Abstract
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a major cause of salmonellosis, and the emergence of multidrug-resistant pathovariants has become a growing concern. Here, we investigate a distinct rough colony variant exhibiting a strong biofilm-forming ability isolated in China. Whole-genome sequencing on 2,212 Chinese isolates and 1,739 publicly available genomes reveals the population structure and evolutionary history of the rough colony variants. Characterized by macro, red, dry, and rough (mrdar) colonies, these variants demonstrate enhanced biofilm formation at 28 °C and 37 °C compared to typical rdar colonies. The mrdar variants exhibit extensive multidrug resistance, with significantly higher resistance to at least five classes of antimicrobial agents compared to non-mrdar variants. This resistance is primarily conferred by an IncHI2 plasmid harboring 19 antimicrobial resistance genes. Phylogenomic analysis divides the global collections into six lineages. The majority of mrdar variants belong to sublineage L6.5, which originated from Chinese smooth colony strains and possibly emerged circa 1977. Among the mrdar variants, upregulation of the csgDEFG operons is observed, probably due to a distinct point mutation (−44G > T) in the csgD gene promoter. Pangenome and genome-wide association analyses identify 87 specific accessory genes and 72 distinct single nucleotide polymorphisms associated with the mrdar morphotype.
Subject terms: Bacterial infection, Bacteria
In this study, the authors identify and characterize distinct Salmonella Typhimurium rough colony strains, likely originating from Chinese smooth colony strains around 1977 that predominantly belong to sublineage L6.5 and carry a specific csgD promoter mutation.
Introduction
Salmonella are among the most common enteric pathogens in humans and animals worldwide and cause typhoidal or nontyphoidal Salmonella (NTS) infections. NTS serovars, which can cause salmonellosis, are responsible for an estimated 153 million illnesses and 56,969 deaths among humans worldwide each year1, representing a significant global public health burden. Salmonella enterica serovar Typhimurium (S. Typhimurium), one of the most common NTSs, is a leading cause of human salmonellosis worldwide2,3. In the United States, S. Typhimurium is the most common Salmonella serovar associated with sporadic infections and foodborne outbreaks4. In Europe, S. Typhimurium is the second most common serovar isolated from humans5. In Asia, especially in China, S. Typhimurium is also among the most frequently encountered serovars5–7.
S. Typhimurium has shown remarkable genetic diversity and the ability to sense and respond to environmental stress. Mutation and evolution are primary drivers of its adaptation to environments8. Whole-genome sequencing technology has revolutionized Salmonella epidemiological research, enabling the identification of sequence types, the prediction of key genes, the tracking of transmission, the analysis of mutation evolution, and the examination of pathogen evolutionary patterns9,10. In recent decades, several dominant S. Typhimurium variants, including definitive phage types DT9, DT204, DT29, DT104, and DT193, the monophasic variant 1,4,[5],12:i:-, and invasive NTS (iNTS), have been associated with successive human salmonellosis epidemics2,3,11–14. Moreover, studies have investigated S. Typhimurium genomic epidemiology at global and regional levels and identified several phylogenetic lineages characterized by multilocus sequence types (STs), such as ST19, ST34, ST36, and ST3132–4,11–16. Recently, Salmonella enterica serovar 1,4,[5],12:i:-, a monophasic variant of S. Typhimurium belonging to ST34, has emerged as a major global cause of NTS disease in animals and humans. Whole-genome evolutionary analysis has shown that this variant evolved into an independent clone and acquired a new genomic island SGI-4, which conferred resistance to multiple antibiotics and the heavy metal copper. Additionally, gene insertion and deletion events have played important roles in the microevolution of this clone3. Furthermore, whole-genome analysis of ST313 African isolates has indicated the emergence of two different lineages from independent origins. Lineage I prevailed in the southwest region of Africa, while Lineage II prevailed in Malawi2. Later research identified a sub-lineage, II.1, in Congo, which carried IncHI1 plasmids that conferred resistance to azithromycin and extended-spectrum beta-lactamases (ESBLs)17. Point mutations in the promoter region of the virulence gene pgtE have been found to upregulate its expression in ST313, contributing to increased virulence and thus distinguishing it from ST19 in phenotype18. These dominant pathovariants and epidemic lineages exhibit distinct host ranges, niche adaptations, levels of pathogenicity, and risks to food safety. Their infections are associated with a higher probability of hospitalization and treatment failure, leading to prolonged infection and potential onward transmission. Therefore, they pose serious public health threats11,13,14,17,19. The emergence of new pathovariants is an ongoing process as new hosts or environmental niches arise because of natural processes or human intervention20. It has been reported that epidemic lineages dominate for 4–15 years before being replaced by a new dominant variant3,21.
In this work, we identify a rough colony variant that has become increasingly prevalent in China in recent years. These rough colony variants exhibit an enhanced biofilm-forming ability and extensive multidrug resistance (MDR), defined as resistance to at least five classes of antimicrobial agents. This indicates a potentially improved capacity for environmental persistence. To understand the evolutionary trajectory and genomic events mediating the microevolution of this variant, phylogenomic analyses are performed by whole-genome sequencing (WGS) of 2212 Chinese isolates contextualized with 1,739 publicly available isolates from multiple geographical regions. We determine a novel phylogenetic lineage characterized by the widespread presence of these rough colony variants. This lineage primarily consists of strains isolated in China and likely originating from Chinese smooth colony strains around 1977. We find that 87 specific accessory genes and 72 distinct single nucleotide polymorphisms (SNPs) are associated with the rough colony variants through pangenome and genome-wide association studies. Phenotypic and molecular investigations are also performed to determine the key factors contributing to the emergence and establishment of this variant. We find that these rough variants show increased expression of csgDEFG operons, due to a mutation in the csgD gene promoter. To avoid potential confusion, in our study, the term “rough colony variants” refers to strains that exhibit a distinct wrinkled morphology when inoculated and cultured on the modified Swarmagar medium. These strains demonstrate a strong biofilm-forming ability as observed through Congo red assays. The term “novel lineage” refers to the L6.5 lineage identified in the phylogenetic analysis.
Results
Emergence and prevalence of a variant of S. Typhimurium with a rough colony morphology
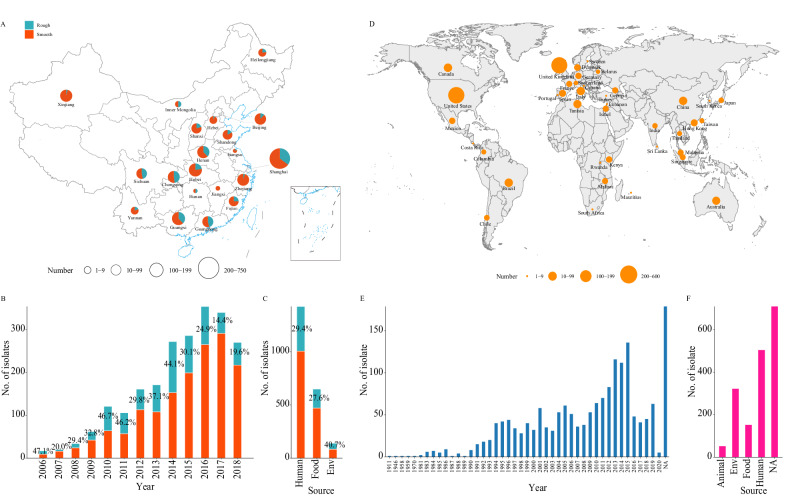
We collected 2212 S. Typhimurium isolates from 20 cities or provinces spanning different geographic regions of China (Northeast, Northwest, Southeast, Southwest, North, South, and Central China) from 2006 to 2018; these isolates were recovered from diverse sources, including humans, food and the environment (Fig. 1A–C, Table 1). We identified a variant of S. Typhimurium with a rough colony morphology. Overall, this variant accounted for 29.6% of all Chinese S. Typhimurium isolates and has demonstrated a persistent prevalence in recent years (Fig. 1B, Table 1). This variant was isolated from 19 of the 20 monitored regions of China and was mainly distributed in the East, South, Southwest, and Central regions of China (Fig. 1A, Table 1). A total of 654 strains exhibiting the rough colony variant were successfully identified and isolated from various samples, including humans (419, 64.1%), food (178, 27.2%), and environment (57, 8.7%). Furthermore, it was observed that the prevalence of the rough colony variant significantly varied across different isolation sources, with environmental sources (40.7%) exhibiting a higher proportion compared to human sources (29.4%) and food sources (27.6%) (P < 0.05) (Fig. 1C, Table 1).
Fig. 1. Distribution of the global S. Typhimurium isolates used in this study.
A Regional distribution of the 2,212 Chinese S. Typhimurium isolates identified in this study. The size of each circle represents the number of strains. The base map was generated using publicly accessible data from the Tianditu Administrative Division Service Center (https://cloudcenter.tianditu.gov.cn/administrativeDivision/). B Temporal distribution of the Chinese S. Typhimurium isolates. C Sources of the Chinese S. Typhimurium isolates. The red and green colors represent the smooth and rough colony strains, respectively. D Geographic distribution of the 1739 S. Typhimurium isolates from the NCBI. The world map data were sourced from the maps package in the R programming language (https://rdocumentation.org/packages/maps). E Temporal distribution of the NCBI S. Typhimurium isolates. F Sources of the NCBI S. Typhimurium isolates. Source data are provided as a Source Data file. Env environment, NA not available.
