Abstract
This prospective, multicentre, non-blinded, open study followed 46 cats with diabetes mellitus during treatment with porcine lente insulin (also known as porcine insulin zinc suspension, Caninsulin®, Intervet) for 16±1 weeks (stabilization phase), with additional monitoring of some cats (n=23) for a variable period. At least three of the following were present at initial presentation: appropriate history of clinical signs consistent with diabetes mellitus, glucosuria, blood glucose greater than 15 mmol/l and fructosamine greater than 380 μmol/l. Insulin treatment was started at a dose rate of 0.25–0.5 IU/kg body weight twice daily, with a maximum starting dose of 2 IU/injection. Twenty-eight of the cats were classed as reaching clinical stability during the study, in 23 of these cats this was during the stabilization phase. Seven cats went into remission during the stabilization phase and one of the cats in week 56. Clinical signs of hypoglycaemia, significantly associated with a dose of 3 units or 0.5 IU/kg or more per cat (twice daily), were observed in nine of the 46 cats during the stabilization phase and concomitant biochemical hypoglycaemia was recorded in most cases. Biochemical hypoglycaemia, recorded in 6% of the blood glucose curves performed during the stabilization phase, was significantly associated with a dose rate of 0.75 IU/kg or more twice daily. This further highlights the need for cautious stepwise changes in insulin dose. The protocol used in the present study is suitable for and easy to use in practice. This study confirmed the efficacy and safety of porcine lente insulin (Caninsulin®) in diabetic cats under field conditions.
Although several pathogenic processes are involved in the development of diabetes, the vast majority of cases of diabetes fall into two broad aetiopathogenic categories (type 1 and type 2), where there is an absolute deficiency of insulin secretion (type 1) or a combination of resistance to insulin action and an inadequate compensatory insulin secretory response (type 2) (American Diabetes Association 2003). Diabetes mellitus, one of the most common endocrine disorders of middle aged and old cats (Rand and Martin 2001), is characterized by hyperglycaemia resulting from defects in insulin secretion, insulin action, or both (type 2 diabetes).
Diabetic cats most commonly have partial pancreatic islet destruction associated with pancreatic amyloidosis and are insulin-deficient (Stogdale 1986). Although cats with detectable insulin concentrations at the time of diagnosis can potentially be treated with oral hypoglycaemic agents, such as glipizide (Nelson et al 1993), the majority of diabetic cats require exogenous insulin administration to control hyperglycaemia. Chronic exposure to hyperglycaemia can lead to cellular dysfunction that may become irreversible, a process that is termed glucose toxicity (Robertson et al 2003). In cats, clinical remission of diabetes mellitus can occur even after months of treatment, but is likely dependent upon the underlying pathogenesis, together with early therapy to minimize glucose toxicity, and is only possible if the beta cell mass has not been destroyed (Rand 1998, Feldman and Nelson 2004).
A number of studies have looked retrospectively at cats with diabetes mellitus treated with a variety of different insulins and different regimes (insulin dose, insulin treatment regime and diet) (Bertoy et al 1995, Crenshaw and Peterson 1996, Kraus et al 1997, Goossens et al 1998). Only two published studies have attempted to apply standardized criteria for both diagnosis and treatment of diabetes mellitus in cats from presentation (Nelson et al 2001, Martin and Rand 2007a). The present multicentre, prospective study in veterinary practices in Europe aimed to confirm the efficacy and safety of twice daily administration of porcine lente insulin (Caninsulin®, Intervet) to cats.
Materials and methods
Animals
Cats newly diagnosed or previously treated but poorly controlled with uncomplicated diabetes mellitus were admitted to the study and their clinical status assessed at admission (week 0, day 0), on days 1–3 and in weeks 1, 3, 6, 9 and either 12 or 16 (stabilization phase). Additional monitoring beyond 16 weeks was optional, with examinations performed roughly every 3 months for a variable period at the investigator's discretion. At admission, at least three of the following should have been present: appropriate history of clinical signs consistent with diabetes mellitus, glucosuria, blood glucose concentrations greater than 15 mmol/l and fructosamine concentrations greater than 380 μmol/l. Uncomplicated diabetes mellitus was defined as cats with diabetes mellitus without identifiable co-existing primary disease (eg, hyperthyroidism, major infection or organ failure, acromegaly, hyperadrenocorticism) that had not received steroid hormone treatment (short-acting corticosteroids within 14 days or long-acting corticosteroids or progestogens within 6 weeks). Cats presented with diabetic ketoacidosis (DKA) and/or with urinary tract infection could be included in the study following appropriate initial treatment (eg, fluid therapy, rapid acting insulin, antimicrobial therapy) for stabilization or elimination of infection.
Treatment
Porcine lente insulin (40 IU/ml porcine insulin zinc suspension, Caninsulin® (also known as Vetsulin®), Intervet International bv, Boxmeer, The Netherlands), an intermediate acting, porcine insulin zinc suspension, was administered by subcutaneous injection using 40 IU/ml syringes. The starting dose was based on the initial blood glucose concentration of each cat; 0.25 IU/kg if the blood glucose concentration was less than 20 mmol/l and 0.5 IU/kg if greater than 20 mmol/l. The maximum starting dose was not to exceed 2 IU/dose and dose rates greater than 0.5 IU/kg twice daily were not recommended during the first 3 weeks of treatment. Concurrent treatment with steroid hormones, oral hypoglycaemic agents or α2 agonists, since stimulation of α2-adrenoceptors depresses insulin secretion producing transient hyperglycaemia, was not permitted. Each cat was fed a standard feline diet based on its usual routine. The type of diet, amount of food, feeding regime and any change of diet were documented.
