003, Adamantos et al 2005). Oxyglobin® can cause vasoconstriction that may result in an increase in systemic arterial pressure. One suggested mechanism is that Oxyglobin® blocks the vasodilatatory effect of nitric oxide, which is produced by endothelial cells (Muir and Wellmann 2003). Moreover, Hb molecules can cause renal failure due to obstruction of renal tubuli (Spahn 2000). Other side effects include dose-related discoloration of mucous membranes and urine at infusion volumes of more than 15 ml/kg. Red discoloration of plasma could interfere with colorimetric laboratory analyses (eg, liver enzymes, bilirubin) (Wall 1998, Rentko and Sharpe 2000, Gibson et al 2002).
Whereas concentrations of Hb increase after administration of Oxyglobin®, the haematocrit (Hct) can decrease due to haemodilution. Thus, the Hct does not represent a suitable parameter for follow-up assessments (Callan and Rentko 2003).
While there are numerous studies regarding the use of Oxyglobin® in dogs (Bosman et al 1992, Harringer et al 1992, Standl et al 1996, Rentko et al 1996), only one retrospective study in cats (Gibson et al 2002) and a few case reports are available (Crystal et al 1999, Nolte and Crovatto 1999, Estrin et al 2006). The objective of this study was to evaluate Oxyglobin® infusions administered to cats between November 2002 and December 2006 at the Clinic for Small Animals of the Free University of Berlin regarding indication, number of infusions, infusion volume, side effects, and survival rate.
Material and methods
Cats that had received infusions of Oxyglobin® between November 2002 and December 2006 were included in this retrospective study. As Oxyglobin® is not licensed for cats in Germany, it was administered under off-label use with owner consent. The decision for administration of Oxyglobin® was based on the general condition of the patient, on the Hct or Hb concentration, and the underlying disease. Cats which had to undergo general anaesthesia received Oxyglobin® if their Hct was below 0.20 l/l. EDTA-anticoagulated blood was used to assess the complete blood cell count by impedance cell counting (Medonic CA 620, Boule Medical, Stockholm; Cell-Dyn 3500, Abbott Diagnostika, Wiesbaden). In case of red blood cell agglutination, the Hct was also established by microcentrifugation (Biofuge pico, Heraeus Instrumente, Osterode).
In most cases, Oxyglobin® was administered by a fluid administration pump at maximum rates of 5 ml/kg/h. The cats were monitored for possible side effects during infusion. Opened bags were stored for a maximum of 24 h at 4°C.
Single or multiple infusions administered within a 24-h period were classified as a single infusion event. Multiple infusions administered more than 24 h apart were classified as separate infusion events. Hb concentrations were established prior to and approximately 12–24 h after administration of Oxylobin and changes in Hb were calculated.
Cases were excluded from analysis of Hb changes if they were given additional blood products prior to post-infusion measurement of Hb, or if no post-infusion Hb measurement was available. Further parameters analysed were the indications for administration of Oxyglobin®, number of infusions/cat, volume infused in 24 h, adverse events, 24-h survival rate post-infusion of Oxyglobin®, and number of cats discharged from the hospital. Cats with pre-existing cardiac diseases were described separately in more detail.
Statistics
Descriptive statistics were used to report the results of age, weight, infusion volume, number of infusions, the Hb pre- and post-infusion and the survival rate. Statistical analyses were performed with computer software (SPSS 12, SPSS GmbH Software, Munich, Germany).
Results
Patients
Over a time period of 4 years, 53 cats with anaemia received 70 infusions of Oxyglobin®. Thirty-seven cats were domestic shorthair, seven were Persian, four were British shorthair, and there was one Siamese, Siamese mix, Carthusian, Maine Coon, and Somali cat each. Five cats were excluded from further evaluation because of lack of complete documentation. Thirty-four cats were male, one of them non-castrated; 19 cats were female, 13 of them spayed. The age ranged from 0.3 to 14 years (median 7.5 years). The weight of the cats ranged from 2 to 7.9 kg (median 4.3 kg).
