Abstract
Obesity is one of the most common medical diseases in cats, but there remains little information on success of weight loss regimes in obese client-owned cats. No information currently exists on body composition changes during weight loss in clinical cases. Twelve obese client-owned cats undertook a weight loss programme incorporating a high-protein low fat diet. Body composition was quantified by dual-energy X-ray absorptiometry, before and after weight loss. Mean (±standard deviation) weight loss was 27±6.8% of starting weight, and mean rate of weight loss was 0.8±0.32% per week. Mean energy allocation during weight loss was 32±7.0 kcal/kg target weight. Mean composition of tissue lost was 86:13:1 (fat:lean:bone mineral). The proportion of lean tissue loss was positively associated with overall percentage of weight loss (simple linear regression, r 2=44.2%, P=0.026). Conventional weight loss programmes produce safe weight loss, but lean tissue loss is an inevitable consequence in cats that lose significant proportions of their starting body weight.
Obesity is defined as an accumulation of excessive amounts of body fat, and is common in cats with prevalence reported to be between 22 and 50% (Sloth 1992, Robertson 1999, Russell et al 2000, Harper et al 2001, Lund et al 2005). Given that overweight cats are predisposed to a variety of associated diseases (Lund et al 2005, German 2006) treatment is important. Pharmaceutical interventions are now available for the treatment of canine obesity (Wren et al 2007), but conventional obesity management, involving dietary energy restriction and increasing activity levels, remains the treatment of choice for cats. A number of research studies have examined the success of weight loss in cats (Butterwick et al 1994, Butterwick and Markwell 1996, Nguyen et al 2004, Laflamme and Hannah 2005, Michel et al 2005), but such data may not be fully representative of weight loss in a practice setting, where the variables are less controlled than in a colony environment.
Ideally, adipose tissue alone is lost during weight loss, whilst lean tissue and bone mineral are preserved. A previous study in cats demonstrated that lean tissue loss is directly proportional both to the degree of caloric energy restriction and to the rate of weight loss (Butterwick and Markwell 1996). This is similar to findings in humans (Forbes 1987, Prentice et al 1991) whilst a recent study in dogs has demonstrated that lean tissue loss is proportional to the overall percentage of weight lost (German et al 2007). Further, work in both dogs and cats has shown dietary composition (eg, high-protein diet) to be important in the relative amounts of the various body tissues which are lost (Diez et al 2002, Nguyen et al 2004, Laflamme and Hannah 2005). However, to date, all studies have been experimental rather than clinical.
The purpose of this study was to examine the factors associated with two key determinants of outcome during weight loss (eg, mean energy allocation required and relative changes in body composition) in a cohort of client-owned cats with naturally occurring obesity. Factors of particular interest included rate of weight loss, degree of energy restriction required for weight loss, and the relative changes in body composition that resulted.
Materials and methods
Patients
Twelve cats participated in this study, all of which were referred to the Royal Canin Weight Management Clinic (RCMWC), University of Liverpool, UK, for the investigation and management of obesity. For inclusion in the study, cats had to be systemically well, and have no significant abnormalities on routine haematological analysis, serum biochemical analysis and urinalysis. Cats were enrolled between December 2004 and April 2007, and their period of weight loss was completed between April 2005 and September 2007. The study was performed in adherence to the University of Liverpool Animal Ethics Guidelines and was approved by the Waltham ethical review committee. The owners of all participating animals gave informed written consent.
Evaluation prior to weight loss
All patients were weighed, body condition scored (Laflamme 1997), and body composition assessed by dual-energy X-ray absorptiometry (DXA). An individually tailored weight management protocol was then instigated (see below).
