Abstract
The pharmacological effects of glargine administered once or twice daily were compared in six healthy cats. A two-way crossover study was performed with insulin and glucose concentrations measured following subcutaneous administration of glargine once daily (0.5 U/kg) or twice daily (0.25 U/kg, repeated after 12 h). Nadir glucose concentration and mean daily glucose concentration did not differ significantly following insulin administration once daily or twice daily in divided doses. Time to reach last glucose nadir differed, with longer intervals occurring following twice daily dosing. Blood glucose failed to return to baseline concentration by 24 h in three of six cats in each treatment group. Insulin variables were not significantly different following once or twice daily dosing. This study in healthy cats demonstrates that glargine has a long duration of action with carry-over effects to the next day likely, regardless of dosing regimen. A study in diabetic cats is required to determine the best dosing regimen.
Insulin therapy is the most effective method of obtaining glycaemic control in diabetic cats. Many different insulin preparations are commercially available, with differing blood glucose-lowering potency, duration of effect and rate of absorption (Rand and Marshall 2004). Most insulins provide less than ideal glycaemic control in cats, mainly because of their short duration of effect. Results from recent research suggest that the long-acting insulin glargine, when administered twice daily and combined with a low carbohydrate diet, provides better glycaemic control and reduced risk of clinical hypoglycaemia compared to insulin with intermediate duration of activity (Marshall and Rand 2005).
Glargine is a synthetic human insulin analogue with very long duration of action. Designed for once daily administration in humans, glargine is marketed as long-acting and ‘peakless’ with respect to its glucose-lowering effect. Glargine was approved by the United States Food and Drug Administration, for use in treating type 1 and type 2 diabetes in humans in 2000.
In healthy cats, glargine has a longer duration of action than lente insulin with a peak action, on average, 14 h after administration, and suppression of blood glucose concentrations for at least 24 h in the majority of cats after a single dose (Marshall et al 2008). In newly diagnosed diabetic cats, glycaemic control is improved and remission rates higher amongst cats treated with glargine twice daily compared with cats treated with lente insulin twice daily (Marshall and Rand 2005). A trial involving both long-term and newly diagnosed diabetic cats showed that glargine administered once daily had a similar efficacy, as assessed by blood glucose and fructosamine concentrations, to lente insulin administered twice daily (Weaver et al 2006). However, it is unclear whether glargine should be administered once or twice daily as there have been no controlled studies comparing glargine administered once daily versus twice daily in domestic animals.
The aims of this study were to compare the effects of administering glargine once daily and twice daily in healthy cats, and to determine if once daily dosing of glargine is indicated based on pharmacokinetic and pharmacodynamic parameters.
Materials and methods
This study used a two-way crossover design, in which each cat received glargine either once or twice daily on the first testing day, then the alternative treatment after 72 h. Plasma insulin and glucose concentrations were measured for 24 h in each cat, after glargine administration.
Animals
Six adult neutered cats (three male, three female) that were clinically healthy and had ideal body condition scores (3 on a scale of 1–5) were obtained from the School of Veterinary Science at The University of Queensland. The study was approved by The University of Queensland Animal Ethics Committee and the cats were rehomed after the trial.
All cats were housed in the research facility 3 weeks prior to the commencement of data collection. During the last week of this period, cats were housed in individual cages. Throughout the trial, cats were fed an extruded dry feline maintenance diet (approximate energy distribution: protein 31%, carbohydrate 28% and fat 42%). Cats were fed once daily in the morning and uneaten food was left in the cages overnight, except prior to and during test days. Prior to test days, uneaten food was removed from the cages after the morning feed, approximately 18 h prior to insulin administration, and food was then withheld until the conclusion of the 24-h testing period.
Insulin
Insulin glargine 100 U/ml (Lantus, Aventis Pharmaceuticals, Frankfurt, Germany) was administered over the dorsal thoracic wall by subcutaneous injection at a dose of 0.5 U/kg once daily or 0.25 U/kg twice daily at 12-h intervals, with doses rounded to the nearest half unit. Insulin was administered by a U-100 syringe with 1 U graduations.
Procedure
At least 48 h prior to the first testing day, the cats were anaesthetised with propofol (Diprivan; Zeneca, UK), dosed to effect, and an 18 g polyurethane central venous catheter was placed in a jugular vein (Cook Veterinary Products, Brisbane, Australia) to facilitate blood sampling. Patency was maintained by twice daily flushing of heparinised saline (20 U/ml heparin in 0.9% NaCl).
