Abstract
Bicyclams are low-molecular-weight anti-human immunodeficiency virus (HIV) agents that have been shown to act as potent and selective CXC chemokine receptor 4 (CXCR4) antagonists. Here, we demonstrate that bicyclams are potent inhibitors of feline immunodeficiency virus (FIV) replication when evaluated in Crandell feline kidney (CRFK) cells. With a series of bicyclam derivatives, 50% inhibitory concentrations (IC50s) against FIV were obtained in this cell system that were comparable to those obtained for HIV-1 IIIB replication in the human CD4+ MT-4 T-cell line. The bicyclams were also able to block FIV replication in feline thymocytes, albeit at higher concentrations than in the CRFK cells. The prototype bicyclam AMD3100, 1-1′-[1,4-phenylene-bis(methylene)]-bis(1,4,8,11-tetraazacyclotetradecane), was only fourfold less active in feline thymocytes (IC50, 62 ng/ml) than in CRFK cells (IC50, 14 ng/ml). AMD2763, 1,1′-propylene-bis(1,4,8,11-tetraazacyclotetradecane), which is a less potent CXCR4 antagonist, was virtually inactive against FIV in feline thymocytes (IC50, >66.5 μg/ml), while it was clearly active in CRFK cells (IC50, 0.9 μg/ml). The CXC chemokine stromal-cell-derived factor 1α had anti-FIV activity in CRFK cells (IC50, 200 ng/ml) but not in feline thymocytes (IC50, >2.5 μg/ml). When primary FIV isolates were evaluated for their drug susceptibility in feline thymocytes, the bicyclams AMD3100 and its Zn2+ complex, AMD3479, inhibited all six primary isolates at equal potency. The marked susceptibility of FIV to the bicyclams suggests that FIV predominantly uses feline CXCR4 for entering its target cells.
Bicyclams represent a new class of human immunodeficiency virus (HIV) inhibitors that have been shown to selectively inhibit HIV type 1 (HIV-1) and HIV-2 but not simian immunodeficiency virus replication (8, 9, 13, 14). These compounds were shown recently to act as potent and selective antagonists of the CXC chemokine receptor 4 (CXCR4) (28, 29), the main coreceptor for syncytium-inducing (SI), T-cell-line-adapted (T-tropic) HIV strains (1, 2, 21, 27). Infection of cells with T-tropic strains of HIV could be potently blocked, whereas no antiviral activity was observed against non-syncytium-inducing (NSI), macrophage-tropic (M-tropic) strains, which mainly use CCR5 as coreceptor (4, 10, 16, 30, 38). A close correlation between anti-HIV-1 activity and interaction with CXCR4 has been found for a series of bicyclam analogues (19).
Feline immunodeficiency virus (FIV) causes a disease in cats that is similar to AIDS in HIV-infected patients and is an adequate model to study the effect of antiviral therapy in vivo (17, 22). Recently, it was shown that FIV strains adapted to grow in Crandell feline kidney (CRFK) cells are able to use CXCR4 for cell fusion and viral entry and that a high degree of homology exists between the human and feline CXCR4 (36). Syncytium formation between persistently FIV-infected CRFK cells and HeLa cells expressing human CXCR4 could be inhibited by human stromal-cell-derived factor 1α (SDF-1α) and by the anti-human CXCR4 monoclonal antibody (MAb) 12G5 (35). Also, SDF-1α was shown to inhibit FIV infection of CRFK cells in a dose-dependent manner as a result of steric hindrance for virus to interact with CXCR4 following the interaction between SDF-1α and feline CXCR4 (24). However, SDF-1α did not inhibit infection of the interleukin-2 (IL-2)-dependent feline T-cell line, called Mya-1, with either the cell-culture-adapted isolate FIV-Petaluma or a primary isolate, indicating the possible existence of a CXCR4-independent pathway of infection in these cells (24). It is currently unknown if receptors other than CXCR4 are necessary for infection with FIV (24, 35). The primary receptor for HIV is CD4 (7), whereas this was shown not to be the receptor for FIV (33), although a progressive depletion of CD4+ T lymphocytes is observed during FIV infection in domestic cats (23). MAbs recognizing feline CD9 have been shown to inhibit FIV infection (33). However, more recent studies suggest that this MAb inhibits viral release but not entry of the virus (12, 34). The relative importance of CXCR4 as a coreceptor for non-cell-culture-adapted strains of FIV and primary isolates is still unknown. Although HIV-1 requires coexpression of both the primary receptor, CD4, and a chemokine receptor, mainly CXCR4 or CCR5, some studies have demonstrated that CD4-independent infection by certain HIV-2 strains can be mediated by CXCR4 alone (18). Other coreceptors for HIV have been described (11, 15, 20, 26), and their importance in HIV-1 infection remains to be established.