Assessment of weight and body composition
True total body weight was measured by electronic weigh scales (range 0.05–100.00 kg; Soehnle Professional, Murrhardt, Germany), which were regularly validated for precision and accuracy using test weights (2–50 kg; guaranteed accuracy ≤0.5%; Blake and Boughton, Thetford, UK). Body composition was analysed by fan-beam DXA (Lunar Prodigy Advance; GE Lunar; Madison, USA), with high precision for repeat analysis in animals (Raffan et al 2006). Patients were either sedated (if DXA alone was performed) or anesthetised (if required for additional procedures, eg, radiographic studies, surgery, routine dentistry), and scanned in dorsal recumbency, as previously described for dogs (Raffan et al 2006). Purpose-designed computer software (enCORE 2004, 8.70.005; GE Lunar) was used for data analysis.
Weight loss regimen
Diet and energy allocation
Prior to weight loss an initial ideal target weight was set, based upon two calculations, one using starting body condition score and one using body composition determined by DXA. For the condition score method, it was assumed that each point on the 9-integer condition scoring system accounted for the cat being 10% above ideal weight (Mawby et al 2004). For the body composition method, the estimated fat mass was compared with a reference range of 15–25%, generated from cats in ideal body condition; and assumed a predicted weight loss of 80:20 fat to non-fat mass whilst on an equivalent high-protein weight loss diet (Nguyen et al 2004). The results from each technique were compared, and the agreed initial target weight was set from a consensus of the two methods.
Initial energy allocation given was calculated as 40 kcalME (metabolisable energy)×estimated target weight (in kg), which aimed to achieve weight loss at 1–2% per week. Further adjustments were then made, based upon other factors, eg, ability to exercise, current energy allocation (in cases referred after failure to lose weight at referring veterinary practice), and owners' request for a gradual rather than sudden acclimatisation to the weight loss programme.
Cats were fed either a dried or moist high-protein, fat-restricted, moderate fibre diet (Table 1). The choice of ration was tailored to the individual patient and depended upon previous dietary preferences. When the moist diet was used, the required amount was calculated and extrapolated to a proportion of the sachet; for the dried ration, the amount was extrapolated to weight in grams. For dried food, owners were asked to weigh food out each day using kitchen scales. To ensure accuracy, a 24-h ration was first weighed on electronic scales (range 1–5000 g; Salter, Tonbridge, UK) at the RCWMC, and then taken home by the client to weigh on their home scales. The RCWMC scales were regularly assessed, for precision and reliability, using test weights (1–100 g; guaranteed accuracy ≤0.1%; Blake and Boughton).
Table 1.
Composition of diets for weight loss
| Criterion | Dried ration * | Moist ration † | ||
|---|---|---|---|---|
| ME content ‡ | 3763 kcal/kgDM | 4010 kcal/kgDM | ||
| Moisture | 7.0 | 83.0 | ||
| Per 100 gDM § | Per 1000 kcal (ME) ∥ | Per 100 gDM § | Per 1000 kcal (ME) ∥ | |
| Crude protein | 45.2 | 120 | 49.3 | 123 |
| Crude fat | 10.8 | 29 | 12.7 | 32 |
| Carbohydrate | 18.6 | 55 | 14.7 | 37 |
| Crude fibre | 9.7 | 26 | 2.7 | 7 |
| Total dietary fibre | 16.6 | 44 | 8.7 | 22 |
| Ash | 9.6 | 26 | 14.7 | 37 |
| l-Carnitine | 22 | 58 | 20 | 50 |
AF=as fed, DM=dry matter.
Obesity management DP 42 (dry), Royal Canin, Aimargues, France.
Obesity management S/O (moist), Royal Canin.
ME measured, on a panel of seven healthy cats, by Royal Canin Research Centre.
All nutrients expressed in g/100 g, except l-carnitine (mg/100 g).
All nutrients expressed as g/1000 kcalME, except l-carnitine (mg/1000 kcalME).
Lifestyle alterations
At the time of initial consultation, the owner was counselled on the lifestyle alterations which would be required to assist in weight loss. This, in part, covered education about the need to prevent excessive feeding, the need to avoid feeding extra food or treats, and information on strategies for providing non-food related rewards. Advice on increasing activity within the home environment was discussed for all cats (eg, moving food bowls prior to meal times and encouraging the cat to follow, organised play sessions), and a tailored exercise plan was devised for each patient. The exact recommendations took account of signalment (eg, breed and age), presence and type of concurrent diseases where applicable, and owner factors (eg, locality, occupation, lifestyle, personal circumstances).
