Abstract
A 14-year-old neutered female domestic shorthair cat was presented for investigation of a non-painful subcutaneous swelling of the nasal dorsum at the site of a scratch injury. Cytological evaluation demonstrated a granulomatous reaction and many variably shaped organisms consistent with yeasts/fungi. Subsequent biopsy and culture yielded a pure growth of a Mucor species. The cat was treated with the second-generation triazole antifungal agent posaconazole for 5 months. Complete resolution was seen with no recurrence 12 months after discontinuing treatment.
A 14-year-old neutered female domestic shorthair cat was presented with a 4-week history of a firm cutaneous/subcutaneous swelling of the dorsum of the nose (Fig 1). The lesion measured approximately 5 mm in diameter, was firm and non-painful and appeared to be associated with a scratch mark. The cat had an indoor–outdoor lifestyle and was an avid hunter of birds and rodents. Previous medical history included treatment for hyperthyroidism 5 years previously with radio-iodine, hypertension and mild renal azotaemia which had developed after radio-iodine therapy but had subsequently resolved. Symptomatic treatment of the swelling with enrofloxaxin (Baytril; Bayer) 5 mg/kg per os q 24 h for 5 days had been administered previously by the referring veterinary surgeon but to no effect.
Fig 1.

Subcutaneous swelling of the nose due to Mucor species infection after local trauma in a 14-year-old cat, prior to treatment.
Physical examination demonstrated no other abnormalities except for the subcutaneous mass which was non-painful and did not appear to be associated with bony distortion or with nasal discharge. Ophthalmic examination was normal and local lymph nodes were not palpably enlarged. A complete blood count and biochemical panel were within reference intervals and a blood sample submitted for cryptococcus capsular antigen latex agglutination testing (LAT). The cat was negative for feline immunodeficiency virus (FIV) antibody and feline leukaemia virus (FeLV) antigen by in-clinic enzyme-linked immunosorbent assay (ELISA). The cat was sedated with diazepam (Diazemuls; Actavis) 0.2 mg/kg and ketamine (Ketaset; Fort Dodge) 5 mg/kg intravenously and fine needle aspirates taken from the nasal swelling. Aspirates demonstrated a pyogranulomatous cytological reaction with multiple organisms of variable morphology ranging from circular/oval to cigar-shaped and demonstrating multiple pauci-septate broad, irregular fungal hyphae and some smaller filamentous forms (Fig 2). Organisms were either free or within macrophages. Initial identification of the organism was not possible, though a yeast or dimorphic mould was suspected based on the morphology of rounded bodies. Cryptococcal LAT returned as negative and so the cat was re-admitted for further investigation under general anaesthesia including thoracic, skull and nasal radiographs and tissue biopsy of the subcutaneous lesion for fungal culture. Wide surgical excision of the affected area was declined by the owner. No clinical important radiographic abnormalities were seen. Biopsies of the lesion were taken with a 3 mm dermal biopsy punch (which resulted in substantial debulking of the swelling) and were submitted for culture at a Health Protection Agency Mycology Reference Laboratory. Pending results, treatment was started with fluconazole (Fluconazole, non-proprietary) 10 mg/kg PO q 24 h as an opportunistic yeast infection was suspected. No adverse effects to fluconazole were seen and serum biochemistry 10 days after starting