Abstract
The triglyceride-glucose (TyG) index is a novel marker of insulin resistance that has been strongly associated with many diseases related to metabolic disorders, such as diabetes, coronary heart disease, myocardial infarction, obesity, nonalcoholic fatty liver disease, and stroke. However, whether the TyG index is associated with the prevalence of gallstones has not been determined. Therefore, the purpose of this study was to evaluate the relationship between the TyG index and the prevalence of gallstones in American adults, as well as the age at which adults in America undergo their first gallstone surgery. We selected individuals from the National Health and Nutrition Examination Survey (NHANES) database from 2017 to March 2020. Based on the goal of our study, comprehensive inclusion and exclusion criteria were created. A logistic regression analysis, dose–response curve, and subgroup analysis were computed to assess the relationship between the TyG index and gallstone prevalence and age at first surgery for gallstone. A total of 3905 participants aged > 20 years were included in our study, of whom 421 had a self-reported history of gallstones. A total of 1884 (48.2%) males and 2021 (51.8%) females were included. After confounders adjustment, it was found single-unit increases in the TyG index were linked with a 25.0% increase in gallstone prevalence (odds ratio [OR] = 1.25, 95% confidence interval [95%CI]: 1.04, 1.51). After conversion of the TyG index values from continuous to categorical variables with tertiles, a marked 48% increase in gallstone incidence was found in tertile 3 relative to tertile 1 (OR = 1.48, 95% CI: 1.09, 1.99). The dose–response curve results indicated positive associations between gallstone prevalence and the TyG index, while the latter was negatively associated with age at first gallstone surgery. Based on subgroup analysis, the positive association between TyG index and high-incidence of gallstones was more significant in females (OR = 1.39, 95% CI: 1.09, 1.77), age < 40 years (OR = 2.02, 95% CI: 1.23, 3.29), and other race (OR = 1.46, 95% CI: 1.06, 2.02). A higher TyG index is associated with a higher incidence of gallstones and may lead to an earlier age of first gallstone surgery. However, a causal relationship between TyG and gallstones cannot be established.
Keywords: Gallstones prevalence, Triglyceride-glucose index, National Health and Nutrition Examination Survey, Cross-sectional study, Metabolic syndrome
Subject terms: Predictive markers, Hepatology, Biliary tract disease
Introduction
Gallstones are a prevalent gastrointestinal condition affecting people worldwide. At the onset of illness, 75% of patients with gallstones are asymptomatic1. As the illness progresses, some patients may experience symptoms such as nausea, discomfort in the upper abdomen, diarrhea, loss of appetite, and other clinical signs. A minority of individuals may have problems such as acute cholangitis, biliary pancreatitis, and other significant issues due to movement of stones1. Gallstones are a non-malignant disease, but increase the likelihood of gallbladder cancer2. Surgery is the primary treatment for gallstones1. Approximately 15% of the population in the United States have gallstones, presenting a significant public health concern3. Epidemiological data indicate significant variations in the incidence of gallstones across various ethnic groups. Its prevalence is as high as 70% among American Indians, whereas it ranges from 10–15% among adult Caucasians4,5. By contrast, Asian populations have a lower prevalence of gallstones6. While prior research has identified several risk factors associated with gallstone development, such as female sex, race, pregnancy, and age > 40 years7, reliable clinical indications for accurately predicting the likelihood of gallstone formation are lacking.
Gallstones can be categorized as cholesterol, pigment, or mixed stones based on their composition8. Previous research has demonstrated that cholesterol is present in over 80% of gallstones and that dyslipidemia is common in the majority of gallstone patients; therefore, gallstones may be viewed as a condition associated with metabolic dysfunction to some degree9,10. Over the past 15 years, the occurrence of metabolic syndrome (MetS) has increased due to improvements in living circumstances. This growth poses increasing difficulties for its prevention and therapy11. Insulin resistance (IR), hyperlipidemia, hyperuricemia, obesity, diabetes, poor glucose control, and hypertension are associated with MetS3,12. Insulin resistance (IR) is the most prevalent cause of MetS and diabetes13. Hence, the prompt and precise detection of IR might facilitate the implementation of early preventive measures and enhance the clinical administration of MetS. Presently, there are several techniques to evaluate IR, including the high insulin clamp test (HEC), frequent sampling intravenous glucose tolerance test (FSIVGTT), homeostasis modeling assessment (HOMA-IR), triglyceride-glucose (TyG) index, and various markers (such as fasting insulin, pancreatic insulin, and pancreatic glucose), each with its own merits and drawbacks14,15. The HEC and FSIVGTT are considered the most reliable methods for evaluating insulin resistance. However, they are not appropriate for widespread screening in the general population because of their high cost, lengthy procedural duration, and invasive nature15.
The TyG index evaluates IR by combining measurements of fasting triglyceride and blood glucose levels. Many studies have shown that the TyG index is a reliable substitute for assessing IR16–18. Furthermore, the TyG index does not need to integrate fasting insulin levels and has the benefit of being straightforward and cost-effective14,15. Associations between the TyG index and diseases such as obesity, nonalcoholic fatty liver disease (NAFLD), hepatic fibrosis, hyperuricemia, atrial fibrillation, hypertension, and diabetes mellitus have been reported19–22. For instance, in the overall population, a raised TyG index was positively associated with greater risk of developing NAFLD and liver fibrosis23,24. Experimental studies demonstrated that IR is a prevalent risk factor for gallstone onset and MetS. Moreover, IR stimulates heightened synthesis of cholesterol within the body, excessive secretion of cholesterol in the bile, and increased production of bile, ultimately fostering gallstone formation25. Hence, we posit that a correlation may exist between the TyG index and the occurrence of gallstones.
Prior to our investigation, there was no assessment of the potential correlation between the TyG index and gallstone incidence. Accordingly, the primary objective of the present study was an investigation of the significance of the TyG index in determining the occurrence of gallstones in adults living in the United States. Additionally, associations between the TyG index and the age at which individuals underwent their first gallstone surgery were evaluated.