Monitoring
All cats were assessed every 14–28 days, depending upon the availability of the owner during the weight loss programme. Body weight measurements were taken and changes made to the dietary plan if necessary. If an appointment was missed, the owner would be contacted by telephone to discuss progress, and to rearrange the appointment. The overall aim was to achieve 1–2% per week weight loss, although slower weight loss was acceptable if progress was steady. Throughout the weight loss period, owners maintained a diary covering diet ration fed, activity, and any additional food that had been consumed (either given as treats or stolen).
At each re-evaluation, changes were made to the weight loss plan, as necessary. If there had been good progress with weight loss, the diet was not altered, but the owner was encouraged to step up activity wherever possible (eg, by increasing intensity and duration of play sessions). If the cat had either gained weight or not lost weight, the potential causes were investigated based upon the information provided by the owner (eg, diary records and discussions during the consultation). If the cause was thought to be related to poor compliance to the diet (eg, administration of extras), the owner was encouraged to return to diet plan, and the amount of food fed was not changed. If activity levels had been decreased, then the owner was encouraged to reinitiate these; however, where there was no obvious reason for poor progress, the amount fed was reduced by a readily calculated amount (eg, 5 g for a cat on the dried ration; ¼ sachet when fed the wet ration) on each occasion. When weight loss was deemed to be too quick (>2% per week) the amount of diet was increased in similar increments. During the programme, the body condition of the patient was continually assessed; the final target weight was altered if, as weight loss progressed, the initial estimate was not thought to be appropriate.
Re-evaluation at the end of weight loss
A detailed evaluation was conducted when target weight was reached. Cats were confirmed to have remained healthy based upon physical examination, routine haematological analysis, serum biochemical analysis and urinalysis. Body weight and body condition were recorded, and body composition assessed by DXA (in 11 of the 12 cats), to confirm that body composition had normalised. In the remaining cat, repeated DXA was not performed, on the request of the owner.
Estimation of supplementary energy intake from additional fed items
The diary information recorded by the owners included a description of additional foodstuffs that the cats had consumed. The caloric value of all items was estimated either from the known energy densities of food (derived from packaging information) or from published information on caloric content of food (Humphries 2002, Kellow 2006).
Statistical analysis
All data are expressed as mean and standard deviation (SD), or as median and range. Daily food allocation is reported as ME intake (in kilocalories, kcal), per kg target body weight (TBW), whilst percentage weight loss calculations are reported as a proportion of starting body weight (SBW).
Statistical analysis was performed with a computer software package (Minitab v14.0; Minitab, State College, PA, USA). Various factors were examined for their effect on two key outcome measures, the mean level of energy allocation required for weight loss and the proportional change in body composition lost during weight loss. Factors tested included signalment (eg, age and gender), stature (based upon starting lean body mass), diet type, percentage of weight lost, mean rate of weight loss and level of reported supplemental feeding. For diet type, cats were assigned to two groups: the first group comprised those cats fed dried diet exclusively during the weight loss period, whilst the second group consisted of cats fed a combination of both types. Other assessments made included comparisons of energy allocation and body composition (fat, lean and bone mineral content [BMC]) at the start and end of weight of loss.
All continuous data were first assessed for normal distribution (for the overall population and all subgroups) using the Anderson–Darling method, and parametric statistical tests were used wherever appropriate. These included paired and unpaired t-tests, and simple linear regression. If data were not normally distributed, non-parametric statistical tests were used, including Mann–Whitney U test and Spearman's correlation coefficient (rs). The level of statistical significance was set at P<0.05.
Results
Study animals
Of the 12 cats, 11 were domestic shorthairs, one was a British Shorthair. Seven and five cats were neutered female and neutered male, respectively, and median age was 128 months (range 78–156 months).