treatment showed no evidence of hepatotoxicity, though substantial change in the size of the subcutaneous mass was not seen. The biopsy sample was cut into small (1 mm) blocks and cultured on Sabourauds dextrose agar at 30°C and 37°C. The mycological culture yielded a large amount of filamentous growth of a yellowish/brown spreading colony identified as a Mucor species by morphological characteristics of the mycelium, sporangiophores and spores. Although a molecular identification was attempted further speciation was not possible. Antifungal susceptibility testing was undertaken according to the National Committee on Clinical Laboratory Standard (NCCLS, now Clinical Laboratory Standards Institute, CLSI) method 38-A for filamentous fungi (NCCLS 2002). Briefly a spore suspension of 0.4×104 to 5×104 cfu/ml was prepared in Roswell Park Memorial Institute (RPMI) medium and inoculated into microtitre trays containing doubling dilutions of the test drugs. After 48 h incubation at 30°C wells were examined for growth inhibition and the minimum inhibitory concentration (MIC) determined. The organism was reported to be sensitive to amphotericin B (MIC 0.5 mg/l) and posaconazole (MIC 0.5 mg/l) but resistant to itraconazole (MIC 2.0 mg/l) and voriconazole (MIC 8.0 mg/l). Moulds of this type are not generally considered to be sensitive to fluconazole and this is, therefore, not routinely tested. The cat's owner declined treatment with amphotericin B on the grounds of not wishing for frequent hospitalisation and risk of nephrotoxicity given previous, albeit temporary, azotaemia. Fluconazole therapy was stopped and treatment with posaconazole was selected after discussion with a consultant microbiologist and after obtaining the owner's informed consent for the use of a non-veterinary-licensed product. Posaconazole oral suspension (Noxafil; Schering-Plough) was administered at a dose of 5 mg/kg PO q 24 h, a dose rate based on a previous report of use in the cat (McLellan et al 2006). The cat was re-examined and a serum biochemistry panel monitored for evidence of hepatotoxicity and electrolyte disturbances after 2, 4, 6 and 8 weeks then every 4 weeks and a complete blood count was assessed after 8 and 16 weeks. The subcutaneous mass decreased in size noticeably by 2 weeks and further over a 3-month period (Fig 3) during which time no evidence of adverse effects, biochemical or haematological abnormalities was seen. From the third month of treatment fine needle aspirate cytology of the area was performed under sedation every 3 weeks. After two consecutive cytology samples which both showed no evidence of mould organisms, a third sample was collected by aspiration and inoculated into Sabouraud medium for fungal culture. Culture and cytology of these samples were negative and treatment with posaconazole was discontinued approximately 5 months after it was started. The cat has continued to display no clinical signs of illness and no recurrence has been seen 12 months after treatment was discontinued.
Fig 2.
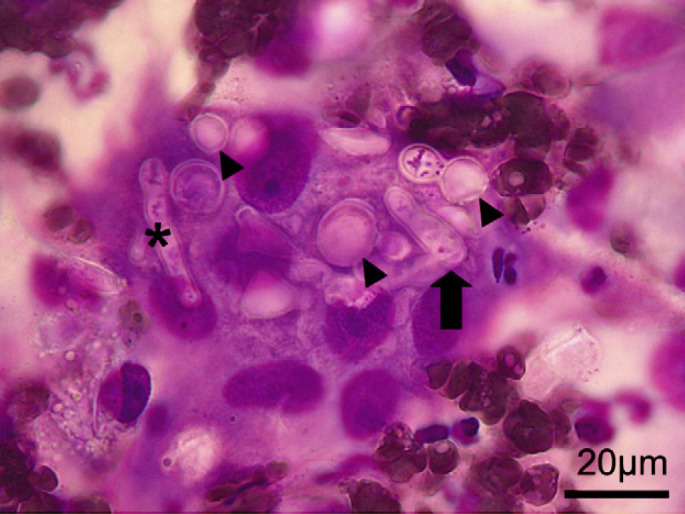
Fine needle aspirate cytology (Romanowski stain) from subcutaneous nasal swelling in a 14-year-old cat. Multiple pleomorphic fungal organisms can be seen varying from broad (approximately 6 μm) irregularly branching pauci-septate hyphae (asterisk) to plumper cigar-shaped (arrow) and rounded yeast-like forms (arrow-heads).
Fig 3.

The region of previous subcutaneous nasal swelling in a 14-year-old cat infected by Mucor species after 5 months of treatment with posaconazole showing resolution of the swelling.