Since FIV binds to both human and feline CXCR4 and given the amino acid sequence homology between the chemokine receptors of both species, we investigated whether the bicyclams would be capable of inhibiting FIV infection. We found that a series of bicyclam analogues inhibit FIV infection in CRFK cells and that their 50% inhibitory concentrations (IC50s) are comparable to those required for inhibiting the replication of HIV-1 IIIB in a T-cell line. Also, infection of primary FIV isolates in IL-2-dependent feline thymocytes could be blocked by the bicyclams, indicating that CXCR4 can function as an essential (co)receptor for primary FIV isolates as well.
MATERIALS AND METHODS
Compounds and chemokines.
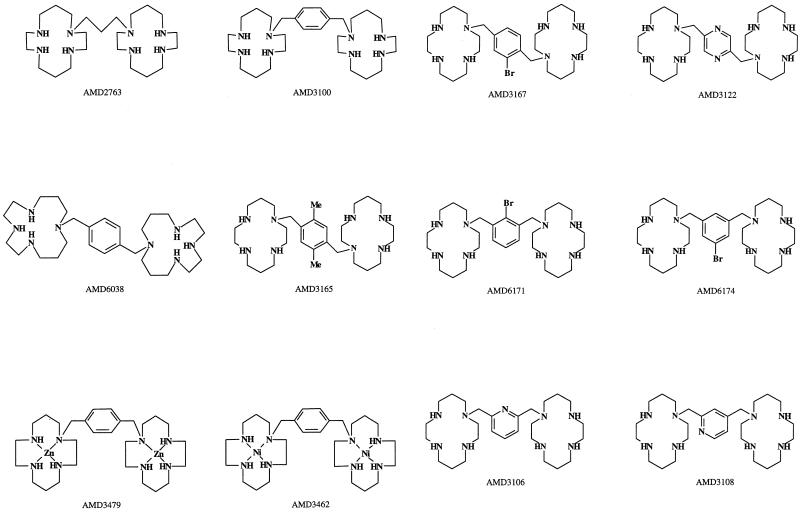
The following bicyclams were evaluated for their anti-FIV activity: AMD2763, AMD3100, AMD3479, AMD3122, AMD3165, AMD3167, AMD3462, AMD6038, AMD6171, AMD3106, AMD3108, and AMD6174. The chemical structures of these compounds have been described in detail elsewhere (3) and are presented in Fig. 1. The CXC chemokine SDF-1α was obtained from R&D Systems Europe Ltd., Abingdon, United Kingdom.
FIG. 1.
Structures of bicyclam analogues.
Cells and viruses.
CRFK cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum (FCS) and antibiotics. Feline thymocytes were collected from specific-pathogen-free cats (Harlan, Zeist, The Netherlands) stimulated with concanavalin A at 2.5 μg/ml and cultured in RPMI 1640 medium containing 10% FCS supplemented with 100 IU of recombinant IL-2/ml. The CRFK-tropic virus FIV-113cr was prepared from a culture of persistently infected CRFK cells. A virus stock of the non-CRFK-tropic, thymocyte-specific strain FIV-113th was made on feline thymocytes. The primary isolates VI-113bm, VI-48liq, VI-194, Katja, Maffie, and VI-156 were obtained from FIV-infected field cats and used as low-passage (two to three passages) virus stocks. The construction of the molecular clones pPET-113th and pPET-113cr was described elsewhere (32). Transfection of DNA from these molecular clones into CRFK cells was performed by the DEAE dextran method as described previously (32). After transfection, the cells were cocultured with feline thymocytes to prepare virus stocks.