Summary of weight loss
Median SBW was 6.90 kg (range 5.45–10.30 kg) whilst median final body weight was 5.05 kg (range 3.95–6.45 kg). The median duration of weight loss was 280 days (range 141–490 days) and the mean±SD percentage weight lost was 27±6.8%. Mean±SD rate of weight loss for all cats was 0.8±0.32% per week (range=0.4–1.3% per week). The median number of examinations (including initial and final examination) was 11 (range 8–22), and the median time between revisits was 20 days (range 14–28 days). Five cats were fed the dried diet exclusively, whilst a mix of dried and moist food was fed in the remaining seven cats. One of these cats was fed a combination of moist and dry throughout weight loss, whilst the remaining six cats were switched between diet types (eg, dried food to mixed ration, n=2; moist food to dried ration, n=1; mixed ration to dry, n=3). All cats required changes to the amount of food fed during the period of weight loss; the amount of food was increased in three cats (once, n=1; twice, n=1; three times, n=1), and decreased in all cats (once, n=4; twice, n=4; three times, n=2; four times, n=1; five times, n=1). On each occasion, the median amount by which the diet was altered was 9% (range 5–25%).
Energy allocation required for weight loss
The mean±SD level of dietary caloric energy allocation (for the whole weight loss period) was 32±7.0 kcal/kgTBW. The energy allocation, required to ensure continuing weight loss, decreased during the programme (mean±SD for allocation at start versus end at of weight loss [eg, 41±3.9 kcal/kgTBW versus 27±6.5 kcal/kgTBW, paired t-test P=0.004]). This corresponded to 55±10.0 kcal/kg0.67TBW and 76±18.7 kcal/kg0.4 SBW. Although starting energy allocation did not differ between cats fed in the two diet types (dried diet exclusively 40±4.0 kcal/kgTBW versus dry/moist combination 39±4.8 kcal/kgTBW; two sample t-test, P=0.724), the mean energy allocation and end energy allocation were both significantly lower for the cats fed a combination of moist and dried food than for those fed dried food exclusively (mean allocation: 29±6.6 kcal/kgTBW versus 36±3.5 kcal/kgTBW, two sample t-test, P=0.049; end allocation: 24±3.3 kcal/kgTBW versus 33±4.7 kcal/kgTBW, two sample t-test, P=0.011). No other factor had any significant effect on the degree of caloric restriction required for weight loss.
Dietary non-compliance
Owners reported dietary non-compliance in nine of 12 cats, either due to the owner treating the cat, or by the cat stealing food. A wide range of additional foodstuffs was eaten including feline foods and snacks (eg, obesity diet ration, standard maintenance diets, dental treats), dog food, meats (chicken, beef, and pork), fish (tuna, salmon, and white fish), and dairy products (milk, cheese and butter). The median estimated contribution to dietary caloric intake from non-compliance was 0.1 kcal/kgTBW (range 0.0–5.0 kcal/kgTBW). This equated to 0.1 kcal/kgTBW0.67 (range 0.0–8.4 kcal/kg0.67TBW) or 0.1 kcal/kgSBW0.4 (range 0.0–11.8 kcal/kg0.4SBW). There was no significant difference in reported dietary non-compliance between different diet types (Mann–Whitney test, P=0.808).
