Abstract
Inhaled medications have proven effective and well tolerated in cats, and inhaled insulin has been used successfully for the management of diabetes mellitus in humans. Thus, we hypothesize that delivery of aerosolized regular insulin can lower blood glucose in healthy cats. Five adult cats were administered aerosolized 0.9% saline (IS), regular insulin intravenously (IV) 0.5 U/kg, and aerosolized regular insulin 15 U/kg (I15) and 25 U/kg (I25) and blood glucose was evaluated. Mean blood glucose was significantly lower at 15, 30 and 45 min in the I25 and IV groups compared to baseline. Similarly, the IV and I25 groups had a significantly greater maximal percent change in blood glucose than the IS group. Significantly more cats developed severe hypoglycemia (<50 mg/dl; 2.7 mmol/l) in the IV and I25 groups than in the IS group. Results of this study demonstrate that aerosolized insulin can effectively lower blood glucose concentrations in healthy cats.
Diabetes mellitus is one of the most common endocrinopathies in cats with one in 500 cats affected (Ettinger and Feldman 2005). Diabetes mellitus is characterized by a relative or absolute insulin deficiency leading to persistent hyperglycemia and glucosuria (Rand 1999). For many feline diabetic patients, injectable insulin therapy is the only effective means of treatment (Martin and Rand 2000). Currently the administration of exogenous insulin involves subcutaneous injections one or more times daily (Martin and Rand 2000, Ettinger and Feldman 2005, Weaver et al 2006). This task may seem intimidating or overly invasive for many cat owners leading to a lack of compliance.
The unpleasant nature of insulin injections has sparked research investigating alternative insulin delivery systems for humans with diabetes mellitus. In January of 2006 the Food and Drug Administration announced approval of the first inhaled insulin (Dunn and Curran 2006). Inhaled insulin is rapidly absorbed into the bloodstream and exerts metabolic effects in a similar manner to subcutaneous insulin (Laube et al 1993, Jendle and Karlberg 1996, Perera et al 2002, Hite et al 2006). In clinical studies, human diabetics have lower hemoglobin A1c concentrations, a marker of glycemic control, when treated with inhaled insulin compared to injectable forms of insulin (Laube et al 1993, Hite et al 2006). Along with excellent efficacy, inhaled insulin is well tolerated by human patients with diabetes mellitus (Laube et al 1993, Jendle and Karlberg 1996, Patton et al 2004).
Inhaled medications are well tolerated and highly efficacious in cats. Use of a radiolabelled pharmaceutical demonstrated that nebulization was an efficient means to deliver drugs to the lower airways of cats (Schulman et al 2004). Clinically, inhaled medications are commonly used in the treatment of inflammatory airway diseases in cats (Padrid 2000, Bay and Johnson 2004, Kirschvink et al 2006). As inhaled medications are well tolerated in cats and inhaled insulin is an efficacious therapy for diabetes mellitus in humans, we investigated the glucose lowering effects of inhaled insulin in healthy cats. We hypothesized that inhaled insulin would effectively lower blood glucose in a manner similar to that of intravenous (IV) insulin.
This was a prospective, placebo controlled, cross-over study design. Five adult cats (Liberty Research, Waverly, NY) from a pathogen-free colony aged 1–2 years weighing 3.5–6.4 kg (mean 4.4 kg) with body condition scores between 3.0 and 3.5/6 were used in this study. Animals were cared for according to the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This study was approved by the University of Missouri Animal Care and Use Committee. Prior to initiation of the study, the cats were acclimated to the aerosolization procedure. On day 1, the cats were sedated for instrumentation with butorphanol (Torbugesic, Fort Dodge, Fort Dodge, IA; 0.2 mg/kg, IV) and medetomidine (Domitor, Pfizer Animal Health, Exton, PA; 10–15 μg/kg, IV). A 4-French jugular catheter (Cook Veterinary Products, Bloomington, IN) was placed for repeated blood sampling and a cephalic IV catheter (Abbott, Abbott Park, IL) was placed for insulin administration and dextrose administration if indicated. There was a 6-h washout between sedation and the administration of treatments.