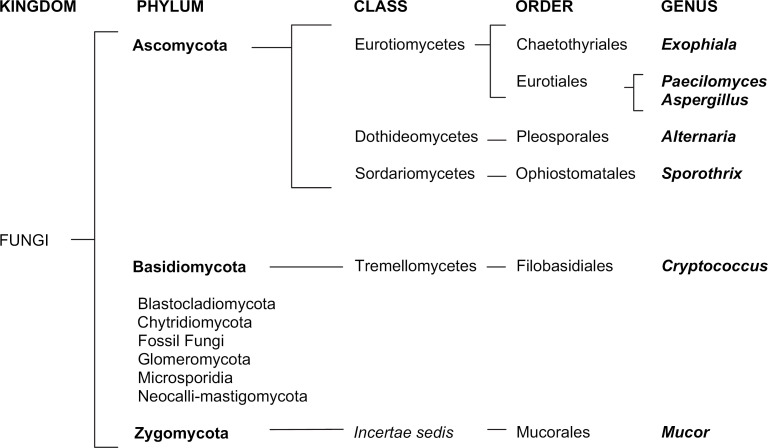
The class Zygomycetes of the phylum Zygomycota comprises two orders of fungi, Mucorales and Entomophthorales. Both can cause invasive clinical disease in man of world-wide importance which is termed zygomycosis, with the term mucormycosis being reserved for infections caused solely by the order Mucorales (Prabhu and Patel 2004). Entomophthoromycoses are considered slowly progressive subcutaneous infections in immunocompetent hosts in tropical regions (Prabhu and Patel 2004, Bouza et al 2006). Mucor is a genus of fungus characterised as a mould due to its filamentous growth and is found in soil, plants, decaying fruit and vegetables (Flagothier et al 2006). Zygomycosis in man has gained recognition as an emerging disease in immunocompromised patients (Bouza et al 2006, Chayakulkeeree et al 2006). There is evidence that widespread prophylactic use of the antifungal agent voriconazole may contribute to its emergence as a problem (Kontoyiannis et al 2005, Glőckner et al 2007). Infection in man is usually caused by inhalation of airborne spores or subcutaneous introduction by contaminated catheters or other devices. Traumatic inoculation in wounds contaminated with water or soil is also reported (Bouza et al 2006). Infection in man may be respiratory, rhino-sinus, cutaneous or involve the central nervous system and is characterised by angio-invasion (Bouza et al 2006, Chayakulkeeree et al 2006).
Naso-ocular lesions of the subcutis in cats appear to be associated with a wide variety of causative agents, including saprophytic fungi, and have been previously reviewed (Malik et al 2004). Most cats, as in this case, did not have evidence of a predisposing immunodeficiency state and it is thought that it is the combination of a large inoculum introduced by traumatic breach of the cutaneous barrier that results in localised disease. In that review, seven cats were reported with subcutaneous infection of the naso-ocular region without concurrent nasal signs and comprised the bacteria Corynebacterium pseudotuberculosis, Mycobacterium avium and Nocardia nova and the fungi Cryptococcus neoformans, Exophiala jeanselmei and Paecilomyces lilacinus. Infection with Alternaria species, Sporothrix schenckii, herpes dermatitis and insect bite hypersensitivity have also been reported to cause similar localised skin and subcutaneous lesions in this area (Kennis et al 1994, Hargis et al 1999, McKay et al 2001, Malik et al 2004, Tennant et al 2004).
Although the precise species of Mucor was not identified in this case, differentiation from other fungi of the order Mucorales could be confidently made on morphological grounds. Reports of Mucor species infections in veterinary species are scant but include disseminated infection in cattle and horses, cutaneous mycosis in the platypus and a single case of cerebral mycosis in a cat (Ravisse et al 1978, Schonmann et al 1997, Connolly et al 2000, Thirion-Delalande et al 2005). The phylogeny and association of Mucor species with other mycoses reported to involve the naso-ocular region of cats are shown in Fig 4. No evidence of retroviral infection by ELISA or of iatrogenic immunosuppression or naturally occurring immunosuppressive disease or of diabetic ketoacidosis was found in the case reported here. It was suspected that localised subcutaneous infection was established traumatically due to the finding of a scratch at the site of the lesion. Mucor species have been isolated from the fur of clinically normal cats and it is possible that the organism may have been traumatically inoculated subcutaneously from an adjacent cutaneous site or by heavy contamination of the perpetrating object such as another cat's claw (Khosravi 1996). It was considered unlikely that the subsequent fungal culture was due to fur-contamination, because of the cytological findings confirming subcutaneous mycosis. It is interesting, considering the organism isolated in this case, that no evidence of an underlying immunocompromising condition was found and that infection was localised rather than displaying the aggressive angio-invasiveness characteristic of zygomycosis with this species in people. This further reinforces the supposition that this was a localised opportunistic infection due to traumatic inoculation of the organism in an immunocompetent host.
Fig 4.
Phylogony of Mucor species in relation to other mycoses reported to affect the sino-orbital region of cats (according to classification of CABI Index Fungorum Database 2008).