Body composition
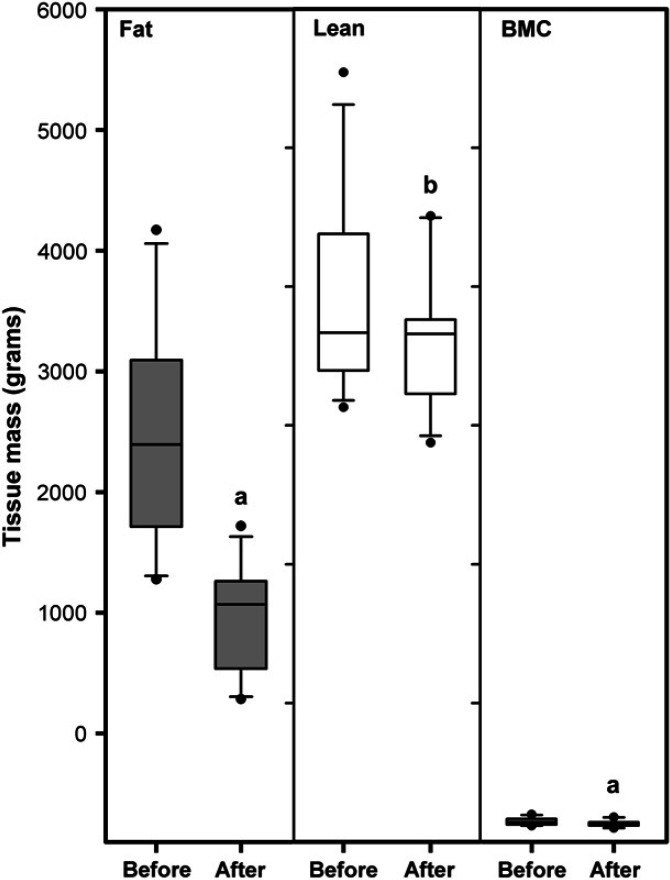
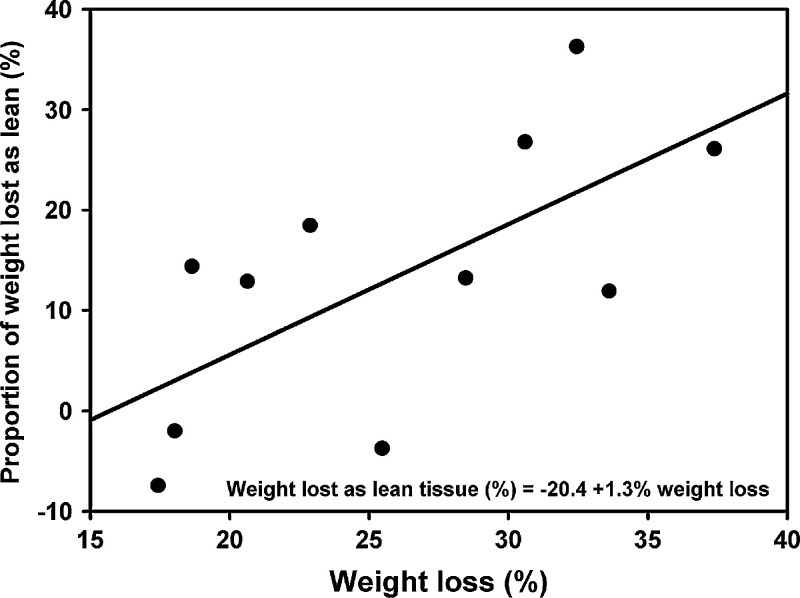
Changes in body composition during weight loss are shown in Fig 1. Significant decreases were noted for fat (median 86%, range 62–105%, P<0.001), lean (median 13%, range −7–36%, P<0.02), and BMC (median 0.9%, range 0.4–2.2%, P<0.001) when compared before and after weight loss (Table 2). There was no association between the proportion of lean tissue lost and either the mean rate of weight loss (rp=−0.315, P=0.346) or the mean daily energy intake (rp=0.464, P=0.151). In fact, the only factor that had a significant effect on the percentage of lean tissue lost was the percentage of body weight lost overall, ie, more lean tissue was lost in the cats that lost the most weight (r2=44.2%, P=0.026; Fig 2).
Fig 1.
Box and whisker plots representing mean change in body composition during weight loss, assessed by DXA, in 11 of the 12 obese cats before and after weight management. The horizontal line represents the median, the box delineates the interquartile range, whilst the whiskers contain 95% of the population whilst the filled circles denote outliers. Significant decreases in all components (lean mass, BMC, and fat mass) were noted (aP<0.0001; bP<0.02), but the greatest loss was in fat mass.
Table 2.