In the first phase of this study, two cats were administered escalating doses of aerosolized regular insulin (Humulin, N, Eli Lilly, Indianapolis, IN; 0.5, 1, 5, 10 and 15 U/kg) with a 30-min washout between treatments to determine the most appropriate dose of insulin for phase 2. Based on these data, aerosolized insulin at 15 and 25 U/kg were selected. There was a 2-week washout period between the phases. In the second phase, five cats were administered aerosolized 0.9% saline (1.5 ml), aerosolized regular insulin (15 U/kg and 25 U/kg, diluted to a volume of 1.5 ml with 0.9% saline) and regular insulin IV (0.5 mgU/kg, diluted to a volume of 1.5 ml with 0.9% saline) over the course of 2 days. Each day, the cats were fasted for 12 h prior to administration of the first treatment. On day 1, the cats received aerosolized saline first followed by IV insulin. On day 2, the cats received aerosolized insulin at 25 U/kg and then 15 U/kg. The aerosolized insulin or saline was administered by gently holding a feline anesthetic mask attached to an Omron vibrating mesh nebulizer (Model NE-U22, Omron Healthcare, Vernon Hills, IL) over the cat's nose and mouth and allowing spontaneous respiration until dryness (approximately 2 min). Between treatments the blood glucose was allowed to plateau for a minimum of 1 h with a total minimum washout period of 2 h. Blood glucose plateau was defined as three or more blood glucose samples at least 15 min apart with less than 10% variation between glucose readings. Cats that had blood glucose concentrations greater than 130 mg/dl (7.2 mmol/l) were given additional washout time until their blood glucose plateaued below 130 mg/dl (7.2 mmol/l). Cats that developed severe hypoglycemia, defined as a blood glucose <50 mg/dl (2.7 mmol/l) and clinical signs of hypoglycemia (staggering, mental dullness or other neurologic signs), were treated with 5% dextrose (Hospira, Inc, Lake Forest, IL) IV to effect.
Blood was collected via jugular catheter at baseline and then at 5, 15, 30 and 45 min after treatment for post-treatment blood glucose evaluation. Additionally, blood samples were collected every 15–30 min between treatments to determine the length of the washout period. Blood glucose was evaluated using a single bedside glucometer (Accucheck Advantage, Roche Diagnostics, Switzerland). Additionally, episodes of severe hypoglycemia [<50 mg/dl (2.7 mmol/l) with clinical signs of hypoglycemia] requiring dextrose administration were recorded. The percent change in blood glucose after each treatment was determined using the following formula:
Statistical analysis was performed using commercially available software (SAS v9, SAS Institute Inc, Cary, NC). A repeated measures analysis was performed using Fisher's least significant difference values obtained from the mixed procedure in SAS for differences between treatments at each time point and differences within treatments between time points. A Fisher's exact test was used to detect differences between groups with respect to the development of severe hypoglycemia. A P-value of less than 0.05 was considered statistically significant.
In the dose escalation phase of this study, aerosolized insulin at doses of 0.5, 1, 5, 10 U/kg had little to no effect on blood glucose concentrations (data not shown). There was a mild glucose lowering effect at 15 U/kg and thus 15 U/kg and a higher dose (25 U/kg) were chosen for the second phase of the study (data not shown).
In the second phase of the study, the aerosol treatments were well tolerated by all cats. Group mean blood glucose was significantly lower at 15, 30 and 45 min in the I25 group (P≤0.018) and the IV group (P<0.001) compared to baseline. A significant decrease in blood glucose was not observed in the IS or I15 groups. Similarly, the IV (P<0.0001) and I25 groups (P=0.0001) (Fig 1) had a significantly greater maximal percent change in blood glucose than the IS group. There was no significant difference between the IS and I15 groups.
Fig 1.
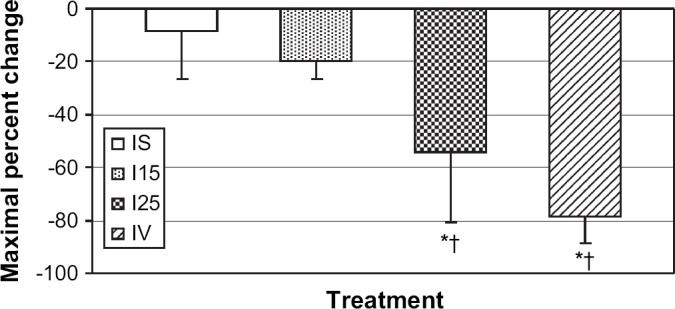
Maximal percent change in blood glucose for cats in the inhaled saline (IS), inhaled insulin 15 U/kg (I15), inhaled insulin 25 U/kg (I25) and IV insulin (IV) groups. *Indicates P<0.05 compared to the IS group. †Indicates P<0.05 compared to I15 group. Mean±standard deviation.
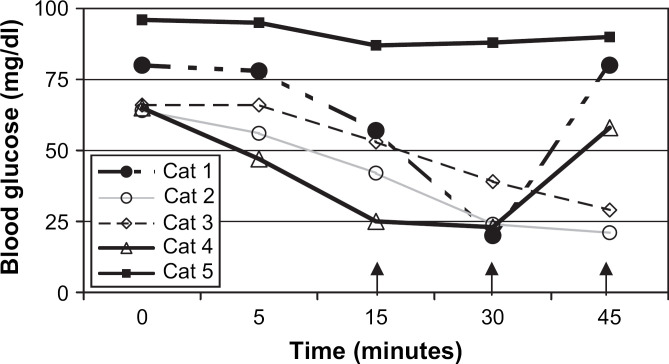
Significantly more cats developed severe hypoglycemia in the IV insulin group (5/5; P=0.008) and I25 group (4/5; P=0.04) than in the IS group (0/5). One cat in the I25 group did not demonstrate a clinically important change in blood glucose (Fig 2). However, all other cats had at least a 50% decrease in their blood glucose concentration after administration of aerosolized insulin at 25 U/kg compared to baseline. There was no significant difference in the development of severe hypoglycemia between the other groups.