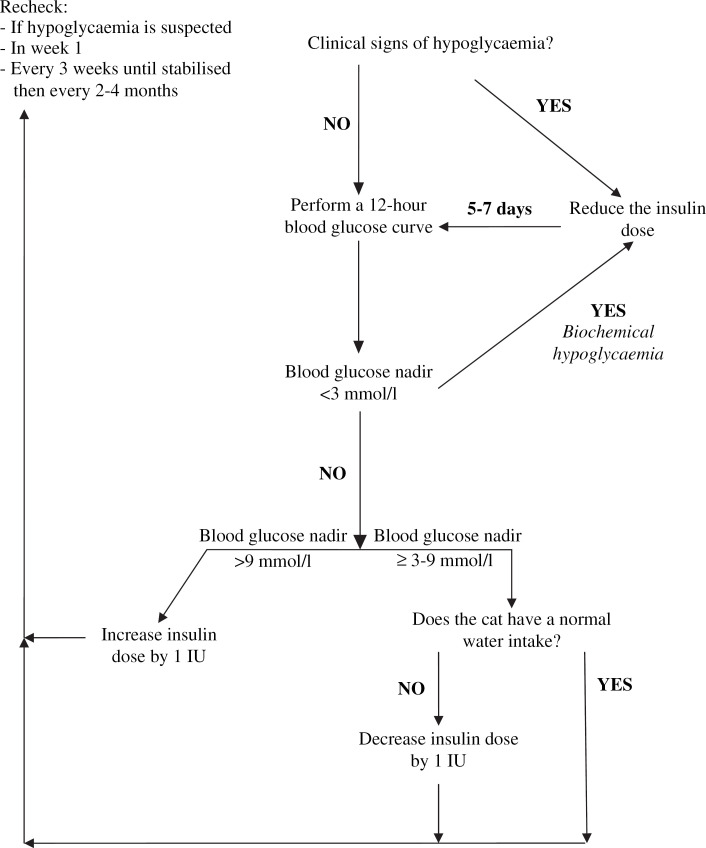
Dose adjustments were based on a dosage algorithm (Fig 1). Cats with hypoglycaemia (showing clinical signs and/or documented biochemical hypoglycaemia (any blood glucose measurement of less than 3 mmol/l during any 12-h test period)) had their insulin dose reduced by at least 1 IU. Cats with a pre-insulin blood glucose concentration less than 9 mmol/l on any examination day and/or clinical signs of hypoglycaemia had insulin administration postponed by 12 h and the insulin dose adjusted as necessary, based on blood glucose concentration. If, after 24 h, the blood glucose concentration was still low, these cats were sent home without insulin treatment and rechecked 1 week later to confirm diabetic clinical remission.
Fig 1.
Algorithm for insulin dosage adjustment in cats (after Rand and Martin 2001).
Clinical examinations
At each visit, the investigator asked each cat owner whether their cat had polyphagia and other relevant clinical signs (polyuria and polydipsia) and whether it had shown clinical signs of hypoglycaemia. This was followed by a thorough physical examination and measurement of body weight. At each visit, the investigator recorded whether in their opinion the cat was stable clinically or not. Clinical stability was defined as a cat that was healthy and interactive at home with a normal appetite and water intake (based on the cat owner's opinion) and stable body weight.
Laboratory evaluations
Blood samples for blood glucose measurement were taken from the cephalic vein and measured using a hand-held, portable blood glucose metre (Elite series, Bayer bv, Mijdrecht, The Netherlands). Each investigator used the same type of portable blood glucose metre. Each portable glucometre was calibrated for each new box of test strips according to manufacturers' recommendations. The blood glucose metre used had been validated in diabetic cats and compared with the gold standard hexokinase method using a Cobas Integra Analyser (Roche, Basel, Switzerland) (Wess and Reusch 2000). Within- and between-day variation was also assessed (Wess and Reusch 2000). This hand-held blood glucose metre measures in the range 1.1–33.3 mmol/l. Blood glucose concentrations above 33.3 mmol/l give a reading of “HI” and below 1.1 mmol/l a reading of “LO”. A nominal value (34.0 mmol/l) was attributed to the “HI” values.
Blood samples were taken for blood glucose measurement at admission, prior to the first insulin dose and at 3, 6, and 9 h afterwards and prior to the second and third insulin doses. At subsequent visits, blood samples were collected in the same manner prior to insulin injection and then every 2 h for 12 h.
Haematology, clinical chemistry (including total bilirubin, fasting bile acids, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, urea, creatinine, sodium, potassium, chloride, calcium and phosphate concentrations) and total thyroxine concentration were evaluated at admission. Fructosamine, total protein, albumin, lipase, triglycerides and cholesterol were measured at admission and at each subsequent visit. Fructosamine values greater than 500 μmol/l were considered to reflect less than adequate control. For other parameters, the results for each cat were assessed based on the reference range for the laboratory where the analysis was performed and values more than 10% outside the reference range were considered to reflect significant organ damage or dysfunction.
At admission and in weeks 1, 3, 6, 9, 12 and/or 16 and thereafter, if deemed necessary by the investigator, a urine sample was collected from each cat by antepubic cystocentesis. This was tested using a urine dipstick (Multistix 10SG, Bayer, Newbury, UK), which includes tests for ketones, and glucose and submitted for bacterial culture and sensitivity testing.
Statistics
The individual animal was the statistical unit. The data were described using descriptive statistics. The level of significance (α) was set at 0.05. The Shapiro–Wilk test was used to test if the data were distributed normally. Data are presented as mean and the standard deviation (mean±SD) and median (range), as appropriate. Data were put in contingency tables to examine whether there was a relationship between the investigator classing the cat as clinically stable or not and relevant biochemistry parameters (fructosamine, fructosamine at admission, mean blood glucose (12 h), blood glucose nadir) and insulin dose using Fisher's exact test. This was also used to examine the differences between cats that went into diabetic clinical remission and those that did not. The relative risk (RR) and 95% confidence interval (CI) are presented, where appropriate.
Results
Animals
Fifty-six cats were included in the study. Ten cats could not be included in the final data analysis due to significant concurrent disease (one hyperthyroidism and one acromegaly), prohibited treatment (one topical corticosteroids), insufficient data (seven cats [two died, three owner compliance, and two difficulty in taking blood samples]). Forty-six cats (32 males [27 castrated], 13 females [10 spayed] and one cat of unspecified sex) aged 10.7±2.9 years (n=44) and weighing 5.0 kg (2.3–11.4 kg) were included in the data analysis. The majority of the cats (n=40, 87%) were non-purebred cats (domestic/European short/long haired): there were six purebred cats (two Maine Coon and one each of Burmese, Siamese, Abyssinian and Foreign shorthair). Thirty-nine of the 46 cats (85%) had just been diagnosed with diabetes mellitus, with clinical signs reported to be present for 5 weeks (range 1–40 weeks, n=35).