Body composition data
| Mean | SD | Range | |
| Before * | |||
| Fat (g) | 2480 | 915.5 | 1270–4170 |
| Lean (g) | 3940 | 685.9 | 3130–5540 |
| BMC (g) | 148 | 24.9 | 117–198 |
| After * | |||
| Fat (g) | 909 | 447.4 | 280–1270 |
| Lean (g) | 3610 | 507.3 | 2880–4510 |
| BMC (g) | 132 | 23.9 | 97–178 |
| Change * (total) | |||
| Fat (g) | 1570 | 600.2 | 880–2910 |
| Lean (g) | 326 | 390.7 | −70–1040 † |
| BMC (g) | 15 | 5.1 | 8–25 |
| Proportional tissue loss | |||
| Fat (%) | 86 | 13.4 | 63–105 † |
| Lean (%) | 13 | 13.6 | −8–36 † |
| BMC (%) | 0.9 | 0.48 | 0.5–2.1 |
Results expressed in grams of tissue.
Negative results for lean tissue and percentage of fat lost in excess of 100% occurred when lean tissue was gained during weight loss.
BMC=bone mineral content, SD=standard deviation.
Fig 2.
Association between percentage body weight lost and composition of the weight loss. Proportion lean tissue lost was positively associated with the overall percentage of weight lost (linear regression, P=0.026).
Discussion
This study shows that conventional weight management therapy can be successful in naturally occurring obesity in client-owned cats. The mean rate of weight loss achieved (0.8% per week) was slower than observed in previous studies, using in colony cats, and feeding diets of similar composition (Butterwick et al 1994, Butterwick and Markwell 1996, Michel et al 2005, Laflamme and Hannah 2005), but was equivalent to that of a similar clinical study in dogs (German et al 2007). This confirms that clinicians should expect slower rates of weight lost in client-owned cats in the clinical setting. A number of reasons could account for this rate of weight loss. First, the proportion of body weight loss in the current study was greater than what is typically observed in colony studies, where ∼15% weight loss is more typical (Butterwick et al 1994, Butterwick and Markwell 1996).
A further reason for slower weight loss observed in this study was the fact that there was non-compliance with the dietary plan, as estimated from diary records. Although overall energy consumption was apparently insignificant in most instances, the true caloric contribution from non-compliance may actually be greater, given the potential for under-reporting from owners; this is an acknowledged limitation of human studies that use such an approach (Heitmann et al 2000). To ensure predictable weight loss, clinicians should ideally strive to prevent owners giving unwanted extras as far as possible. However, as giving treats is an important part of pet ownership, it can be sanctioned, as long as the clinician is aware of it (eg, by maintaining a record) so that it can be factored into the total daily allowance for the patient.
Although these data provide useful information on current weight loss strategies for cats, there may be problems in extrapolating directly the results to practitioners in first-opinion practice. In this respect, a referral population was studied, and some cats had been referred because weight loss had been unsuccessful at the practice of the referring veterinarian. Further, in our weight management clinic, a full-time member of staff (SH) is employed specifically to oversee weight loss programmes, enabling more intensive supervision than is possible in many first-opinion practices. This may, to an extent, counteract the problems faced by the population of cats referred. Thus, the results of the current study may not be completely representative of what may be expected in general practice.
The results of the current study show that conventional weight loss can be successful, but close monitoring of the programme is required. All cats in this study required at least one alteration to their dietary regime. The cats in the mixed ration group required more decreases in food intake in order to maintain weight loss at the same rate as those fed the dry ration exclusively. The reasons for this are not known. One possibility is that the moist ration was more difficult to measure out accurately when proportions of a sachet were fed, leading to overfeeding; in contrast, it would be easier to be accurate when measuring out dried food on weigh scales. A second possibility is that the characteristics of cats in the two diet groups differed. Whilst statistical analysis did not reveal any obvious differences between groups (in terms of signalment, percentage starting body fat, and overall proportion of weight lost), the groups may have differed in other ways that were not directly assessed (eg, activity levels). Thirdly, as the group fed the mixed ration included cats where switches in type of diet were required (eg, from moist to dry, or vice versa), this grouping may have inadvertently selected for cats in which compliance was most likely to be a problem. Fourth, a role for nutrient composition cannot be discounted, as the macronutrient composition varied between the dried and moist rations, especially with regard to carbohydrate content. Finally, and most notably, the study was not designed as a randomised controlled trial comparing diets. Therefore, further studies are required to determine what constitutes the best type of diet for weight loss in cats.
