Abstract
Purpose
In April 2020, the UK Government implemented NHS Test and Trace to provide SARS-CoV-2 quantitative reverse transcription polymerase chain reaction (qRT-PCR) testing for the public, with nose-and-throat swabbing for samples performed by trained staff. Self-swabbing (SS) would allow rapid scale-up of testing capacity and access. Six studies were undertaken to determine whether SS was as effective for detecting SARS-CoV-2 as swabbing performed by trained staff.
Methods
Six prospective studies were conducted between April–October 2020, using six swab/media combinations. Differences between assisted swabbing (AS) and SS were evaluated for concordance, positivity, sensitivity, cycle threshold (Ct) values and void rates. Statistical analysis was performed using 95% confidence intervals (CIs), paired t-tests and model-based methods.
Results
Overall, 3,253 individuals were recruited (median age 37 years, 49% female), with 2,933 having valid paired qRT-PCR results. Pooled concordance rate was 98% (95% CI: 96%, 99%). Positivity rate differences for SS (8.1%) and AS (8.4%) and differences in pooled sensitivities between SS (86%; 95% CI: 78%, 92%) and AS (91%; 95% CI: 78%, 96%) were nonsignificant. Both types of swabbing led to pooled void rates below 2% and strongly correlated Ct values. Age, sex and previous swabbing experience did not have a significant impact on concordance or sensitivity.
Conclusion
The UK adopted a policy to promote self-testing for SARS-CoV-2 based on data demonstrating equivalence of SS versus AS. Positive outcomes with SS are likely generalisable to testing for other respiratory pathogens, and we consider self-sampling and self-testing essential for future pandemic preparedness.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-024-04866-z.
Keywords: COVID-19, Self-testing, Self-swabbing, PCR, United Kingdom, Testing programme
Introduction
As part of the coronavirus disease 2019 (COVID-19) pandemic response, the UK Government established National Health Service (NHS) Test and Trace to deliver the national testing programme [1–4], where quantitative reverse transcription polymerase chain reaction (qRT-PCR, hereafter ‘PCR’) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was provided free to members of the public with or without (self-declared) symptoms. Initially, testing was conducted on nasal (mid-turbinate level, one nostril) and throat (both peri-tonsillar areas) swab samples at in-person testing sites by trained staff [5].
To control SARS-CoV-2 transmission, the testing service needed to scale-up to be available to anyone who self-declared symptoms. A key constraint was availability of trained staff to perform swabbing and, hence, number of appointment slots. A further access limitation was the need to attend testing sites in-person, which was impractical for many (due to shielding, immobility or transport issues). This health inequity was an unintended artefact of the testing service, which required mitigation.
One approach to addressing these challenges was to investigate whether individuals could collect samples themselves [6]. At the time, there was little evidence showing individuals could swab themselves to achieve accurate testing for SARS-CoV-2. Furthermore, in the early pandemic, global supply chain shortages of swabs and collection vials limited the capacity of the COVID-19 testing service.
As no single swab–vial combination was available at volumes to deliver the public health response, six prospective studies—with identical inclusion criteria, but different swab–vial combinations—were undertaken to assess the suitability of use as a self-sampling collection device across the range of devices available. In each study, individuals collected samples by themselves (hereafter, self-swabbing [SS]) and were then swabbed by a trained staff member (hereafter, assisted swabbing [AS]), allowing direct comparison between SS and AS. Here, we report results from a meta-analysis of the six service evaluation studies, to determine whether a difference in performance (measured by ability to detect SARS-CoV-2) between SS and AS could be observed.
Materials and methods
Data collection
Six service evaluation studies were conducted between April and October 2020 at three COVID-19 testing sites in the UK (Chessington, Manchester and Leicester) to compare the performance of SS versus AS for PCR testing. In this context, as was standard practice for routine PCR testing at the time.
Participants aged ≥ 18 years, were required to have read and understood printed SS instructions (provided in English) with illustrations and agreed to participate. Study designs are summarised in Table 1. A minimum study size was determined, so that the 95% confidence intervals (Cis) for estimated concordance rates between AS and SS (as defined in Supplementary Table 1) will fall within a 10% margin. More specifically, CIs were calculated using the Clopper-Pearson method and simulated for a varying number of participants and concordance rates. This led to a minimum number of required participants of 78 per study.
Table 1.