Materials and methods
Study design and participants
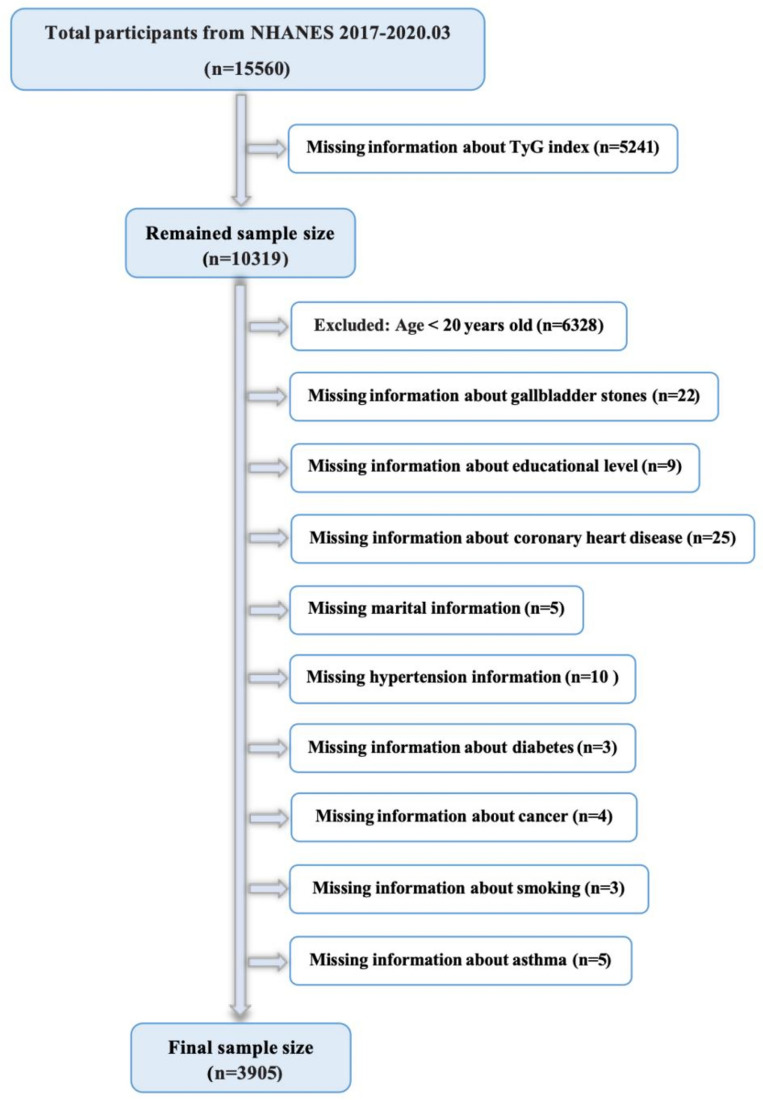
Baseline data from the open-source National Health and Nutrition Examination Survey (NHANES) database, maintained by the Centers for Disease Control and Prevention, were used. The field activities of the NHANES program were suspended in March 2020 due to the COVID-19 pandemic, which began in 2019. Thus, the dataset of the observational research included the period from 2017 to 2020.03, i.e., while the gallstone questionnaire was available. Given that the questionnaire was specifically tailored for those aged 20 years and older, we omitted participants below the age of 20 years. Figure 1 illustrates the specific criteria used for inclusion and exclusion. From a total of 3,905 participants, 421 instances of gallstones were reported by the subjects themselves.
Figure 1.
Flowchart for participants from NHANES 2017–2020.
Data collection
The TyG index was used as the exposure variable.
Triglyceride (TG) and fasting glucose levels were measured on an automatic biochemical analyzer, while serum TG levels were determined using the Roche Modular P and Cobas 6000 chemical analyzers. Gallstone prevalence and the age at which the first surgical removal of the gallstones occurred were assessed using questionnaires. The age at which the first gallstone procedure was performed, and the frequency of gallstones were used as end-measures.
Table 1 details the relevant variables extracted from the NHANES database. Each participant had a 24-h dietary recall between 2017 and 2020.03. The average consumption rate from these two recalls was used in our study.
Table 1.
Covariates extracted in detail from the NHANES database.
| Items | Composition |
|---|---|
| Demographic variables | Age, Gender, Race, BMI |
| Comorbidities | Diabetes, Asthma, Hypertension, Cancer, CHD |
| Dietary intake factors | Energy intake, Fat intake, Sugar intake, Water intake |
| Laboratory variables | TG, FBG, SCr |
| Others | Education level, PIR, Marital status, Alcohol consumption, Physical activity level, Smoking status |
NHANES the National Health and Nutrition Examination Survey, BMI body mass index, CHD coronary heart disease, TG triglyceride, FBG fasting blood glucose, SCr serum creatinine, PIR poverty income ratio.
The NHANES survey protocol was approved by the Ethics Review Committee of the National Center for Health Statistics (NCHS), and all participants provided written informed consent. This study was exempt from ethical clearance because of its public availability in the NHANES database.
Handling of missing values
The presence of several missing values in the continuous variables necessitated their transformation into categorical variables. The missing values were given the label "unclear" and grouped into a separate category represented by a dummy variable. The study variables and measurement methodologies can be found at www.cdc.gov/nchs/nhanes/.
Statistical methods
For normally distributed data, continuous variables were expressed as mean ± SD; for non-normally distributed continuous variables, as median (IQR); and for categorical variables, as numbers (%). Multiple logistic regression models were used, following the recommended guidelines, to examine the associations between gallstone prevalence and different TyG tiles in three separate models26,27. Model 1 was not adjusted for the covariates, while Model 2 was adjusted for sex, age, ethnicity, and marital status, and Model 3 for all variables. To evaluate the relationship between the TyG index and the development of gallstones as well as the age at which gallstone surgery was first performed, we used the penalty spline method to create smooth curves and generalized additive model (GAM) regression. The inflection point values were determined using a natural ratio test after identifying a non-linear relationship. Gender, age, race, diabetes, and hypertension were included as variables in the subgroup analyses. P < 0.05 was considered statistically significant. All analyses were carried out using R version 4.0.2 (http://www.R-project.org, The R Foundation) and Empower software (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA, USA).
