Abstract
Feline primary immune-mediated thrombocytopenia (pIMT) is a rare hematological disorder. Platelet-bound antibody assays for cats have variable specificity and sensitivity and are not widely available. Diagnosis of pIMT is made on the basis of exclusion of other identifiable causes of thrombocytopenia and the response to immunosuppressive therapy. This report describes four cats with severe thrombocytopenia and no detectable underlying disease. One cat was euthanased because of pulmonary hemorrhage, while the other cats had frequent relapses, two of these cats developed diabetes mellitus due to long-term corticosteroid therapy. In these cats IMT had a chronic course and responded poorly to therapy with prednisolone. Alternative immunomodulatory drugs may be considered in the treatment of feline IMT.
In dogs, immune-mediated thrombocytopenia (IMT) can occur as a primary condition in which there is no apparent inciting cause for the production of antiplatelet antibodies, or as a secondary condition due to a presumptive antigenic stimulus (Lewis and Meyers 1996). Platelet-bound antibodies were detected by a flow cytometric assay in thrombocytopenic cats with fat necrosis, feline leukemia virus (FeLV) infection, feline immunodeficiency disease (FIV), feline infectious peritonitis (FIP), lymphosarcoma, leukemia, hyperthyroidism, pyelonephritis, and hepatitis (Kohn et al 2006). Significant thrombocytopenia in cats has also been reported with ehrlichiosis, anaplasmosis, neoplasia, cardiac disease, thromboembolism, disseminated intravascular coagulation (DIC), and several drugs, including griseofulvin, doxorubicin, azathioprine, carboplatin, propylthiouracil, and ribavirin (Povey 1978, Peterson et al 1984, Levy 1991, Beale et al 1992, O'Keefe and Schaeffer 1992, Jordan et al 1993, Peterson et al 1995, Hahn et al 1997, Breitschwerdt et al 2002, Lappin et al 2004, Estrin et al 2006). In most of these conditions, the mechanism of thrombocytopenia is unknown and may represent additional causes of secondary IMT (sIMT).
In contrast to dogs, feline primary IMT (pIMT) is a rare hematological event (Jordan et al 1993, Kohn et al 2006), and has been reported as an isolated disorder in only a few individual case reports (Joshi et al 1979, Tasker et al 1999, Garon et al 1999). Platelet-bound antibody assays for cats have variable specificity and sensitivity and are not widely available so their use has limited clinical utility, and the diagnosis of feline pIMT remains a tentative diagnosis of exclusion (Kohn et al 2006).
The purpose of this report is to describe clinical presentation, treatment and outcome of four cats with a presumptive diagnosis of pIMT. These cases were referred to the Veterinary Medical Center at the University of Minnesota between January 2002 and January 2007.
Case 1: A 6-year-old, 4.6 kg (10.2 lb) spayed female Abyssinian cat was presented for a 1-week history of petechiae on the external ears and epistaxis. The cat was strictly kept indoors, did not have any known exposure to drugs or toxins, and was vaccinated for rabies 5 months before presentation. On physical examination, petechiae of the external ears, ventral abdomen and tongue were noted.
Complete blood count revealed severe thrombocytopenia (12,000 platelets/μl [reference range, 160,000–489,000/μl], Cell-Dyne System 3500, Diagnostics Division, Abbott Laboratories, Santa Clara, CA) with a hematocrit of 30% (reference range, 26.1–46.7%). Blood smears were evaluated for manual platelet counting and evidence of platelet clumping. Results of serum biochemical tests, serum total thyroxine (TT4), and measurements of coagulation (including fibrinogen, prothrombin time, activated partial thromboplastin time, and fibrin degradation products) were within our laboratory reference ranges. Results of screening tests for FIV antibodies, FeLV antigen (FIV/FeLV Combo Plus Test; Idexx Laboratories), and Ehrlichia canis, Rickettsia rickettsii, and Anaplasma phagocytophilum by immunofluorescent antibody assay (IFA) were negative. Thoracic radiography, echocardiogram, and abdominal ultrasonography were unremarkable. Cytological evaluation of a bone marrow aspirate and histological evaluation of core bone marrow biopsy specimens indicated mild to moderate megakaryocytic hyperplasia. Bone marrow was negative for FeLV by IFA.