Overview of the six service evaluation studies, including the demographic characteristics of the study populations
| Study 1 | Study 2 | Study 3 | Study 4 | Study 5 | Study 6 | Total | ||
|---|---|---|---|---|---|---|---|---|
| Product | MW951S Sigma Virocult Kit |
Medline MD202003 dry swab with vial of 0.85% saline |
PROVIR Viral Transport Kit (TS/5–34 A) |
E&O BM1673- M043-3 Vial + Medium, Alphalab SW1040 |
Combination kit with ISS PBT093 tube filled with 3 ml TF saline and Citotest 2122-0008 swab |
BD Improve Medical Instruments 8,110,111 vial, 550,040 A dry swab |
. | |
| Inclusion criteria | ≥ 18 years old; read and understood self-swabbing instructions | ≥ 18 years old; read and understood self-swabbing instructions | ≥ 18 years old; read and understood self-swabbing instructions | ≥ 18 years old; read and understood self-swabbing instructions | ≥ 18 years old; read and understood self-swabbing instructions | ≥ 18 years old; read and understood self-swabbing instructions | . | |
| Location/site | Chessington Regional Test Centre and UK Biocentre | Manchester Airport | Leicester (Birstall Park & Ride) | Leicester (Birstall Park & Ride) | Leicester (Birstall Park & Ride) | Leicester (Birstall Park & Ride) | . | |
| N (recruited) | 97 | 395 | 689 | 492 | 1,005 | 575 | 3,253 | |
| N (with PCR outcome) | 90 | 348 | 654 | 458 | 892 | 491 | 2,933 | |
| Median age (years); (min–max) | 43(19–67) | 40(18–81) | 37(18–84) | 36(18–81) | 38(18–81) | 35(18–84) | 37(18–84) | |
| Sex: male (n,%) | 4 (4) | 113 (33) | 286 (44) | 212 (47) | 447 (50) | 239 (49) | 1,301 (44) | |
| Sex: female (n,%) | 9 (10) | 117 (34) | 362 (55) | 243 (54) | 445 (50) | 252 (51) | 1,428 (49) | |
| Sex: missing (n,%) | 77 (86) | 118 (34) | 6 (1) | 3 (1) | 0 | 0 | 204 (7) | |
| Previous swabbing experience (%) | 39 | 20 | 23 | 20 | 56 | n/a | 29 | |
The original study names were: Study 1: SE-SWTC1/SSES; Study 2: SE-SWTC3/COMBI006; Study 3: TS5-34 A; Study 4: COMBI021; Study 5: COMBI031; and Study 6: COMBI045. PCR, polymerase chain reaction
A throat sample (both peri-tonsillar areas) and then a nasal sample (single nostril to the mid-turbinate level) were collected by participants using a single swab, without trained staff involvement [7]. This swab was placed by the participant into a fresh tube filled with viral transport medium and sealed/packaged and placed in collection boxes. Immediately afterwards, a further throat and nasal sample was collected using a fresh single swab, by a trained staff member. This was placed into a fresh tube filled with viral transport medium as for the SS sample. Although samples were collected by different people, care was taken to ensure handling was as similar as possible. Within studies, paired samples were collected using swabs and viral transport medium from the same manufacturer. Data on selfreported age, sex and previous (professional and self-) swabbing experience were collected on-site at time of attendance.
After sample collection, sealed tubes containing swabs and viral transport medium in collection boxes were batched and transported within 4 h to UK Biocentre, Milton Keynes, where all PCR testing was conducted [8]. For PCR testing, Thermofisher TaqPath COVID-19 CE-IVD PCR assays were used. These assays amplify specific regions (ORF1ab, N and S genes) of the SARS-CoV-2 genome and use a bacteriophage MS2 as internal control for PCR and extraction. In line with manufacturer’s instructions for use, a PCR test was deemed positive if the cycle threshold (Ct) value was < 40 for one or more target genes. The Ct value [8] was also reported and converted to viral concentrations (VCs) based on previous calibration [9]. Samples or PCR traces not meeting specified validity criteria (e.g., due to low sample volume, incorrect amplification curves or control probe not amplifying) were declared void. Processing technicians were not aware whether samples were collected by SS or AS.