Ethic statement
The NHANES survey protocol was approved by the Ethics Review Committee of the National Center for Health Statistics (NCHS), and all participants provided written informed consent. This study was exempt from ethical clearance because of its public availability in the NHANES database.
Results
Participant characteristics
The baseline demographic information on the participants is shown in Table 2. The TyG index was 8.83 (8.42, 9.21) in patients with stones, markedly higher than the value of 8.60 (8.20, 9.03) observed in those without stones, P < 0.001.
Table 2.
Baseline characteristics of participants.
| Characteristic | Non-stone formers (n = 3484) | Stone formers (n = 421) | P-value |
|---|---|---|---|
| TyG index | 8.60 (8.20, 9.03) | 8.83 (8.42, 9.21) | < 0.001 |
| Age (years) | 51.00 (35.00, 64.00) | 60.00 (46.00, 71.00) | < 0.001 |
| Gender (%) | < 0.001 | ||
| Female | 1718 (49.3) | 303 (72.0) | |
| Male | 1766 (50.7) | 118 (28.0) | |
| Race (%) | < 0.001 | ||
| White | 1148 (33.0) | 168 (39.9) | |
| Black | 912 (26.2) | 73 (17.3) | |
| Mexican American | 439 (12.6) | 60 (14.3) | |
| Other Race | 985 (28.3) | 120 (28.5) | |
| BMI | 28.30 (24.60–33.10) | 31.70 (27.40–37.60) | < 0.001 |
| Physical Activity (%) | 0.001 | ||
| Never | 1054 (30.3) | 158 (37.5) | |
| Moderate | 1169 (33.6) | 146 (34.7) | |
| Vigorous | 1261 (36.2) | 117 (27.8) | |
| Marital Status (%) | < 0.001 | ||
| Cohabitation | 2045 (58.7) | 244 (58.0) | |
| Solitude | 743 (21.3) | 125 (29.7) | |
| Never married | 696 (20.0) | 52 (12.4) | |
| Educational level (%) | 0.69 | ||
| Less than high school | 675 (19.4) | 88 (20.9) | |
| High school | 821 (23.6) | 101 (24.0) | |
| More than high school | 1988 (57.1) | 232 (55.1) | |
| Alcohol consumption (%) | < 0.001 | ||
| Never | 289 (8.3) | 32 (7.6) | |
| Ever | 647 (18.6) | 127 (30.2) | |
| Now | 2354 (67.6) | 231 (54.9) | |
| Unclear | 194 (5.6) | 31 (7.4) | |
| Hypertension (%) | < 0.001 | ||
| No | 2193 (62.9) | 188 (44.7) | |
| Yes | 1286 (36.9) | 233 (55.3) | |
| Unclear | 5 (0.1) | 0 (0.0) | |
| Diabetes (%) | < 0.001 | ||
| No | 2860 (82.1) | 291 (69.1) | |
| Yes | 515 (14.8) | 119 (28.3) | |
| Borderline | 107 (3.1) | 11 (2.6) | |
| Unclear | 2 (0.1) | 0 (0.0) | |
| Asthma (%) | < 0.001 | ||
| No | 2950 (84.7) | 323 (76.7) | |
| Yes | 530 (15.2) | 98 (23.3) | |
| Unclear | 4 (0.1) | 0 (0.0) | |
| CHD (%) | 0.001 | ||
| No | 3331 (95.6) | 385 (91.4) | |
| Yes | 139 (4.0) | 34 (8.1) | |
| Unclear | 14 (0.4) | 2 (0.5) | |
| Cancers (%) | < 0.001 | ||
| No | 3149 (90.4) | 343 (81.5) | |
| Yes | 333 (9.6) | 77 (18.3) | |
| Unclear | 2 (0.1) | 1 (0.2) | |
| Smoking status (%) | 0.005 | ||
| Never | 1998 (57.3) | 231 (54.9) | |
| Ever | 821 (23.6) | 127 (30.2) | |
| Now | 665 (19.1) | 63 (15.0) | |
| PIR | 0.043 | ||
| < 1.3 | 820 (23.5) | 93 (22.1) | |
| ≥ 1.3– < 3.5 | 1183 (34.0) | 172 (40.9) | |
| ≥ 3.5 | 992 (28.5) | 104 (24.7) | |
| Unclear | 489 (14.0) | 52 (12.4) | |
| Total Sugar (%) | 88.69 (54.08, 137.14) | 84.26 (53.97, 124.98) | 0.202 |
| Total Kcal (%) | 1,980.00 (1,438.00, 2,670.00) | 1,762.00 (1,318.00, 2,385.00) | < 0.001 |
| Total Fat (%) | 77.84 (52.74, 112.16) | 72.05 (49.75, 102.34) | 0.007 |
| Total Water (%) | 1,920.00 (720.00, 3,480.00) | 1,710.00 (630.00, 3,549.00) | 0.447 |
| SCr (mg/dl) | 74.26 (61.88, 88.40) | 70.72 (59.23, 86.63) | 0.034 |
Logistic regression results between the TyG index and gallstones prevalence
The crude, minimally adjusted, and fully adjusted multiple regression models all showed that the TyG index was positively associated with gallstone prevalence, with varying adjustments made to the impact of variables on the correlation. The fully adjusted model showed that a single-unit increase in the TyG index was associated with a 25.0% greater likelihood of gallstone development (odds ratio [OR] = 1.25, 95% CI: 1.04, 1.51) (Table 3). We also converted the continuous variables of the TyG index to categorical variables (tertiles) for sensitivity analysis. The results indicated a marked increase of 48% in gallstone prevalence relative to tertile 1 (OR = 1.48, 95% CI: 1.09, 1.99) (Table 3).
Table 3.
Logistic regression analysis between the TyG index with gallstones prevalence.
| Characteristic | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) |
|---|---|---|---|
| TyG index | 1.58 (1.36–1.83) | 1.46 (1.23–1.73) | 1.25 (1.04–1.51) |
| Categories | |||
| Tertile 1 | 1 | 1 | 1 |
| Tertile 2 | 1.54 (1.17–2.03) | 1.28 (0.96–1.71) | 1.22 (0.91–1.63) |
| Tertile 3 | 2.22 (1.71–2.88) | 1.77 (1.33–2.34) | 1.48 (1.09–1.99) |
Model 1 = no covariates were adjusted.