A presumptive diagnosis of pIMT was made and immunosuppressive therapy with oral prednisolone (Prednisolone tablets; Watson Labs Corona, 5 mg twice daily) in combination with antibiotic therapy (Doxycycline tablets; Ivax Pharmaceuticals Miami, 5 mg/kg twice daily, pending vector-borne disease testing) was prescribed. After 5 days of therapy, the platelet count was 21,000/μl, the hematocrit decreased to 14.5%, and hematochezia was noted. An intravenous (IV) transfusion of cross-matched packed red blood cells (pRBCs) at 10 ml/kg over 4 h was administered, oral prednisolone was discontinued, and dexamethasone (dexamethasone sodium phosphate injection; Sicor Pharmaceuticals, 1 mg/kg once daily) was started intravenously. Two days later, the platelet count was within our laboratory reference range, and the cat was discharged from the hospital with instructions to give dexamethasone orally at 1 mg/kg on a tapering schedule. Four months after initiation of corticosteroid therapy, the platelet count was 327,000/μl. At that time, the owner reported that the cat had increased thirst and some urinary accidents outside the litterbox in the last few weeks. A chemistry profile disclosed hyperglycemia (420 mg/dl [reference range, 74–143 mg/dl]) and urinalysis revealed glucosuria. The cat was diagnosed with diabetes mellitus and treatment was begun with insulin zinc suspension (Humulin L; Eli Lilly, 3 units SC twice daily). The cat's diabetes went into remission after 1 year of insulin therapy and a decrease in the dexamethasone to 0.5 mg/kg every other day was recommended. At that time, the platelet count was stable at 365,000/μl. Over a 5-year follow-up, the cat experienced three episodes of relapse with platelet counts <50,000/μl and recurrence of clinical signs, and is still treated with dexamethasone (0.5 mg/kg SC every 72 h) at the time of writing.
Case 2: A 12-year-old, 5.2 kg (11.5 lb) neutered male domestic shorthair cat was evaluated for a 4-day history of epistaxis. The cat was allowed to go outdoors under the owners' supervision, and did not receive any drugs or vaccination in the last 3 months before presentation.
Complete blood count disclosed severe thrombocytopenia (2000/μl [reference range, 160,000–489,000/μl], Cell-Dyne System, Diagnostics Division, Abbott Laboratories, Santa Clara, CA) with a hematocrit of 34%. Blood smears were evaluated for manual platelet counting and evidence of platelet clumping. Serum biochemical tests detected an increased creatinine (1.8 mg/dl; reference range, 0.6–1.4 mg/dl). A midstream free catch urine sample was collected into a sterile container, and urinalysis was consistent with hematuria (>50 RBCs/high power field, 0–5 white blood cells [WBCs]/high power field, and 1+ protein using the sulfosalicylic acid [SSA] precipitation test), with a urine specific gravity of 1.029. A urine protein/creatinine ratio was 0.2 (reference range, <0.42). There was no bacterial growth on urine culture. The cat was normotensive, and fundoscopic examination was unremarkable. Measurement of coagulation and serum TT4 were within our laboratory reference ranges. Results of screening tests for FIV antibodies, FeLV antigen, and Ehrlichia canis, Rickettsia rickettsii, and Anaplasma phagocytophilum by IFA were negative. Thoracic radiography and abdominal ultrasonography were unremarkable. Cytological evaluation of a bone marrow aspirate and histological evaluation of core bone marrow biopsy specimens indicated moderate megakaryocytic hyperplasia (see Fig 1). Bone marrow was negative for FeLV by IFA.
Fig 1.