Statistical analysis
To compare outcomes between SS and AS, contingency tables were constructed for each study and the pooled dataset. Subsequently, concordance and Cohen’s kappa coefficients were calculated, alongside positive and negative percentage agreement (PPV/NPV). Definitions of statistical measures are provided in Supplementary Table 1. Contingency tables were further assessed using McNemar’s tests. Additionally, void and positivity rates for SS and AS were calculated. For sensitivity derivation, participants were regarded as COVID-19 positive if either swab or both swabs showed a positive outcome. 95% CIs for proportions were derived using the Clopper–Pearson (exact) method and proportions were compared using two-sided chi-squared tests with Yates’ continuity correction. Where p-values were calculated for all studies simultaneously, adjustment for multiple testing (N = 6) using the Holm method was performed. Ct values for the three target genes were averaged and compared between SS and AS using paired t-tests and Spearman correlation. The probability of a concordant positive result of SS was modelled by logistic regression with SS Ct value, age group (18–40, 41–60 and ≥ 61 years), sex and previous swabbing experience as independent variables. Finally, a random-effects meta-analysis of the six studies was performed applying the R package meta [10]. Pooled estimates of rate or proportions (concordance, sensitivity, void rates) and rate differences were obtained using the inverse variance method and the DerSimonian–Laird estimator for between-study variance [11]. This approach gives weights to each study, which are the inverse of the variance of the rate estimates. For calculation of the pooled sensitivity, for example, larger studies with respect to number of positive cases (such as study 6) were given more weight than smaller studies (such as study 1). 95% CI were derived using the Clopper–Pearson method and heterogeneity was reported based on the I2 statistics [12]. Statistical analyses were conducted in R (version 4.2.1).
Results
Between April and October 2020, 3,253 individuals were recruited, of whom 211 withdrew consent after sample collection (Table 1 and Supplementary Fig. 1). Void results were recorded for 109 participants. Pooled void rates of SS and AS were 1.8% (95% CI: 0.9%, 3.7%) and 1.3% (95% CI: 0.6%, 3.0%) respectively (difference: 0.2%; 95% CI: −0.6%, 1.1%; Supplementary Fig. 2).
Valid paired PCR samples were recorded for 2,933 individuals included in further analysis. Participant age ranged 18–84 years, with median age of 37 years; individuals ≥ 61 years of age constituted 8% of the total population with available age information (Table 1). In the population with evaluable swabs, 49% of participants were females and 44% were males; no information on sex was available for 7% (Table 1). Full age and sex distributions of study participants are given in Supplementary Tables 2 and 3, respectively. Of the total recruited population, 29% had previous swabbing experience.
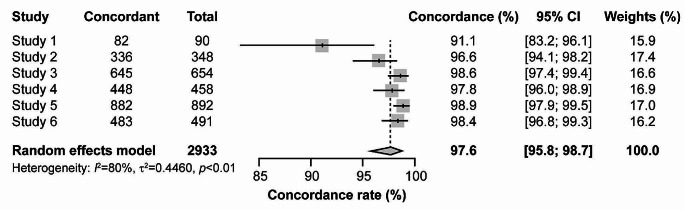
Concordance between SS and AS was > 90% for all studies, and ≥ 95% in all except Study 1 (Fig. 1).
Fig. 1.
Forest plot of rate of concordance between SS and AS
95% CI of single studies are shown as horizontal lines. Diamond indicates pooled estimate with left and right sides corresponding to lower and upper 95% CI.
AS, assisted swabbing; CI, confidence interval; SS, self-swabbing.
Pooled concordance across all studies was 98% (95% CI: 96%, 99%). Cohen’s kappa coefficients ranged 0.72–0.95, indicating substantial to almost perfect agreement (Supplementary Table 4). With respect to AS test results, SS showed a mean PPV of 87% and a mean NPV of 99%. Subgroup analysis indicated neither sex nor age had a statistically significant impact on concordance (Supplementary Tables 5 and 6).