Admission
The clinical signs at admission are shown in Table 2. Cats that had been treated previously (seven cats, 15%) or those that met only three of the four inclusion criteria are shown in Table 1. The initial blood glucose concentration was 24.0±6.1 mmol/l (n=44) and fructosamine (Table 3) was 581±147 μmol/l (n=44). Glucosuria was reported in all cats where it was measured (n=44). Ketonuria was present in 12 of these cats (27%).
Table 2.
Clinical signs at admission
| Clinical sign | Number of cats (%) |
| Polydipsia | 38 (83) |
| Polyuria | 37 (80) |
| Weight loss | 27 (59) |
| Depression | 19 (41) |
| Polyphagia | 17 (37) |
| Poor appetite/anorexia | 14 (30) |
| Vomiting | 8 (17) |
| Weakness | 7 (15) |
| Lethargy | 6 (13) |
| Diarrhoea | 5 (11) |
| Anxiety | 3 (6) |
| Obesity | 2 (4) |
| Plantigrade stance | 2 (4) |
| Underweight | 1 (2) |
| Weight gain | 1 (2) |
| Dull hair coat/dry skin | 1 (2) |
Table 1.
Cats meeting only three of the four inclusion criteria or that had been treated for diabetes prior to admission to the trial
| Cat | Duration of clinical signs | Previous treatment for diabetes | Clinical signs | Glucosuria | Blood glucose >15 mmol/l | Fructosamine >380 μmol/l |
|---|---|---|---|---|---|---|
| #1 | 2 days | Bovine protamine zinc insulin (Insuvet PZI, Schering Plough Animal Health, UK) | + | + | − | + |
| #14 | 3 days | Porcine lente insulin (Caninsulin, Intervet) | + | + | − | + |
| #15 | 3 weeks | None | + | + | − | + |
| #18 | 5 months | Porcine lente insulin (Caninsulin, Intervet), glipizide | + | + | + | + |
| #19 | 3 months | Oral hypoglycaemic agent (unspecified) | + | + | + | + |
| #20 | 5 months | None | + | + | − * | + |
| #24 | 3 months | Porcine lente insulin (Caninsulin, Intervet), recombinant human lente insulin (Humulin, Eli Lilly) and glipizide | + | NS | + | + |
| #26 | 19 months | Treatment with various insulins (unspecified) | + | + | + | − |
| #28 | 6 months | None | + | + | + | NS |
| #33 | Short history (unspecified) | None | + | + | + | NS |
| #36 | 18 months | Recombinant human insulin crystalline zinc suspension (Ultratard, Novo Nordisk) | + | + | + | + |
| #38 | 1 week | None | + | + | + | − |
| #41 | 1 year | None | + | + | + | − † |
| #48 | 6 days | None | + | + | + | – |
NS, No sample.
Cat #20, initial blood glucose 14.4 mmol/l, second pre-treatment sample 1 day later 22.9 mmol/l. Cat did not go into clinical remission.
Cat #41, initial fructosamine 367 μmol/l, week 1: 367 μmol/l, week 3: 417 μmol/l and week 6: 367 μmol/l. Remission in week 8: fructosamine 220 μmol/l.
Table 3.
Clinical chemistry parameters in diabetic cats before and during treatment with porcine lente insulin
| Fructosamine (μmol/l) (mean±SD) | Cholesterol (mmol/l) (mean±SD) | Triglycerides (mmol/l) (median (range)) | Lipase (IU/l) (median (range)) | Total protein (g/l) (mean±SD) | Albumin (g/l) (mean±SD) | |
| Admission n, % | 581±147, 44, 68 | 6.69±2.86, 45, 56 | 0.87 (0.24–12.90), 42, 40 | 95 (5–1788), 40, 37 | 76±9, 46, 11 | 34±4, 46, 4 |
| Week 1±0, n, % | 525±149, 41, 54 | 5.46±2.83, 40, 60 | 1.31 (0.37–12.56), 42, 45 | 125 (95–1919), 37, 35 | 77±9, 43, 12 | 34±4, 42, 0 |
| Week 3±1, n, % | 553±171, 40, 60 | 4.58±2.00, 33, 39 | 1.00 (0.18–17.19), 37, 19 | 123 (8–2199), 34, 47 | 80±8, 40, 15 | 34±5, 39, 3 |
| Week 7±1, n, % | 532±141, 40, 62 | 5.18±2.48, 35, 57 | 1.20 (0.37–4.68), 38, 21 | 80 (8–1948), 27, 41 | 81±9, 39, 15 | 35±4, 40, 5 |
| Week 9±1, n, % | 505±162, 31, 58 | 5.10±3.02, 28, 61 | 0.94 (0.30–3.20), 31, 26 | 63 (7–2506), 23, 43 | 79±6, 31, 6 | 34±3, 32, 0 |
| Week 13±1, n, % | 521±163, 36, 58 | 4.68±2.47, 30, 53 | 1.31 (0.20–7.66), 34, 23 | 90 (1–3011), 28, 43 | 78±8, 33, 1 | 35±4, 35, 0 |
| Week 16±1, n, % | 536±167, 31, 64 | 4.96±2.30, 26, 31 | 1.04 (0.20–6.20), 31, 10 | 79 (1–2625), 27, 30 | 81±6, 8, 10 | 34±3, 30, 0 |
| Week 19±1, n, % | 551±116, 11, 73 | 5.34±1.75, 9, 33 | 0.83 (0.50–2.65), 9, 0 | 96 (5–2625), 8, 62 | 81±7, 6, 25 | 33±3, 8, 0 |
| Week 24±1, n, % | 626±70, 6, 100 | 5.60±1.75, 6, 83 | 1.18 (0.52–1.69), 6, 0 | 156 (37–2031), 6, 67 | 84±6, 6, 33 | 33±4, 6, 0 |
| Week 32±3, n, % | 482±185, 17, 53 | 4.25±2.48, 13, 46 | 1.09 (0.44–6.10), 12, 17 | 110 (1–2275), 12, 50 | 79±6, 15, 7 | 33±4, 15, 0 |
| Week 49±5, n, % | 482±114, 7, 53 | 4.58±1.75, 5, 20 | 1.40 (0.30–6.80), 7, 29 | 159 (25–300), 5, 60 | 77±6, 7, 14 | 35±3, 7, 0 |
Data expressed as mean±SD if distributed normally or as median (range) if not distributed normally. Number of samples and percent of samples >499 μmol/l (fructosamine) or more than 10% outside of the reference range for the laboratory where the measurement was performed. Cats went into clinical remission in weeks 2, 3, 8 (three cats), 18, 20 and 56.