Fig 2.
Blood glucose concentrations in individual cats after inhaled insulin (25 U/kg). Arrows indicate time points at which dextrose was administered to cats that developed severe hypoglycemia.
Administration of regular insulin via aerosol significantly lowered blood glucose in a similar manner to IV insulin. The doses of inhaled insulin used in this study were based on a preliminary dose escalation study indicating that lower doses were not effective. The most effective dose of aerosolized insulin used in this study was 25 U/kg. Based on these data, it appears that aerosolized insulin is systemically bioavailable and physiologically active in cats.
The maximal percent change in blood glucose from baseline was used for statistical purposes in this study. Because many of the cats required dextrose administration due to hypoglycemia, this calculation was used to circumvent the interference from dextrose administration at various time points. Because of this interference, it was impossible to determine the duration of action of the inhaled insulin. However, blood glucose was significantly lower in the I25 group at 15 min compared to baseline indicating that the onset of action was between 5 and 15 min.
This study did not evaluate the impact of short-acting insulin on diabetic regulation. Ideally, traditional longer acting insulin would be used to treat diabetic cats. However, longer acting insulin requires a specific environment (ie, the subcutaneous space) for proper absorption. While it is unlikely that short-acting insulin will be an efficacious sole treatment for diabetes mellitus in cats, it may offer advantages in some patients where injectable insulin is not an option compared to other treatments like diet change or hypoglycemic agents alone. Further study is warranted to evaluate the potential use of aerosolized insulin in cats with diabetes mellitus as an adjunctive treatment.
Acknowledgements
This research was supported by a grant from the Phi Zeta Chapter at the University of Missouri, College of Veterinary Medicine. The authors wish to thank Mr Matthew Haight for his technical assistance with this investigation.
References
- Bay J., Johnson L. Feline bronchial disease/asthma. King L. Textbook of Respiratory Disease in Dogs and Cats, 2004, Saunders: Philadelphia. [Google Scholar]
- Dunn C., Curran M. Inhaled human insulin (Exubera): a review of its use in adult patients with diabetes mellitus, Drugs 66, 2006, 1013–1032. [DOI] [PubMed] [Google Scholar]
- Ettinger S., Feldman E. Textbook of Veterinary Internal Medicine, 6th edn, 2005, Elsevier Saunders: St. Louis. [Google Scholar]
- Hite P., Barnes A., Johnston P. Exuberance over exubera, Clinical Diabetes 24, 2006, 110–114. [Google Scholar]
- Jendle J., Karlberg B. Intrapulmonary administration of insulin to healthy volunteers, Journal of Internal Medicine 240, 1996, 93–98. [DOI] [PubMed] [Google Scholar]
- Kirschvink N., Leemans J., Delvaux F., Snaps F., Jaspart S., Evrard B., Delattre L., Cambier C., Clercx C., Gustin P. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis, Journal of Feline Medicine and Surgery 8, 2006, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B., Georgopoulos A., Adams G. Preliminary study of the efficacy of insulin aerosol delivered by oral inhalation in diabetic patients, Journal of the American Medical Association 269, 1993, 2106–2109. [PubMed] [Google Scholar]
- Martin G., Rand J. Current understanding of feline diabetes: part 2, treatment, Journal of Feline Medicine and Surgery 2, 2000, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrid P. Feline asthma, Veterinary Clinics of North America Small Animal Practice 30, 2000, 1279–1293. [DOI] [PubMed] [Google Scholar]
- Patton J.S., Bukar J.G., Eldon M.A. Clinical pharmacokinetics and pharmacodynamics of inhaled insulin, Clinical Pharmacokinetics 43, 2004, 781–801. [DOI] [PubMed] [Google Scholar]
- Perera A., Kapitza C., Nosek L., Fishman R.S., Shapiro D.A., Heise T., Heinemann L. Absorption and metabolic effect of inhaled insulin: intrapatient variability after inhalation via the Aerodose insulin inhaler in patients with type 2 diabetes, Diabetes Care 25, 2002, 2276–2281. [DOI] [PubMed] [Google Scholar]
- Rand J. Current understanding of feline diabetes: part 1, pathogenesis, Journal of Feline Medicine and Surgery 1, 1999, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman R., Crochik S., Kneller S., McKiernan B.C., Schaeffer D.J., Marks S.L. Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats, American Journal of Veterinary Research 65, 2004, 806–809. [DOI] [PubMed] [Google Scholar]
- Weaver K., Rozanski E., Mahony O.M., Chan D.L., Freeman L.M. Use of glargine and lente insulins in cats with diabetes mellitus, Journal of Veterinary Internal Medicine 20, 2006, 234–238. [DOI] [PubMed] [Google Scholar]