This study is the first to document the changes in body composition that occur during weight loss in a clinical setting. In keeping with the results of experimental studies using a diet of similar composition, the majority (>80%) of tissue mass lost was adipose tissue, with smaller (although significant) losses of lean tissue (Nguyen et al 2004). In the current study, the main factor associated with the amount of lean tissue lost was the overall percentage weight loss, eg, cats which lost a greater proportion of body weight lost more lean tissue. This is similar to a recent study in dogs (German et al 2007). However, in contrast to previous work in cats (Butterwick and Markwell 1996), the rate of weight loss and degree of caloric restriction did not have a significant impact on the proportion of lean and fat lost. The reasons for this discrepancy are not clear, but could relate to the relatively slower rate of weight loss for cats in the current study in comparison with the previous work. Incorporating exercise in weight programmes has also been shown to assist in lean tissue preservation during weight loss in human patients (Ross et al 1995, 1996). However, this factor was not examined during the current study. Given that, in some cases, energy intake required for weight loss was low, another possible reason for lean tissue loss could have been that protein intake in these cats might have been insufficient. Against this possibility, there was no correlation between the proportion of lean tissue lost and daily energy (and therefore protein) intake. Further, the daily protein consumption in all cats exceeded the minimum requirements for maintenance in adult cats, as recommended in the 2006 NRC guidelines (data not shown).
In summary, the current study has demonstrated that successful weight loss in obese cats is feasible in clinical practice, using a conventional approach with dietary caloric energy restriction and increased activity. However, marked caloric energy restriction is required, and the rate of weight loss is slower than in equivalent experimental studies. The main predictor of amount of lean tissue lost was the overall percentage weight loss, suggesting that this may be inevitable in animals required to lose a large proportion of their body mass.
Acknowledgements
The authors wish to acknowledge the referring veterinarians for referring cases, and the clinical staff at the University of Liverpool for assistance with case management. Clare Atherton, Katie Burton, Eric Servet, Renaud Sergheraert, Rachel Hackett and John Rawlings are all acknowledged for their assistance. AG's senior lectureship is funded by Royal Canin. This study was funded by a grant from Waltham.
References
- Butterwick R.F., Wills J.M., Sloth C., Markwell P.J. A study of obese cats on a calorie-controlled weight reduction programme, Veterinary Record 134, 1994, 372–377. [DOI] [PubMed] [Google Scholar]
- Butterwick R.F., Markwell P.J. Changes in the body composition of cats during weight reduction by controlled dietary energy restriction, Veterinary Record 138, 1996, 354–357. [DOI] [PubMed] [Google Scholar]
- Diez M., Nguyen P., Jeusette I., Devois C., Istasse L., Biourge V. Weight loss in obese dogs: evaluation of a high-protein, low-carbohydrate diet, Journal of Nutrition 132, 2002, 1685S–1687S. [DOI] [PubMed] [Google Scholar]
- Forbes G.B. Lean body mass–body fat interrelationships in humans, Nutrition Reviews 45, 1987, 225–231. [DOI] [PubMed] [Google Scholar]
- German A.J. The growing problem of obesity in dogs and cats, Journal of Nutrition 136, 2006, 1940S–1946S. [DOI] [PubMed] [Google Scholar]
- German A.J., Holden S.L., Bissot T., Hackett R.M., Biourge V. Dietary energy restriction and successful weight loss in obese client-owned dogs, Journal of Veterinary Internal Medicine 21, 2007, 1174–1180. [DOI] [PubMed] [Google Scholar]