Cats were assigned to one of the two treatment sequences (with three cats assigned to each sequence), with each cat receiving either a single dose of 0.5 U glargine/kg or two doses of 0.25 U glargine/kg 12 h apart on the first test day, and the other regimen on the second test day 72 h later. No insulin was administered between test days. Blood samples were collected via jugular catheter before (−30 and −5 min) and 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h after first administration of insulin. Blood samples were placed into ethylenediaminetetraacetic acid (EDTA) tubes on ice immediately after collection and centrifuged within 1 h to prevent in vitro glycolysis (Christopher and O'Neill 2000). The resultant plasma was stored at −70°C until assayed. Remaining red cells were aseptically washed and re-suspended in saline before auto transfusion to maintain red cell mass (Appleton et al 2001).
Sample analysis
Plasma glucose concentrations were determined using a YSI stat 2300 glucose analyzer (YSI Inc, Yellow Springs, Ohio, USA). Plasma insulin concentrations were measured using a commercially available radioimmunoassay kit (Phadeseph Insulin RIA; Pharmacia and Upjohn Diagnostics AB, Uppsala, Sweden) validated for measurement of feline insulin (Lutz and Rand 1993).
Statistical analysis
Duration of insulin action was defined for each cat-treatment combination as the time from insulin administration to the first timepoint after the last glucose nadir when blood glucose concentration was not significantly different from baseline (P>0.05). Blood glucose concentrations at specific timepoints were considered to differ significantly from baseline glucose concentrations when they were less than baseline for that cat-treatment combination minus the 90% range of differences. The 90% range of differences was estimated using the methodology reported by Marshall et al (2008) and was calculated to be 0.94 mmol/l.
Mean glucose concentration and area under the curve for glucose concentration were calculated for each cat-treatment combination using the linear trapezoidal method (Rowland and Tozer 1989). For insulin, the area under the curve above baseline up to return to baseline was also calculated using this method.
Effects of dosing regimen were assessed using analysis of variance after accounting for treatment sequence, cat within treatment sequence and test day. Following twice daily treatment, nadir glucose and mean glucose concentrations were compared for the first and second 12-h periods using paired t-tests. Analysis of variance for calculating the 90% range of differences and statistical analyses of outcome variables were performed using Stata version 9.2 (Statacorp, College Station, TX).
Results
There was substantial variation between cats in response to glargine and a difference in response between 0.25 and 0.5 U/kg dosing (Table 1, Fig 1). The blood glucose-lowering effect continued in some cats for at least 24 h with both dosing regimens. Blood glucose concentration did not return to baseline by 24 h in three of six cats following either once or twice daily administration, with two cats failing to return to baseline after both once and twice daily treatment. For cats that did return to baseline by 24 h, times to return to baseline were 14, 16 and 22 h for once daily treatment and 18, 22 and 22 h for twice daily treatment. At 12 h after insulin administration, all the cats receiving glargine once daily had blood glucose concentrations below baseline. For cats receiving divided doses of glargine given twice daily, at 12 h after initial insulin administration, blood glucose concentrations were significantly lower than baseline glucose concentrations in two cats and not significantly lower than baseline in four cats. For one of these four cats, blood glucose concentration was never significantly less than baseline during the first 12-h interval, indicating that for this cat, 0.25 U/kg of glargine was insufficient to result in a significant glucose-lowering effect in the first 12 h. The combined results at 12 and 24 h for once and twice daily dosing suggest a dose-dependent effect on duration of action.
Table 1.