Model 2 = Model 1 + age, gender, race, and marital status were adjusted.
Model 3 = Model 2 + education level, physical activity, smoking status, alcohol consumption, diabetes, hypertension, asthma, cancers, coronary heart disease, poverty income ratio, total kcal, total fat, total sugar, and serum creatinine were adjusted.
Dose–response and threshold effects of the TyG index on gallstones prevalence
TyG index and gallstones prevalence were further explored using generalized additive models and smoothed curve fitting. Our findings indicated a nonlinear positive correlation between the TyG index and gallstones prevalence (Fig. 2). A threshold effect was found on the likelihood ratio test for the influence of the TyG index and gallstones, with an optimal inflection point value of 8.32 (Table 4).
Figure 2.
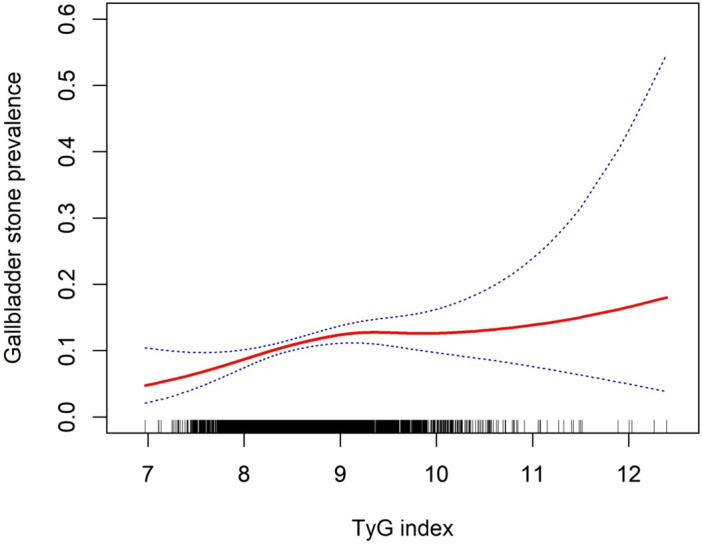
Density dose–response relationship between TyG index with gallstones prevalence. The area between the upper and lower dashed lines is represented as 95% CI. Each point shows the magnitude of the TyG index and is connected to form a continuous line. Adjusted for all covariates except effect modifier.
Table 4.
Two-piecewise linear regression and logarithmic likelihood ratio test explained the threshold effect analysis of the TyG index with gallstones prevalence.
| TyG index | ULR Test | PLR Test | LRT Test |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | P-value | |
| < 8.32 | 1.25 (1.04–1.51) | 3.13 (1.31–7.49) | 0.027 |
| > = 8.32 | 1.09 (0.87–1.37) |
ULR univariate linear regression, PLR piecewise linear regression, LRT logarithmic likelihood ratio test, statistically significant: P < 0.05.
Subgroup analysis
Subgroup analyses were undertaken to evaluate the robustness of the association between the TyG index and gallstones development. The results are summarized in Table 5. Significant OR values were found for certain demographic characteristics: 1.39 (95% CI: 1.09, 1.77) for females, 2.02 (95% CI: 1.23, 3.29) for participants below the age of 40, and 1.46 (95% CI: 1.06, 2.02) for other ethnic groups. The influence of hypertension and diabetes on the relationships between the TyG index and gallstone prevalence were also evaluated (Table 5).
Table 5.
Subgroup regression analysis between the TyG index with gallstones prevalence.
| Characteristic | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) |
|---|---|---|---|
| Stratified by age (years) | |||
| 20–39 | 1.76 (1.22–2.53) | 2.70 (1.76–4.12) | 2.02 (1.23–3.29) |
| 40–59 | 1.29 (1.01–1.65) | 1.36 (1.05–1.77) | 1.24 (0.91–1.68) |
| 60–80 | 1.42 (1.11–1.81) | 1.31 (1.01–1.70) | 1.09 (0.81–1.45) |
| Stratified by gender | |||
| Female | 2.00 (1.64–2.43) | 1.64 (1.33–2.03) | 1.39 (1.09–1.77) |
| Male | 1.41 (1.09–1.82) | 1.26 (0.94–1.67) | 1.12 (0.82–1.52) |
| Stratified by race | |||
| White | 1.62 (1.26–2.07) | 1.63 (1.25–2.14) | 1.31 (0.96–1.81) |
| Black | 1.67 (1.13–2.48) | 1.52 (1.00–2.33) | 1.02 (0.63–1.66) |
| Mexican American | 1.13 (0.76–1.68) | 0.94 (0.58–1.52) | 0.85 (0.46–1.57) |
| Other Race | 1.53 (1.17–2.01) | 1.47 (1.09–1.97) | 1.46 (1.06–2.02) |
| Stratified by BMI | |||
| ≤ 24.9 | 1.87 (1.21–2.89) | 1.27 (0.75–2.16) | 1.24 (0.68–2.25) |
| 25–29.9 | 1.28 (0.94–1.73) | 1.06 (0.74–1.50) | 0.89 (0.60–1.32) |
| ≥ 30 | 1.30 (1.06–1.60) | 1.24 (0.98–1.58) | 1.07 (0.83–1.39) |
| Stratified by diabetes | |||
| No | 1.56 (1.27–1.91) | 1.44 (1.14–1.81) | 1.37 (1.07–1.74) |
| Yes | 1.08 (0.82–1.43) | 1.10 (0.80–1.50) | 1.05 (0.76–1.46) |
Model 1 = no covariates were adjusted.
Model 2 = Model 1 + age, gender, race, and marital status were adjusted.
Model 3 = Model 2 + education level, physical activity, smoking status, alcohol consumption, diabetes, hypertension, asthma, cancers, coronary heart disease, poverty income ratio, total kcal, total fat, total sugar, and serum creatinine were adjusted.