Indications
Reasons for administration of Oxyglobin® instead of feline whole blood were documented in 43 cases: lack of feline blood (n=16), lack of compatible blood donors for cats with blood type B (six), difficulties in blood typing due to severe agglutination of red blood cells (one). In 20 cases Oxyglobin® from a bag opened within the previous 24 h was used instead of feline blood or packed red blood cells, in order to reduce costs and limit the use of stored blood or feline donors.
In all cases, indication for use of Oxyglobin® was anaemia. Of the 48 cats, 25 suffered from blood loss anaemia, 13 from haemolysis, and eight from ineffective erythropoiesis. In two cats, the cause of anaemia remained unknown.
Thirty cats also received whole blood (n=29) or packed red blood cells (n=1) in addition to Oxyglobin®. Forty-nine infusions of Oxyglobin® in 36 cats were evaluated regarding the Hb change pre- and post-administration. Fourteen infusions in 12 cats were excluded from analysis due to the administration of blood products, and two infusions (two cats) were excluded as post-infusion Hb measurements were not available.
All cats received infusions with Ringer's lactate due to shock, dehydration, or lack of water intake.
All cats in the study displayed red discoloration of their plasma after having received Oxyglobin® (Fig 1).
Fig 1.
Feline serum sample before (right) and after (left) Oxyglobin® infusion; red discoloration is visible.
Cats with blood loss anaemia
Twenty-four cats suffered from acute blood loss anaemia. One cat displayed chronic blood loss anaemia due to uraemic ulcerative gastritis (Table 1).
Table 1.
Indications for Oxyglobin® infusion, infusion volume, Hb pre-infusion, Hb change post-infusion, survival rate, and discharge from the hospital of 48 cats
| Oxyglobin® ml/kg (median) | Hb pre g/dl (median) | Hb change g/dl (median) | Survival rate 24 h/discharge (n) | |
|---|---|---|---|---|
| Blood loss anaemia | ||||
| Trauma (n=9) | 7.6–20 (10.3) | 3.3–7.8 (5.2) | −0.8–2.3 (0.65) | 6/5 |
| FLUTD (n=7) | 4.5–9.8 (6.7) | 4–7 (5.2) | −0.1–3.1 (0.9) | 6/4 |
| Haemostatic disorder (n=4) | 6.9–13.1 (8.3) | 3.8–7.1 (6.5) | 0.9–3.3 (1.9) | 3/2 |
| Haemoabdomen (n=2) | 12; 25 | 4.4; 4.8 | Excluded | 1/1 |
| Gastric ulcer (n=1) | 13.3 | 3.1 | Excluded | 1/0 |
| Nephrectomy (n=1) | 20 | 5.5 | 0.5 | 1/1 |
| Epistaxis (n=1) | 6.3 | 3.6 | 1.1 | 0 |
| Haemolytic anaemia | ||||
| IMHA (n=2) | 11.3; 14.8 (14.5) | 2–3 (2.1) | 0.9–2.5 (2.2) | 2/2 |
| Haemoplasmosis (n=2) | 7.1; 21.1 | 3.3; 6.7 | 2.2; 5.2 | 2/2 |
| Heinz body anaemia (n=3) | 8.2–16.5 (12) | 4.2–5.3 (4.9) | 1.2; 3.7 | 3/3 |
| Unknown (n=6) | 5.9–20 (14.6) | 2.1–7.1 (3.6) | 0.4–2.6 (0.8) | 4/2 |
| Ineffective erythropoiesis | ||||
| AID (n=5) | 4.4–17.7 (7.85) | 4.9–6.4 (5.7) | −0.5–2.4 (0.8) | 4/2 |
| Bone marrow disorder (n=2) | 6.7–16.7 (10.3) | 2.5–4.5 (3.1) | 0.5–1.6 (0.9) | 2/1 |
| CRF (n=1) | 8.5 | 5.3 | Excluded | 1/0 |
| Aetiology unknown (n=2) | 8.1; 10.1 | 3.2; 5.8 | Excluded; 7.1 | 1/1 |
Range and median values are given for the Oxyglobin® volume administered, Hb pre-infusion and Hb change. n=Number of cats, IMHA=immune-mediated haemolytic anaemia
Eighteen cats received one infusion of Oxyglobin® and seven cats received two over a time period of several days. The volumes ranged from 4.5 to 25 ml/kg/24 h (median 9.6 ml/kg/24 h). In eight of the 25 cats, the daily Oxyglobin® volume was split into two doses. The volume per infusion ranged from 3.6 to 13.1 ml/kg (median 6.6 ml/kg). Values of Hb prior to administration of Oxyglobin® ranged from 3.1 to 7.8 g/dl (median 5.1 g/dl). Additional whole blood transfusions were given in 18 cases: 14 and four cats received one and two transfusions, respectively.