Virus infection assay.
Replication of a cell-culture-adapted strain (FIV-113cr) in CRFK cells and a thymocyte-specific strain (FIV-113th) in feline thymocytes was monitored in the presence of different concentrations of compounds. Viral replication was measured by determining p24 in the supernatant by a viral core antigen enzyme-linked immunosorbent assay established in our own laboratory (31). The IC50s and the drug concentrations reducing the number of viable mock-infected cells by 50% were calculated.
Cell fusion assay.
The inhibition of syncytium formation was studied in a fusion assay involving FIV-infected CRFK cells and HeLa cells expressing human CXCR4. The drug concentration reducing the number of syncytia by 50% was determined microscopically.
Analysis of CXCR4 expression.
Feline thymocytes were incubated with AMD3100 at different concentrations for 15 min at room temperature. The anti-human CXCR4 MAb (#173; R&D Systems) was then added for 30 min at room temperature. The cells were washed twice and then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse MAb for 30 min at room temperature. The cells were analyzed with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The percentage of inhibition of MAb binding in the presence of different concentrations of compound was calculated by using the mean fluorescence intensity values, as described previously (28).
RESULTS
Inhibition of FIV infection by the bicyclams.
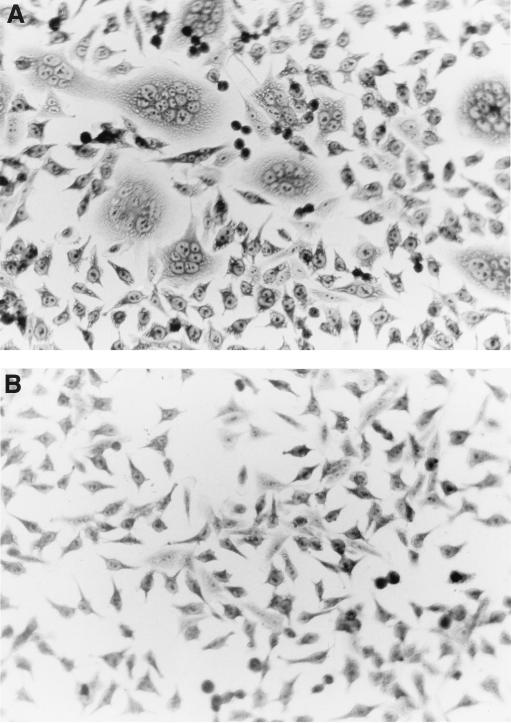
Recently, it was demonstrated that cell-culture-adapted strains of FIV can bind to the human and feline α-chemokine receptor CXCR4, after which fusion and entry occur (24). We therefore examined whether fusion and entry of FIV could be inhibited by using several bicyclam derivatives. These compounds are known to inhibit replication with T-cell-tropic strains of HIV-1 and HIV-2 through a selective interaction with the human CXCR4 receptor (28). The efficacy of these drugs against FIV was first studied in a fusion inhibition assay with persistently FIV-infected CRFK cells and HeLa cells. HeLa cells were incubated with increasing concentrations of AMD3100, 1-1′-[1,4-phenylene-bis(methylene)]-bis(1,4,8,11-tetraazacyclotetradecane), for 1 h, after which CRFK cells persistently infected with FIV-113cr were added. One day after cocultivation, the cells were fixed and stained, and the number of syncytia per well was quantified by light microscopy. The concentration of the compound reducing the number of syncytia by 50% as compared to the untreated control cultures was then calculated.