Glucose and insulin concentrations and indices following subcutaneous administration of glargine insulin once daily (0.5 U/kg) or twice daily (0.25 U/kg repeated after 12 h) in a two-way crossover study in 6 healthy cats
| Once daily administration (0.5 U/kg) | Twice daily administration (0.25 U/kg) | P-Value | Difference between means (twice daily−once daily) | 95% confidence interval for difference | |
|---|---|---|---|---|---|
| Glucose concentration at nadir (mmol/l) | 1.6±0.3 (1.1–2.9) | 1.6±0.2 (1.2–2.3) | 1.000 | 0.0 | −0.8, 0.8 |
| Nadir glucose concentration during 0–12 h (mmol/l) | 1.9±0.4 (1.0–3.9) | 2.6±0.4 (1.5–3.7) | 0.082 | 0.7 | −0.1, 1.5 |
| Time to first nadir (h) | 11.7±1.4 (8–16) | 13.3±2.9 (4–22) | 0.526 | 1.6 | −5.1, 8.3 |
| Time to last nadir (h) | 12.7±1.2 (8–16) | 17.0±1.1 (14–22) | 0.048 | 4.3 | 0.2, 8.4 |
| Duration of action – proportion of cats not returned to baseline glucose concentration by 24 h (number of six cats) | 0.5 (3) | 0.5 (3) | |||
| Mean daily glucose concentration (mmol/l) | 3.4±0.2 (2.8–4.3) | 3.3±0.2 (2.8–4.2) | 0.793 | −0.1 | −0.8, 0.6 |
| Mean 0–12 h glucose concentration (mmol/l) | 3.0±0.4 (1.9–4.2) | 3.6±0.2 (2.9–4.2) | 0.079 | 0.6 | −0.1, 1.3 |
| Mean 12–24 h glucose concentration (mmol/l) | 3.8±0.4 (2.6–5.1) | 3.0±0.3 (2.0–4.2) | 0.121 | −0.8 | −1.9, 0.3 |
| Peak insulin concentration (μU/ml) | 48±6 (33–68) | 56±13 (21–111) | 0.585 | 8 | −27, 43 |
| Area under 24 h insulin concentration curve (μUh/ml) | 486±37 (398–616) | 525±62 (365–784) | 0.625 | 39 | −162, 240 |
Data reported as mean±SEM. Values in parentheses indicate range.
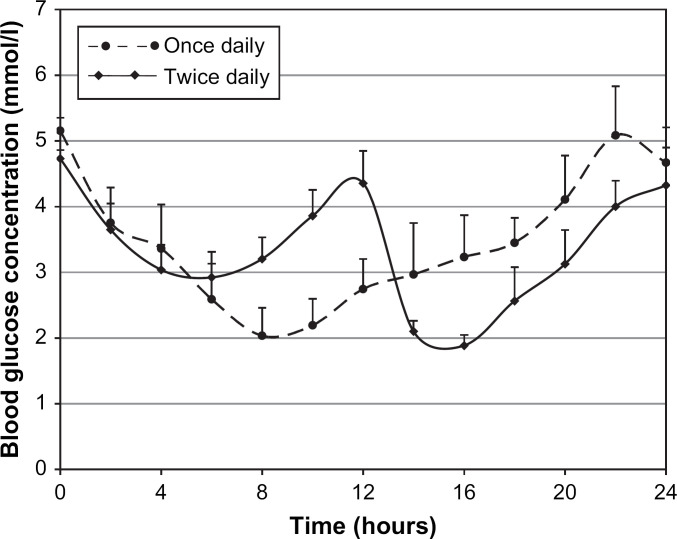
Fig 1.
Mean plasma glucose concentrations (and standard errors of means) after subcutaneous administration of glargine once daily (0.5 U/kg) or twice daily (0.25 U/kg repeated after 12 h) in a two-way crossover study in six healthy cats.
Mean glucose concentration over 24 h did not differ significantly between once and twice daily dosing (P=0.793). When mean glucose concentration during the first 12 h was examined, there was a trend that approached significance (P=0.079) suggesting that once daily administration resulted in lower mean glucose concentration (3.0±0.4 mmol/l, mean±SEM) than when the dose was divided and administered twice daily (3.6±0.2 mmol/l), suggesting a dose-dependent glucose-lowering effect (Table 1, Fig 1).
The lowest nadir glucose concentration did not differ significantly (P=1.000) between once and twice daily dosing, nor did time to first nadir (P=0.526) (Table 1, Fig 1). However, time to last nadir differed between treatments (P=0.048), with longer intervals following twice daily (17.1±1.1 h) compared with once daily (12.7±1.2 h) treatment (Table 1, Fig 1). There was a trend which approached significance (P=0.082) suggesting that the nadir during the first 12 h was lower for once daily (1.9±0.4 mmol/l) compared to twice daily (2.6±0.4 mmol/l) treatment, consistent with a dose-dependent glucose-lowering effect (Table 1, Fig 1).
When glargine was administered twice daily, the nadir in the second 12 h (1.7±0.2 mmol/l) was significantly lower than the nadir during the first 12 h (2.6±0.4 mmol/l; P=0.049) indicating a carry-over effect from the first injection (Table 2, Fig 1). There was also a trend which was not statistically significant (P=0.093) suggesting that following glargine administration twice daily, mean glucose concentration was lower during the 12 h after the second injection (3.0±0.3 mmol/l) than during the 12 h after the first injection (3.6±0.2 mmol/l), consistent with a carry-over effect (Table 2, Fig 1).