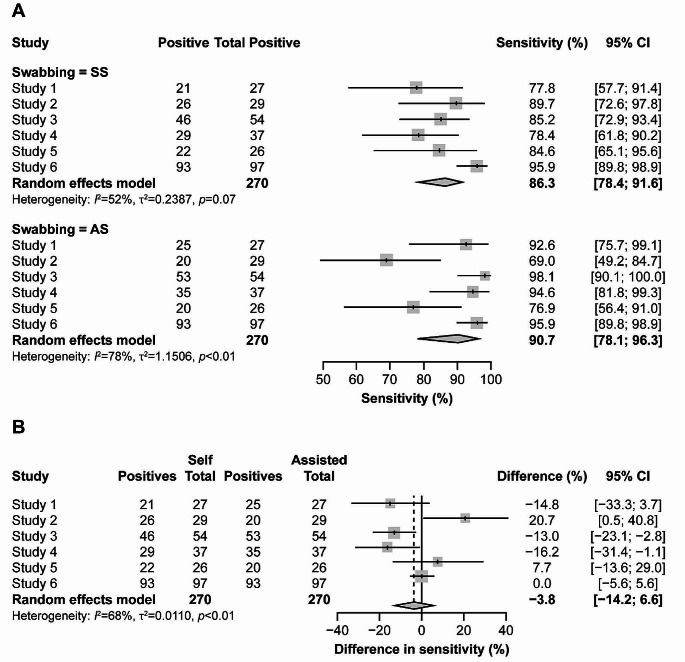
Positivity rates for SS (8.1%) and AS (8.4%) were not statistically significantly different (p = 0.70) (Supplementary Table 7); however, 10% and 13% of positive tests for SS and AS were negative for the other approach. Assuming any positive outcome by SS and AS is a true positive result, we derived a pooled sensitivity of 86% (95% CI: 78%, 92%) for SS and 91% (95% CI: 78%, 96%) for AS (Fig. 2A).
Fig. 2.
Forest plot comparing sensitivity in SARS-CoV-2 detection between SS and AS (a) Sensitivity of SS and AS across the six studies. (b) Differences in sensitivity between SS and AS. 95% CI of single studies are shown as horizontal lines. Diamond indicates pooled estimate with left and right sides corresponding to lower and upper 95% CI. Dashed line indicates pooled difference in sensitivity. AS, assisted swabbing; CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SS, self-swabbing
Heterogeneity was observed between studies, with studies 2 and 5 indicating higher sensitivity of SS and studies 1, 3 and 4 indicating higher sensitivity of AS. However, the meta-analysis showed pooled difference in sensitivity was not statistically significant (− 3.8%; 95% CI: −14.2%, 6.6%; Fig. 2B).
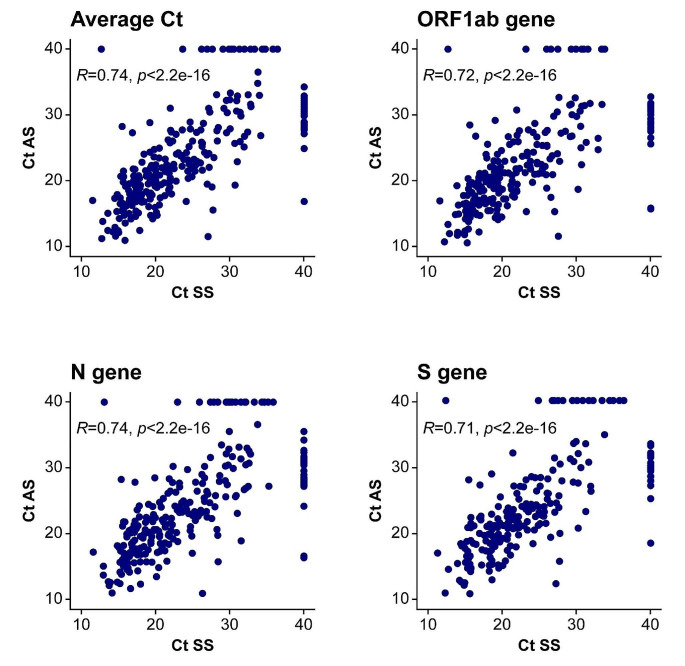
A Spearman coefficient > 0.7 between AS and SS was observed for average Ct values and those for individual PCR target genes (Fig. 3).
Fig. 3.
Scatterplots of Ct values measured in paired AS and SS samples For visualisation purposes, Ct values were set to 40 when SARS-CoV-2 was undetectable by qPCR in one of the paired samples. Thus, dots on the Ct = 40 horizontal or vertical lines represent samples which were positive either for the AS (vertical line) or SS (horizontal line) only. These values were not included for calculation of the Spearman correlation coefficient R and its significance. AS, assisted swabbing; Ct, cycle threshold; qPCR, quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SS, self-swabbing
Overall mean Ct values were 21.5 and 21.4 for SS and AS respectively (Supplementary Table 7) and paired Student’s t-test did not indicate statistically significant differences for the average Ct values or those of individual genes (Supplementary Fig. 3). Visualisation of Ct values of paired samples with only one positive test suggested the majority of discordant samples had high Ct values (Fig. 3). Of 33 positive AS samples with a paired negative SS sample, only two (6%) had a Ct value < 25; of 24 positive SS samples with a paired AS negative, only two (8%) had a Ct value < 25.