As shown in Fig. 2, addition of the prototype bicyclam AMD3100 clearly inhibited the formation of syncytia. AMD3100 reduced the number of syncytia by 50% at a concentration of 0.055 μg/ml. A total of 12 different bicyclam derivatives were studied in the syncytium inhibition assay, and their IC50s are presented in Table 1. AMD2763, which contains an n-propyl bridge between the 1,4,8,11-tetraazamacrocyclic (cyclam) ring systems, has lower anti-HIV activity (8) than AMD3100, in which the two cyclam rings are connected by an aromatic phenylene-bis(methylene) bridge, and also is less active against FIV (IC50, 0.56 μg/ml). The AMD3100 Zn2+ complex, AMD3479, had an IC50 of 0.007 μg/ml, whereas the AMD3100 Ni2+ complex, AMD3462, had an IC50 of 0.03 μg/ml. No difference in IC50s of 2-bromo AMD6171 and 5-bromo AMD6174 was noted, whereas AMD3108 was about 170-fold less active than AMD3106. AMD3167, the meta-linked analogue of AMD3106, was clearly more active (IC50, 0.008 μg/ml) than AMD3106 (IC50, 0.063 μg/ml). SDF-1α inhibited the fusion between FIV-infected cells and HeLa cells at an IC50 of 0.17 μg/ml.
FIG. 2.
Fusion of FIV-infected CRFK cells with uninfected HeLa cells (A) and inhibition of fusion in the presence of AMD3100 at a concentration of 1 μg/ml (B).
TABLE 1.
Activity of bicyclam derivatives and SDF-1α against fusion between FIV-infected CRFK cells and HeLa cells, against HIV-1 IIIB replication in MT-4 cells, against replication of FIV-113cr in CRFK cells, and against FIV-113th replication in feline thymocytes
| Compound | IC50 (μg/ml) for:
|
Ratio of IC50 of FIV-113th to IC50 of FIV-113cr | |||
|---|---|---|---|---|---|
| Fusion of FIV-infected CRFK and HeLa cells | HIV-1 IIIB in MT-4 cells | FIV-113cr in CRFK cells | FIV-113th in thymocytes | ||
| AMD2763 | 0.56 | 0.78 | 0.94 | 66.5 | 77 |
| AMD3100 | 0.055 | 0.009 | 0.014 | 0.062 | 4 |
| AMD3479 | 0.007 | 0.008 | 0.008 | 0.139 | 17 |
| AMD3122 | 15.6 | 20.9 | 47.6 | >100 | >2 |
| AMD3165 | 0.065 | 0.006 | 0.013 | 0.40 | 31 |
| AMD3167 | 0.008 | 0.006 | 0.002 | 0.34 | 170 |
| AMD3462 | 0.030 | 0.02 | 0.02 | 0.64 | 32 |
| AMD6038 | 0.039 | 0.07 | 0.057 | 6.0 | 105 |
| AMD6171 | 0.036 | 0.14 | 0.109 | 4.4 | 40 |
| AMD3106 | 0.063 | 0.04 | 0.108 | 6.2 | 57 |
| AMD3108 | 10.7 | 3.1 | 6.4 | 50.4 | 8 |
| AMD6174 | 0.038 | 0.06 | 0.043 | 1.9 | 44 |
| SDF-1α | 0.170 | 0.150 | 0.200 | >2.5 | >12 |
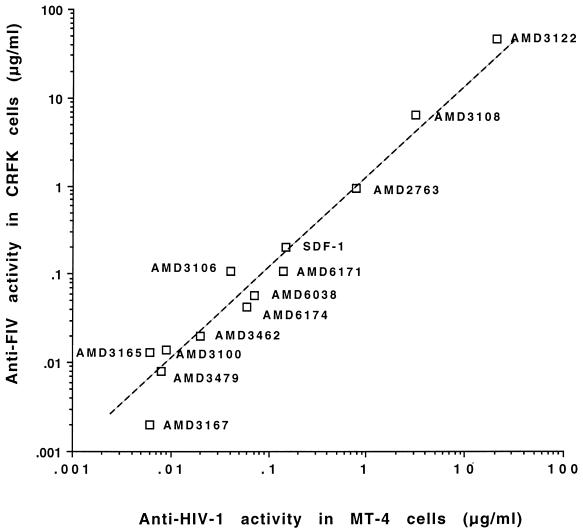
The efficacy of the antiviral drugs against a CRFK-adapted FIV strain (FIV-113cr) on CRFK cells and a T-cell-tropic FIV strain (FIV-113th) on feline thymocytes has also been evaluated. In Table 1, the IC50s for FIV infection in CRFK cells and feline thymocytes are listed. For comparison, the IC50s of the bicyclams for the HIV IIIB strain in MT-4 cells are also listed. Besides activity in the fusion inhibition assay, all bicyclams showed inhibition of FIV replication in CRFK cells (Table 1). The IC50s obtained in the syncytium inhibition assay were comparable to the IC50s for inhibiting FIV replication in CRFK cells. Higher concentrations (4- to 170-fold greater than the IC50 in CRFK cells) were needed to block infection of the thymocyte-adapted strain, FIV-113th, in feline thymocytes. However, antiviral activity of the bicyclams in the latter cells could still