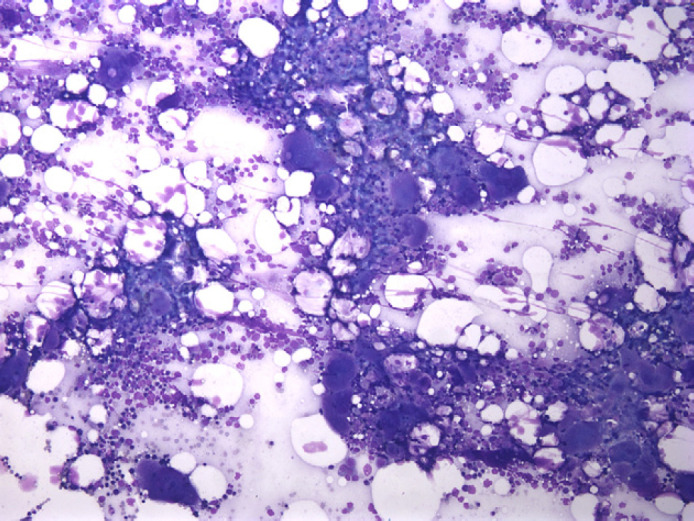
Moderate megakaryocytic hyperplasia in case 2.
Diagnoses of stage 2 minimal risk hypertensive (MNH), non-proteinuric (NP) chronic kidney disease (CKD) and pIMT were made. A renal formula diet (Hill's Prescription Diet Feline k/d brand pet food; Hill's Pet Nutrition Inc, Topeka, Kansas), and immunosuppressive therapy with oral prednisolone (5 mg twice daily) in combination with antibiotic therapy (Doxycycline tablets; Ivax Pharmaceuticals Miami, 5 mg/kg twice daily, pending vector-borne disease testing) was prescribed. After 1 week of therapy, the platelet count was 46,000/μl, and epistaxis resolved. After 11 months of corticosteroid therapy on a tapering schedule, the cat was presented as an emergency for diabetic ketoacidosis and hepatic lipidosis. At that time, the cat was still treated with oral prednisolone at 1 mg/kg once daily, and the platelet count was 312,000/μl. The cat was discharged 6 days later with instructions to decrease oral prednisolone to 0.5 mg/kg every other day and administer insulin therapy (Insulin zinc suspension, Humulin L; Eli Lilly, 4 units SC twice daily).
Over a 5-year follow-up, the cat experienced seven episodes of relapse with platelet counts <50,000/μl and recurrence of epistaxis at any attempts to decrease the prednisolone dose to <0.5 mg/kg/day. Five years following the diagnosis, the cat remained on treatment with oral prednisolone (1 mg/kg once daily) because the owner declined alternative immunomodulatory therapy. The cat has remained a well-regulated diabetic with insulin therapy (Glargine insulin, Lantus; Sanofi–Aventis Pharmaceuticals, 4 units SC twice daily), and has stable stage 2 CKD MNH/NP (creatinine at 2.2 mg/dl).
Case 3: A 7-year-old, 3.5 kg (7.7 lb) spayed female domestic shorthair cat was presented as an emergency for acute onset of hemoptysis. The cat did not have any known exposure to drugs or toxins, and was vaccinated for rabies 7 months before presentation. On physical examination, the cat had pale mucous membranes, a grade 3/6 systolic heart murmur with point of maximal intensity at the right parasternal border, and hemoptysis was noted.
Hematological abnormalities included severe normochromic normocytic regenerative anemia (hematocrit, 13.5%; mean corpuscular volume, 42.6 fl [reference range, 39.2–50.6 fl]; mean corpuscular hemoglobin, 16.2 pg [reference range, 12.9–17.7 pg]; aggregate reticulocytes, 7400/μl; punctuated reticulocytes, 15,200/μl) and severe thrombocytopenia (1000 platelets/μl). The cat was blood type A (Slide method, agglutination test, University of Pennsylvania, Philadelphia, PA) and a direct Coombs' test was negative. A midstream free catch urine sample was collected into a sterile container, and there was no subsequent bacterial growth on urine culture. Results of biochemical tests, urinalysis, measures of coagulation, and serum TT4 were within our laboratory reference ranges. Results of screening tests for FIV antibodies, FeLV antigen, and Ehrlichia canis, Rickettsia rickettsii, and Anaplasma phagocytophilum by IFA were negative. Echocardiogram, abdominal radiography and ultrasonography were unremarkable. Thoracic radiography disclosed a diffuse interstitial pattern.