Table 1.
Characteristics of the Chinese S. Typhimurium strains in this study
| Characteristic | Total (n = 2212) | Rough (n = 654) | Smooth (n = 1558) |
|---|---|---|---|
| Year, n (%) | |||
| 2006 | 17 (0.8) | 8 (1.2) | 9 (0.6) |
| 2007 | 20 (0.9) | 4 (0.6) | 16 (1.0) |
| 2008 | 34 (1.5) | 10 (1.5) | 24 (1.5) |
| 2009 | 61 (2.8) | 20 (3.1) | 41 (2.6) |
| 2010 | 120 (5.4) | 56 (8.6) | 64 (4.1) |
| 2011 | 106 (4.8) | 49 (7.5) | 57 (3.7) |
| 2012 | 161 (7.3) | 48 (7.3) | 113 (7.3) |
| 2013 | 170 (7.7) | 63 (9.6) | 107 (6.9) |
| 2014 | 270 (12.2) | 119 (18.2) | 151 (9.7) |
| 2015 | 286 (12.9) | 86 (13.1) | 200 (12.8) |
| 2016 | 357 (16.1) | 89 (13.6) | 268 (17.2) |
| 2017 | 340 (15.4) | 49 (7.5) | 291 (18.7) |
| 2018 | 270 (12.2) | 53 (8.1) | 217 (13.9) |
| Region, n (%) | |||
| Central China | 276 (12.5) | 86 (13.1) | 190 (12.2) |
| Eastern China | 1028 (46.5) | 289 (44.2) | 739 (47.4) |
| Northeast China | 39 (1.8) | 9 (1.4) | 30 (1.9) |
| Northern China | 214 (9.7) | 32 (4.9) | 182 (11.7) |
| Northwest China | 117 (5.3) | 5 (0.8) | 112 (7.2) |
| Southern China | 312 (14.1) | 133 (20.3) | 179 (11.5) |
| Southwest China | 226 (10.2) | 100 (15.3) | 126 (8.1) |
| Source, n (%) | |||
| Human | 1426 (64.5) | 419 (64.1) | 1007 (64.6) |
| Food | 646 (29.2) | 178 (27.2) | 468 (30.1) |
| Environment | 140 (6.3) | 57 (8.7) | 83 (5.3) |
| Sex, n (%) | 1426 | 419 | 1007 |
| Female | 551 (38.6) | 163 (38.9) | 388 (38.5) |
| Male | 763 (53.5) | 244 (58.2) | 519 (51.5) |
| NA | 112 (7.9) | 12 (2.9) | 100 (9.9) |
| Age in years, n (%) | 1426 | 419 | 1007 |
| 0–6 | 819 (57.4) | 303 (72.3) | 516 (51.2) |
| 7–17 | 47 (3.3) | 17 (4.1) | 30 (3.0) |
| 18–40 | 195 (13.7) | 36 (8.6) | 159 (15.8) |
| 41–65 | 167 (11.7) | 38 (9.1) | 129 (12.8) |
| ≥66 | 82 (5.8) | 13 (3.1) | 69 (6.9) |
| NA | 116 (8.1) | 12 (2.9) | 104 (10.3) |
NA not available.
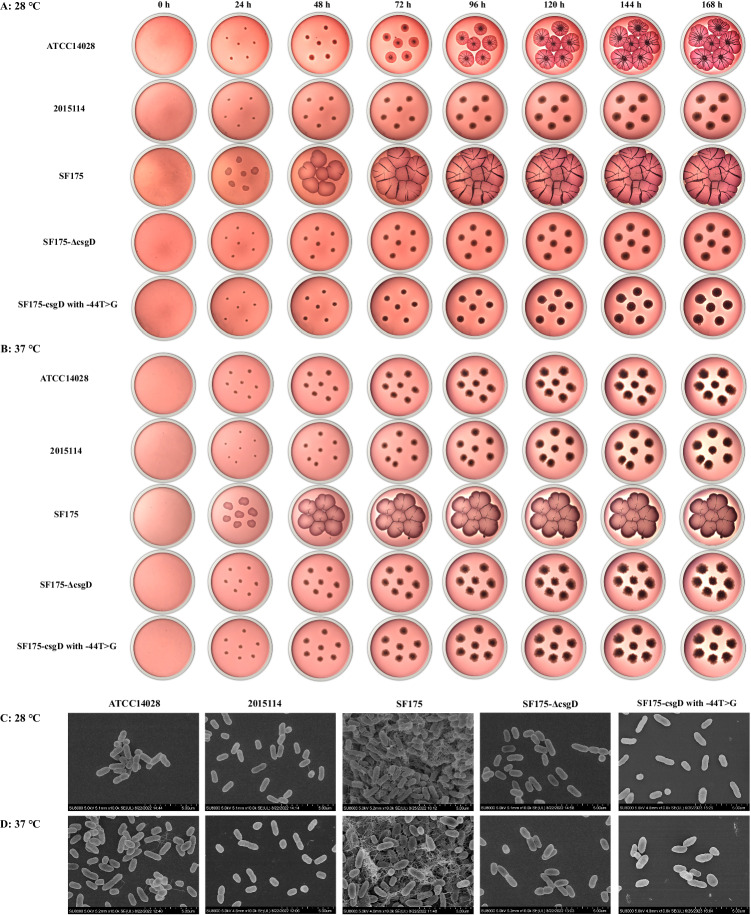
When the strains were inoculated onto a modified Swarmagar medium for cultivation, the rough colonies exhibited a film-like and wrinkled morphology, while the control S. Typhimurium (ATCC 14028) did not display such morphology characteristics. When incubated at 28 °C on Luria-Bertani (LB) agar containing Congo red and Coomassie blue without salt, the control S. Typhimurium (ATCC 14028) formed a typical red, dry, and rough (rdar) colony with the formation of concentric rings and a wrinkled appearance after 3 days. In comparison, the rough variants identified in this study (represented by SH15SF175, hereafter abbreviated as SF175) appeared as macro-rdar colonies (designated as mrdar colonies) with more pronounced wrinkles, while the smooth colony strains (represented by 2015114) formed small and white (saw) colonies with no obvious corrugations or wrinkles (Fig. 2A). Of particular note, the rough colony variants remained mrdar-positive with wrinkles at 37 °C, while the control ATCC 14028 and the smooth 2015114 isolates were rdar-negative (Fig. 2B). Furthermore, over extended cultivation periods, the mrdar colony variants developed increasingly larger colonies with more pronounced wrinkles than the control ATCC 14028 strain and the smooth isolates (Fig. 2A, B). Scanning electron microscopy (SEM) examinations revealed that the SF175 mrdar colony formed thick layers of cells that were densely embedded in extracellular polymeric substances (EPSs) with the more extensive formation of the cross-linked fibril matrix (Fig. 2C, D). The EPS and fibrillar network formed a highly resilient structure that appeared to bind the cells together. Notably, at 28 °C, these structures appeared to be thicker and denser compared to those at 37 °C. The control strain and the smooth 2015114 isolate formed monolayers of cells under the same conditions, and these cells were separated from each other.
Fig. 2. Morphology of the rough and smooth colonies of the representative S. Typhimurium isolates in this study.
In this study, the rough colony strain SF175, smooth colony strain 2015114, control strain ATCC 14028, and mutants SF175-ΔcsgD and SF175-csgD with -44 T > G were selected as representatives to observe the colony morphology. A, B Colony morphology of the representative S. Typhimurium isolates grown for seven consecutive days on Luria-Bertani agar containing Congo red and Coomassie blue without salt at 28 °C and 37 °C. C, D Scanning electron micrographs of the representative S. Typhimurium isolates cultured for 24 h at 28 °C and 37 °C. Representative scanning electron micrograph for one replicate. Scale bar, 5 mm. Source data are provided as a Source Data file.
Biofilm formation
Crystal violet biofilm assays and pellicle formation assays showed that the mrdar variants examined in this study had greater biofilm-forming ability than the smooth variants; for example, the mrdar colony from SF175 generated significantly more biofilm than the control ATCC strain and strain 2015114 at both 28 and 37 °C (Fig. S1, S2, S3). Moreover, confocal laser scanning microscopy (CLSM) observations showed that the biofilm of the mrdar colony morphotype had more EPSs (green color) than the smooth morphotype and ATCC 14028. Overall, the mrdar variants exhibited greater biofilm-forming ability at 28 °C than at 37 °C (Fig. S1).