At admission, 34 of the cats had uncomplicated diabetes mellitus, nine DKA and two hyperosmolar, hyperglycaemic, non-ketotic (HHNK) syndrome. With the exception of one cat with DKA, all of the cats with DKA or HHNK syndrome and five other cats were managed initially using fluid therapy alone, rapid acting insulin alone or a combination of these. Three cats (7%) had positive urine culture (Escherichia coli) at admission. Sixteen urine samples were taken from cats for bacterial culture for suspected urinary tract infection at later dates. One cat had a positive urine culture (E coli and alpha haemolytic Streptococcus species) after 9 weeks of treatment.
Haematology and clinical chemistry findings included leucocytosis (32%), elevated fasting bile acids (32%), elevated liver enzymes (AST 74%; ALT 53% and alkaline phosphatase 27%) and abnormal electrolyte concentrations (including hypo- (13%) and hyper-kalaemia (4%) and hypo- (12%), hyper-phosphataemia (12%) and hypertriglyceridaemia (33%)). Only one cat had total thyroxine concentrations above the upper limit of the reference range but the cat did not have clinical signs consistent with a diagnosis of hyperthyroidism and the total thyroxine concentration measurement was repeated and was within the reference range on all other occasions. Twenty cats (48%) had evidence of euthyroid sick syndrome: total thyroxine or free thyroxine below the lower limit of the reference range.
Where the type of food was specified (n=41), 10 cats were fed dry food only, 11 moist food only and 20 a mixture of dry and moist food. All but one of these cats were fed commercial cat food. Only nine cats (20%) were fed a diet that may help manage body weight (Hill's w/d or r/d, Hill's Pet Nutrition Ltd or Whiskas low calorie, Masterfoods). Where specified (n=43), 28 cats (65%) were fed ad libitum and 15 cats (35%) had a controlled number of meals per day.
Stabilization
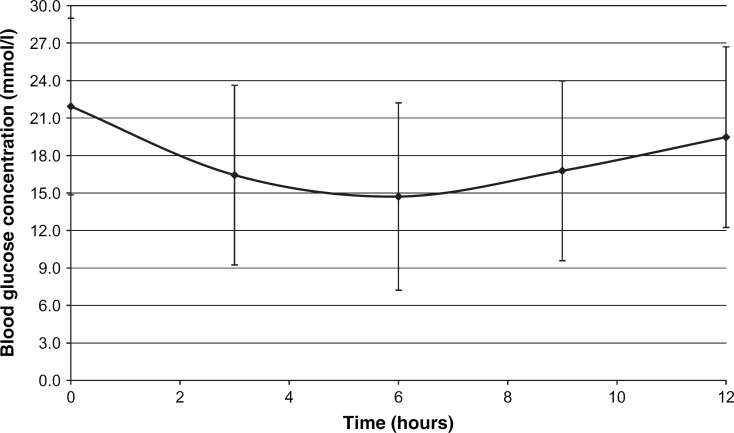
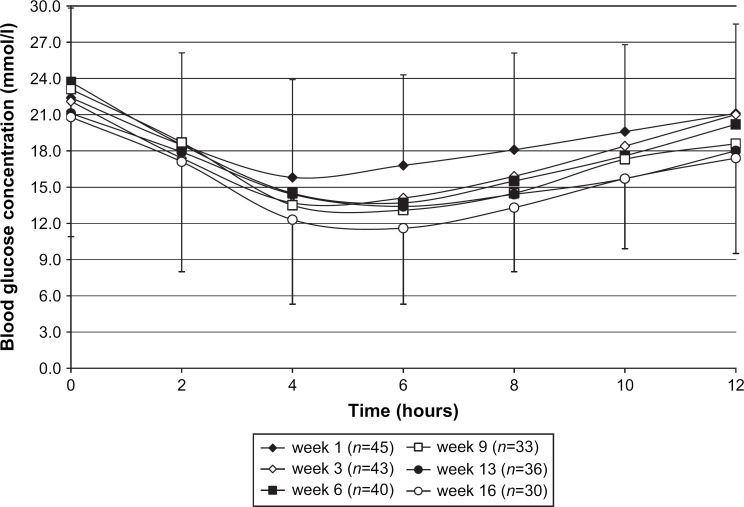
The 12-h blood glucose curve after the first insulin dose is shown in Fig 2. The clinical signs improved and the percentage of cats with normal attitude and appetite increased markedly during the first few weeks of the study. The 12-h blood glucose curves during the stabilization phase are shown in Fig 3. One cat in week 3 and another in week 12 had clear evidence of rebound hyperglycaemia (Somogyi effect). Insulin dose, mean blood glucose and peak insulin action are shown in Table 4.
Fig 2.
Blood glucose curve (mean±SD, n=46) after initial treatment with porcine lente insulin.
Fig 3.
Mean blood glucose curves during the stabilization phase. Error bars represent the standard deviation of the mean in weeks 1 and 16. Values in other weeks (not shown) are numerically similar.
Table 4.