Dose–response and threshold effects of the TyG index on age at first gallstone surgery
A generalized additive model with smooth curve fitting was used for further evaluation of the association between the TyG index and age at first gallstone surgery. The threshold for the influence of the TyG index on age at first gallstone surgery was 9.26, shown by a similar natural ratio test (Table 6). When TyG ≥ 9.26, the age at first gallstone surgery in American adults became younger with the increasing TyG (Fig. 3).
Table 6.
Two-piecewise linear regression and logarithmic likelihood ratio test explained the threshold effect analysis of the TyG index with age at the first gallstone surgery.
| TyG index | ULR test | PLR test | LRT test |
|---|---|---|---|
| β (95% CI) | β (95% CI) | P-value | |
| < 9.26 | − 0.44(-3.10, 2.22) | 3.34 (− 0.64, 7.32) | 0.010 |
| > = 9.26 | − 5.87 (− 10.89, − 0.85) |
ULR univariate linear regression, PLR piecewise linear regression, LRT logarithmic likelihood ratio test.
Statistically significant: P < 0.05.
Figure 3.
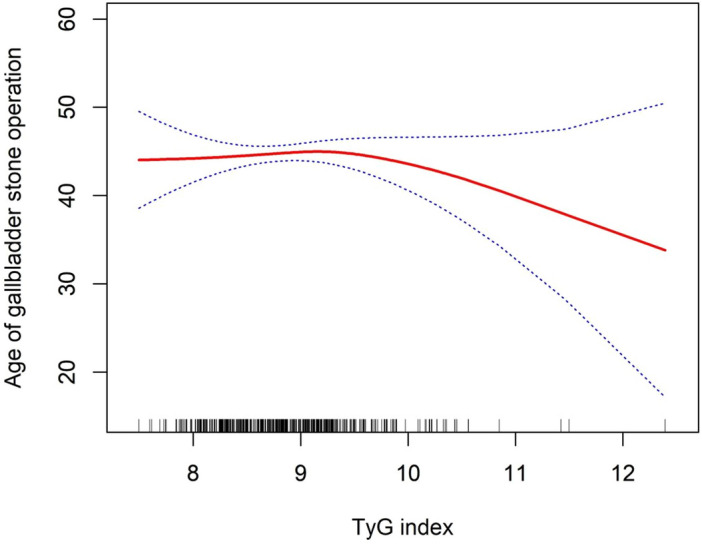
Density dose–response relationship between the TyG index and age at first gallstone surgery. The area between the upper and lower dashed lines is represented as the 95% CI. Each point shows the magnitude of the TyG index and is connected to form a continuous line. Adjusted for all covariates except the effect modifier.
Discussion
To the best of our knowledge, this is the first comprehensive analysis of the association between the TyG index and gallstone prevalence using data from a large-sample, prospective, and nationally representative investigation of the population of the USA. It was found that the TyG index and gallstones were linked, with unit increases in the index associated with 25.0% increases in gallstone incidence (OR = 1.25, 95% CI: 1.04, 1.51). After conversion of the TyG index values from continuous to categorical variables with tertiles, a marked 48% increase in gallstone incidence was found in tertile 3 relative to tertile 1 (OR = 1.48, 95% CI: 1.09, 1.99). It was also observed that when TyG ≥ 9.26, the higher the TyG index, the younger the age at first gallstone surgery. Additional subgroup analysis showed a greater risk of gallstone development in females (OR = 1.39, 95% CI: 1.09, 1.77), people aged between 20 and 39 years (OR = 2.02, 95% CI: 1.23, 3.29), and specific ethnicities (OR = 1.46, 95% CI: 1.06, 2.02). Thus, these findings indicate the usefulness of the TyG index in predicting gallstone development.
Gallstones are common in the US; in 2015 alone, 1.5 million individuals sought medical attention for the condition28. MetS is known to be potentially associated with a wide range of diseases (including NAFLD, cardiovascular disease, diabetes mellitus, and gallstones, among others), and represents a significant public health challenge to the world's population. Simultaneously, the incidence of MetS has increased dramatically and is currently at an unprecedented level29,30.
The pathophysiology of gallstone formation is not completely understood. Gallstone initiation is currently thought to be controlled by a combination of environmental and genetic variables31. Previous studies have indicated that gallstones are linked to factors such as age, sex, female physiological condition, obesity, cardiovascular illness, microbiota composition, glucose metabolism, and environmental exposures1,32–34. Genetic factors increase the probability of gallstone formation. In the USA, Caucasian Americans are more likely to develop gallstones than African Americans; specifically, gallstones have been reported in 16.6% and 8.6% of white women and men, respectively, relative to 13.9% and 5.3% of black women and men, respectively35. Our findings also confirm the existence of disparities in the prevalence of gallstones across various racial groups. In our study, we found that Mexican American was insignificant about OR in TyG index with gallstones prevalence. The potential reasons for this may be related to the insufficient sample size of Mexican Americans included and the different genetic, socioeconomic, and lifestyle1,6,7. It is necessary for future studies to incorporate data from larger sample sizes of the population and to give due consideration to other potential influencing factors in order to obtain more definitive conclusions. Advanced age significantly increases the likelihood of developing gallstones36,37. The prevalence of gallstones increases dramatically beyond 40 years of age, and in elderly populations is multiplied by a factor of 4–104. Our study found that TyG index was more strongly associated with the prevalence of gallstones in the age group of 20–39 years as compared to other age groups. The mechanisms behind this may be the following. Firstly, with the development of social economy and improvement of living standard, the dietary structure of young people generally tends to be high in calories, fat and cholesterol, along with insufficient dietary fiber intake. This change in dietary structure may lead to an increase in cholesterol saturation, which promotes the formation of gallstones38,39. Secondly, the increasing prevalence of obesity among young people is closely related to negative lifestyles, which can cause disorders of lipid metabolism and thus increase the risk of gallstone formation40. Moreover, several studies have found that family history plays an important role in the development of gallstones in young people. Genetic factors may lead to abnormal gallbladder function or impaired gallbladder emptying, increasing the risk of stone formation41. In addition, specific hormonal level changes, such as changes in female hormone levels (e.g., progesterone, birth control pills, etc.), have also been linked to gallstone formation. Notably, these changes may be more prominent in younger people, especially in women39. This is also consistent with the results in our subgroup analysis. However, the exact mechanism is not clear and more studies are necessary to explore it. Previous research indicates that women have a greater incidence of gallstones than males42. Our results support this finding. Extensive clinical data indicate that oral contraceptive steroids and a combination of estrogens significantly contribute to the development of cholesterol stones in premenopausal women43–45. The classical estrogen regulatory mechanisms include the hormone 17β-estradiol (e2) which activates the "e2-esr1-srebp-2" route. This pathway stimulates the production and release of cholesterol from the liver into bile. Furthermore, estrogen enhances the process of biliary cholesterol release by increasing the activity of ABCG5/G8, resulting in excessive accumulation of cholesterol in the bile46. Animal studies have demonstrated that estrogen can stimulate the activation of G protein-coupled receptor 30 (GPR30) via the epidermal growth factor receptor signaling cascade. This activation inhibits hepatic cholesterol 7α-hydroxylase and bile acid synthesis, ultimately resulting in increased hepatic cholesterol secretion and the promotion of gallstone formation47.