Twenty-two of 32 Oxyglobin® infusions (given to 18 cats) could be evaluated regarding the development of Hb: changes in Hb ranged from −0.8 to 3.3 g/dl (median 0.9 g/dl). A decrease in Hb was present in five cases (median −0.2 g/dl). In 17 cases, the Hb concentration increased (0.2–3.3 g/dl, median 1.1 g/dl).
The 24-h survival rate was 72% (18/25). Four cats were euthanased (epistaxis due to rhinitis, feline lower urinary tract disease (FLUTD), liver tumour, pancreatitis and disseminated intravascular coagulation (DIC)) and three died (traumatic inguinal hernia and DIC, pelvic fracture in two cats) due to their underlying diseases. Another cat died 28 h after Oxyglobin® infusion due to the underlying disease (uraemic ulcerative gastritis). Two cats with hypertrophic cardiomyopathy (HCMP) suffering from FLUTD and one cat with thrombocytopenia, haematuria and pericardial effusion died 30–48 h after administration of Oxyglobin® presumably due to volume overload.
Fourteen of the 25 cats suffering from blood loss anaemia were discharged from the hospital after 3–19 days.
Cats with haemolytic anaemia
Thirteen cats suffered from haemolytic anaemia (Table 1).
These 13 cats received 18 Oxyglobin® infusions, with eight cats receiving only one infusion each. Five cats were transfused twice with Oxyglobin® over a time period of several days. The cats received 5.9–21.1 mlOxyglobin®/kg/24 h (median 12.3 ml/kg/24 h). In 10 cats, the overall infusion volume was divided and given approximately 12 h apart: volumes administered ranged from 3.8 to 10.7 ml/kg (median 7.2 ml/kg) per dose. Hb concentrations prior to administration were between 2 and 7.1 g/dl (median 4 g/dl). Four, two, and one cat, respectively, received one, two, and six transfusions of whole blood in addition to the Hb solution. Sixteen Oxyglobin® infusions in 12 cats were evaluated regarding the Hb change post-infusion. Increases in Hb after administration of Oxyglobin® ranged from 0.2 to 5.2 g/dl (median 1.2 g/dl). A decrease in Hb did not occur in any of the cases.
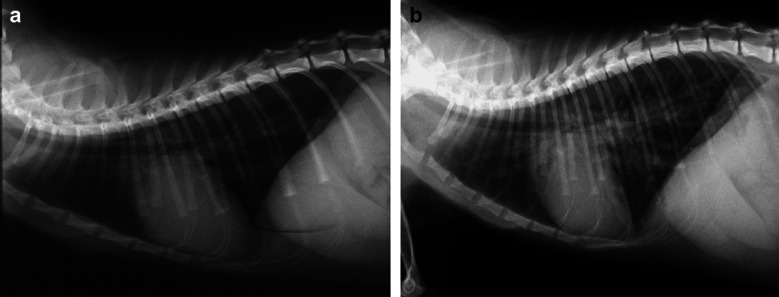
The 24-h survival rate was 84% (11/13). One cat suffering from sepsis died within 24 h after Oxyglobin® administration due to severe underlying disease. One cat which suffered from HCMP died 6 h after Oxyglobin® infusion because of pulmonary oedema. Another cat with HCMP and haemolytic anaemia was euthanased 30 h after Oxyglobin® infusion because of volume overload (Fig 2).