Differential diagnosis for diffuse interstitial pattern includes artifact, lymphosarcoma, diffuse pulmonary metastasis, pneumonitis, fibrosis, and disease in transition, such as edema, bronchopneumonia, and hemorrhage. As the cat had severe thrombocytopenia, diffuse pulmonary hemorrhage was suspected. Cytological evaluation of a bone marrow aspirate and histological evaluation of core bone marrow biopsy specimens revealed marked megakaryocytic hyperplasia, and 20% of megakaryocytes had one or two intracytoplasmatic or surface-associated neutrophils, consistent with emperipolesis (see Fig 2). Bone marrow was negative for FeLV by IFA.
Fig 2.
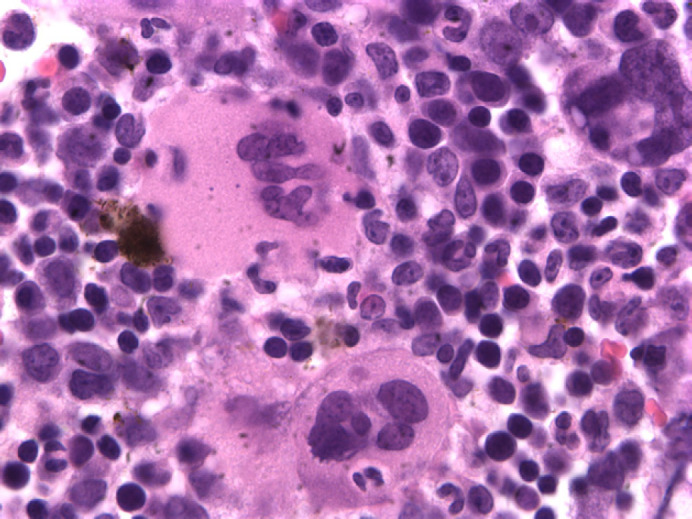
Megakaryocyte with intracytoplasmatic neutrophil(emperipolesis) in case 3.
A presumptive diagnosis of pIMT was made and immunosuppressive therapy with dexamethasone sodium phosphate (0.3 mg/kg IV bid) in combination with antibiotic therapy (Doxycycline; 5 mg/kg twice daily, pending vector-borne disease testing) was started. An IV infusion of pRBCs at 10 ml/kg over 4 h was also administered. On day 2, hematocrit was 19%, platelet count was <1000/μl and the cat became dyspneic. Oxygen therapy (40%) was provided via oxygen chamber enrichment, and a single IV injection of vincristine (Vincristine injectable; Mayne Pharma, 0.02 mg/kg) was administered. On day 3, respiratory rate and effort were still increased, platelet count was 1000/μl, and hematocrit decreased to 12%. Another transfusion of pRBCs (10 ml/kg IV over 4 h) was administered. After the transfusion, hematocrit was 21%, but the cat continued to be extremely dyspneic. Because of poor response to therapy and clinical deterioration, the owner elected humane euthanasia. Necropsy examination revealed multiple foci of alveolar hemorrhages in the lungs, submucosal hemorrhages of the urinary bladder, and few hemorrhages in the myocardium. An anticoagulant toxicology screen on hepatic tissue was negative.
Case 4: A 5-year-old, 5.1 kg (11.2 lb) neutered male domestic shorthair cat was evaluated for a 3-week history of decreased appetite, weight loss, hematuria and dysuria. The cat did not have any known exposure to drugs, toxins, or vaccines. Physical examination was non-remarkable.