be demonstrated. Interestingly, SDF-1α had no antiviral activity up to a concentration of 2.5 μg/ml when evaluated against FIV replication in thymocytes, whereas it had an IC50 of 0.2 μg/ml in the CRFK cells. In general, compounds with less activity in CRFK cells were shown to be less active in thymocytes as well. From the comparable IC50s obtained for inhibition of HIV replication in MT-4 cells and FIV infection, it can be concluded that the bicyclams bind not only to human CXCR4 but also to feline CXCR4. Fig. 3 shows the correlation for the 12 bicyclam derivatives and SDF-1α between the antiviral activity for HIV-1 IIIB and FIV-113cr (IC50s). The correlation coefficient was 0.9 and the calculated r2 value was 0.81.
FIG. 3.
Correlation of anti-HIV-1 (strain IIIB) activity in MT-4 cells and anti-FIV (strain 113cr) activity in CRFK cells of the different bicyclam analogues and SDF-1α.
Primary FIV isolates use CXCR4 in feline thymocytes.
To verify whether primary isolates of FIV could use CXCR4 as a (co)receptor, different primary isolates were used for infection of feline thymocytes. These isolates did not replicate in CRFK cells and were used after two to three passages in thymocytes. The results are summarized in Table 2. The bicyclams AMD3100 and AMD3479 clearly blocked infection of primary isolates evaluated in thymocyte cultures, suggesting that the use of CXCR4 as a (co)receptor is not just a property of the CRFK-cell-adapted strains of FIV but that CXCR4 is also the main (co)receptor used by FIV isolates to infect their target cells. In addition, not much variation in the activity of both AMD3100 and AMD3479 against the different FIV isolates was seen. Clone pPET-113th replicates only in thymocytes and pPET-113cr replicates in both CRFK cells and thymocytes (32). Both clones were inhibited by AMD3100 at comparable IC50s.
TABLE 2.
Anti-FIV activity of AMD3100 and AMD3479 against six different primary isolates and two molecular clones of FIV
| Isolate or clone | IC50 (μg/ml) of:
|
|
|---|---|---|
| AMD3100 | AMD3479 | |
| Primary FIV isolates | ||
| VI-113bm | 0.021 | 0.049 |
| Katja | 0.022 | 0.053 |
| Maffie | 0.060 | 0.127 |
| VI-48liq | 0.027 | 0.072 |
| VI-194 | 0.038 | 0.120 |
| VI-156 | 0.068 | 0.032 |
| Molecular clones | ||
| pPET-113th | 0.26 | NDa |
| pPET-113cr | 0.26 | ND |
ND, not determined.
AMD3100 interacts with feline CXCR4.
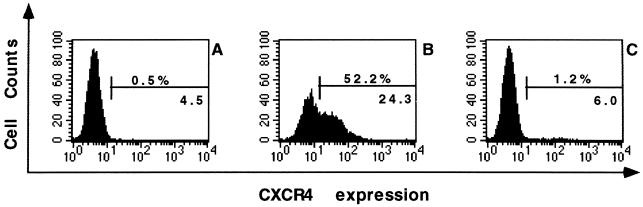
AMD3100 was found to dose-dependently inhibit the binding of anti-CXCR4 MAb to feline thymocytes. The human anti-CXCR4 MAb (#173) was used because it cross-reacts with feline CXCR4 and recognizes the second extracellular loop of human CXCR4. Although the 12G5 MAb also recognizes the second extracellular loop of human CXCR4, it does not bind to feline CXCR4 (reference 24 and our own observations). AMD3100 at 1 μg/ml almost completely blocked the binding of the MAb #173 to CXCR4 on feline thymocytes (Fig. 4). The IC50 of AMD3100 to inhibit this MAb on feline thymocytes was 20 ng/ml, which is comparable to its IC50 obtained with the 12G5 MAb on human SUPT1 cells (28). AMD3100 had no inhibitory effect (at 100 μg/ml) on the binding of feline anti-CD4 MAb (MCA 1347; Serotec, Oxford, United Kingdom) and anti-CD8 MAb (MCA 1350; Serotec) in feline thymocytes.