Fig 2.
Lateral thoracic radiograph of a cat displaying HCMP and anaemia before (a) and after (b) receiving a bovine Hb solution (Oxyglobin®); the cat developed severe pulmonary oedema and died 6 h after infusion.
Nine cats (69%) were discharged within 4–14 days after having received infusions of Oxyglobin.
Cats with anaemia due to ineffective erythropoiesis
Eight cats suffered from anaemia due to ineffective erythropoiesis (Table 1).
Four cats received one infusion. Three and one cat(s) received two and three Oxyglobin® infusions in the course of several days, respectively. The volume of Oxyglobin® administered ranged from 4.4 to 20 ml/kg/24 h (median 8.5 ml/kg/24 h). In four cases the overall infusion volume was divided and given approximately 12 h apart; volumes administered ranged from 4.4 to 8.9 ml/kg (median 7.0 ml/kg) per dose. Hb concentrations were 2.5–6.4 g/dl (median 5.25 g/dl) prior to Oxyglobin® administration. Five cats also received blood transfusions, two of which received one. Two cats received two transfusions. One cat received four blood transfusions.
Hb changes (10 infusions) ranged from −0.5 to 2.4 g/dl (median 0.8 g/dl). After two infusions, a decrease in Hb of 1 or 0.5 g/dl was observed.
The 24-h survival rate was 88% (7/8). One cat suffering from fat tissue necrosis died within 24 h due to the underlying disease. Another two cats with anaemia of inflammatory disease (AID) due to polytrauma and hepatopathy were euthanased 24 and 48 h, respectively, post-Oxyglobin® infusion, due to their underlying diseases. Two anaemic cats had a HCMP in addition: one of them was euthanased due to chronic renal failure (CRF) 2 days after Oxyglobin® infusion; the second cat (bone marrow disorder) died with signs of volume overload 2 days post-Oxyglobin® administration.
Three cats were discharged from the hospital within 3–8 days after administration of Oxyglobin®.
Cats with anaemia of unknown aetiology
Two cats with anaemia of unknown aetiology received Oxyglobin® infusions (8.1 and 10.1 ml/kg) (Table 1). The pre-infusion Hb concentrations were 3.2–5.8 g/dl. One cat died 6 h after Oxyglobin® infusion, and the other cat was euthanased 30 h thereafter, respectively, due to their underlying diseases (bile peritonitis, lymphocytic cholangitis).
Cats with anaemia and cardiac disease
Eleven of 48 cats suffered from cardiac disease (HCMP [10], pericardial effusion [one]) diagnosed by echocardiography or histopathology. Of these 11 cats, four suffered from blood loss anaemia, five from haemolytic anaemia, and two from ineffective erythropoiesis. In four cats the clinicians were aware of a pre-existing heart disease, but no clinical or radiological signs of fluid overload prior to Oxyglobin® administration were present.
Eight cats received one infusion of Oxyglobin® and three cats received two over a time period of several days. The volume administered ranged from 6.7 to 19.8 ml/kg/24 h (median 12.2 ml/kg/24 h). In three cats the daily Oxyglobin® volume was split into two doses. Values of Hb prior to administration of Oxyglobin® ranged from 2 to 7.1 g/dl (median 3.9 g/dl). One additional blood transfusion was given in five cats. Increases in Hb after 12 Oxyglobin® infusions ranged from 0.5 to 3.3 g/dl (median 1.0 g/dl).