Insulin dose, mean blood glucose and peak insulin action in diabetic cats during treatment with porcine lente insulin
| Insulin dose (IU/kg twice daily) (mean±SD) | Insulin dose (IU/cat twice daily) (median (range)) | Mean 12-h blood glucose (mmol/l) (mean±SD) | Blood glucose nadir (mmol/l) (mean±SD) | Time to nadir (h) (median (range)) | |
| Week 0 (admission) | 0.34±0.14 (n=46) | 1 (1–4) (n=46) | 18.0±6.2 (n=46) | 13.0±6.9 (n=46) | 6 (3–12) (n=46) |
| Week 0 (discharge) | 0.34±0.18 (n=41) | 1 (1–4) (n=41) | 17.8±6.1 (n=46) | ||
| Week 1±0 | 0.45±0.18 (n=45) | 2 (1–5) (n=45) | 19.2±6.1 (n=45) | 14.0±7.2 (n=45) | 5 (2–10) (n=45) |
| Week 3±1 | 0.52±0.26 (n=37) | 3 (1–7) (n=38) | 18.5±6.3 (n=43) | 10.9±6.1 (n=43) | 5 (2–12) (n=42) |
| Week 7±1 | 0.60±0.24 (n=36) | 3 (1–7) (n=38) | 18.2±6.0 (n=40) | 11.8±6.6 (n=40) | 6 (2–10) (n=38) |
| Week 9±1 | 0.67±0.24 (n=32) | 3 (1–8) (n=32) | 17.1±5.9 (n=33) | 11.3±6.6 (n=33) | 6 (2–12) (n=33) |
| Week 13±1 | 0.74±0.28 (n=30) | 3 (1–9) (n=32) | 16.6±6.5 (n=36) | 10.9±7.0 (n=36) | 6 (2–12) (n=34) |
| Week 16±1 | 0.64±0.32 (n=30) | 4 (1–7) (n=30) | 15.4±6.1 (n=30) | 9.4±6.0 (n=30) | 6 (2–12) (n=30) |
| Week 19±1 | 0.55±0.21 (n=10) | 3 (1–7) (n=10) | 15.2±5.5 (n=10) | 9.9±6.5 (n=10) | 6 (4–12) (n=10) |
| Week 24±1 | 0.63±0.25 (n=8) | 4 (1–8) (n=8) | 18.3±7.4 (n=8) | 11.2±7.3 (n=8) | 6 (4–10) (n=8) |
| Week 32±3 | 0.64±0.22 (n=17) | 3 (1–6) (n=17) | 16.5±6.6 (n=17) | 10.2±6.6 (n=17) | 5 (2–9) (n=17) |
| Week 49±5 | 0.69±0.31 (n=8) | 4 (1–11) (n=8) | 13.8±6.8 (n=8) | 7.4±5.3 (n=8) | 6 (3–10) (n=8) |
Data are expressed as mean±SD if distributed normally or as median (range) if not distributed normally. Cats went into clinical remission in weeks 2, 3, 8 (three cats), 18, 20 and 56.
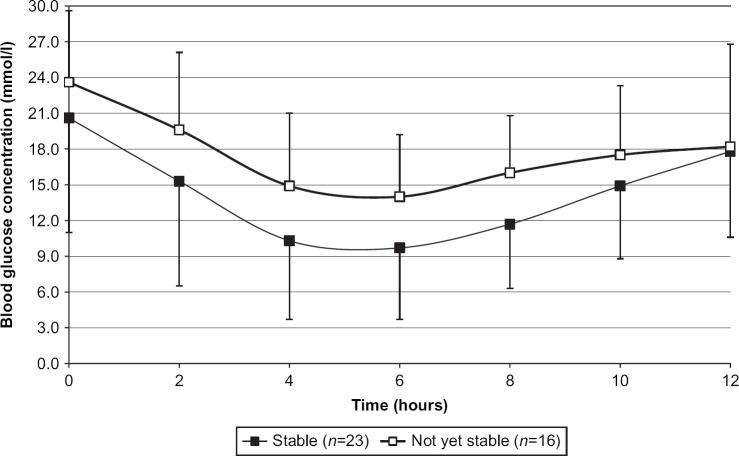
Twenty-three cats, excluding the seven cats that had gone into remission during the stabilization phase, were recorded by the investigators as being clinically stable by week 16±1 (Table 5). The time until each cat was first recorded as being clinically stable was quite variable (10±5 weeks). Cats classified as clinically stable no longer had clinical signs of diabetes (polyuria and polydipsia), had normal attitude and usually had normal appetite. These cats were significantly less likely to have a nadir blood glucose concentration of greater than 13.9 mmol/l (P=0.031, RR 0.382 95% CI 0.141–1.031) or a mean blood glucose concentration of greater than 13.9 mmol/l (P=0.012, RR 0.485 95% CI 0.308–0.764) at the end of the stabilization phase. There was no significant association (P>0.05) between the investigators' classification of a cat as stable clinically and previous treatment for diabetes mellitus, or admission blood glucose, admission fructosamine or body weight at admission, or time to blood glucose nadir or maximum blood glucose concentration, fructosamine, insulin dose, or body weight at the end of the stabilization phase or change in body weight from admission to the end of the stabilization phase. The mean 12-h blood glucose curves in cats classified as stable or not are shown in Fig 4. Insulin dose, mean blood glucose and peak insulin action are shown in Table 4.
Table 5.
Insulin dose, mean blood glucose, peak insulin action and fructosamine in cats still requiring porcine lente insulin treatment classified as stable (n=23) or not yet stable (n=16) at the end of the stabilization phase
| Clinical status | Body weight (kg) (mean±SD) | Insulin dose (IU/kg twice daily) (mean±SD) | Insulin dose (IU/cat twice daily) (median (range)) | Mean 12-h blood glucose (mmol/l) (mean±SD) | Blood glucose nadir (mmol/l) (mean±SD) | Time to nadir (h) (median (range)) | Fructosamine (μmol/l) (mean±SD) |
| Stable | 5.2±1.0 (n=23) | 0.64±0.32 (n=22) | 3 (1–6) (n=22) | 14.0±5.7 (n=23) | 7.7±5.1 (n=23) | 4 (2–12) (n=23) | 504±145 (n=23) |
| Not yet stable | 5.6±1.2 (n=16) | 0.72±0.40 (n=14) | 4 (1–9) (n=14) | 17.2±5.4 (n=16) | 11.9±5.7 (n=16) | 6 (2–12) (n=16) | 598±160 (n=16) |
Data are expressed as mean±SD if distributed normally or as median (range) if not distributed normally.
Fig 4.
Blood glucose curves (mean±SD) from cats classified as clinically stable or not after the stabilization phase.
Twenty-three of the 39 cats (59%) that had not gone into remission by the end of the stabilization phase were re-examined at a later date (Tables 3 and 4). Clinical chemistry results from these cats in Table 3 and insulin dose, mean blood glucose and peak insulin action are shown in Table 4. Five of these cats were recorded as reaching clinical stability in weeks 20, 29, 30, 37 and 54, respectively, and one further cat went into diabetic clinical remission (in week 56).