FIG. 4.
Inhibition of binding of the anti-CXCR4 MAb to feline thymocytes in the presence (C) or absence (B) of AMD3100 at 1 μg/ml. In panel A, results obtained with an isotype control MAb are shown. The percentages of fluorescence-positive cells and the mean fluorescence intensity values are indicated in each histogram.
DISCUSSION
Bicyclams were shown to inhibit the replication of SI, T-tropic strains of HIV through a selective and potent binding to CXCR4. It has been recently demonstrated that CXCR4 is also used by cell-culture-adapted strains of FIV for binding and entry into the cells (24, 36, 37). In view of the homology between the human and feline CXCR4, the antiviral activity of the bicyclams against FIV replication was determined. Inhibition of fusion and entry of FIV was clearly demonstrated in the presence of the bicyclams. Although the bicyclams are somewhat less inhibitory against infection of feline thymocytes by a non-cell-culture-adapted FIV strain, our data indicate that CXCR4 also functions as an important (co)receptor for FIV in these cells. Therefore, we can conclude that binding to CXCR4 is not a property that is exclusive to CRFK-adapted virus strains.
In addition, the bicyclams can be used as specific probes for the feline chemokine receptor to assess whether primary isolates (and non-cell-culture-adapted strains) of FIV use CXCR4 for infection. In this study, we demonstrated that the bicyclams AMD3100 and AMD3479 could block infection of thymocytes by both primary isolates and a lymphocyte-specific infectious clone of FIV, suggesting that CXCR4 is the main receptor for FIV to enter its target cells. No antiviral effect of AMD3100 at 1 μg/ml was noted against feline herpes virus, which was included as a control.
It was demonstrated that human SDF-1α inhibits FIV infection of CRFK cells, while SDF-1α had no effect on FIV infection of the IL-2-dependent feline T-cell line Mya-1 (24). This was interpreted as evidence for the existence of a CXCR4-independent mechanism of FIV infection for this T-cell line. However, CCR5-dependent entry could be ruled out, since none of the human β-chemokines, such as RANTES, MIP-1α, or MIP-1β, had an antiviral effect against FIV infection (24), whereas these chemokines have potent anti-HIV activity in human peripheral blood mononuclear cells (5).
Interestingly, FIV infection of CRFK cells can be not only inhibited but also enhanced by SDF-1α due to the upregulation of CXCR4 in these cells (24). This phenomenon is not seen with the bicyclams because their mechanism of antiviral activity is based on a direct binding with the CXCR4 receptor. Bicyclams act as pure antagonists at the CXCR4 level because even at 100 μg/ml (100,000-fold anti-HIV activity), they do not induce Ca2+ flux in a CXCR4+ T-cell line (e.g., SUPT1), a monocytic cell line (e.g., THP-1), or a CXCR4-transfected cell line (e.g., HOS.CD4.CXCR4) (29).
We have demonstrated that SDF-1α inhibits FIV replication in CRFK cells but not in thymocytes (Table 1) or Mya-1 cells (data not shown). This is in contrast with the bicyclams, which could be considered a reliable probe for CXCR4 and which proved inhibitory to FIV in thymocytes (Table 1 and 2) as well as Mya-1 cells (data not shown). There was no synergistic or additive effect when AMD3100 was combined with SDF-1α. This was also not expected, as SDF-1α at 2.5 μg/ml had no antiviral activity by itself in feline thymocytes (data not shown). In addition, no other human chemokine receptor tested so far (e.g., CCR1, -2, -3, -4, and -5) supports fusion mediated by FIV, suggesting that the interaction of envelope glycoprotein of FIV with CXCR4 is highly specific (37). As feline CCR5 also failed to support fusion or infection with CRFK-tropic viruses and primary isolates (37), we propose that CXCR4 may act as the main, if not the sole, receptor for FIV.