Leptin is also closely linked to the development of gallstones48. The development of gallstones is closely linked to disruption of the ability of the gallbladder to regulate the absorption and release of ions and water. Previous studies have shown that leptin may enhance the probability of stone formation by affecting gallbladder motility49–51. Leptin-deficient obese mice show decreased reactions to acetylcholine, neuropeptide Y, and cholecystokinin, resulting in reduced gallbladder movement and an elevated likelihood of developing gallstones52. Lee et al.53 discovered a notable increase in the expression of leptin and leptin receptors in gallbladder tissues of patients with cholelithiasis. Subsequent investigations demonstrated a strong correlation between elevated leptin levels in the blood and disrupted lipid metabolism, specifically hyperlipidemia and hypercholesterolemia. These imbalances in lipid levels may have contributed to gallstone formation53. Moreover, a recent study has shown that leptin can affect the development of gallstones by controlling the metabolism of bile acids via the OB-Rb/AMPKa2/BSEP signaling pathway54.
The precise mechanism underlying raised TyG indices and increased gallstone development remains incompletely understood. Based on previous studies, IR may serve as a fundamental mechanism. MetS, obesity, and gallstones are intricately linked55,56, and IR is the primary mechanism underlying MetS and obesity57. Previous studies have shown that IR in high-risk Hispanic populations results in the synthesis of bile that is saturated with cholesterol58. This in turn disrupts the normal functioning of the gallbladder and promotes the development of gallstones58. Animal studies have identified at least two distinct pathways that connect gallstones to MetS59. Biddinger et al.59 discovered that mice with hepatic insulin resistance, specifically caused by disruption of the insulin receptor in the liver (LIRKO mice), exhibited elevated expression of the cholesterol transporters ABCG5 and ABCG8. This increase was attributed to inhibition of the forkhead transcription factor FoxO1. Consequently, cholesterol secretion increases. In addition, LIRKO mice exhibited decreased expression of bile acid synthase, namely Cyp7b1, and the farnesoid X receptor. As a result, there was a reduction in the concentration of bile salts, which increased the probability of gallstone formation59. A study conducted in Japan showed that mice fed a diet high in protein and fat have accelerated gallstone formation because of their heightened nutritional condition. Moreover, the study revealed that metabolic factors had diverse effects on mice of different sexes25.
This study has several strengths. First, the data were acquired from the NHANES database, which consists of a representative sample of individuals from the USA. The participants strictly adhered to a meticulously designed study protocol accompanied by comprehensive quality control and assurance measures, ensuring the reliability of the findings. Furthermore, NHANES offers comprehensive demographic and metabolic data, which allowed us to account for significant confounding factors in our multivariate models. We accounted for confounding factors and conducted subgroup analysis to verify the generalizability of our findings to a broader population. Nevertheless, our study has some limitations. First, the study was cross-sectional, and thus causal relationships between the TyG index and gallstones could not be determined. Furthermore, the data on gallstones in this study were obtained using questionnaires, which are inherently subject to recall bias. Finally, the NHANES database lacks information on clinical factors, including medication history and the precise composition of stones. Notwithstanding these constraints, this study unveils, for the first time, the association between the TyG index and gallstone incidence, providing evidence for the efficacy of the index for predicting gallstone development.
Conclusions
This study revealed that an elevated TyG index was associated with an increased prevalence of gallstones and a younger age at first gallstone surgery in American adults. Furthermore, our identification of TyG index as a robust and easily measured indicator of the likelihood of gallstones should serve to catalyze improvement in levels of engagement with this issue between healthcare providers and the general population. Nevertheless, further multicenter prospective cohort studies are needed to substantiate our results.
Acknowledgements
We are grateful to the NHANES participants and staff. We appreciate all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Abbreviations
- TyG
Triglyceride-glucose index
- NHANES
National health and nutrition examination survey
- OR
Odds ratio
- CI
Confidence interval
- MetS
Metabolic syndrome
- IR
Insulin resistance
- HEC
High insulin clamp test
- FSIVGTT
Frequent sampling intravenous glucose tolerance test
- HOMA-IR
Homeostasis modeling assessment
- NAFLD
Nonalcoholic fatty liver disease
- TG
Triglyceride
- NCHS
National center for health statistics
- GAM
Generalized additive model
- GPR30
G protein-coupled receptor 30
Author contributions
(I) Conception and design: J.W., D.W.; (II) Administrative support: J.W., P.Y., J.H.; (III) Provision of study material or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: J.W., H.L.; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. (J.W., H.L.and J.H. contributed equally to this work and share first authorship. P.Y. and D.W. contributed equally to this work and share corresponding authorship).
Funding
This study was supported by NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital) (Grant No.2023HYX032), Medical Research Youth Innovation Project of Sichuan Province, China (Grant No. Q23046), and the Scientific Research Project of Mianyang Health Commission (Grant No. 202341).
Data availability
The raw data supporting the conclusions of this paper will be made available by the authors without reservation and can be obtained from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jianjun Wang, Han Li and Junchao Hu.