Seven cats developed complications such as pulmonary oedema (three), pleural effusion (one), or both (two), and respiratory distress (one). Lung oedema and pleural effusion were confirmed in six cats either by pathological (three) or radiological (three) examination. One of these seven cats died 6 h after the Oxyglobin® infusion. Four of the seven cats died 25–48 h and one cat was euthanased 30 h after the infusion due to volume overload. Only four cats did not develop complications. The Oxyglobin® volume in the six cats which died ranged from 6.7 to 19.8 ml/kg/24 h (median 12.3 ml/kg/24 h).
Discussion
Anaemia was the indication for administration of Oxyglobin® in all 53 cats examined in this study. In another study, anaemia was also the indication in the majority of cats (70/72, 97%); in two cats, Oxyglobin® was administered in an attempt to deliver oxygen to sites of thromboses (Gibson et al 2002).
In a study of 46 cats suffering from DIC, seven cats received Oxyglobin® in addition to blood products, while 23 cats were only treated with transfusions of feline plasma or red blood cells. There was no information regarding the reason for administration of Oxyglobin® instead of blood products (Estrin et al 2006). Reasons for choosing Oxyglobin® rather than feline blood products in our study were a lack of available feline blood in the majority of the cases or a lack of compatible type B blood. In some cases, Oxyglobin® was used instead of whole blood, as only approximately one-quarter to one-third of one bag is needed for an infusion for one cat; and the product is too expensive to discard the rest. In the study by Gibson et al (2002) Oxyglobin® was administered because of a lack of readily available feline blood (50 of 60 documented indications) and the lack of a compatible blood donor in three cases. Five cats were given Oxyglobin® in an attempt to limit the use of feline blood.
In this study, one or two infusions of Oxyglobin® were given over a time period of 1–5 days. The median number of infusions was one/cat which is similar to the information given in another study (Gibson et al 2002).
The volume of the 65 Oxyglobin® infusions ranged from 4.4 to 25 ml/kg/24 h (median 9.8 ml/kg/24 h) and was slightly lower compared to the volume administered by Gibson et al (2002) (median infusion volume 11 ml/kg/24 h). Lower volumes could have been the reason for additional administration of blood products, which were used more frequently (63%) in this compared to the other study (54%) (Gibson et al 2002).
Hb changes after administration of Oxyglobin® ranged from −1.5 to 5.2 g/dl (median 0.9 g/dl). In eight of the 49 infusions, which could be evaluated in regard to Hb measurement post-infusion, a decrease in Hb was detected. After six, 13, 11, and 11 administrations of Oxyglobin® an increase of <0.5 g/dl, 0.5 to <1 g/dl, 1 to <2 g/dl, and ≥2 g/dl was detected, respectively. As in the study by Gibson et al (2002), increases of Hb were related to the volume of Oxyglobin® administered. The average change in Hb was 1.54±1.63 g/dl in the study by Gibson et al (2002) and was higher than the values obtained here (1.09±1.26 g/dl). This can be explained by larger volumes used for infusion. The decrease in Hb after administration of Oxyglobin® observed in eight cats might be explained by ongoing blood loss or extravascular haemolysis. Three cats suffering from ineffective erythropoiesis also displayed a decrease in Hb. This might be due to haemodilution caused by additional crystalloid infusion therapy.