Antimicrobial resistance (AMR) analysis of the mrdar variants of S. Typhimurium
The antimicrobial susceptibility testing revealed that the Chinese S. Typhimurium isolates exhibited extensive AMR profiles (Table 2). The majority of these isolates exhibited MDR, with 79.5% demonstrating resistance to three or more Clinical and Laboratory Standards Institute (CLSI) classes, including MDR phenotypes such as ASSuT (45.5%, resistance to at least ampicillin, streptomycin, sulfisoxazole, and tetracycline) and ACSSuT (22.0%, resistance to at least ampicillin, chloramphenicol, streptomycin, sulfisoxazole, and tetracycline) (Table 2). Notably, the mrdar variants exhibited higher rates of resistance to multiple antibiotics than the smooth colony isolates, particularly for older antibiotics, including tetracycline (90.5% vs. 71.1%), ampicillin (89.4% vs. 70.7%), sulfisoxazole (93.7% vs. 68.5%), nalidixic acid (95.3% vs. 34.3%), chloramphenicol (71.6% vs. 36.4%), trimethoprim/sulfamethoxazole (58.3% vs. 31.3%), and gentamicin (57.3% vs. 17.0%). A clear difference in MDR was also observed among the rough and smooth colony isolates, including those with resistance to ≥3 classes (95.4% vs. 72.8%), ≥4 classes (92.5% vs. 65.7%), ≥5 classes (83.9% vs. 28.9%) and ≥6 classes (58.1% vs. 14.8%) and the ACSSuT phenotype (34.6% vs. 16.8%) (Table 2).
Table 2.
Antimicrobial resistance of the Chinese S. Typhimurium strains in this study
| Antimicrobial | Antimicrobial resistance (%) | P value* | ||
|---|---|---|---|---|
| Total (n = 2212) | Rough (n = 654) | Smooth (n = 1558) | ||
| Ceftriaxone | 15.6 | 17.7 | 14.7 | 0.087 |
| Tetracycline | 76.7 | 90.5 | 71.1 | 9.384E-23 |
| Ceftiofur | 16.0 | 17.7 | 15.2 | 0.163 |
| Cefoxitin | 3.7 | 2.4 | 4.2 | 0.063 |
| Gentamicin | 28.9 | 57.3 | 17.0 | 8.329E-81 |
| Ampicillin | 76.2 | 89.4 | 70.7 | 3.725E-21 |
| Chloramphenicol | 46.8 | 71.6 | 36.4 | 2.197E-51 |
| Ciprofloxacin | 6.2 | 4.0 | 7.2 | 0.006 |
| Trimethoprim/sulfamethoxazole | 39.3 | 58.3 | 31.3 | 4.450E-32 |
| Sulfisoxazole | 75.9 | 93.7 | 68.5 | 1.573E-36 |
| Nalidixic acid | 52.3 | 95.3 | 34.3 | 4.454E-150 |
| Streptomycin | 54.2 | 56.0 | 53.4 | 0.272 |
| Azithromycin | 9.7 | 9.2 | 9.9 | 0.651 |
| Amoxicillin/clavulanic | 2.9 | 3.2 | 2.8 | 0.730 |
| ≥3 CLSI classes | 79.5 | 95.4 | 72.8 | 4.240E-21 |
| ≥4 CLSI classes | 73.6 | 92.5 | 65.7 | 0.009 |
| ≥5 CLSI classes | 45.2 | 83.9 | 28.9 | 1.706E-65 |
| ≥6 CLSI classes | 27.6 | 58.1 | 14.8 | 2.223E-95 |
| ASSuT | 45.5 | 49.7 | 43.8 | 0.011 |
| ACSSuT | 22.0 | 34.6 | 16.8 | 7.111E-20 |
| ACSSuTCRO | 5.8 | 6.1 | 5.6 | 0.935 |
| ACSSuTCIP | 4.1 | 2.0 | 5.0 | 0.002 |
| ACSSuTAZI | 3.7 | 4.1 | 3.5 | 0.533 |
Two-sided chi-square tests were performed without adjusting for multiple comparisons.
CLSI Clinical and Laboratory Standard Institute, ASSuT resistance to ampicillin, streptomycin, sulfamethoxazole/sulfisoxazole, and tetracycline, ACSSuT resistance to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole/sulfisoxazole, and tetracycline, CRO ceftriaxone, CIP ciprofloxacin, AZI azithromycin.
*P < 0.05 denotes that there is a clear significant difference in resistance to the corresponding antimicrobials.
Population structure of global S. Typhimurium, including the Chinese isolates
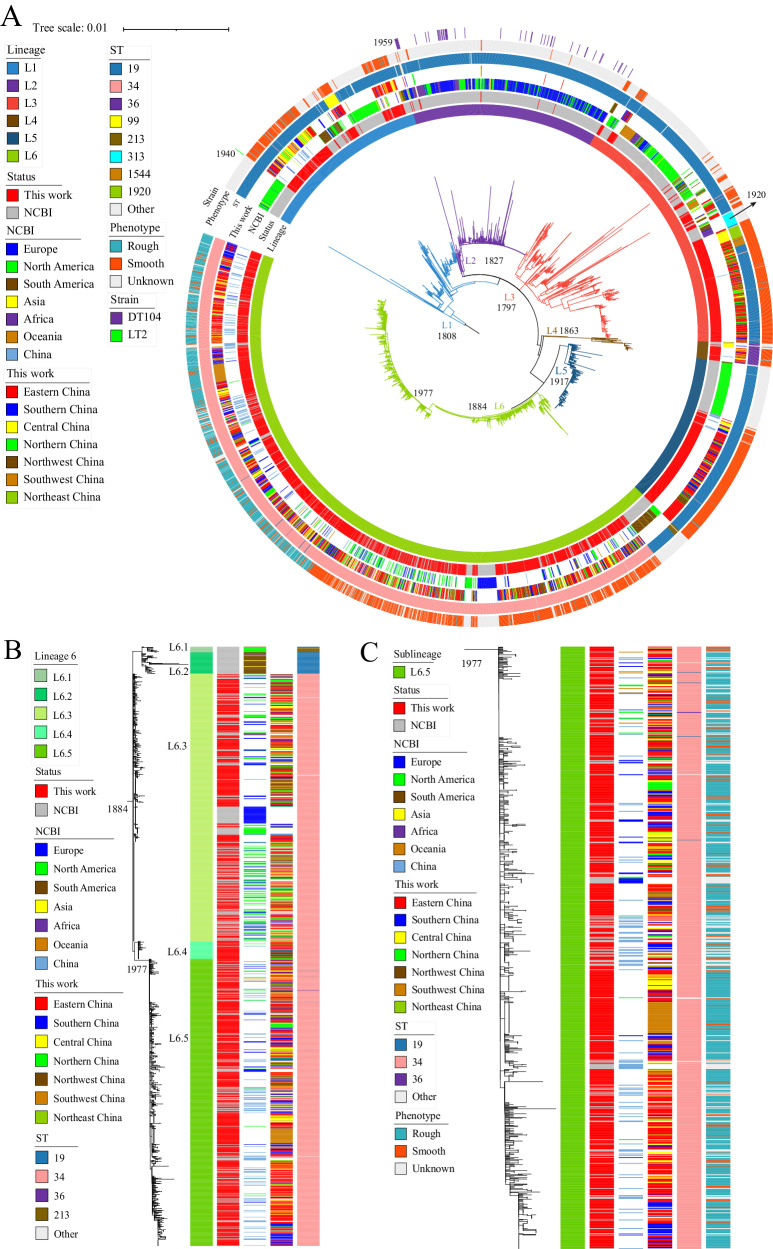
The maximum likelihood (ML) phylogenetic tree was constructed based on 85,508 core SNPs identified from a dataset of 3951 genomes. This dataset comprised 2212 strains that we sequenced and analyzed ourselves, and 1739 strains whose sequencing data were downloaded from the NCBI Salmonella Typhimurium Genome database (Supplementary Data 1). We downloaded all available WGS data of Salmonella Typhimurium from the NCBI database in August 2021, and after excluding records with incomplete strain background information, such as location and collection dates, we were left with data for 1739 strains. The cgMLST analysis showed that the rough colony strains formed distinct clusters separate from other branches, suggesting considerable genetic divergence (Fig. S4).
The global S. Typhimurium populations, rooted with S. Paratyphi A270 (accession ERR326600), could be divided into six distinct lineages (L1 to L6) in hierBAPS analysis. Among these lineages, L6 was the largest lineage, and L4 was the smallest (Fig. 3A). The multilocus sequence typing (MLST) analysis showed that the global S. Typhimurium populations contained two predominant STs, ST19 (52.3%) and ST34 (42.3%) (Fig. 3A, Supplementary Data 2). ST19 was mainly distributed in Lineages L1, L2, L3, and L5, ST34 was mainly distributed in Lineage L6, and the isolates in Lineage L4 mainly belonged to ST36. ST19 (73.4%) was the main ST among the NCBI sequences, but ST34 (59.3%) was the main ST among the Chinese isolates in this study (Supplementary Data 2).
Fig. 3. Population structure of the 3951 S. Typhimurium genomes analyzed in this study.