Diabetic clinical remission
Seven cats (15%) were in clinical remission by the end of the stabilization phase of the study. Only one of the cats followed for a variable period thereafter was recorded as going into clinical remission. Insulin treatment was stopped in weeks 2, 3, 8 (n=3), 18, 20 and 56. Blood glucose concentrations ranged between 1.1 and 11.2 mmol/l (43 samples) at the time remission was suspected and 3.2 and 8.3 mmol/l (25 samples) at the time remission was confirmed. None of the cats that went into clinical remission had been treated previously for diabetes mellitus. All but one of these cats had fructosamine concentrations less than 500 μmol/l when remission was suspected (306±135 μmol/l, n=7, one missing value) and all of the cats had a fructosamine concentration less than 500 μmol/l (254±62 μmol/l, n=8) when remission was confirmed 5 weeks later (median, range 1–9 weeks). The lipase, cholesterol, triglycerides and total protein concentrations from all of these cats were within 10% of the laboratories reference ranges.
Cats that did not go into remission by the end of the stabilization period were significantly more likely to have a maximum blood glucose greater than 22.9 mmol/l (P=0.039), mean 12-h blood glucose of greater than 8.9 mmol/l (P=0.0002), nadir blood glucose concentration greater than 5.9 mmol/l (P=0.003) or a fructosamine concentration of greater than 460 μmol/l (P=0.021, RR 12.000 95% CI 1.585–90.844) at this point. There was no statistically significant association between fructosamine concentration at admission, initial (admission) blood glucose, time to blood glucose nadir, body weight or change in body weight from admission and a cat going into clinical diabetic remission.
Hypoglycaemia
Biochemical hypoglycaemia (any blood glucose measurement less than 3 mmol/l during a 12-h test period) was recorded in 25 of the 396 (6%) blood glucose curves from 19 cats performed during the stabilization phase (Table 6). A dose rate of 0.75 IU/kg or more twice daily was significantly more likely to be associated with biochemical hypoglycaemia (P=0.041, RR 2.346 95% CI 1.155–4.768). Clinical hypoglycaemia (n=8, Table 6) was significantly likely to be associated with a dose of greater than or equal to 3 units (P=0.023, RR 9.040 95% CI 1.142–71.565) or greater than or equal to 0.5 IU/kg (P=0.037, RR 8.000 95% CI 1.010–63.361) per cat twice daily.
Table 6.
Cats with biochemical (n=19) and/or clinical hypoglycaemia (n=8) during treatment with porcine lente insulin
| Hypoglycaemia | Insulin dose (IU/cat twice daily) | Insulin dose (IU/kg twice daily) | Prior to clinical remission, n | Statistics*, P |
| Biochemical (n=19) | 3 (1–9) | 0.62±0.33 | 4 | 0.866 |
| Clinical (n=8) | 6 (1–9) | 0.91±0.44 | 2 | 0.994 |
Fisher's exact test was used to look at the relationship between the occurrence of biochemical or clinical hypoglycaemia and the onset of clinical diabetic remission.
Discussion
This prospective, multicentre, non-blinded, open study confirmed that twice daily porcine lente insulin is safe and effective in the management of uncomplicated diabetes in cats. The protocol used is suitable for and easy to use in practice. Not all cats with diabetes mellitus necessarily have elevated fructosamine concentrations at diagnosis, although clinical signs and glucosuria are usually present. Achieving diabetic stability may take at least 3–4 months and although clinical remission usually develops in this period it may take considerably longer. Insulin treatment of diabetic cats without dietary modification will probably result in clinical remission in around one-fifth and stability in two-thirds or more of uncomplicated cases. Hypoglycaemia is not an infrequent finding in diabetic cats. This is usually associated with a high insulin dose, care should be taken when increasing insulin dose and doses greater than 2 units/cat twice daily should be used with caution, particularly in the first few weeks of treatment.
The majority of cats included in the present study had not been treated previously for diabetes mellitus. The seven cats that had been treated for diabetes mellitus were not stable on that treatment and met the inclusion criteria and did not go into clinical remission (Table 1). It has been shown that it is possible to reverse glucose toxicity and restore sufficient beta cell function to produce clinical remission in cats that have been diabetic for 14–30 months but this appears less likely in cats that have been diabetic for 36–72 months (Bennett et al 2006). In the present study, the one cat that had not been treated previously for diabetes mellitus went into clinical remission after 56 weeks of treatment, underlying the need for perseverance in the management of feline diabetics.
The results of the present study (around 60% clinically stable after 3–4 months of treatment) are in line with studies published previously, using a variety of different insulins, whether clinical assessment or blood glucose measurements were used to assess stability (Bertoy et al 1995, Goossens et al 1998, Martin and Rand 2007a).
Clinical remission (15% after the stabilization phase of 3–4 months) in the present study is in line with previous studies (Goossens et al 1998, Martin and Rand 2007a). More recently, Weaver et al (2006) reported a remission rate of 43% in cats administered a different lente insulin twice daily and fed a high-protein, low-carbohydrate diet for 12 weeks. Insulin glargine, which is not approved for veterinary use, in combination with a high-protein, low-carbohydrate diet, has been reported to result in higher clinical remission rates when administered twice daily (Rand 2006) but not once daily (Weaver et al 2006). The regulations in many countries (eg, Europe) mean that veterinary surgeons have a legal responsibility to prescribe an approved veterinary medicinal product as the treatment of first choice for diabetic cats. More recently, Bennett et al (2006) showed that insulin (NPH, a different lente insulin, ultralente or protamine zinc insulin) could be discontinued in 41–68% of the 63 cats they studied when combined with dietary change (to a moderate carbohydrate, high-fibre or low-carbohydrate, low-fibre food), exercise and weight loss. This is in line with previous studies (Mazzaferro et al 2003) and emphasizes the importance of diet on glycaemic control in diabetic cats.
Each investigator used the same type of portable blood glucose metre, which has been evaluated and shown to give accurate readings (relative to the hexokinase method) in blood samples from cats (Wess and Reusch 2000), so that blood glucose measurements could be compared between centres. The low end and high end ranges of portable blood glucose metres are notoriously inaccurate. In particular, portable blood glucose metres generally measure erroneously low, so as to warn of impending and potentially life threatening hypoglycaemia. It is, therefore, possible that the present study overestimated the number of cats with biochemical hypoglycaemia. In practice, a low reading from a portable blood glucose metre should be confirmed using a reference analyser. In the present study, all of the blood glucose curves were performed in an in hospital situation and therefore a degree of stress hyperglycaemia cannot be ruled out. Ideally blood glucose concentrations should be maintained below the renal threshold to prevent glucosuria and subsequent polyuria and polydipsia whilst avoiding a too rapid decline in blood glucose concentration and hypoglycaemia and resultant clinical signs and/or rebound hyperglycaemia.