Contributor Information
Pei Yang, Email: 305827337@qq.com.
Decai Wang, Email: decaiwang_2020@163.com.
References
- 1.Sun, H. et al. Factors Influencing Gallstone Formation: A Review of the Literature. Biomolecules12(4), 550 (2022). 10.3390/biom12040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundal, R. & Shaffer, E. A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol.6, 99–109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinton, L. M. & Shaffer, E. A. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver6(2), 172–187 (2012). 10.5009/gnl.2012.6.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaffer, E. A. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol.20(6), 981–996 (2006). 10.1016/j.bpg.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Shabanzadeh, D. M. Incidence of gallstone disease and complications. Curr. Opin. Gastroenterol.34(2), 81–89 (2018). 10.1097/MOG.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 6.Stinton, L. M., Myers, R. P. & Shaffer, E. A. Epidemiology of gallstones. Gastroenterol. Clin. North America.39(2), 157–vii (2010). 10.1016/j.gtc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo, J. C. et al. Sex and ethnic/racial-specific risk factors for gallbladder disease. BMC Gastroenterol.17(1), 153 (2017). 10.1186/s12876-017-0678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portincasa, P. et al. Management of gallstones and its related complications. Expert Rev.Gastroenterol. Hepatol.10(1), 93–112 (2016). 10.1586/17474124.2016.1109445 [DOI] [PubMed] [Google Scholar]
- 9.Taylor, D. R. et al. Calcium carbonate in cholesterol gallstones: polymorphism, distribution, and hypotheses about pathogenesis. Hepatology22(2), 488–496 (1995). [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol.65(1), 146–181 (2016). 10.1016/j.jhep.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Kassi, E., Pervanidou, P., Kaltsas, G. & Chrousos, G. Metabolic syndrome: definitions and controversies. BMC Med.9, 48 (2011). 10.1186/1741-7015-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao, F. et al. Prevalence and influencing factors of metabolic Syndrome among adults in China from 2015 to 2017. Nutrients13(12), 4475 (2021). 10.3390/nu13124475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, W. et al. The association of metabolic syndrome components and diabetes mellitus: evidence from China National Stroke Screening and Prevention Project. BMC Public Health19(1), 192 (2019). 10.1186/s12889-019-6415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borai, A., Livingstone, C., Kaddam, I. & Ferns, G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med. Res. Methodol.11, 158 (2011). 10.1186/1471-2288-11-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borai, A., Livingstone, C. & Ferns, G. A. The biochemical assessment of insulin resistance. Annal. Clin. Biochem.44(Pt 4), 324–342 (2007). 10.1258/000456307780945778 [DOI] [PubMed] [Google Scholar]
- 16.Wang, S. et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc. Diabetol.20(1), 82 (2021). 10.1186/s12933-021-01274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramdas Nayak, V. K., Satheesh, P., Shenoy, M. T. & Kalra, S. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance. J. Pakistan Med. Assoc.72(5), 986–988 (2022). 10.47391/JPMA.22-63 [DOI] [PubMed] [Google Scholar]
- 18.Jeong, S. & Lee, J. H. The verification of the reliability of a triglyceride-glucose index and its availability as an advanced tool. Metabolomics17(11), 97 (2021). 10.1007/s11306-021-01837-9 [DOI] [PubMed] [Google Scholar]
- 19.Li, X., Zhan, F., Peng, T., Xia, Z. & Li, J. Association between the Triglyceride-Glucose Index and Non-Alcoholic Fatty Liver Disease in patients with Atrial Fibrillation. Eur. J. Med. Res.28(1), 355 (2023). 10.1186/s40001-023-01188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Y. et al. Correlation between the triglyceride-glucose index and the onset of atrial fibrillation in patients with non-alcoholic fatty liver disease. Diabetol. Metab. Syndr.15(1), 94 (2023). 10.1186/s13098-023-01012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song, S., Son, D. H., Baik, S. J., Cho, W. J. & Lee, Y. J. Triglyceride Glucose-Waist Circumference (TyG-WC) Is a Reliable Marker to Predict Non-Alcoholic Fatty Liver Disease. Biomedicines10(9), 2251 (2022). 10.3390/biomedicines10092251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng, G. et al. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Disease.20(1), 134 (2021). 10.1186/s12944-021-01561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, Y. et al. Baseline level and change trajectory of the triglyceride-glucose index in relation to the development of NAFLD: a large population-based cohort study. Front. Endocrinol.14, 1137098 (2023). 10.3389/fendo.2023.1137098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling, Q. et al. The triglyceride and glucose index and risk of nonalcoholic fatty liver disease: A dose-response meta-analysis. Front. Endocrinol.13, 1043169 (2023). 10.3389/fendo.2022.1043169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyasaka, K. et al. Susceptibility to obesity and gallbladder stasis produced by a protein- and fat-enriched diet in male mice compared with female mice. Nutr. Metab.4, 14 (2007). 10.1186/1743-7075-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, Y. et al. Relationship between hepatitis C and kidney stone in US females: Results from the national health and nutrition examination survey in 2007–2018. Front. Public Health.10, 940905 (2022). 10.3389/fpubh.2022.940905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, Y. et al. Association between aldehyde exposure and kidney stones in adults. Front. Public Health10, 978338 (2022). 10.3389/fpubh.2022.978338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peery, A. F. et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology162(2), 621–644 (2022). 10.1053/j.gastro.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan, K. E. et al. Longitudinal Outcomes Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-analysis of 129 Studies. Clin. Gastroenterol. Hepatol.S1542–3565(23), 00754–1 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Kuckuck, S. et al. Long-term glucocorticoids in relation to the metabolic syndrome and cardiovascular disease: A systematic review and meta-analysis. J. Int. Med.295(1), 2–19 (2024). 10.1111/joim.13739 [DOI] [PubMed] [Google Scholar]