The triazole antifungal agent fluconazole was initially selected in this case as a yeast infection (specifically Cryptococcus neoformans) was initially suspected. In retrospect, this probably represented a poor initial choice as broad hyphae were identifiable on the initial cytology and a zygomycosis could have been suspected on this basis. Fluconazole is considered largely ineffective for treating moulds (Glőckner et al 2007). Mucormycosis in man is generally treated with either a lipid formulation of amphotericin B and/or with posaconazole (Prabhu and Patel 2004, Chandrasekar 2006, Chayakulkeeree et al 2006). The organism identified here showed sensitivity to both of these agents but not to itraconazole or voriconazole. Amphotericin B lipid complex (ABLC) has been used to treat dogs and cats with susceptible fungal infections though pharmacokinetics data in these species appear unavailable (Plumb 2005). Lipid-complexed products are associated with less risk of nephrotoxicity than amphotericin deoxycholate; however, they are very expensive, still mandate frequent monitoring for nephrotoxicity and attention to patient hydration and are recommended to be given three times weekly by intravenous infusion (Plumb 2005). A method of subcutaneous depot administration has also been reported that appears well tolerated (Malik et al 1996). Posaconazole is a broad-spectrum triazole antimycotic agent which is similar in chemical structure to itraconazole and in common with all azoles binds to lanosterol-14α-demethylase, an enzyme responsible for oxidative demethylation of lanosterol to ergosterol, an essential lipid component of fungal cell membranes (Hof 2006). It has proven to be highly effective in treating zygomycosis in humans, including cases that are refractory to treatment with ABLC and response rates compare favourably to those seen with ABLC (Kauffman 2006). Posaconazole, unlike the lipid formulations of amphotericin B, is available in an orally administered form and has a broad spectrum of activity including dimorphic fungi (such as Blastomyces, Coccidioides and Histoplasma species), both in their yeast and filamentous forms, Aspergillus species, Candida species, dematiaceous fungi and zygomycetes (Hof 2006). Whilst antifungal efficacy and pharmacodynamics of posaconazole in experimental animal models of fungal infection in mice, rats, rabbits, dogs and monkeys are reported (Nomeir et al 2000) and summarised by Groll and Walsh (2006), at present pharmacokinetics data for the cat are not published. Oral bioavailability in dogs is high and is increased by feeding; half-life in this species is 23 h with Tmax being reached at 8.3–15 h depending on dose, and serum concentration reached steady state by day 8 with once daily dosing (Nomeir et al 2000). Clinical veterinary experience with this drug is limited, there being but one published case report of use in a cat with invasive orbital aspergillosis (McLellan et al 2006). In the case reported here posaconazole was selected over ABLC or conventional amphotericin given intravenously or subcutaneously due to owner concerns regarding very frequent hospital admissions and the risk of nephrotoxicity where previous azotaemia had been documented.
Although likely uncommon, opportunist Mucor species infections should be added to the differential diagnosis list of organisms which may cause focal subcutaneous lesions of the naso-orbital area in cats. In this case, posaconazole at this dose appeared safe and was well tolerated, resulting in clinical, cytological and culture cure of the infection with no relapse 12 months after discontinuation of treatment.
Acknowledgements
The authors would like to thank Dr Gill McLellan for helpful advice regarding her experience of use of posaconazole in the cat and Dr Steve Shaw for his photographic skills.
References
- Bouza E., Munoz P., Guinea J. Mucormycosis: an emerging disease?, Clinical Microbiology and Infection 12 (suppl 7), 2006, 7–23. [Google Scholar]
- Chandrasekar P. Selection criteria for antifungals: the right patients and the right reasons, International Journal of Antimicrobial Agents 27 (suppl 1), 2006, 17–20. [DOI] [PubMed] [Google Scholar]
- Chayakulkeeree M., Ghannoum M.A., Perfect J.R. Zygomycosis: the re-emerging fungal infection, European Journal of Clinical Microbiology and Infectious Disease 25, 2006, 215–229. [DOI] [PubMed] [Google Scholar]
- Commonwealth Advisory Bureaux (CABI) Index Fungorum. Available from: <http://www.indexfungorum.org>.