The interaction between FIV and CXCR4 has been investigated by using a series of chimeric CXCR4 molecules (36). As with HIV, the major determinant of CXCR4 for FIV entry is the second extracellular loop of CXCR4 (36), although the first and third loops of CXCR4 also contribute to the FIV envelope binding site, which is comparable to the situation with HIV. Thus, FIV and HIV follow a very similar mechanism of interacting with CXCR4. In this context, it is not surprising that the bicyclams have comparable activity against FIV and HIV. The CRFK-cell-adapted strains of FIV show a shift in the net charge of the V3 loop of the envelope protein (32) that is comparable to changes that can be observed in the V3 loop of HIV following the switch from the NSI to the SI phenotype (6), and this switch correlates with disease progression in humans. Since SI strains of HIV use CXCR4 as coreceptor, it was postulated that CRFK cell tropism of FIV was determined by the ability of the virus to use CXCR4 as coreceptor (24). Based on our results, we can further conclude that the use of CXCR4 is not just a property of cell-culture-adapted FIV strains but also extends to primary FIV isolates.
Relative resistance to AMD3100 is conferred by different single amino acid substitutions in the second extracellular loop of human CXCR4 (25). In general, the positive charge of the bicyclams might block HIV and FIV entry by preventing the electrostatic interactions between CXCR4 and the HIV-FIV envelope. AMD3100 concentration-dependently inhibited the binding of anti-CXCR4 MAb (such as 12G5 or #173) to human CXCR4 (reference 28 and our unpublished data) at concentrations which were comparable to those required for its anti-HIV activity. The MAb (#173) binds to the second extracellular loop of human CXCR4, cross-reacts with feline CXCR4, and was also dose-dependently inhibited by AMD3100.
In conclusion, we have demonstrated that FIV predominantly uses CXCR4 for entering its target cells. The high anti-FIV potency and selectivity of the bicyclams further support the use of the bicyclams in the treatment of FIV infections. While this approach may allow the establishment of an appropriate therapy for the treatment of FIV infections in cats, it may also prove most valuable as a model to delineate novel strategies to prevent AIDS progression in humans.
ACKNOWLEDGMENTS
We thank Sandra Claes, Erik Fonteyn for excellent technical assistance, and Dominique Brabants and Inge Aerts for fine editorial assistance.
This work was supported by grant G.0104.98 from the Fonds voor Wetenschappelijk Onderzoek (FWO)-Vlaanderen, by project 95/5 from the Geconcerteerde Onderzoeksacties (GOA)-Vlaamse Gemeenschap, and by the European Commission.