- Harper E.F., Stack D.M., Watson T.D.G., Moxham G. Effects of feeding regimens on body weight, composition and condition score in cats following ovariohysterectomy, Journal of Small Animal Practice 42, 2001, 433–438. [DOI] [PubMed] [Google Scholar]
- Heitmann B.L., Lissner L., Osler M. Do we eat less fat, or just report so?, International Journal of Obesity 24, 2000, 435–442. [DOI] [PubMed] [Google Scholar]
- Humphries C. The Hugely Better Calorie Counter, 2002, Foulsham: Slough, UK. [Google Scholar]
- Kellow J.W.R. The Calorie, Carb and Fat Bible, 2006, Weight Loss Resources: Peterborough, UK. [Google Scholar]
- Laflamme D.P. Development and validation of a body condition score for cats: a clinical tool, Feline Practice 25, 1997, 13–18. [Google Scholar]
- Laflamme D.P., Hannah S.S. Increased dietary protein promotes fat loss and reduces loss of lean body mass during weight loss in cats, International Journal of Applied Research in Veterinary Medicine 3, 2005, 62–68. [Google Scholar]
- Lund E.M., Armstrong P.J., Kirk C.A., Klausner J.S. Prevalence and risk factors for obesity in adult cats from private US veterinary practices, International Journal of Applied Research in Veterinary Medicine 3, 2005, 88–96. [Google Scholar]
- Mawby D.I., Bartges J.W., d'Avignon A., Laflamme D.P., Moyers T.D., Cottrell T. Comparison of various methods for estimating body fat in dogs, Journal of the American Animal Hospital Association 40, 2004, 109–114. [DOI] [PubMed] [Google Scholar]
- Michel K.E., Bader A., Shofer F.S. Impact of time-limited feeding and dietary carbohydrate content on weight loss in group-housed cats, Journal of Feline Medicine and Surgery 7, 2005, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P., Leray V., Dumon H., Martin L., Siliart B., Diez M., Biourge V. High protein intake affects lean body mass but not energy expenditure in non-obese neutered cats, Journal of Nutrition 134, 2004, 2084S–2086S. [DOI] [PubMed] [Google Scholar]
- Prentice A.M., Goldberg G.R., Jebb S.A., Black A.E. Physiological-responses to slimming, Proceedings of the Nutrition Society 50, 1991, 441–458. [DOI] [PubMed] [Google Scholar]
- Raffan E., Holden S.L., Cullingham F., Hackett R.M., Rawlings J.M., German A.J. Standardized positioning is essential for precise determination of body composition using dual-energy x-ray absorptiometry in dogs, Journal of Nutrition 136, 2006, 1976S–1978S. [DOI] [PubMed] [Google Scholar]
- Robertson I.D. The influence of diet and other factors on owner-perceived obesity in privately owned cats from metropolitan Perth Western Australia, Preventive Veterinary Medicine 40, 1999, 75–85. [DOI] [PubMed] [Google Scholar]
- Ross R., Pedwell H., Rissanen J. Effects of energy restriction and exercise on skeletal-muscle and adipose-tissue in women as measured by magnetic-resonance-imaging, American Journal of Clinical Nutrition 61, 1995, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Ross R., Rissanen J., Pedwell H., Clifford J., Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men, Journal of Applied Physiology 81, 1996, 2445–2455. [DOI] [PubMed] [Google Scholar]
- Russell K., Sabin R., Holt S., Bradley R., Harper E.J. Influence of feeding regimen on body condition in the cat, Journal of Small Animal Practice 41, 2000, 12–17. [DOI] [PubMed] [Google Scholar]
- Sloth C. Practical management of obesity in dogs and cats, Journal of Small Animal Practice 33, 1992, 178–182. [Google Scholar]
- Wren J.A., Gosselin J.A., Sunderland S.J. Dirlotapide: a review of its properties and role in the management of obesity in dogs, Journal of Veterinary Pharmacology and Therapeutics 30, 2007, 11–16. [DOI] [PubMed] [Google Scholar]