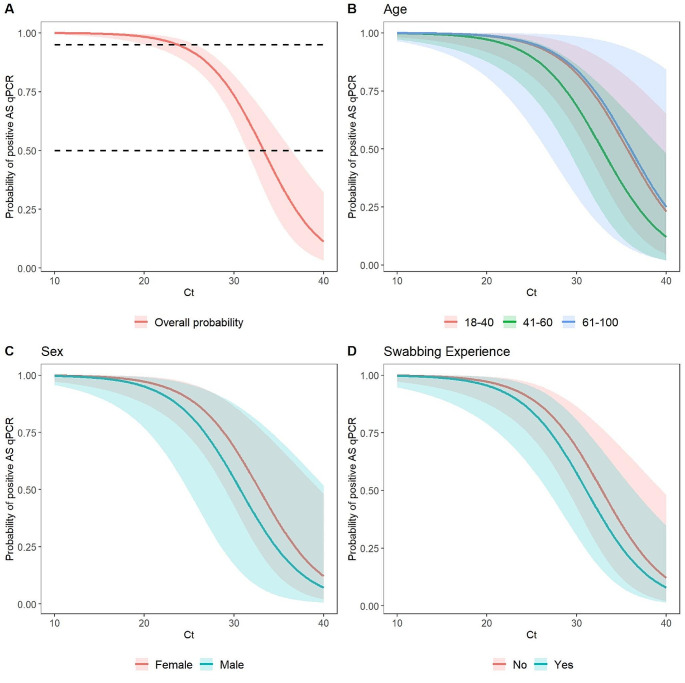
There was strong statistical evidence for the association between SS-based Ct value and positive AS outcome (p < 0.001), while age, sex and previous swabbing experience were not significant predictors of a positive AS-based outcome (Supplementary Table 8). The predicted probability of a positive AS outcome showed probability remained higher than 95% up to a Ct value of 20 and dropped below 50% only for Ct values > 30 (Fig. 4).
Fig. 4.
Predicted probability of a concordant positive AS-based SARS-CoV-2 test for a positive SS-based test, based on Ct value and stratified by age brackets (B), sex (C) and previous swabbing experience (D). Probabilities displayed were predicted based on the full cohort. Dashed lines indicate 50% and 95% probabilities (A). Probabilities were predicted for female participants with no previous swabbing experience (B), for participants 41–60 years of age and no previous swabbing experience (C), and for female participants 41–60 years of age (D). Shaded bands indicate the 95% CI. AS, assisted swabbing; CI, confidence interval; Ct, cycle threshold; qPCR, quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SS, self-swabbing
Discussion
Data collected from six studies during the early COVID-19 pandemic in the UK were used to assess suitability of swab-vial combinations as SS devices within the COVID-19 testing programme. The high concordance of SS and AS regarding test results, positivity rate or Ct values based on over 2,900 participants suggested SS did not disproportionately miss individuals with lower viral concentration which, hypothetically, may have been characteristic of poorer first-time user technique. These findings supported decision-making regarding the use of SS during the COVID-19 pandemic.
Study populations were well-balanced, with similar proportions of males and females recruited overall. There was no statistically significant difference in concordance rates when stratified by sex. When our studies were conducted, emphasis was on testing people from the UK National Health Service, other key workers and their household contacts, most of whom were working age. Most participants were 18–40 years of age, with ~ 1/3 being 41–60 years of age, and < 10% were ≥ 61 years of age. These demographics align with participant populations in similar studies [13–15]. There was no statistically significant difference in concordance between age groups, which aligns with a previous study of South Korean patients that reported older age did not affect concordance between SS and AS results [16].
Sensitivity results reported here align with those published previously. A 2020 analysis among participants in Washington, USA, found sensitivities of 94.0% (97.5% CI: 83.8%, 100%) and 96.2% (97.5% CI: 87.0%, 100%) for participant-collected nasal and mid-turbinate samples, respectively [17], overlapping with the pooled sensitivity rates for SS (86%) and AS (91%) we observed. Other studies report sensitivities for participant-collected nasal and oropharyngeal samples of 80–99%, respectively [13, 14, 18–20].