A Maximum likelihood phylogeny was performed on 3,951 global S. Typhimurium genomes, including the 2212 Chinese strains in this study (marked in red) and 1739 public genomes from the NCBI (marked in gray). The population structure was estimated using hierBAPS, and lineages or sublineages are labeled with different colors as LX or LX.Y, where X and Y are the lineage and sublineage numbers, respectively. The rings, from inner to outer, labeled with different colors indicate the lineages, sublineages, geographical origin, isolation regions, sequence types, and colony phenotypes. B Maximum likelihood tree of S. Typhimurium Lineage L6. C Maximum likelihood tree of S. Typhimurium sublineage L6.5. Divergence dates are given on the major nodes in the tree. Source data are provided as a Source Data file. ST sequence type.
All lineages appeared to have a global distribution (Fig. 3A, Fig. S5). Organisms from North America were widely distributed across all six lineages, indicating their basal position within the current S. Typhimurium populations. Although the Chinese isolates in this study were also present in all six lineages, they were primarily observed in Lineage L6 (59.0%). The Chinese organisms in each lineage were clustered together and formed locally established groups that likely originated from abroad, predominantly from North America (Fig. 3A, B, Fig. S5).
From the rooted phylogenetic tree and BEAST analysis, we estimated that the current S. Typhimurium population descended from a most recent common ancestor (MRCA) that existed circa 1696 (95% confidence interval [CI]: 1686–1706). It then diversified into six distinct lineages during the 1800s (L1), 1820s (L2), 1790s (L3), 1860s (L4), 1910s (L5), and 1880s (L6) (Fig. 3A, Fig. S6). These findings suggest that the current S. Typhimurium population descended from a common ancestor and has successfully dispersed and evolved into several phylogenetic lineages that are now circulating globally. The locally established Chinese groups can probably be traced back to ancestors from abroad, especially from North America.
Phylogenetic analysis of the mrdar variants of S. Typhimurium
To determine the phylogeny of the mrdar variants, a separate ML tree was constructed based on 21,168 core SNPs identified from 1754 isolates of Lineage L6 (Fig. 3B). The L6 lineage, a recently derived clade that developed circa 1884 (95% CI: 1874–1894), comprised 44.4% of all isolates in this study. Among the 654 mrdar colony variants, the majority were typed as ST34 (621, 95.0%) and located in Lineage L6 (625, 95.6%), particularly sublineage L6.5 (614, 93.9%). It was estimated that the mrdar variants of L6.5 could be traced back to 1977 (95% CI: 1966–1987) and likely originated from the smooth phenotype of S. Typhimurium in China (Fig. 3B). Based on the regional distribution data, the sublineage L6.5 was primarily composed of strains from China, including strains from various regions within the country. Additionally, this sublineage included strains from other parts of the world, such as Europe (23 isolates), North America (11 isolates), Oceania (four isolates), and South America (one isolate) (Fig. 3C, Supplementary Data 1).
Distribution of the AMR determinants among the global S. Typhimurium lineages
We examined the presence of genetic determinants of AMR, including the acquired resistance gene content and the presence of QRDR mutations22. A total of 152 different AMR determinants were identified, and each of the isolates contained numerous AMR determinants (ranging from 20 to 50; Fig. S7A–C, Supplementary Data 1). The six lineages contained different contents of AMR determinants, and the isolates in Lineage L6 contained more AMR determinants than those in other lineages (Fig. S7A, Fig. S8). Moreover, the mrdar variants carried more AMR determinants than the smooth colony isolates (Fig. S7B), and the mrdar variants in Lineage L6 carried more AMR determinants than the smooth colony strains in L6 (Fig. S7C). In particular, the rough colony variants in sublineage L6.5 carried more AMR determinants, including 12 aminoglycoside resistance genes (AAC(3)-IV, AAC(6’)-Ib7, AAC(6’)-Ib-cr6, aadA1, aadA2, aadA3, aadA12, aadA25, strA, strB, APH(3’)-Ia, and APH(4)-Ia), three beta-lactam resistance genes (blaOXA-1, blaTEM-1, and blaTEM-60), two efflux pump-mediated genes with decreased susceptibility to disinfectants (qacEdelta1 and qacL), three phenicol resistance genes (catB3, floR, and cmlA1), one rifamycin resistance gene (arr-3), three sulfonamide resistance genes (sul1, sul2, and sul3), two tetracycline resistance genes (tetB and tetR), one trimethoprim resistance gene (dfrA12), and two quinolone resistance genes (oqxA and oqxB) (Fig. S8). The differences in resistance phenotypes between mrdar variants and smooth strains were largely due to the presence of specific resistance genes. For example, aminoglycoside resistance genes can confer resistance to gentamicin, and beta-lactam resistance genes can confer resistance to ampicillin. Moreover, the identification of resistance genes such as floR and arr3 suggests that there may be differences in resistance to other antibiotics that have not been tested yet, such as fluphenicol and rifampicin. Resistance to fluoroquinolones is commonly associated with point mutations in quinolone resistance-determining regions (QRDRs) of the gyrA, gyrB, parC, and parE genes23. The QRDR mutations were mainly observed in Chinese strains, particularly within sublineages L6.5 and L1.3. Within sublineage L6.5, the mrdar variants mostly harbored the D87N or D87Y mutation in the gyrA gene, while sublineage L1.3 strains, characterized by their smooth morphology, carried mutations not only in the gyrA gene (S83L and/or D87N) but also in the parC gene (S80R) (Fig. S8). The presence of double or triple mutations in the QRDR is correlated with a high level of resistance to ciprofloxacin, and single mutations are associated with decreased sensitivity to this antibiotic24. Consequently, compared to smooth colony strains, the mrdar variants exhibited a lower resistance rate (3.98% vs. 7.18%) and a higher intermediate rate (10.70% vs. 2.50%) to ciprofloxacin.
Distribution of virulence factors (VFs) among global S. Typhimurium lineages
A total of 265 different virulence genes were identified from the VF database (VFDB), and each of the isolates contained numerous VFs (ranging from 61 to 264; Supplementary Data 1). The six lineages contained different contents of virulence genes, with the isolates in Lineage L2 harboring the most, followed by L3, L1, L5, L6, and L4 (Fig. S7D, Fig. S9). Furthermore, we found that some VFs were associated with the S. Typhimurium lineages; for example, the plasmid-encoded fimbriae gene pefABCD, the type III secretion system (T3SS)-secreted effector gene spvBCD (Salmonella pathogenicity island 2 [SPI-2] encoding), and genes mig-5 and rck were present in the strains in Lineages L2 and L3 and some of the strains in Lineage L1 but absent in the strains in Lineages L4, L5 and L6 (Fig. S9). Overall, mrdar variant strains had a lower presence of virulence genes compared to non-variant strains (Fig. S7E). Nevertheless, within the L6 lineage, mrdar variants showed a higher abundance of virulence genes compared to smooth isolates (Fig. S7F). Additionally, the mrdar variants in sublineage L6.5 primarily carried the fljAB genes (Fig. S9).
Identification of AMR- and virulence-associated plasmids
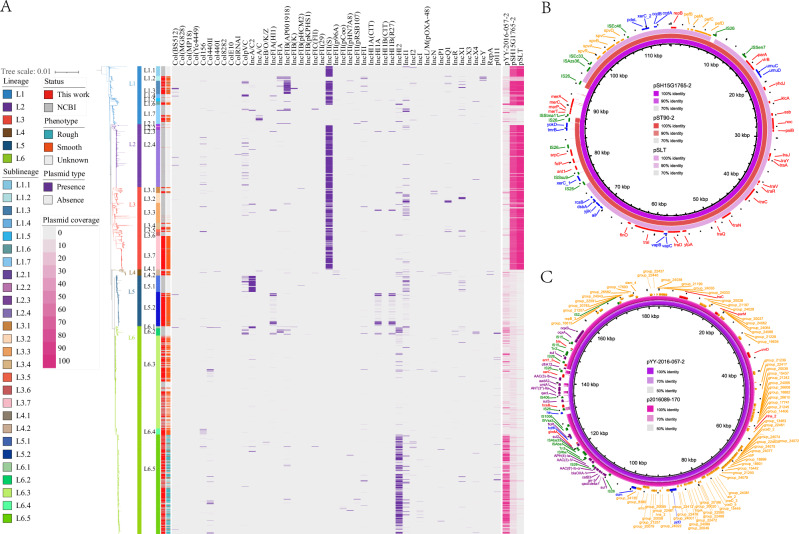
The plasmid replicon prediction results showed that a total of 47 plasmid replicons were predicted. The IncFII(S) replicon was present in lineages L1, L2, and L3, and the IncHI2 replicon was detected in sublineage L6.5, where the rough colony strains were mainly distributed (Fig. 4A). The combination of Illumina and Nanopore sequencing data led to the generation of two complete plasmids, specifically pSH15G1765-2 and pYY-2016-057-2 (Fig. 4B, C). The plasmid pSH15G1765-2, which belonged to the IncFII(S) replicon and measured 114,289 bp in size, was prevalent in lineages L1, L2, and L3. This plasmid was highly similar to plasmid pST90-2 (accession CP050736) identified from a Chinese S. Typhimurium in 2012 and the virulence plasmid pSLT (accession AE006471)25. Similar to pSLT and pST90-2, the plasmid pSH15G1765-2 carried the virulence genes pefABCD, spvBCD, and rck (Fig. 4B); compared with these two plasmids, pSH15G1765-2 acquired ISStma11 and the mercury resistance (mer) operon merTPCA, which is associated with mercury resistance26. The plasmid pYY-2016-057-2, which was frequently found in the L6.5 sublineage, measured 183,430 bp and belonged to the IncHI2 replicon. Its structure was similar to that of plasmid p2016089-170 (accession CP090536), which was previously identified from a Salmonella 4,[5],12:i:- strain in China. The pYY-2016-057-2 plasmid carried 19 AMR genes, sul1, qacEdelta1, arr-3, catB3, blaOXA-1, aac(6’)-Ib-cr, aac(3)-IV, aph(4)-Ia, sul2, floR, sul3, qacL, ANT(3”)-IIa, cmlA1, aadA2, AAC(3)-Ib, dfrA12, oqxA, and oqxB (Fig. 4C). Compared with p2016089-170, pYY-2016-057-2 acquired sul3, qacL, ANT(3”)-IIa, cmlA1, aadA2, AAC(3)-Ib, and dfrA12.