Fructosamine concentrations in cats can be corrected for total protein (Reusch and Haberer 2001) but this is only essential if there is a problem with protein turnover or loss and was not performed in the present study. It should also be remembered that there is a lag between blood glucose concentrations and their reflection in the fructosamine concentrations due to the half-life of serum proteins (Elliott et al 1999). The closer fructosamine concentration is to the upper limit of the reference range for non-diabetic cats, the better the stability. Only one of the 14 cats (three of which had been treated previously for diabetes mellitus) with admission fructosamine less than 500 μmol/l went into clinical remission, after 8 weeks of treatment.
Nelson et al (2001) reported that fructosamine concentrations decreased from 598±110 to 419±128 μmol/l after 45 days of treatment, with values remaining above the 500 μmol/l cut-off in 15% of cats at this point. Martin and Rand (2007b) concluded that in the laboratory that they used fructosamine concentrations below 422 μmol/l reflect exemplary control. In the present study, although average fructosamine concentration remained above 500 μmol/l, the number of cats with elevated fructosamine decreased (from 30 to 20 cats) with time and 10 cats had fructosamine below 422 μmol/l. There was an apparent worsening of the average fructosamine concentration (and a number of the clinical chemistry parameters) at the 24±1 week's visit in the present study but is not thought to be a reflection of loss of control of diabetes. The effect may in part be due to the low number of cats examined at this time point. Only one of the cats examined at 24±1 weeks had a fructosamine concentration less than 500 μmol/l at its previous visit.
The present study followed cats with uncomplicated diabetes mellitus and attempted to exclude concurrent diseases such as hyperthyroidism, acromegaly, hyperadrenocorticism, major infection and major organ failure. The diagnosis of intercurrent disorders in cats with diabetes mellitus is important in particular with respect to guiding expected response to treatment and prognosis. However, not all concurrent diseases are simple to diagnose and this is particularly challenging in older cats where concurrent conditions are common and where a number of conditions have non-specific clinical signs and where making a definitive diagnosis is difficult, eg, pancreatitis (Zoran 2006). It is, therefore, not possible within the confines of the diagnostic tests used in the present study to conclude that all of the cats included were in fact uncomplicated cases.
In cats, a number of risk factors for the development of diabetes mellitus have long been known, namely age, obesity and sex (Panciera et al 1990). The cats included in the present study are similar to the feline populations that have been studied previously in that they were predominately middle aged or older, male, neutered cats. In the present study, few cats were reported as being obese, although this may relate to the time of presentation. Some authors have described over-representation of Burmese cats in the diabetic population (Rand et al 1997, McCann et al 2007). Although far more domestic/European shorthair cats were included in the present study, no conclusions can be drawn since the study was multicentre and referral centre-based and as such may represent a skewed sample of the cat population as a whole.
In their prospective study, Nelson et al (2001) reported biochemical hypoglycaemia in 31% of 67 cats. Clinical signs of hypoglycaemia were seen in five of the cats (7%) in that study (Nelson et al 2001). Hypoglycaemia was observed in cats in the present study. Four of the cats with biochemical hypoglycaemia, but no clinical hypoglycaemia, were on the point of going into clinical diabetic remission. In general, clinical signs of hypoglycaemia require that the patient has been hypoglycaemic for a long period. There was no statistically significant association between hypoglycaemia and clinical diabetic remission in the present study (Table 6), but this may be due to the relatively low number of cats in the latter category. As a general rule, clinical hypoglycaemia appeared to occur later in the present study than biochemical hypoglycaemia (14 weeks (8–18 weeks) compared with 9 weeks (6–16 weeks)). The present study confirmed that there was a significant association between high insulin dose rates and hypoglycaemia. This further underlines the need for care when using high doses of insulin in cats with diabetes mellitus.
The traditional goal of diabetes treatment was the resolution of clinical signs (since this is the main complaint of the pet owner) while avoiding death due to hypoglycaemia or DKA. This aim has shifted towards achieving diabetic remission by addressing the causes of overt diabetes such as glucose toxicity and hypertriglyceridemia. Exogenous insulin may decrease endogenous insulin secretion thereby reducing the likelihood of islet amyloid deposition (Standl 2007). The management of diabetes mellitus involves controlling blood glucose concentrations using a combination of insulin administration and diet and regulation of activity. The present study focussed on only one aspect of this – insulin treatment. It has been shown that diets high in protein and low in carbohydrate (Mazzaferro et al 2003, Bennett et al 2006) reduce insulin requirements and that other dietary elements, such as chromium (Appleton et al 2002) and vanadium (Greco 1999), might improve glucose tolerance. However, cats are notoriously fussy eaters (Mugford and Thorne 1980) and will not always eat a prescribed diet. Specific diets were not enforced in the present study and the diets fed reflect the situation in practice. Although this could be seen as a serious limitation of the present study, it accurately reflects the effect of porcine lente insulin treatment alone on diabetic cats and as such perhaps further underlines the need to combine the three approaches outlined above, with special emphasis on diet and insulin, to manage diabetic cats adequately.
The present study is the first, multicentre, prospective clinical study that examined and confirmed the efficacy and safety of porcine lente insulin in diabetic cats under field conditions. The protocol used is suitable and easy to use in practice. The study further highlights the need for cautious stepwise changes in dose. Further study examining the effect of porcine lente insulin treatment in combination with a low-carbohydrate, high-protein diet is warranted.
Acknowledgements
The authors would like to thank all of the cat owners and their cats for participating in this study. Thanks also to Intervet International bv for supporting the study: special thanks go to J. Bergman for initial coordination of the study and to R. Frenais and S. Burgaud for their comments on the manuscript.