We observed correlation of Ct value for SS vs. AS (r = 0.74) within the range reported previously. Two studies conducted in the USA in 2020 reported Pearson correlations from 0.78 to 0.86 between AS nasopharyngeal and SS nasal and mid-turbinate swabs [17, 18]. An analysis conducted in Bangladesh in 2021 found correlations of 0.82 and 0.81 for the COVID-19 N and ORF1ab genes, respectively, between AS nasopharyngeal samples and SS nasal samples [13]. Other studies conducted in India and Denmark have reported weaker correlations (0.356 and 0.4534, respectively) [15, 19]. We also observed most discordant samples had high Ct values for either the AS or SS sample. Similar associations between Ct values/viral concentration and SS sensitivity have been reported in other studies [13, 15–18].
While efficacy and accessibility of SS had been demonstrated for other respiratory illnesses before the pandemic [21], there was uncertainty this would translate to SARS-CoV-2. It is notable that at the start of the pandemic, there were no PCR respiratory sample collection kits approved for SS identified by the UK national testing programme. Our results demonstrated it is possible for individuals to reliably self-swab to detect SARS-CoV-2. This directly led to policy changes regarding SARS-CoV-2 testing, resulting in larger in-person testing centre throughput, opening of a home testing channel and a mass asymptomatic self-testing programme. However, results from these studies were not used to select swab/vial combinations for widespread use; in these six studies, assessed swab/vial combinations were designed for professional use and repurposed for self-use without undergoing extensive usability studies. Other swab/vial combinations had to pass additional laboratory, clinical and other compatibility tests to be included in the national testing programme. It should be noted that clear instructions must be provided in a format the person presenting for testing can understand, and this should be (and was) considered when planning wider implementation.
Technologies developed to help diagnose COVID-19 have been applied elsewhere, with growth in self-testing kit availability for various pathogens [22–25]. Our findings demonstrating comparability between SS and AS provide further support for the perspective that self-sampling and self-testing are likely to become an increasingly important healthcare component, outside of pandemic responses. Self-sampling has the potential to impact on clinical care, for example it could aid in managing conditions for vulnerable individuals by offering convenience, reducing exposure risks, and empowering individuals to take control of their health. Results supporting a paradigm shift in self-sampling have also been reported in other studies examining various swabbing approaches [13–19, 26]. Additionally, the global market for healthcare-associated self-testing is valued at ~ 20 billion US$ and expected to reach > 39 billion US$ by 2030 [27]. While our study showed SS can replace AS for a large part of the public in the case of COVID-19 testing, it remains essential from a regulatory perspective to validate reliability and performance of SS for future applications through comprehensive studies like ours. Taken alongside other changes in healthcare provision observed during the pandemic, including widespread telehealth uptake, this suggests an increasing shift to home-based paradigms of healthcare [28].
This meta-analysis has several strengths. To our knowledge, this is the largest analysis to date comparing performance of SS versus AS in a real-world setting, with > 2,900 participants. This participant volume enabled development of detailed sub-group analyses and model-based approaches to detect any factor which influenced the outcome measures. Additionally, we assessed for potential effects of previous swabbing experience in participants, enabling statistical analysis of any association between first-time use and testing performance.
There were several limitations of this meta-analysis. Participation used a convenience sampling approach of only asking site attendees to be tested, leading to study populations that were not representative of the general population, but instead of those willing and able to attend COVID-19 testing sites. Individuals unable to consent were not included, including those who could not understand English. There was an ongoing programme of improvement incorporating user feedback throughout the testing programme to improve swabbing instructions and other aspects of user experience. While this was received positively, although this may have impacted on the comparability of studies, there was no evidence this affected performance over time. The presence of an observer may have impacted on generalisability of the self-swabbing performance; however, observers were instructed not to interfere with the self-swabbing process, minimising this risk. This study took place in the context of the early pandemic phase, utilising rapidly implemented testing infrastructure whose primary purpose was public health testing. No information was collected for people who declined to participate. The impact of the order of testing was not examined.
Conclusion
The results reported here support the use of SARS-CoV-2 SS as a viable alternative to AS and endorse the benefits of a broader self-testing strategy. Demonstrating that SS was a viable approach triggered a step change in the UK’s COVID-19 pandemic response, confirming mass testing is possible. Looking ahead, the positive results in these studies support the suggestion that self-sampling and self-testing are essential for pandemic preparedness and will become a standard requirement for healthcare services in general.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The views expressed are those of the author(s) and not necessarily those of the NIHR, UKHSA or the Department of Health and Social Care. We thank the staff of the Regional Testing Services, data managers and all participants in the six service evaluation studies. Particular acknowledgement is owed to Raghavendran Kulasegaran-Shylini, Mark Stockbridge, George Walker, Toi Neibler, Matt Rowe and Mark Hughes. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Edward Potts, Jin Heppell and Alexandra Newby of Ashfield MedComms, an Inizio company, and funded by the UKHSA.