Fig. 4. Profiles and comparisons of plasmids associated with antimicrobial resistance and virulence factor genes.
A Plasmid profiles among the global S. Typhimurium isolates. The bands to the left of the heatmap indicate the lineage, sublineage, source, and colony morphology of the strains. Purple stripes represent the presence of different plasmid replicons, and red stripes represent the presence of the complete plasmids identified in this study. A total of 47 plasmid replicons were predicted, and two distinct plasmids harboring antimicrobial resistance or virulence factor genes were identified by a combination of Nanopore long-read sequencing and Illumina short-read sequencing. B, C Pairwise comparisons of two distinct, complete plasmids identified in this study. The plasmid pSH15G1765-2 belonging to the IncFII(S) replicon (114 kb in size) is highly similar to the plasmid pST90-2 (accession CP050736) identified from a Chinese S. Typhimurium strain in 2012 and the virulence plasmid pSLT (accession AE006471) from S. Typhimurium strain LT2. pYY-2016-057-2, belonging to the IncHI2 replicon (183 kb in size), is similar to a previously reported plasmid p2016089-170 (accession CP090536) identified from a Salmonella 4,[5],12:i:-strain in China. Source data are provided as a Source Data file.
Pangenome analysis
Pangenome analysis based on the 3951 isolates identified a total of 41,024 genes, including 1821 core genes, 1453 soft-core genes, 2094 shell genes, and 35,636 cloud genes (Fig. S10, Fig. S11). Among the accessory genes, 89 specific genes that are significantly associated with the S. Typhimurium lineages with P values below 1e-300 were selected, of which 87 were distributed in the rough isolates in Lineage L6.5 (including addA, alx_2, dam_4, hha_2, hipA, hns_2, pcoC, repB_3, smc, stiP, yceD_2, yceD_3, yceD_5) and 74 were hypothetical protein genes, while the remaining two genes, dsbD_2 and dcuA_2, were distributed in the smooth isolates in other lineages (Fig. S11A, Supplementary Data 3). Notably, 78 of the significantly associated accessory genes such as alx_2, hha_2, hipA, hns_2, repB_3, smc, stiP, yceD_2, yceD_3, yceD_5 were located in the pYY-2016-057-2 plasmid (Fig. 4C). Clusters of Orthologous Groups (COG) functional classification showed that 29 genes mapped to 32 COG clusters, which are mainly involved in cell cycle control, cell division and chromosome partitioning, signal transduction mechanisms, coenzyme transport and metabolism, and replication, recombination, and repair (Fig. S11B).
Genome-wide association study (GWAS)
GWAS analysis identified a total of 72 SNPs significantly (−log10(P) > 200) associated with the rough colony variants, of which 65 were located within protein-coding regions and seven within intergenic regions. Among the coding region SNPs, 31 (43.1%) resulted in missense mutations, one (1.4%) was a nonsense mutation, and 33 (55.6%) were synonymous variants (Fig. S12A, Supplementary Data 4). COG analysis showed that the nonsynonymous SNPs affected 31 genes in 35 COG function classes, and these were mainly associated with amino acid transport and metabolism, transcription, cell wall/membrane/envelope biogenesis, inorganic ion transport and metabolism, and intracellular trafficking, secretion, and vesicular transport (Fig. S12B, Supplementary Data 4).
Multi-omics analysis of the rough and smooth colony strains
To explore the potential genetic variations mediating morphotype conversion, multi-omics, including genomic and proteomic sequencing, was performed based on the 20 rough and 20 smooth selected colony organisms (Supplementary Data 5). WGS analysis showed that the 20 rough colony isolates clustered together with some differential core SNPs compared to the 20 smooth colony organisms (Fig. 5A). No obvious recombination events were identified among the 20 rough and 20 smooth colony isolates. Data-independent acquisition mass spectrometry (DIA-MS) proteomic analysis showed that a total of 2998 proteins were shared by the rough and smooth colony organisms, and 108 and 116 proteins were identified to be unique to the rough and smooth colony isolates, respectively (Fig. S13A, Supplementary Data 6). A total of 424 differential proteins were identified in the rough colony isolates versus the smooth colony isolates, among which 326 showed increased abundance and 98 showed decreased abundance (Fig. S13B, Supplementary Data 7). Heatmap analysis exhibited clear hierarchical clustering of these proteins based on the abundance correlated with rough or smooth colony morphotypes (Fig. S13C). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses indicated that ATP-binding cassette (ABC) transporters, flagellar assembly, bacterial chemotaxis, biofilm formation, Glyoxylate and dicarboxylate metabolism, and Pyruvate metabolism were among the top 20 KEGG pathways, and these are involved in the functions of signal transduction, membrane transport, cell motility, and cellular community (Fig. S13D, Supplementary Data 8). In the biofilm formation pathway, the abundance of CsgD, CsgC, FliA, and FlgM were upregulated, and those of CsrA and RcsB were downregulated (Fig. S13E). In the bacterial chemotaxis pathway, the abundance of methyl-accepting chemotaxis proteins (MCPs), including Tsr, Tar, Trg, and MglB, and other chemotaxis proteins, including FliGMN, were upregulated (Fig. S13F). In the flagellar assembly pathway, the abundance of FliACDGKLMN and FlgBCDEGIKLM was upregulated (Fig. S13G).
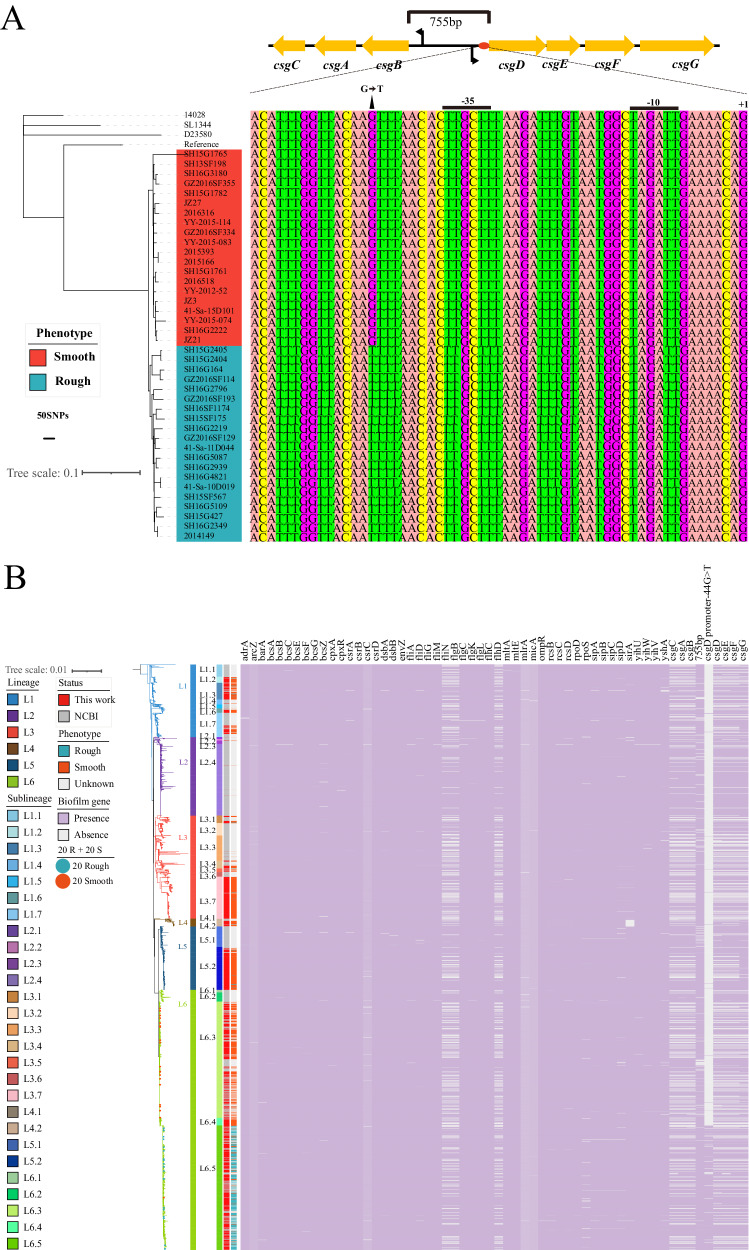
Fig. 5. Maximum likelihood phylogeny of the global S. Typhimurium isolates with a heatmap showing the distribution of biofilm formation-associated genes or mutations.