- 31.Zdanowicz, K., Daniluk, J., Lebensztejn, D. M. & Daniluk, U. The Etiology of Cholelithiasis in Children and Adolescents-A Literature Review. Int. J. Mol. sci.23(21), 13376 (2022). 10.3390/ijms232113376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, M. H. et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet.27(1), 79–83 (2001). 10.1038/83799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigor’eva, I. N. & Romanova, T. I. Gallstone Disease and Microbiome. Microorganisms8(6), 835 (2020). 10.3390/microorganisms8060835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurer, K. J. et al. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology128(4), 1023–1033 (2005). 10.1053/j.gastro.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Everhart, J. E., Khare, M., Hill, M. & Maurer, K. R. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology117(3), 632–639 (1999). 10.1016/S0016-5085(99)70456-7 [DOI] [PubMed] [Google Scholar]
- 36.Liew, P. L. et al. Fatty liver disease: predictors of nonalcoholic steatohepatitis and gallbladder disease in morbid obesity. Obesity Surg.18(7), 847–853 (2008). 10.1007/s11695-007-9355-0 [DOI] [PubMed] [Google Scholar]
- 37.Festi, D. et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J. Gastroenterol.14(34), 5282–5289 (2008). 10.3748/wjg.14.5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su, P. Y., Hsu, Y. C., Cheng, Y. F., Kor, C. T. & Su, W. W. Strong association between metabolically-abnormal obesity and gallstone disease in adults under 50 years. BMC Gastroenterology19(1), 117 (2019). 10.1186/s12876-019-1032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan, S., Gill, D., Giovannucci, E. L. & Larsson, S. C. Obesity, Type 2 Diabetes, Lifestyle Factors, and Risk of Gallstone Disease: A Mendelian randomization investigation. Clin. Gastroenterol. Hepatol.20(3), e529–e537 (2022). 10.1016/j.cgh.2020.12.034 [DOI] [PubMed] [Google Scholar]
- 40.Cheng, J. et al. Association of pro-inflammatory diet with increased risk of gallstone disease: A cross-sectional study of NHANES January 2017-March 2020. Front. Nutr.11, 1344699 (2024). 10.3389/fnut.2024.1344699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mi, N. et al. Genetic risk, adherence to healthy lifestyle behaviors, and risk of cholelithiasis: A population-based cohort study. Prevent. Med.182, 107942 (2024). 10.1016/j.ypmed.2024.107942 [DOI] [PubMed] [Google Scholar]
- 42.Tazuma, S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract. Res. Clin. Gastroenterol.20(6), 1075–1083 (2006). 10.1016/j.bpg.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 43.Bennion, L. J., Ginsberg, R. L., Gernick, M. B. & Bennett, P. H. Effects of oral contraceptives on the gallbladder bile of normal women. N. Engl. J. Med.294(4), 189–192 (1976). 10.1056/NEJM197601222940403 [DOI] [PubMed] [Google Scholar]
- 44.Grodstein, F. et al. A prospective study of symptomatic gallstones in women: relation with oral contraceptives and other risk factors. Obstetr. Gynecol.84(2), 207–214 (1994). [PubMed] [Google Scholar]
- 45.Petitti, D. B., Sidney, S. & Perlman, J. A. Increased risk of cholecystectomy in users of supplemental estrogen. Gastroenterology94(1), 91–95 (1988). 10.1016/0016-5085(88)90614-2 [DOI] [PubMed] [Google Scholar]
- 46.Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science290(5497), 1771–1775 (2000). 10.1126/science.290.5497.1771 [DOI] [PubMed] [Google Scholar]
- 47.de Bari, O., Wang, T. Y., Liu, M., Portincasa, P. & Wang, D. Q. Estrogen induces two distinct cholesterol crystallization pathways by activating ERα and GPR30 in female mice. J. Lipid Res.56(9), 1691–1700 (2015). 10.1194/jlr.M059121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graewin, S. J. et al. Leptin regulates gallbladder genes related to gallstone pathogenesis in leptin-deficient mice. J. Am. Coll. Surg.206(3), 503–510 (2008). 10.1016/j.jamcollsurg.2007.09.015 [DOI] [PubMed] [Google Scholar]
- 49.Graewin, S. J. et al. Diminished gallbladder motility in Rotund leptin-resistant obese mice. HPB7(2), 139–143 (2005). 10.1080/13651820510028800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swartz-Basile, D. A. et al. Biliary lipids and cholesterol crystal formation in leptin-deficient obese mice. HPB8(5), 386–392 (2006). 10.1080/13651820600641233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, S. N. et al. Hyperleptinaemia and hypoadiponectinaemia are associated with gallstone disease. Eur. J. Clin. Investig.36(3), 176–180 (2006). 10.1111/j.1365-2362.2006.01611.x [DOI] [PubMed] [Google Scholar]
- 52.Goldblatt, M. I., Swartz-Basile, D. A., Svatek, C. L., Nakeeb, A. & Pitt, H. A. Decreased gallbladder response in leptin-deficient obese mice. J. Gastr. Surg.6(3), 438–444 (2002). 10.1016/S1091-255X(01)00046-4 [DOI] [PubMed] [Google Scholar]
- 53.Lee, S., Kweon, O. K. & Kim, W. H. Associations between serum leptin levels, hyperlipidemia, and cholelithiasis in dogs. PloS One12(10), e0187315 (2017). 10.1371/journal.pone.0187315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen, J. et al. Leptin Influence Cholelithiasis Formation by Regulating Bile Acid Metabolism. Turkish J. Gastroenterol.32(1), 97–105 (2021). 10.5152/tjg.2020.19594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106(25), 3143–3421 (2002). [PubMed]
- 56.Diehl, A. K. Cholelithiasis and the insulin resistance syndrome. Hepatology31(2), 528–530 (2000). 10.1002/hep.510310238 [DOI] [PubMed] [Google Scholar]
- 57.Wang, J. et al. Association of METS-IR index with prevalence of gallbladder stones and the age at the first gallbladder stone surgery in US adults: A cross-sectional study. Front. Endocrinol.13, 1025854 (2022). 10.3389/fendo.2022.1025854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nervi, F. et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J. Hepatol.45(2), 299–305 (2006). 10.1016/j.jhep.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 59.Biddinger, S. B. et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med.14(7), 778–782 (2008). 10.1038/nm1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this paper will be made available by the authors without reservation and can be obtained from the corresponding author.