REFERENCES
- 1.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 3.Bridger G J, Skerlj R T, Thornton D, Padmanabhan S, Martellucci S A, Henson G W, Abrams M J, Yamamoto N, De Vreese K, Pauwels R, De Clercq E. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J Med Chem. 1995;38:366–378. doi: 10.1021/jm00002a019. [DOI] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 6.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalgleish A G, Beverley P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E, Yamamoto N, Pauwels R, Baba M, Schols D, Nakashima H, Balzarini J, Murrer B A, Schwartz D, Thornton D, Bridger G, Fricker S, Henson G, Abrams M, Picker D. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc Natl Acad Sci USA. 1992;78:5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, Thornton D, Skerlj R, Gaul F, Padmanabhan S, Bridger G, Henson G, Abrams M. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38:668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 12.de Parseval A, Lerner D L, Borrow P, Willett B J, Elder J H. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J Virol. 1997;71:5742–5749. doi: 10.1128/jvi.71.8.5742-5749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vreese K, Kofler-Mongold V, Leutgeb C, Weber V, Vermeire K, Schacht S, Anné J, De Clercq E, Datema R, Werner G. The molecular target of bicyclams, potent inhibitors of human immunodeficiency virus replication. J Virol. 1996;70:689–696. doi: 10.1128/jvi.70.2.689-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vreese K, Reymen D, Griffin P, Steinkasserer A, Werner G, Bridger G J, Esté J, James W, Henson G, Desmyter J, Anné J, De Clercq E. The bicyclams, a new class of potent human immunodeficiency virus inhibitors, block viral entry after binding. Antiviral Res. 1996;29:209–219. doi: 10.1016/0166-3542(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 15.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 16.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 17.Egberink H, Borst M, Niphuis H, Balzarini J, Neu H, Schellekens H, De Clercq E, Horzinek M, Koolen M. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc Natl Acad Sci USA. 1990;87:3087–3091. doi: 10.1073/pnas.87.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 19.Esté J A, Labrera C, De Clercq E, Struyf S, Van Damme J, Bridger G, Sherlj R T, Abrams M J, Henson G, Gutierrez A, Clotet B, Schols D. Activity of different bicyclam derivatives against human immunodeficiency virus depends on their interaction with the CXCR4 chemokine receptor. Mol Pharmacol. 1999;55:67–73. doi: 10.1124/mol.55.1.67. [DOI] [PubMed] [Google Scholar]
- 20.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann K, Kuffer M, Balzarini J, Naesens L, Goldberg M, Erfle V, Goebel F D, De Clercq E, Jindrich J, Holý A, Bischofberger N, Kraft W. Efficacy of the acyclic nucleoside phosphonates (S)-9-(3-fluoro-2-phosphonylmethoxypropyl)-adenine (FPMPA) and 9-(2-phosphonylmethoxyethyl)adenine (PMEA) against feline immunodeficiency virus. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;17:120–128. doi: 10.1097/00042560-199802010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann-Fezer G, Thum J, Ackley C, Herbold M, Mysliwietz J, Thefeld S, Hartmann K, Kraft W. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol. 1992;66:1484–1488. doi: 10.1128/jvi.66.3.1484-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosie M J, Broere N, Hesselgesser J, Turner J D, Hoxie J A, Neil J C, Willett B J. Modulation of feline immunodeficiency virus infection by stromal cell-derived factor. J Virol. 1998;72:2097–2104. doi: 10.1128/jvi.72.3.2097-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, De Clercq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusion and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 28.Schols D, Esté J A, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res. 1997;35:147–156. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 29.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 31.Vahlenkamp T W, Egberink H F, van Eijk M J T, Slotboom-Kamphorst A M E, Verschoor E J, Horzinek M C, De Ronde A. Competitive reverse transcription-polymerase chain reaction for quantitation of feline immunodeficiency virus. J Virol Methods. 1995;52:335–346. doi: 10.1016/0166-0934(94)00169-h. [DOI] [PubMed] [Google Scholar]
- 32.Verschoor E J, Boven L A, Blaak H, van Vliet A R W, Horzinek M C, De Ronde A. A single mutation in the V3 envelope neutralization domain of feline immunodeficiency virus determines its tropism for CRFK cells. J Virol. 1995;69:4752–4757. doi: 10.1128/jvi.69.8.4752-4757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willett B J, Hosie M J, Jarrett O, Neil J C. Identification of a putative cellular receptor for feline immunodeficiency virus as the feline homologue of CD9. Immunology. 1994;81:228–233. [PMC free article] [PubMed] [Google Scholar]
- 34.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 35.Willett B, Hosie M, Shaw A, Neil J. Inhibition of feline immunodeficiency virus infection by CD9 antibody operates after virus entry and is independent of virus tropism. J Gen Virol. 1997;78:611–618. doi: 10.1099/0022-1317-78-3-611. [DOI] [PubMed] [Google Scholar]
- 36.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willett B J, Adema K, Heveker N, Brelot A, Picard L, Alizon M, Turner J D, Hoxie J A, Peiper S, Neil J C, Hosie M J. The second extracellular loop of CXCR4 determines its function as a receptor for feline immunodeficiency virus. J Virol. 1998;72:6475–6481. doi: 10.1128/jvi.72.8.6475-6481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]