A Maximum likelihood phylogeny of the 20 rough and 20 smooth S. Typhimurium isolates with a heatmap showing the nucleotide mutations of csgBAC-csgDEFG operons. The reference genomes S. Typhimurium ATCC 14028 (GenBank accession CP043907.1), LT2 (GenBank accession NC_003197), SL1344 (GenBank accession FQ312003), and D23580 (GenBank accession FN424405) are also included. B Maximum likelihood phylogeny of the 3,951 global S. Typhimurium genomes with a heatmap showing the distribution of biofilm formation-associated genes or mutations. The presence of a biofilm gene or mutation is indicated in purple and the absence of a gene is indicated in gray. The selected 20 rough and 20 smooth S. Typhimurium isolates are indicated in blue and red, respectively. Source data are provided as a Source Data file. SNP single nucleotide polymorphism.
To verify the reliability of the proteomic data, the mRNA abundance of the csgBAC-csgDEFG operons was detected, and the results showed that the rough colony isolate SF175 had notably increased mRNA levels of the csgDEFG operons compared with the control strain ATCC 14028 and the smooth colony isolate 2015114, which is consistent with the proteomic data (Fig. S13H, Supplementary Data 9). According to the genomic and proteomic analyses, it can be inferred that biofilm formation may be associated with the mrdar morphotype.
The csgD knockout mutant is unable to form the mrdar morphotype
To further determine the molecular mechanism conferring mrdar conversion, we investigated whether the majority of the global S. Typhimurium populations, irrespective of having the rough or smooth colony morphotype, contained biofilm formation-associated genes, including the csgBAC-csgDEFG operons, the bcsABZC-bcsEFG operons, and flagellar genes, and shared identical gene sequences (Fig. 5B). However, when comparing the intergenic region between the csgBAC-csgDEFG operons of the rough and smooth colony isolates, a distinct point mutation (−44G > T) was identified in the promoter of the csgD gene. This mutation was widely distributed in the rough colony isolates, with a prevalence rate of 95.3% (623/654) (Fig. 5A, B). Notably, among the rough colony strains of the L6 lineage, 98.8% (618/625) carried this point mutation. To further determine the function of the csgD gene and its promoter mutation in conferring rdar conversion, we constructed a csgD knockout mutant (SF175-ΔcsgD) and a single point mutant with −44 T > G substitution in the csgD promoter (SF175-csgD with −44 T > G) (Supplementary Data 9). We observed that these two mutants were unable to form rdar colonies, showing substantially reduced biofilm formation at 28 °C and 37 °C, in contrast to the wild-type SF175 isolate (Fig. 2, Fig. S1). These results indicated the important regulatory role of the csgD gene and its promoter, and a single point mutation in the promoter of csgD can influence the CsgD-regulated rough colony and biofilm formation.
Discussion
The major finding of this study is the identification of a macro-rdar colony morphology of S. Typhimurium that emerged and became prevalent in China. We provided a comprehensive description of the genomic and proteomic characteristics of these mrdar variants and observed that most of them clustered in a novel sublineage on the phylogenetic tree. The typical mrdar morphotype was first described by Römling et al. during their investigation of the multicellular behavior of two unrelated S. Typhimurium strains27. They observed that these two strains spontaneously developed this phenotypic variation during laboratory passage and identified mutations in the csgD promoter as the underlying cause. Specifically, one strain had a single T insertion after position -17, while the other had a −44 T > G mutation in the csgD promoter. In our study, we report a novel lineage of S. Typhimurium carrying the −44 T > G mutation in the csgD promoter within a wild S. Typhimurium population. Notably, this lineage appeared to be spreading not only within China but also across multiple regions of the world.
Our present study demonstrated that wild-type ATCC 14028 formed a typical rdar morphotype at 28 °C but not at 37 °C; thus, the typical rdar morphotype seems to be temperature-restricted28,29. However, the mrdar variants identified in this study seem to be more tolerant to temperature variations than ATCC 14028 with the typical rdar morphology and the smooth colony variants, as these display stronger biofilm-forming ability at 28 °C and 37 °C. Furthermore, the mrdar variants were more commonly MDR than the non-mrdar variants, and, of concern, a greater proportion of mrdar variants were isolated from the environment than the proportions of these variants isolated from humans and food. It has been demonstrated that Salmonella rdar biofilm formation can protect the attached bacterial cells from desiccation, disinfection, antimicrobials, host immune responses, and other environmental stresses and facilitate their transmission, colonization, and persistence on diverse biotic and abiotic surfaces in the environment and inside hosts30,31. These findings indicate that this mrdar variant represents an evolutionary transition to a distinct form of colony behavior by S. Typhimurium, which is not influenced by temperature and may possess an augmented ability to adapt to the environment.
This study provides a snapshot of the large phylogeny of Chinese S. Typhimurium, especially the mrdar variants within the global phylogeny. Using a global collection of 3,951 genomes, we found that the global collections were divided into six distinct lineages, all of which contained both the Chinese isolates and those from other regions of the world. Within each lineage, the organisms isolated abroad were basal to the Chinese isolate. These results suggest that the contemporary Chinese S. Typhimurium isolates were probably sourced from several ancestors circulating worldwide and introduced into China possibly through multiple transmission events. Notably, the majority of the mrdar variants were found in sublineage L6.5, which probably evolved from smooth Chinese isolates in L6. The L6.5 sublineage consisted of 840 strains, including 708 strains tested in this study and 132 strains downloaded from NCBI. Among them, 777 strains carried the −44G > T substitution in the csgD gene promoter, accounting for a high proportion of 92.50%. The substitution was also observed in strains collected outside of China, suggesting the potential global distribution of mrdar variants of S. Typhimurium carrying the −44G > T substitution in the csgD gene promoter. The dates of lineage divergence were provided by Bayesian phylogenetic inference. In this study, reference S. Typhimurium LT2 was predicted to date to 1940 (95% CI: 1929–1950) (Fig. 3, Fig. S6) and was isolated in the 1940s25. Moreover, the MRCA of S. Typhimurium DT104 was predicted to date to 1959 (95% CI: 1948–1969) (Fig. 3, Fig. S6), consistent with the first reports of DT104 strains since the 1960s in England and Wales14,32. It is estimated that the current global S. Typhimurium population shares an MRCA that existed circa 1696 (95% CI: 1686–1706) and then spread and localized into several circulating lineages. The ancestor of the L6.5 strains was traced back to circa 1977 (95% CI: 1966–1987), showing that the clonal expansion of mrdar variants occurred in recent evolutionary history. The cgMLST analysis showed that the rough colony strains formed distinct clusters separate from other branches, suggesting a considerable genetic uniqueness of the rough colony strains. These findings indicated that the mrdar variants had evolved into a more recently emergent clone and underwent a contemporary localized clonal expansion and global dispersal, thus with potentially great public health significance.
It has been reported that both gene acquisition and genome degradation are linked with the emergence and niche adaptation of several important pathovariants with great public health significance11,19,20. For example, the iNTS variants of ST313 S. Typhimurium presented with the loss of multi-cellular stress resistance associated with the rdar-negative phenotype, which was caused by the inactivation of BcsG cellulose biosynthetic enzyme and two nucleotide changes (−80A > C, −189A > G) in the csgD gene promoter region19,28,29. Consequently, the iNTS variants of ST313 S. Typhimurium have become more host-adapted to humans and become a frequent cause of bloodstream infections in sub-Saharan Africa. Here, we identified a mrdar colonial phenotype with a stronger ability of biofilm formation, which means better tolerance and resistance33. The rdar colonies display a distinct form of colony behavior that allows biofilm formation and enhances Salmonella stress resistance in the environment29. Acquisition of the rdar biofilm morphotype is a complex process influenced by structural compositions, the bacterial genome, environmental signals, and stress factors. The typical Salmonella biofilm matrix is mainly composed of curli fimbriae, cellulose, biofilm-associated protein BapA, and O-antigen capsule30,31. Genes for curli production are found in the csg operon29,34. Divergent csg operons, comprising curli biosynthesis machinery (csgBAC), transcriptional regulation (csgD), and curli assembly machinery (csgEFG), are central to curli biosynthesis. CsgD is involved in activating the transcription of both operons and acts as the primary transcriptional activator of the rdar biofilm morphotype27,28. In this study we investigated whether the mrdar phenotype strains contained csg operons with sequences identical to the smooth isolates, but with a distinct and dominant point mutation (−44G > T) in the promoter region of the csgD gene in the mrdar phenotype strains. Data from the proteomics and transcriptomics analysis confirmed that the mrdar variants had a higher abundance of csgDEFG operons than the smooth colony strains. In addition, we constructed a csgD knockout mutant (SF175-ΔcsgD) and a single point mutant (SF175-csgD with −44 T > G), and the mutants lost the rough colony morphotype. These findings define the key role of the csgD gene and the −44G > T mutation in its promoter region in rdar biofilm formation. It is worth noting that previous studies have indicated a strong correlation between rpoS and the ability to form biofilms35,36. However, in our analysis of proteomic data from 40 strains, we found no statistically significant difference in the abundance of RpoS between rough and smooth colony strains. Out of the 654 mrdar variants confirmed through experiments, only 27 had nonsynonymous mutations in the rpoS gene. Furthermore, these 27 strains also had mutations in the csgD promoter, suggesting that RpoS was not the cause of the variants in our study. Furthermore, we constructed an rpoS knockout strain, which showed a decrease in biofilm formation ability, but still exhibited the characteristics of a rough colony on the modified Swarmagar medium (Fig. S14).