Gibson et al (2002) described pulmonary oedema (eight) and/or thoracic effusion (21) in 25 of 72 cats that were treated with Oxyglobin®. However, a pleural effusion and pulmonary oedema were present in eight and two cats, respectively, prior to the treatment. In our study, life-threatening side effects such as pulmonary oedema, pleural effusion and respiratory distress occurred in seven cats (15%) and all of these cats suffered from heart diseases. Four of these seven cats received additional blood transfusions on the same day. Six of them died and only one cat survived. The administration of blood products and crystalloids in addition to Oxyglobin® may be an additional risk factor for circulatory overload. In the study of Gibson et al (2002) the median dose of Oxyglobin® given to cats which developed circulatory overload was 20.6 ml/kg whereas in this study it was only 12.3 ml/kg/24 h. However, these results suggest that a significantly lower dose is recommended especially in cats with pre-existing heart diseases. Therefore, the Oxyglobin® volume and rate of infusion needs to be adjusted to the patient. However, as the exact rate was not recorded this parameter could not be evaluated. But the risk of volume overload might be decreased if a considerably lower infusion rate than 5 ml/kg/h is administered. A careful examination prior to administration of Oxyglobin® is very important, as many cats suffering from cardiomyopathy do not display clinical signs (Fox 1999). Monitoring of central venous pressure during and immediately after infusion of Oxyglobin® is recommended. This is, however, difficult in cats. Clinical assessment of respiratory rate, jugular venous distention, and diuresis may be used as general indicators of expanded blood volume (Callan and Rentko 2003). A limiting factor of the present study was its retrospective nature, and clinical parameters such as respiratory rate, heart rate and clinical improvement of activity were not recorded in all cats.
In a study by Hamilton et al (2001), dogs displayed antibody formation against Hb after repeated administration of Oxyglobin®. However, this was of no clinical relevance. Anaphylactic reactions did not occur in any of the dogs. One dog developed an angioedema after the seventh Hb infusion, which did not require treatment. Corresponding data are not available for cats. However, the manufacturer recommends the use of Oxyglobin® only if the patient had not been treated with the product earlier on. In both our study and that by Gibson et al (2002), an anaphylactic reaction could not be observed in any of the cats. Yet, the interval between the individual infusions was only 1–5 days (median 2) in our study which might be too short to develop antibodies.
Thirty-seven of 48 cats survived the initial 24 h after administration of Oxyglobin®. The 24-h survival rate of 77% (n=37) was somewhat lower than the survival rate of 88% (n=80) in a study on 91 anaemic cats that were treated with whole blood transfusions (Weingart et al 2004). One cat died 6 h after Oxyglobin® infusion because of pulmonary oedema that might have been due to administration of Oxyglobin®. In 10 cats that had died within 24 h, a pathological examination could not be performed. However, all of these cats suffered from a severe underlying disease, and a correlation between the administration of Oxyglobin® and the death was unlikely. However, Oxyglobin® related volume overload was suspected in six other cats which died 6–48 h after infusion. Twenty-six of the 48 cats (54%) included in this study were discharged from the hospital.
In the study by Gibson et al (2002), the 24-h survival rate was 47%. This is noticeably less compared to our study. Only 23 cats (32%) were discharged from the hospital. In 23 cases, a pathological examination was performed: 14 cats displayed pulmonary oedema or thoracic effusion; of these cats, seven or nine cats suffered from lung or cardiac diseases, respectively. Despite the mortality rate in that study, the authors saw no clinical or pathological evidence that death or euthanasia was attributable to administration of the Oxyglobin® solution.
Administration of Oxyglobin® is efficient and safe for treatment of anaemia in cats; however, the volume and rate of the infusion have to be carefully adjusted to the patient. It should be given very cautiously to cats with cardiac (or respiratory) diseases especially with concurrent blood transfusion or crystalloid infusion to avoid life-threatening circulatory overload.
Prospective studies with appropriate end-point targets might be useful to further clarify the correct dosage and rate of administration in cats not only with anaemia but also shock syndromes.