Table 2.
Mean plasma glucose concentrations 0–12 h and 12–24 h following subcutaneous administration of glargine insulin twice daily (0.25 U/kg repeated after 12 h) in a two-way crossover study in six healthy cats
| 0–12 h | 12–24 h | P-Value | Difference between means (12–24 h minus 0–12 h) | 95% confidence interval for difference | |
| Nadir glucose concentration (mmol/l) | 2.6±0.4 (1.5–3.7) | 1.7±0.2 (1.2–2.3) | 0.049 | −0.9 | −1.8, 0.0 |
| Mean glucose concentration (mmol/l) | 3.6±0.2 (2.9–4.2) | 3.0±0.3 (2–4.2) | 0.093 | −0.6 | −1.3, 0.1 |
Data reported as mean±SEM. Values in parentheses indicate range.
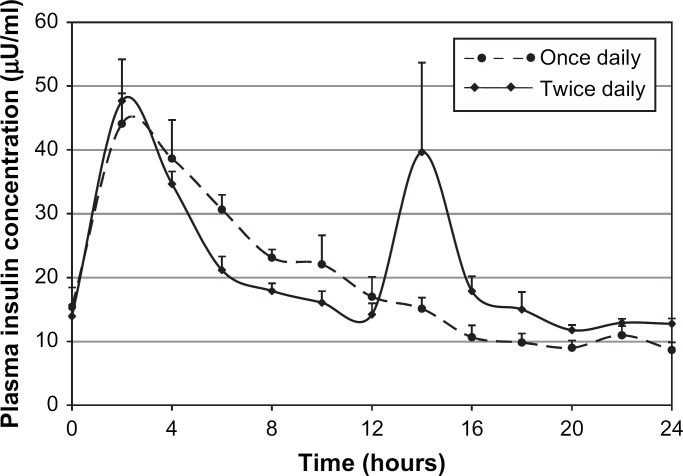
Peak insulin concentration did not differ significantly between treatments (P=0.585) nor did area under the 24-h insulin curve (P=0.625) (Table 1, Fig 2).
Fig 2.
Mean plasma insulin concentrations (and standard errors of means) after subcutaneous administration of glargine once daily (0.5 U/kg) or twice daily (0.25 U/kg repeated after 12 h) in a two-way crossover study in six healthy cats.
Discussion
This is the first reported study in domestic animals comparing the effects of insulin glargine administered once or twice daily. The most important finding from this study in healthy cats is that glargine has a long duration of action with blood glucose concentrations at 24 h suppressed below baseline in a substantial proportion of cats regardless of whether insulin is administered once daily (0.5 U/kg) or twice daily in divided doses (0.25 U/kg repeated after 12 h).
There was evidence of a carry-over effect after 12 h following twice daily administration, and at 24 h regardless of whether insulin was administered once or twice daily. This is likely to be advantageous in treating diabetic cats because it increases the likelihood that exogenous insulin effects will continue until the next insulin injection, regardless of the dosing regimen. In contrast, because of the short duration of effect with lente insulin, when administered every 12 h there is, on average, 4 h prior to each injection (ie, 8 h each day) where there is minimal exogenous insulin action, leading to marked hyperglycaemia in many diabetic cats (Martin and Rand 2001). Because of the long duration of insulin action and carry-over effects seen in this study in healthy cats, diabetic cats treated with glargine, should have blood glucose measured initially just prior to insulin administration when using serial blood glucose monitoring to assess insulin dose. Measuring blood glucose prior to insulin injection is important to ensure that cats with low pre-insulin blood glucose concentrations are not overdosed.
The blood glucose-lowering effect and duration of action of glargine in this study in healthy cats was similar to that reported in previous studies in healthy cats (Marshall et al 2008) and diabetic humans (Owens et al 2000). Glargine is marketed for human use as very long-acting and ‘peakless’. The peakless claim is potentially misleading in that it refers to glargine's glucose utilisation rate being ‘peakless’, and does not imply that the blood glucose concentration curve after administration has no nadir. The glucose utilisation rate is the amount of intravenous glucose required to maintain a constant plasma glucose concentration after subcutaneous injection of insulin. In this study, there were definite nadirs and peaks in glucose and insulin concentrations, but the glucose utilisation rate was not studied.