The known biofilm formation pathways include curli expression, cellulose synthesis, BapA secretion, O-antigen capsule, and others30,31. In addition to the notable upregulation of the csg operon in the mrdar variants, no obvious changes in the abundance of the bcsABZC-bcsEFG operons responsible for cellulose synthesis were observed among the rough and smooth colony strains. Furthermore, the bapA gene responsible for BapA secretion was downregulated among the mrdar variants. Therefore, the mrdar biofilm formation observed in the present study was seemingly not fully dependent on the typical known biofilm pathways, and there may be other regulatory pathways responsible for rdar biofilm formation. The biofilm formation process includes several stages: surface attachment, growth and maturation of matrix-embedded communities, and finally, biofilm dispersion37,38. It has been demonstrated that flagellar motility plays an important role in Salmonella biofilm initiation and maturation39. Bacteria sense the chemical stimuli and swim toward the nutrient, attaching to the substrate through flagellar motility. Flagella can serve as adhesins, directly promoting surface attachment. In the mature biofilm, motility is repressed, but the flagella could be repurposed as important structural elements of the biofilm matrix39,40. A previous study showed that hyperexpression of the T3SS encoded by SPI-1 facilitates the biofilm phenotype, which is not dependent on established biofilm mechanisms such as curli and cellulose but dependent on the SPI-1 T3SS secreted proteins SipA, SipB, SipC, SopB, SopE, and SptP, which are enriched and present in the biofilms41. In this study, the proteomic analysis showed that there were differential proteins among the rough and smooth colony strains, which are mainly involved in biofilm formation, flagellar assembly, the two-component system, ABC transporters, bacterial chemotaxis, and quorum sensing pathways. We observed clear upregulation of the abundance of flagellar-associated proteins, including FliACDGKLMN and FlgBCDEGIKLM; MCPs, including Tsr, Tar, Trg, and MglB; and secreted proteins, including SipABCD, sopB, SopE, SopE2, SptP, and InvG (Supplementary Data 7). Moreover, Hfq, an RNA chaperone, was also upregulated, which is one of the major global regulators that influences gene expression in response to environmental stimuli and plays a diverse role in bacterial physiology, including biofilm formation, motility, and the T3SS42,43. Hfq has been identified as a global activator of biofilm formation in S. Typhimurium and positively affects csgD expression and biofilm formation31. According to the above analyses, mrdar biofilm formation may be a complex and rigorous regulatory process involving multiple pathways, thus mediating better bacterial adaptation among mrdar variants.
In addition to gene acquisition and genome degradation, other genetic factors, such as accessory genome elements and SNPs, are considered to be associated with the evolutionary success of the variants of S. Typhimurium. Accessory genome elements and loss-of-function SNPs have been demonstrated to be involved in the host adaptation of the iNTS variants of S. Typhimurium ST31319,44. Here, we performed pangenome and GWAS analyses to identify the accessory genes and SNPs significantly associated with the evolutionary success of the mrdar variants. We found 87 accessory genes and 72 SNPs that are significantly associated with the mrdar variants in sublineage L6.5, and COG functional analysis showed that the accessory genes and nonsynonymous SNP-affected genes mapped to the COG clusters are mainly involved in replication, recombination and repair, signal transduction mechanisms, inorganic ion transport and metabolism, amino acid transport and metabolism, intracellular trafficking, secretion and vesicular transport, and cell wall/membrane/envelope biogenesis (Fig. S11, Fig. S12). Among the affected genes with COG functional clusters, the hipBA operon was unique to the mrdar variants (Supplementary Data 3 and 6). HipA, encoding a serine/threonine kinase, is the only toxin that has been validated as a biofilm tolerance factor and is a critical persistence factor that induces bacterial persistence, and the hipBA operon, categorized as a toxin-antitoxin module, has been linked to the persistent phenotype45–47. The above analyses provide strong evidence to support the adaptive evolution of mrdar variants by the accumulation of genomic signatures, which may be associated with the local establishment and niche adaptation of these variants.
Antibiotic use might have provided the selection pressure that drove the emergence and microevolution of the MDR dominant pathovariants, and the acquisition of AMR is conferred largely by either horizontal gene transfer or chromosomal gene mutations of AMR determinants2,11,17,19. The acquisition of AMR determinants may act as a major driving force for the adaptive evolution of MDR S. Typhimurium lineages. Here, among the mrdar variants, we observed AMR and a higher rate of MDR, such as the ASSuT and ACSSuT resistance patterns, compared to what was observed among the smooth colony strains. Moreover, several ESBL genes, including blaCTX-M, blaOXA, and blaTEM, macrolide resistance genes, including mphA, mef(B), ermX, and ereA2, and the plasmid-mediated colistin resistance (mcr) gene were also detected in the mrdar variants48, and point mutations (D87N or D87Y) in the gyrA gene occurred in most of the mrdar variants. These AMR determinants conferred additional resistance to first-line antibiotics for the treatment of Salmonella infections, including third-generation cephalosporins, azithromycin, and ciprofloxacin, and also resistance to colistin, the last treatment option for multidrug-resistant gram-negative bacteria. Although certain resistance genes, such as mcr-9, do not necessarily confer polymyxin-resistant phenotypes in Salmonella, the majority of these genes are located on plasmids and transposable elements49. This facilitates the transfer of these genes to distantly related bacteria through mechanisms such as conjugation, transduction, or transformation, ultimately leading to the dissemination of antibiotic resistance50,51. One plausible explanation for the severe MDR of this specific variant is the easy availability and enrichment of AMR determinants, particularly the distinct plasmid pYY-2016-057-2 belonging to the IncHI2 replicon, which carries 19 AMR genes that confer resistance to aminoglycosides, sulfonamides, trimethoprim, phenicol, cephalosporins, quinolones, rifamycin, and disinfecting agents. Of concern, antibiotic usage in China is complex and widespread and has presented a new and serious antibiotic crisis, as severe antibiotic pollution in the environment has been found52,53. Therefore, the severe antibiotic pollution in the environment may be another driving force for the emergence of severe MDR, as this variant was present in a higher proportion of environmental strains and showed stronger environmental adaptability.
This study has several limitations. While the global genome dataset was partially obtained from the NCBI database, the colonial phenotypes (rough or smooth) of the isolates from abroad were not determined, and thus, we can only make a preliminary judgment by analyzing the point mutation of the csgD promoter and provide some preliminary insights into the global distribution of mrdar variants. Further international epidemiological studies are required to understand the spread of the mrdar variant, assess the selective benefits and drawbacks of the −44 T > G mutation, and elucidate the role of other SNPs identified in the L6.5 lineage. It has been demonstrated that the csgD gene and mutations in the csgD promoter region are essential for rdar biofilm formation, but the regulation mechanism of the −44G > T point mutation in the promoter region of the csgD gene on the phenotype conversion and biofilm formation requires further study.
In conclusion, this study reports a distinct variant of the rdar colony morphology of S. Typhimurium that has emerged and become prevalent in China. This variant exhibited a potentially enhanced capacity for environmental adaptation, including a high proportion of MDR and enhanced biofilm-forming ability. Furthermore, the variant strains clustered together to form a novel lineage on the phylogenetic tree. This study also highlights the potential national and international spread of this variant through a large-scale genomic epidemiology study. The acquisition of distinct phenotypic characteristics and genomic changes probably resulted in the emergence of this new lineage and prevalence of this variant, contributing to its competitive advantages and genome adaptation.
Methods
Bacterial isolates
A collection of 2212 Chinese S. Typhimurium isolates with different spatial and temporal contexts were included in this study (Fig. 1A–C, Table 1, Supplementary Data 1). The S. Typhimurium isolates were identified with API 20E biochemical tests (bioMerieux, Marcy l’Etoile, France) and serotyped with a commercial antiserum kit (SSI, Copenhagen, Denmark) following the Kauffmann-White scheme. In cases where autoagglutinating strains could not be serotyped by the Kauffmann-White scheme, retrospective serotype confirmation was performed through WGS. An additional collection of 1739 globally distributed isolates was included, which were obtained from the NCBI database and span six continents (Asia, Africa, North America, South America, Europe, and Oceania) (Fig. 1D–F, Supplementary Data 2). This study was approved by the institutional review board of the Chinese PLA Center for Disease Control and Prevention, Beijing, China.