Abbreviations
- AS
Assisted swabbing
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- Ct
Cycle threshold
- NHS
National Health Service
- NPV
Negative percentage agreement
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- PPV
Positive percentage agreement
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SS
Self-swabbing
- UK
United Kingdom
- VC
Viral concentration
Author contributions
This research was conceptualised by TF, DC, EB, PK, MS, TP, LD and SHi. Data curation was performed by MEF, ET and OK. Formal analysis was conducted by MEF, ET and SSC. Funding acquisition was secured by TF and EB. Investigation was conducted by MEF and SSC. Methodology was developed by TF, DC, MEF, SSC, AD, PK and MS. Project administration was the responsibility of TF. Provision of study resources was the responsibility of EB and SHo. The research was supervised by TF. Data visualisation was conducted by MEF. The original draft of this manuscript was developed by SAT and EB. Review and editing of subsequent drafts were conducted by all authors.
Funding
The project was funded by UKHSA’s predecessor organisation NHS Test and Trace (part of the Department of Health and Social Care).
Data availability
Data collected for the study, including de-identified individual participant data and a data dictionary defining each field in the set, will be available from NHS Digital’s Data Access Request Service with publication of this Article. Details of how to apply for access to the data via the Data Access Request Service are provided at https://digital.nhs.uk/services/data-access-request-service-dars.
Declarations
Competing interests
TF, MEF, SAT, EB, OK, SSC, AD, LD and SHo are employees of the UK Health Security Agency, which succeeded the NHS Test and Trace service in the United Kingdom. SHi is an employee of NHS England. MS is a consultant for Synnovis and has previously received consulting fees from CSL Seqirus, support for attending meetings and/or travel from AMP Europe and Velsera. SHo and TP are supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance (NIHR200915), a partnership between the UK Health Security Agency (UKHSA) and the University of Oxford. DC, ET and PK declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Department of Health and Social Care. Coronavirus (COVID-19): scaling up testing programmes (2020) https://www.gov.uk/government/publications/coronavirus-covid-19-scaling-up-testing-programmes (accessed 30 January 2024)
- 2.Department of Health and Social Care Government launches NHS Test and Trace service. 27 May 2020 2020. https://www.gov.uk/government/news/government-launches-nhs-test-and-trace-service (accessed 30 January 2024).
- 3.Department of Health and Social Care. Coronavirus testing extended to all essential workers in England who have symptoms (2020) https://www.gov.uk/government/news/coronavirus-testing-extended-to-all-essential-workers-in-england-who-have-symptoms (accessed 30 January 2024)
- 4.Department of Health and Social Care, The Rt Hon Matt Hancock MP. Health and Social Care Secretary’s statement on coronavirus (COVID-19): 2 April 2020 (2020) https://www.gov.uk/government/speeches/health-and-social-care-secretarys-statement-on-coronavirus-covid-19-2-april-2020 (accessed 30 January 2024)
- 5.Kurniawan SJ, Kaisar MMM, Kristin H, Ali S (2024) Comparable performance of antigen-detecting rapid test by healthcare worker-collected and self-collected swabs for SARS-CoV-2 diagnostic: a systematic review and meta-analysis. Rev Med Virol 34:e2492 10.1002/rmv.2492 [DOI] [PubMed] [Google Scholar]
- 6.Public Health England COVID-19: guidance for taking swab samples. How to take a combined throat and nose swab (postal version). 2 July 2020 2020. https://www.gov.uk/government/publications/covid-19-guidance-for-taking-swab-samples?msclkid=8159e4a1d07511ecb6443cc46c75522c (accessed 03 September 2023)
- 7.Gov UK (2020) COVID-19 home PCR test instructions. 27 June 2023 https://www.gov.uk/government/publications/testing-for-coronavirus-at-home/covid-19-home-pcr-test-instructions (accessed 03 September 2023).
- 8.UK Health Security Agency (2023) PCR testing for SARS-CoV-2 during the COVID-19 pandemic. https://www.gov.uk/government/publications/pcr-testing-for-sars-cov-2-during-the-covid-19-pandemic (accessed 30 January 2024).