Studies have shown that human patients with type 1 or type 2 diabetes treated with glargine have similar to improved glycaemic control compared with patients treated with neutral protamine Hagedorn insulin, but more importantly there are fewer hypoglycaemic episodes (Fonseca et al 2004, Fulcher et al 2005). Hypoglycaemic episodes in diabetic cats treated with insulin can be life-threatening. Preliminary results from a small study in diabetic cats suggest that clinical hypoglycaemia is less frequent in glargine-treated cats than cats treated with lente or protamine zinc insulin (PZI) (Marshall and Rand 2005), but a larger study is needed to provide statistical evidence that the incidence of clinical hypoglycaemia in diabetic cats treated with glargine is lower than for cats treated with other types of insulin.
Reducing the time on a daily basis that β cells are exposed to marked hyperglycaemia is thought to be important for recovery of β cell function from the suppressive effects of glucose toxicity. In humans and cats, recovery of β cell function is critical for subsequent diabetic remission (Robertson et al 2000, Link 2001). We predict that better glycaemic control and reduced periods of hyperglycaemia would be achieved in a greater proportion of diabetic cats if glargine is administered twice rather than once daily. Clinical observations suggest that remission rates in newly diagnosed diabetic cats are higher when glargine is administered twice daily compared to once daily dosing (Marshall and Rand 2005, Weaver et al 2006). Diabetic remission is highly advantageous, resulting in improved health and quality of life, and should be one of the main goals of therapy for diabetic cats. A prospective controlled trial in diabetic cats is indicated to determine whether glycaemic control and remission rates are improved with glargine administered twice rather than once daily.
There was large individual variation in plasma insulin concentrations for a given calculated insulin dose, and associated large variation in blood glucose concentrations. The source of the variation in this study is likely to be multifactorial including differences in rates and percentage of absorption of insulin from the subcutaneous injection site, variation in insulin sensitivity between cats, and variation in the actual dose received per kg of metabolic weight. Similar variation in glucose responses is also reported in human (Bantle and Laine 1988, Bantle et al 1990, Moberg et al 1995) and dog studies (Fleeman and Rand 2003). Errors with insulin dosing were a potential source of variation in insulin and glucose concentrations between study cats, particularly for twice daily administration, and this may also be important in clinical practice. Most study cats receiving twice daily treatment at a dose of 0.25 U/kg received only 1 U at each injection. This very small volume of insulin was administered by a U-100 syringe with 1 U graduations making dosage errors likely. It has been shown that paediatric nurses using similar syringes to those used in this study were unable to accurately dose any amount under 2 U (Casella et al 1993). Glargine should not be mixed or diluted, so accurate dosing is difficult in most cats. These errors are likely to be minimised by use of insulin syringes designed for 100 U/ml insulin with 0.5 U gradations.
This study in healthy cats demonstrates that there are large variations between cats in glucose responses to a calculated dose of glargine. Similar large individual variations in glucose response to the same calculated dose of glargine are likely to occur in diabetic cats, making it essential to monitor blood glucose concentration, especially for the first few days after initiating therapy with glargine. This variation may be between or within cat. Research using glargine in diabetic cats is required to evaluate the day-to-day variation in glucose response, and to assess the effects of this variation on monitoring and adjusting insulin dose. Detemir, a new long-acting insulin analogue with a different mode of action to glargine, is being marketed for use in humans as being more predictable in its action than glargine (Klein et al 2007). However, there are no published studies assessing effects of detemir in cats.
In summary, this study in healthy cats demonstrates that glargine has a long duration of action, with carry-over effects to the next day likely, regardless of once or twice daily dosing. With twice daily treatment, this long duration of action tends to produce a lower nadir glucose concentration after the second injection, compared to the first. A study involving diabetic cats is required to compare these dosing regimens, and to fully evaluate the potential of glargine for treatment of diabetes mellitus in cats.
Acknowledgements
The authors would like to thank the Australian Companion Animal Health Foundation for funding this study and the valued support from all the veterinary research technicians at The University of Queensland.