On laboratory examination, mild normochromic normocytic regenerative anemia (hematocrit, 21.1%; mean corpuscular volume, 43.3 fl; mean corpuscular hemoglobin, 15.6 pg; aggregate reticulocytes, 118,600/μl; punctuated reticulocytes, 236,800/μl) and severe thrombocytopenia (5000 platelets/μl) were detected. A direct Coombs' test was negative. A midstream free catch urine sample was collected into a sterile container, and urinalysis was consistent with hematuria (too numerous to count RBCs/high power field, 5–20 WBCs/high power field 4+ protein on SSA). There was no bacterial growth on urine culture. Results of biochemical tests, measurements of coagulation, and serum TT4 were within our laboratory reference ranges. Results of serological tests for FIV antibodies, FeLV antigen, and Ehrlichia canis, Rickettsia rickettsii, and Anaplasma phagocytophilum by IFA were negative. Thoracic radiography and abdominal ultrasonography were unremarkable. Cytological evaluation of a bone marrow aspirate and histological evaluation of core bone marrow biopsy specimens revealed mild megakaryocytic and erythroid hyperplasia. Bone marrow was negative for FeLV by IFA.
A presumptive diagnosis of pIMT was made and immunosuppressive therapy with oral prednisolone (5 mg twice daily) in combination with antibiotic therapy (Doxycycline; 5 mg/kg twice daily, pending vector-borne disease testing) was started. On day 2, platelet count was 8000/μl and hematuria continued. On day 3, platelet count was 20,000/μl, the cat ate voluntarily and was discharged from the hospital. Eleven days after discharge, platelet count was within reference range, but 4 weeks later after decreasing the prednisolone dose by 25%, the platelet count was 30,000/μl. At that time, oral cyclosporine (Cyclosporine capsules; Pliva, 25 mg twice daily) was added to the therapeutic protocol. One week later, the platelet count was 90,000/μl, within reference range after two additional weeks of therapy. Over a 16-month follow-up, the cat had three episodes of lower urinary tract infections, and experienced two episodes of relapse with platelet count <50,000/μl at any attempt to decrease the cyclosporine dose <5 mg/kg/day. Eighteen months after the diagnosis, the cat was still being treated with prednisolone at 1 mg/kg/day and cyclosporine at 5 mg/kg/day orally.
In cats, decreased platelet counts are a common laboratory finding during automated counting due to the tendency for feline platelets to aggregate and the difficulty in differentiating between the similar size of some feline platelets and RBCs (Zelmanovich and Hetherington 1998). Thrombocytopenia in cats should always be confirmed by manual counting of platelets and blood smears should be evaluated for evidence of platelet clumping.
All four cats in these case series had severe thrombocytopenia attributable to an underlying immune-mediated mechanism on the basis of the following criteria: marked thrombocytopenia on presentation with increased numbers of megakaryocytes demonstrable in bone marrow aspirates and core biopsies; and exclusion of other causes of thrombocytopenia, such as drug exposure, neoplasia, DIC, infectious disease, and pseudothrombocytopenia due to machine error. Furthermore, three cats responded to immunosuppressive therapy. As molecular biology diagnostic techniques, such as polymerase chain reaction assays or Western blot analysis, for vector-borne diseases, FIV, and FeLV on bone marrow samples were not performed in the cases presented here, infectious diseases cannot be completely ruled out.
A mild to moderate, transient and self-limiting thrombocytopenia has been reported in dogs within 3–10 days after modified-live virus vaccination (Stokol and Parry 1997). In our study, two cats (cases 1 and 3) were relatively recently vaccinated for rabies before the presentation, but the time relationship between vaccination and onset of clinical signs makes it an unlikely triggering factor for the severe thrombocytopenia detected in these two cases. Platelet-bound antibodies assays were not used in these cases because they are not widely available and lack proven specificity for feline pIMT (Kohn et al 2006).