References
- American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus, Diabetes Care 26, 2003, S5–S20. [DOI] [PubMed] [Google Scholar]
- Appleton D.J., Rand J.S., Sunvold G.D., Priest J. Dietary chromium tripicolinate supplementation reduces glucose concentrations and improves glucose tolerance in normal-weight cats, Journal of Feline Medicine and Surgery 4, 2002, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N., Greco D.S., Peterson M.E., Kirk C., Mathes M., Fettman M.J. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus, Journal of Feline Medicine and Surgery 8, 2006, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoy E.H., Nelson R.W., Feldman E.C. Effect of lente insulin for treatment of diabetes mellitus in 12 cats, Journal of the American Veterinary Medical Association 206, 1995, 1729–1731. [PubMed] [Google Scholar]
- Crenshaw K.L., Peterson M.E. Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992–1994), Journal of the American Veterinary Medical Association 209, 1996, 943–949. [PubMed] [Google Scholar]
- Elliott D.A., Nelson R.W., Reusch C.E., Feldman E.C., Neal L.A. Comparison of serum fructosamine and blood glycosylated hemoglobin concentrations for assessment of glycemic control in cats with diabetes mellitus, Journal of the American Veterinary Medical Association 214, 1999, 1794–1798. [PubMed] [Google Scholar]
- Feldman E.C., Nelson R.W. Diabetes mellitus. Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, 3rd edn, 2004, Saunders: St Louis, 539–579. [Google Scholar]
- Goossens M.M., Nelson R.W., Feldman E.C., Griffey S.M. Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985–1995), Journal of Veterinary Internal Medicine 12, 1998, 1–6. [DOI] [PubMed] [Google Scholar]
- Greco DS. (1999) Oral hypoglycaemic therapy in cats. In: Proceedings of American College of Veterinary Internal Medicine 17th Annual Veterinary Medical Forum, Chicago, USA, June 10–13, 1999. pp. 647–649.
- Kraus M.S., Calvert C.A., Jacobs G.J., Brown J. Feline diabetes mellitus: a retrospective mortality study of 55 cats (1982–1994), Journal of the American Animal Hospital Association 33, 1997, 107–111. [DOI] [PubMed] [Google Scholar]
- McCann T.M., Simpson K.E., Shaw D.J., Butt J.A., Gunn-Moore D.A. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire based-putative risk factor analysis, Journal of Feline Medicine and Surgery 9, 2007, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.J., Rand J.S. Control of diabetes mellitus in cats with porcine insulin zinc suspension, Veterinary Record 161, 2007a, 88–94. [DOI] [PubMed] [Google Scholar]
- Martin G.J., Rand J.S. Comparisons of different measurements for monitoring diabetic cats treated with porcine insulin zinc suspension, Veterinary Record 161, 2007b, 52–58. [DOI] [PubMed] [Google Scholar]
- Mazzaferro E.M., Greco D.S., Turner A.S., Fettman M.J. Treatment of feline diabetes mellitus using an alpha-glucosidase inhibitor and a low-carbohydrate diet, Journal of Feline Medicine and Surgery 5, 2003, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford R.A., Thorne C. Comparative studies of meal patterns in pet and laboratory housed dogs and cats. Anderson R.S. Nutrition of the Dog and Cat, 1980, Pergamon Press: Oxford, UK, 3–14. [Google Scholar]
- Nelson R.W., Feldman E.C., Ford S.L., Roemer O.P. Effect of an orally administered sulfonylurea, glipizide, for treatment of diabetes mellitus in cats, Journal of the American Veterinary Medical Association 203, 1993, 821–827. [PubMed] [Google Scholar]
- Nelson R.W., Lynn R.C., Wagner-Mann C.C., Michels G.M. Efficacy of protamine zinc insulin for treatment of diabetes mellitus in cats, Journal of the American Veterinary Medical Association 218, 2001, 38–42. [DOI] [PubMed] [Google Scholar]
- Panciera D.L., Thomas C.B., Eicker S.W., Atkins C.E. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986), Journal of the American Veterinary Medical Association 197, 1990, 1504–1508. [PubMed] [Google Scholar]
- Rand J. Editorial: glargine, a new long-acting insulin analog for diabetic cats, Journal of Veterinary Internal Medicine 20, 2006, 219–220. [DOI] [PubMed] [Google Scholar]
- Rand J., Martin G. Management of feline diabetes mellitus, Veterinary Clinics of North America Small Animal Practice 31 (5), 2001, 881–914. [DOI] [PubMed] [Google Scholar]
- Rand J.S., Bobbermien L.M., Hendrikz J.K., Copland M. Over representation of Burmese cats with diabetes mellitus, Australian Veterinary Journal 75, 1997, 402–405. [DOI] [PubMed] [Google Scholar]
- Rand J.S. Understanding feline diabetes: pathogenesis and management, Veterinary Quarterly 20 (suppl 1), 1998, S35–S37. [DOI] [PubMed] [Google Scholar]
- Reusch C.E., Haberer B. Evaluation of fructosamine in dogs and cats with hypo- or hyperproteinaemia, azotaemia, hyperlipidaemia and hyperbilirubinaemia, Veterinary Record 148, 2001, 370–376. [DOI] [PubMed] [Google Scholar]
- Robertson R.P., Harmon J., Tran P.O., Tanaka Y., Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection, Diabetes 52, 2003, 581–587. [DOI] [PubMed] [Google Scholar]
- Standl E. The importance of beta-cell management in type 2 diabetes, International Journal of Clinical Practice. Supplement 153, 2007, 10–19. [DOI] [PubMed] [Google Scholar]
- Stogdale L. Definition of diabetes mellitus, Cornell Veterinarian 76 (2), 1986, 156–174. [PubMed] [Google Scholar]
- Weaver K.E., Rozanski E.A., Mahonay O.M., Chan D.L., Freeman L.M. Use of glargine and lente insulins in cats with diabetes mellitus, Journal of Veterinary Internal Medicine 20, 2006, 234–238. [DOI] [PubMed] [Google Scholar]
- Wess G., Reusch C. Assessment of five portable blood glucose meters for use in cats, American Journal of Veterinary Research 61, 2000, 1587–1592. [DOI] [PubMed] [Google Scholar]
- Zoran D.L. Pancreatitis in cats: diagnosis and management of a challenging disease, Journal of the American Animal Hospital Association 42, 2006, 1–9. [DOI] [PubMed] [Google Scholar]