References
- Appleton D.J., Rand J.S., Sunvold G.D., Priest J. Determination of reference values for glucose tolerance, insulin tolerance and insulin sensitivity tests in clinically normal cats, American Journal of Veterinary Research 62, 2001, 630–636. [DOI] [PubMed] [Google Scholar]
- Bantle J.P., Laine D.C. Day-to-day variation in glycaemic control in type I and type II diabetes mellitus, Diabetes Research 8, 1988, 147–149. [PubMed] [Google Scholar]
- Bantle J.P., Weber M.S., Rao S.M.S., Chattopadhyay M.K., Robertson R.P. Rotation of the anatomic regions used for insulin injections and day-to-day variability of plasma glucose in type I diabetic subjects, Journal of the American Medical Association 263, 1990, 1802–1806. [PubMed] [Google Scholar]
- Casella S.J., Mongilio M.K., Plotnick L.P., Hesterberg M.P., Long C.A. Accuracy and precision of low-dose insulin administration, Pediatrics 91, 1993, 1155–1157. [PubMed] [Google Scholar]
- Christopher M.M., O'Neill S. Effect of specimen collection and storage on blood glucose and lactate concentration in healthy, hyperthyroid, and diabetic cats, Veterinary Clinical Pathology 29, 2000, 22–28. [DOI] [PubMed] [Google Scholar]
- Fleeman L.M., Rand J.S. Evaluation of day to day variability of serial blood glucose concentration curves, Journal of the American Veterinary Medical Association 3, 2003, 317–321. [DOI] [PubMed] [Google Scholar]
- Fonseca V., Bell D.S., Berger S., Thomson S., Mecca T.E. A comparison of bedtime insulin glargine with bedtime neutral protamine Hagedorn in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study, American Journal of the Medical Sciences 328, 2004, 274–280. [DOI] [PubMed] [Google Scholar]
- Fulcher G.R., Gilbert R.E., Yue D.K. Glargine is superior to NPH for improving glycated hemoglobin and fasting blood glucose levels during intensive insulin therapy, Internal Medicine Journal 35, 2005, 536–542. [DOI] [PubMed] [Google Scholar]
- Klein O., Lynge J., Endahl L., Damholt B., Nosek L., Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes, Diabetes, Obesity and Metabolism 9, 2007, 290–299. [DOI] [PubMed] [Google Scholar]
- Link KRJ. (2001) Feline Diabetes: Diagnostics and Experimental Modelling [doctoral thesis]. The University of Queensland, Brisbane.
- Lutz T.A., Rand J.S. Comparison of five commercial radioimmunoassay kits for the measurement of feline insulin, Research in Veterinary Science 55, 1993, 64–69. [DOI] [PubMed] [Google Scholar]
- Marshall R.D., Rand J.S. Treatment with insulin glargine results in higher remission rates than lente or protamine zinc insulins in newly diagnosed diabetic cats (abstract), Journal of Veterinary Internal Medicine 19, 2005, 425. [Google Scholar]
- Marshall R.D., Rand J.S., Morton J.M. Glargine and protamine zinc insulin have a longer duration of action and result in lower mean daily glucose concentrations than lente insulin in healthy cats, Journal of Veterinary Pharmacology and Therapeutics 31, 2008, 205–212. [DOI] [PubMed] [Google Scholar]
- Martin G., Rand J.S. Pharmacology of a 40IU/ml porcine lente insulin preparation in diabetic cats. Findings during the first week and after 5 or 9 weeks of therapy, Journal of Feline Medicine and Surgery 3, 2001, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg E., Kollind M., Lins P.E., Adamson U. Day-to-day variation of insulin sensitivity in patients with type 1 diabetes: role of gender and menstrual cycle, Diabetic Medicine 12, 1995, 224–228. [DOI] [PubMed] [Google Scholar]
- Owens D.R., Coates P.A., Luzio S.D., Tinbergen J.P., Kurzhals R. Pharmacokinetics of 125I-labeled insulin glargine in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites, Diabetes Care 23, 2000, 813–819. [DOI] [PubMed] [Google Scholar]
- Rand J.S., Marshall R.D. Feline diabetes mellitus. Mooney, Peterson BSAVA Manual of Canine and Feline Endocrinology, 2004, British Small Animal Veterinary Association: Gloucester, 129–141. [Google Scholar]
- Robertson R.P., Tanaka Y., Sacchi G., Tran P.O.T., Gleason C., Poitout V. Glucose toxicity of the β-cell: cellular and molecular mechanisms. LeRoith, Olefsky, Taylor Diabetes Mellitus, 2000, Lippincott Williams & Wilkins: New York, 125–132. [Google Scholar]
- Rowland M., Tozer T.N.. Rowland, Tozer Clinical Pharmacokinetics: Concepts and Applications, 2nd edn, 1989, Lea & Febiger: Philadelphia, 459–471. [Google Scholar]
- Weaver K.E., Rozanski E.A., Mahony O.M., Chan D.L., Freeman L.M. Use of glargine and lente insulins in cats with diabetes mellitus, Journal of Veterinary Internal Medicine 20, 2006, 234–238. [DOI] [PubMed] [Google Scholar]