In the cases presented here, clinical history, physical examination, and clinicopathological findings were similar to those previously described in the few individual case reports of cats with pIMT (Joshi et al 1979, Tasker et al 1999, Garon et al 1999). In one cat (case 3), a bone marrow examination disclosed many megakaryocyte-associated neutrophils, interpreted as emperipolesis. Bone marrow emperipolesis has been previously reported in one cat with suspected pIMT (Garon et al 1999), and in many humans with idiopathic thrombocytopenic purpura, but it is a non-specific finding (Cashell and Buss 1992).
Immunosuppressive doses of corticosteroids have been the mainstay of treatment of canine pIMT (Lewis and Meyers 1996). In this report, two cats (cases 2 and 4) initially responded to oral prednisolone alone but became unresponsive after the dose was tapered. There was an apparent response to cyclosporine at doses of at least 5 mg/kg/day in one cat (case 4). Another cat (case 1) had a rapid platelet count recovery using dexamethasone intravenously. In previous reports, two cats with pIMT had a poor response to prednisolone orally, but a marked increase in platelet count that was sustained long-term using either dexamethasone or cyclosporine orally (Tasker et al 1999, Garon et al 1999).
The development of transient diabetes mellitus in cats associated with the use of glucocorticoids is well documented (Lien et al 2006). In this case series, the chronic use of corticosteroids was suspected to cause diabetes mellitus in two cats (cases 1 and 2), and recurrent bacterial urinary tract infections in another cat (case 4). One cat (case 3) was euthanased because of clinical deterioration and poor response to IV therapy with dexamethasone. This cat was presented for hemoptysis and required two pRBCs' transfusions. Necropsy examination detected pulmonary hemorrhage, and no underlying disease explaining the severe thrombocytopenia. In a recent retrospective study, dogs with IMT presenting with hemoptysis appeared to have the poorest outcomes, as 100% were euthanased during their visit due to difficulties in management and rapid progression of their disease (Bailiff and Norris 2002). In a previous report, one cat with suspected pIMT did not recover after surgical removal of a benign gastric mass infected with Nocardia species due to chronic immunosuppression, 15 months after initial diagnosis (Garon et al 1999).
In our study, the overall survival rate was 75% of cases at the time of discharge and 1 year after the initial diagnosis. This finding is similar to the three previous single case reports with only one cat euthanased on presentation (Joshi et al 1979), and two other cats alive at 1 year after the diagnosis (Tasker et al 1999, Garon et al 1999).
To the authors' knowledge, this represents the largest case series of cats with presumed pIMT reported in the veterinary literature. The cases presented here suggest that feline pIMT may have a chronic course and responds poorly to oral therapy with immunosuppressive doses of prednisolone. Because of the rare nature of this disorder, multicenter prospective studies are needed to evaluate the response to alternative immunomodulatory drug protocols in cats with pIMT.
References
- Bailiff N.L., Norris C.R. Clinical signs, clinicopathological findings, etiology, and outcome associated with hemoptysis in dogs: 36 cases (1990-1999), Journal of the American Animal Hospital Association 38, 2002, 125–133. [DOI] [PubMed] [Google Scholar]
- Beale K.M., Altman D., Clemmons R.R., Bolon B. Systemic toxicosis associated with azathioprine administration in domestic cats, American Journal of Veterinary Research 53, 1992, 1236–1240. [PubMed] [Google Scholar]