References
- Adamantos S., Boag A., Hughes D. Clinical use of a haemoglobin-based oxygen carrying solution in dogs and cats, In Practice 27, 2005, 399–405. [Google Scholar]
- Bosman R.J., Minton J., Ju H.J., et al. Free polymerized hemoglobin versus hydroxyethyl starch in resuscitation of hypovolemic dogs, Anesthesia and Analgesia 75, 1992, 811–817. [DOI] [PubMed] [Google Scholar]
- Callan M.B., Rentko V.T. Clinical application of a haemoglobin-based oxygen carrying solution, Veterinary Clinics of North America Small Animal Practice 33, 2003, 1277–1293. [DOI] [PubMed] [Google Scholar]
- Crystal M.A., Mott J., Van der Veldt P. Blood loss and no matching donor, Veterinary Forum 16, 1999, 72–73. [Google Scholar]
- Estrin M., Wehausen C.E., Jessen C.R., Justine A.L. Disseminated intravascular coagulation in cats, Journal of Veterinary Internal Medicine 20, 2006, 1334–1339. [DOI] [PubMed] [Google Scholar]
- Fox P.R. Feline cardiomyopathies, Textbook of Canine and Feline Cardiology. Principles and Clinical Practice, 2nd edn, 1999, WB Saunders: Philadelphia, 621–678. [Google Scholar]
- Gibson G.R., Callan M.B., Hoffmann V., Giger U. Use of haemoglobin based oxygen carrying solutions in cats: 72 cases (1998–2000), Journal of the American Veterinary Medical Association 221, 2002, 96–102. [DOI] [PubMed] [Google Scholar]
- Hamilton R., Kelly N., Gawryl M., Rentko V.T. Absence of immunopathology associated with repeated administration of a Hb-based oxygen carrier in dogs, Transfusion 41, 2001, 219–225. [DOI] [PubMed] [Google Scholar]
- Harringer W., Hodakowski G.T., Svizzero T., Jacobs E.E., Vlahakes G.J. Acute effects of massive transfusion of a bovine hemoglobin blood substitute in a canine model of hemorrhagic shock, European Journal of Cardio-Thoracic Surgery 6, 1992, 649–654. [DOI] [PubMed] [Google Scholar]
- Muir W.W., Wellmann M.L. Hemoglobin solutions and tissue oxygenation, Journal of Veterinary Internal Medicine 17, 2003, 127–135. [DOI] [PubMed] [Google Scholar]
- Nolte D.M., Crovatto A. Cyanosis from acetaminophen toxicosis, Veterinary Forum 16, 1999, 55–57. [Google Scholar]
- Rentko V.T., Wohl J., Murtaugh R., Cotter S., Hohenhaus A., Callan M.B., Hansen B., Jacobson J., Couto G., Gawryl M.S. A clinical trial of hemoglobin-based oxygen-carrying (HBOC) fluid in the treatment of anemia in dogs, Journal of Veterinary Internal Medicine 10, 1996, 177. [Google Scholar]
- Rentko V.T., Sharpe T.A. Red blood cell substitutes. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Hematology, 2000, Lippincott Williams and Wilkins: Baltimore, 874–878. [Google Scholar]
- Spahn D.R. Current status of artificial oxygen carriers, Advanced Drug Delivery Reviews 40, 2000, 143–151. [DOI] [PubMed] [Google Scholar]
- Standl T.G., Horn P., Wilhelm S., Greim C., Freitag M., Freitag U. Bovine hemoglobin is more potent than autologous red blood cells in restoring tissue oxygenation after profound isovolemic hemodilution in dogs, Canadian Journal of Anaesthesia 43, 1996, 714–723. [DOI] [PubMed] [Google Scholar]
- Wall R.E. Synthetic colloids and blood substitutes, Supplement of the Compendium on Continuing Education in Veterinary Practice 20, 1998, 4–9. [Google Scholar]
- Wardrop K.J., Reine N., Birkenheuer A., Hale A., Hohenhaus A., Crawford C., Lappin M.R. Canine and feline blood donor screening for infectious disease, Journal of Veterinary Internal Medicine 19, 2005, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart C., Giger U., Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation, Journal of Feline Medicine and Surgery 6, 2004, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein N.W., Blais M.C., Harris K., Oakley D.A., Aronson L.R., Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen, Journal of Veterinary Internal Medicine 21, 2007, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]