- 9.Lee LYW, Rozmanowski S, Pang M et al (2022) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infectivity by viral load, S gene variants and demographic factors, and the utility of lateral Flow devices to prevent transmission. Clin Infect Dis 74:407–415 10.1093/cid/ciab421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzer G, Carpenter JR, Rücker G (2015) Meta-analysis with R. 1 ed. Springer Cham, Springer [Google Scholar]
- 11.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannan N, Raihan R, Parvin US et al (2022) Detection of SARS-CoV-2 RNA by reverse transcription-polymerase chain reaction (RT-PCR) on self-collected nasal swab compared with professionally collected nasopharyngeal swab. Cureus 14:e25618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therchilsen JH, von Buchwald C, Koch A et al (2020) Self-collected versus Healthcare Worker-Collected swabs in the diagnosis of severe Acute Respiratory Syndrome Coronavirus 2. Diagnostics (Basel) 10:678 10.3390/diagnostics10090678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mane A, Jain S, Jain A et al (2022) Diagnostic performance of patient self-collected oral swab (tongue and cheek) in comparison with healthcare worker-collected nasopharyngeal swab for severe acute respiratory syndrome coronavirus-2 detection. APMIS 130:671–677 10.1111/apm.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SJ, Jung J, Kim ES et al (2022) Diagnostic Performance, Stability, and usability of self-collected combo swabs and saliva for Coronavirus Disease 2019 diagnosis: a case-control study. Infect Chemother 54:517–528 10.3947/ic.2022.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu YP, Jennings R, Hart B et al (2020) Swabs collected by patients or Health Care workers for SARS-CoV-2 testing. N Engl J Med 383:494–496 10.1056/NEJMc2016321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCulloch DJ, Kim AE, Wilcox NC et al (2020) Comparison of Unsupervised Home Self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open 3:e2016382 10.1001/jamanetworkopen.2020.16382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horlyck S, Nielsen SH, Gress T et al (2023) Combined nasal- and oropharyngeal self-swab provides equivalent performance compared to professionally collected oropharyngeal swabs in detecting SARS-CoV-2 in a real-life setting. J Virol Methods 313:114667 10.1016/j.jviromet.2022.114667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hempel EM, Bharmal A, Li G et al (2024) Prospective, clinical comparison of self-collected throat-bilateral nares swabs and saline gargle compared to health care provider collected nasopharyngeal swabs among symptomatic outpatients with potential SARS-CoV-2 infection. J Assoc Med Microbiol Infect Dis Can 8:283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seaman CP, Tran LTT, Cowling BJ, Sullivan SG (2019) Self-collected compared with professional-collected swabbing in the diagnosis of influenza in symptomatic individuals: a meta-analysis and assessment of validity. J Clin Virol 118:28–35 10.1016/j.jcv.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 22.Bacon A, Wang W, Lee H et al (2023) Review of HIV Self Testing technologies and Promising approaches for the Next Generation. Biosens (Basel) 13:298 10.3390/bios13020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran NK, Albahra S, Rashidi H, May L (2023) Innovations in infectious disease testing: leveraging COVID-19 pandemic technologies for the future. Clin Biochem 117:10–15 10.1016/j.clinbiochem.2021.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budd J, Miller BS, Weckman NE et al (2023) Lateral flow test engineering and lessons learned from COVID-19. Nat Reviews Bioeng 1:13–31 10.1038/s44222-022-00007-3 [DOI] [Google Scholar]
- 25.Van Der Pol B (2023) Testing technologies as Enhancers of Disease intervention specialist activities: applying lessons from COVID-19. Sex Transm Dis 50:S53–S56 10.1097/OLQ.0000000000001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waggoner JJ, Vos MB, Tyburski EA et al (2022) Concordance of SARS-CoV-2 results in self-collected nasal swabs vs swabs collected by Health Care Workers in children and adolescents. JAMA 328:935–940 10.1001/jama.2022.14877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coherent Market Insights, SELF-TESTING MARKET, ANALYSIS (2022). https://www.coherentmarketinsights.com/market-insight/self-testing-market-4902 (accessed 31 January 2024).
- 28.Bestsennyy O, Chmielewski M, Koffel A, Shah A From facility to home: how healthcare could shift by 2025. 15/01/2024 2022. https://www.mckinsey.com/industries/healthcare/our-insights/from-facility-to-home-how-healthcare-could-shift-by-2025 (accessed 15 January 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study, including de-identified individual participant data and a data dictionary defining each field in the set, will be available from NHS Digital’s Data Access Request Service with publication of this Article. Details of how to apply for access to the data via the Data Access Request Service are provided at https://digital.nhs.uk/services/data-access-request-service-dars.