- Breitschwerdt E.B., Abrams-Ogg A.C., Lappin M.R., Bienzle D., Hancock S.I., Cowan S.M., Clooten J.K., Hegarty B.C. Molecular evidence supporting Ehrlichia canis-like infection in cats, Journal of Veterinary Internal Medicine 16, 2002, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashell A.W., Buss D.H. The frequency and significance of megakaryocytic emperipolesis in myeloproliferative and reactive states, Annals of Hematology 64, 1992, 274–276. [DOI] [PubMed] [Google Scholar]
- Estrin M.A., Wehausen C.E., Jessen C.R., Lee J.A. Disseminated intravascular coagulation in cats, Journal of Veterinary Internal Medicine 20, 2006, 1334–1339. [DOI] [PubMed] [Google Scholar]
- Garon C.L., Scott M.A., Selting K.A., Cohn L.A. Idiopathic thrombocytopenic purpura in a cat, Journal of the American Animal Hospital Association 35, 1999, 464–470. [DOI] [PubMed] [Google Scholar]
- Hahn K.A., McEntee M.F., Daniel G.B., Legendre A.M., Nolan M.L. Hematologic and systemic toxicoses associated with carboplatin administration in cats, American Journal of Veterinary Research 58, 1997, 677–679. [PubMed] [Google Scholar]
- Jordan H.L., Grindem C.B., Breitschwerdt E.B. Thrombocytopenia in cats: a retrospective study of 41 cases, Journal of Veterinary Internal Medicine 9, 1993, 261–265. [DOI] [PubMed] [Google Scholar]
- Joshi B.C., Raplee R.G., Powell A.L., Hancock F. Autoimmune thrombocytopenia in a cat, Journal of the American Animal Hospital Association 15, 1979, 585–588. [Google Scholar]
- Kohn B., Linden T., Leibold W. Platelet-bound antibodies detected by a flow cytometric assay in cats with thrombocytopenia, Journal of Feline Medicine and Surgery 8, 2006, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Breitschwerdt E.B., Jensen W.A., Dunnigan B., Rha J.Y., Williams C.R., Brewer M., Fall M. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America, Journal of the American Veterinary Medical Association 225, 2004, 893–896, 879. [DOI] [PubMed] [Google Scholar]
- Levy J.K. Ataxia in a kitten treated with griseofulvin, Journal of the American Veterinary Medical Association 198, 1991, 105–106. [PubMed] [Google Scholar]
- Lewis D.C., Meyers K.M. Canine idiopathic thrombocytopenic purpura, Journal of Veterinary Internal Medicine 10, 1996, 207–218. [DOI] [PubMed] [Google Scholar]
- Lien Y.H., Huang H.P., Chang P.H. Iatrogenic hyperadrenocorticism in 12 cats, Journal of the American Animal Hospital Association 42, 2006, 414–423. [DOI] [PubMed] [Google Scholar]
- O'Keefe D.A., Schaeffer D.J. Hematologic toxicosis associated with doxorubicin administration in cats, Journal of Veterinary Internal Medicine 6, 1992, 276–282. [DOI] [PubMed] [Google Scholar]
- Peterson J.L., Couto C.G., Wellman M.L. Hemostatic disorders in cats: a retrospective study and literature review, Journal of Veterinary Internal Medicine 9, 1995, 298–303. [DOI] [PubMed] [Google Scholar]
- Peterson M.E., Hurvitz A.I., Leib M.S., Cavanaugh P.G., Dutton R.E. Propylthiouracil-associated hemolytic anemia, thrombocytopenia, and antinuclear antibodies in cats with hyperthyroidism, Journal of the American Veterinary Medical Association 184, 1984, 806–808. [PubMed] [Google Scholar]
- Povey R.C. Effect of orally administered ribavirin on experimental feline calicivirus infection in cats, American Journal of Veterinary Research 39, 1978, 1337–1341. [PubMed] [Google Scholar]
- Stokol T., Parry B.W. The effect of modified-live virus vaccination on von-Willebrand factor antigen concentrations and platelet counts in dogs, Veterinary Clinical Pathology 26, 1997, 135–137. [DOI] [PubMed] [Google Scholar]
- Tasker S., Mackin A.J., Day M.J. Primary immune-mediated thrombocytopenia in a cat, Journal of Small Animal Practice 40, 1999, 127–131. [DOI] [PubMed] [Google Scholar]
- Zelmanovich D., Hetherington E.J. Automated analysis of feline platelets in whole blood, including platelet count, mean platelet volume, and activation state, Veterinary Clinical Pathology 27, 1998, 2–9. [DOI] [PubMed] [Google Scholar]