- Connolly J.H., Canfield P.J., Obendorf D.L. Gross, histological and immunohistochemical features of mucormycosis in the platypus, Journal of Comparative Pathology 123, 2000, 36–46. [DOI] [PubMed] [Google Scholar]
- Flagothier C., Arrese J.E., Quatresooz P., Piérard G.E. General review: cutaneous mucormycosis, Journal de Mycologie Médicale 16, 2006, 77–81. [Google Scholar]
- Glőckner A., Vehreschild J.J., Cornely O.A. Zygomycosis –current epidemiological aspects, Mycoses 50 (suppl 1), 2007, 50–55. [DOI] [PubMed] [Google Scholar]
- Groll A.H., Walsh T.J. Antifungal efficacy and pharmacodynamics of posaconazole in experimental models of invasive fungal infections, Mycoses 49 (suppl 1), 2006, 7–16. [DOI] [PubMed] [Google Scholar]
- Hargis A.M., Ginn P.E., Mansell J., Garber R.L. Ulcerative facial and nasal dermatitis and stomatitis in cats associated with feline herpesvirus 1, Veterinary Dermatology 10 (4), 1999, 267–274. [DOI] [PubMed] [Google Scholar]
- Hof H. A new, broad-spectrum azole antifungal: posaconazole – mechanisms of action and resistance, spectrum of activity, Mycoses 49 (suppl 1), 2006, 2–6. [DOI] [PubMed] [Google Scholar]
- Kauffman C.A. Clinical efficacy of new antifungal agents, Current Opinion in Microbiology 9, 2006, 483–488. [DOI] [PubMed] [Google Scholar]
- Kennis R.A., Rosser E.J., Jr., Dunstan R.W. Difficult dermatologic diagnosis, Journal of the American Veterinary Medical Association 204, 1994, 51–52. [PubMed] [Google Scholar]
- Khosravi A.R. Fungal flora of the hair coat of stray cats in Iran, Mycoses 39, 1996, 241–243. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D.P., Lionakis M.S., Lewis R.E., Chamilos G., Healy M., Perego C., Safdar A., Kantarjian H., Champlin R., Walsh T.J., Raad I.I. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case–control observational study of 27 recent cases, Journal of Infectious Diseases 191, 2005, 1350–1360. [DOI] [PubMed] [Google Scholar]
- Malik R., Craig A.J., Wigney D.I., Martin P., Love D.N. Combination chemotherapy of canine and feline cryptococcosis using subcutaneously administered amphotericin B, Australian Veterinary Journal 73, 1996, 124–128. [DOI] [PubMed] [Google Scholar]
- Malik R., Vogelnest L., O'Brien C.R., White J., Hawke C., Wigney D.I., Martin P., Norris J.M. Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats–clinical experience 1987–2003, Journal of Feline Medicine and Surgery 6, 2004, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay J.S., Cox C.L., Foster A.P. Cutaneous alternariosis in a cat, Journal of Small Animal Practice 42, 2001, 75–78. [DOI] [PubMed] [Google Scholar]
- McLellan G.J., Aquino S.M., Mason D.R., Kinyon J.M., Myers R.K. Use of posaconazole in the management of invasive orbital aspergillosis in a cat, Journal of the American Animal Hospital Association 42, 2006, 302–307. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard. NCCLS Document M38-A, 2002, National Committee for Clinical Laboratory Standards: Wayne, PA. [Google Scholar]
- Nomeir A.A., Kumari P., Hilbert M.J., Gupta S., Loebenberg D., Cacciapuoti A., Hare R., Miller G.H., Lin C.C., Cayen M.N. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys, Antimicrobial Agents and Chemotherapy 44, 2000, 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb D.C. Amphotericin B desoxycholate/amphotericin B lipid-based. Plumb D.C. Plumb's Veterinary Drug Handbook, 5th edn, 2005, Blackwell: Iowa, 45–50. [Google Scholar]
- Prabhu R.M., Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment, Clinical Microbiology and Infection 10 (suppl 1), 2004, 31–47. [DOI] [PubMed] [Google Scholar]
- Ravisse P., Fromentin H., Destombes P., Mariat F. Cerebral mucormycosis in the cat caused by Mucor pusillus, Sabouraudia 16, 1978, 291–298. [PubMed] [Google Scholar]
- Schonmann M., Thoma R., Braun U. A case of acute disseminated mucor encephalitis in a heifer, Schweizer Archiv für Tierheilkunde 139, 1997, 490–494. [PubMed] [Google Scholar]
- Tennant K., Patterson-Kane J., Boag A.K., Rycroft A.N. Nasal mycosis in two cats caused by Alternaria species, Veterinary Record 155, 2004, 368–370. [DOI] [PubMed] [Google Scholar]
- Thirion-Delalande C., Guillot J., Jensen H.E., Crespeau F.L., Bernex F. Disseminated acute concomitant aspergillosis and mucormycosis in a pony, Journal of Veterinary Medicine Series A 52, 2005, 121–124. [DOI] [PubMed] [Google Scholar]