Characterization of the rough and smooth colony morphology of S. Typhimurium
For colony morphology characterization, modified Swarmagar medium culturing and Congo red assays were performed (Supplementary Methods). The S. Typhimurium isolate ATCC 14028 was selected as a control strain due to its typical rdar colony morphology28,29. The rough and smooth morphotypes were also observed through SEM examinations (Supplementary Methods). One rough colony strain (SF175), one smooth colony strain (2015114), and the control strain (ATCC 14028) were selected as representatives in this study.
Biofilm formation
To determine the biofilm formation ability of the rough colony variants, we selected 20 representative rough colony strains and 20 representative smooth colony strains (Supplementary Data 5) for comparison, and the S. Typhimurium isolate ATCC 14028 was used as a control. These 40 representative strains were randomly selected from the L6 strains based on the results of phylogenetic analysis. Biofilm formation was examined by crystal violet assays, pellicle formation assays, and CLSM imaging (Supplementary Methods).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by broth microdilution using a 96-well microtiter plate (Sensititre CMV3AGNF, Trek Diagnostic Systems; Thermo Fisher Scientific, Inc., West Sussex, United Kingdom) according to the recommendations of the CLSI guidelines54. Fourteen different antibiotics were included in this experiment: ceftriaxone (CRO), tetracycline (TET), ceftiofur (XNL), cefoxitin (FOX), gentamicin (GEN), ampicillin (AMP), chloramphenicol (CHL), ciprofloxacin (CIP), trimethoprim/sulfamethoxazole (SXT), sulfisoxazole (FIS), nalidixic acid (NAL), streptomycin (STR), azithromycin (AZI), and amoxicillin/clavulanic acid in a 2:1 ratio (AUG2). Escherichia coli ATCC 25922 was used for quality control.
Whole-genome sequencing and genome assembly
In this study, the genomes of 2212 Chinese S. Typhimurium isolates were sequenced (Supplementary Methods). The 20 rough and 20 smooth colony strains selected were also submitted for long-read sequencing to obtain their complete genomes (Supplementary Methods). Genomic comparisons were performed on the complete genomes from the 20 rough and 20 smooth colony strains to analyze their genetic differences in chromosomal regions such as SNPs, indels, and rearrangements with BLAST, Snippy (v4.6.0) (https://github.com/tseemann/snippy), and Gubbins (v 2.4.1)55.
SNP calling and phylogenetic analysis
A genome dataset that included the 2,212 Chinese strains sequenced in this study and 1739 public genomes from NCBI was constructed (Supplementary Data 1). Among these genomes, the core genome SNPs were identified using the Snippy (v4.6.0) pipeline, and the population structure was estimated using hierBAPS (hierarchical Bayesian analysis of population structure)56 (Supplementary Methods).
MLST analysis
MLST analysis was performed by scanning the sequences of seven housekeeping genes (aroC, dnaN, hemD, hisD, purE, sucA, and thrA) against PubMLST typing schemes using MLST.
cgMLST analysis
We used the CHEWBBACA software to construct a local cgMLST core gene database of Salmonella enterica, which contained 3000 core allelic genes57. We then ran the AlleleCall function of chewBBACA.py to align the bacterial genomes with the core gene loci and obtain the allelic profiles. We applied the ExtractCgMLST function of chewBBACA.py to filter out the accessory genes based on a presence threshold of 0.99 and obtained a final matrix of 2661 core genes. We used the phyloviz software58 and the goeBURST Full MST algorithm to construct a minimum spanning tree based on the cgMLST results and show the genetic relationships and the clonal complexes of the Salmonella enterica strains59.
AMR gene and VF detection
AMR genes were detected using ABRicate (https://github.com/tseemann/abricate) against the Comprehensive Antibiotic Resistance Database (CARD)60, and thresholds of 80% were selected for minimum coverage and minimum identity. Point mutations in the QRDRs of the gyrA, gyrB, parC, and parE genes were investigated using Snippy. The virulence genes were aligned to the VFDB61 using BLAST (v 2.5.0)62 with thresholds of 80% for minimum coverage and minimum identity.
Plasmid detection
Complete plasmid sequences were generated from the 20 rough and 20 smooth colony strains sequenced by Nanopore long-read sequencing in combination with Illumina short-read sequencing (Supplementary Methods). Known plasmid replicons were screened using ABRicate (https://github.com/tseemann/abricate), and the genomic assemblies from Illumina short-read sequencing were mapped against the selected plasmids using BLAST to determine whether the strains carried corresponding plasmids with minimum identity and minimum coverage thresholds of 95%.
Pangenome analysis
The assembled genomes were annotated with Prokka63. Based on the annotated assemblies, a pangenome was constructed for all 3951 isolates using Roary64. The Chi-square test selected P values below 1e-300 from the accessory genes, and significantly related genes were defined as those present in more than 75% of the isolates in sublineage 6.5 and less than 25% in the other lineages or those present in less than 25% of the isolates in sublineage 6.5 and more than 75% in the other lineages. COG classification of the genes identified by pangenome analysis was performed by mapping to the COG function database using BLAST, followed by visualization using R.
GWAS analysis
A GWAS was also performed on the 2212 Chinese strains to identify significant SNPs associated with rough or smooth colony morphology (Supplementary Methods).
Distribution of the biofilm-associated genes and mutations
The typical rdar morphotype is the best-studied Salmonella biofilm phenotype27,30,31. In this study, 58 biofilm-associated genes, such as curli fimbriae-encoding genes (csgBAC-csgDEFG operons) and cellulose synthesis genes (bcsABZC-bcsEFG operons), were included to search for their distribution and identify the presence of point mutations by sequence comparisons within the rough and smooth colony strains. In addition, the csgBAC-csgDEFG operons and promoters of csgD were compared with those of LT2 and ATCC 14028 using the Snippy pipeline (Supplementary Methods).
Proteomic analysis of the rough and smooth colony strains
To compare the proteome profiles of the rough and smooth colony strains, the selected 20 rough and 20 smooth colony strains were subjected to data-independent acquisition (DIA)-based proteomics sequencing (Supplementary Data 5) (Supplementary Methods). No imputation method was used in this analysis. Differential proteins were considered if the fold change between the rough strains and smooth controls was >1.5 or <0.67 and the P value of the t-test was <0.05. The differential proteins were then analyzed by hierarchical clustering with Cluster 3.0 (http://bonsai.hgc.jp/mdehoon/software/cluster/software.htm) and Java Treeview software (http://jtreeview.sourceforge.net). Subsequently, the differential proteins were subjected to GO analysis with Blast2GO (https://www.blast2go.com/) and matched against the KEGG database (http://www.kegg.jp/) by the KEGG Automatic Annotation Server (KAAS, https://www.genome.jp/tools/kaas/) to obtain their KEGG orthology identifications. P < 0.05 by Fisher’s exact test was considered to indicate significance.
Based on the proteomic results, differential proteins, including the csgDEFG operons, were selected to assess their mRNA abundance using real-time quantitative polymerase chain reaction (RT‒qPCR) assays (Supplementary Methods). In addition, recombination analysis was conducted on the 20 smooth and 20 rough colony strains from the proteomic analysis (Supplementary Methods).
Construction of the mutants with csgD gene knockout and a single nucleotide replacement (−44 T > G) in its promoter
To determine the possible role of the csgD gene and its promoter in mediating morphotype conversion and biofilm formation of the mrdar variants, a mutant with the knockout of the csgD gene and a mutant with a single nucleotide replacement (−44 T > G) in its promoter were constructed for the rough colony strain SF175 (Supplementary Methods).
Statistical analysis
Between-group comparisons were performed using the chi-square test. The Kruskal‒Wallis test was performed between lineages for the carried AMR genes and VFs, while the Wilcoxon test was performed between rough and smooth phenotype isolates. Other descriptive data are summarized using descriptive statistics. P values of less than 0.05 were considered to indicate statistical significance. Statistical analysis was performed using R (v 4.0.3), and statistical plots were visualized using ggplot2 (v 3.3.5).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the National Nature Science Foundation of China (no. 82173580 to S.F.Q. and no. 82202538 to Y.X.) and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-044 to J.T.Y.). We would like to thank Chunli Li (Institute of Microbiology, Chinese Academy of Sciences) for sample preparation and SEM examination.
Author contributions
S.F.Q., J.T.Y., X.B.X., and H.B.S. conceived and designed the experiments. Y.X., Y.W.Z., Y.R.H., X.G.L., X.W., X.P.L., Y.Q.P., C.J.Y., H.b.L., X.Y.D., H.B.L., X.Y.L., H.W., C.W., Y.H., Q.W., H.Q.J., and M.J.Y. performed the experiments. S.F.Q., Y.X., K.P.Z., K.Y.M., Y.W.Z., J.F.L., K.K.L., Y.R.W., Y.J.C., and F.C. analyzed the data. Y.X., Y.W.Z., K.P.Z., K.K.L., Y.R.H., X.G.L., X.W., X.P.L., Y.Q.P., Y.R.W., C.J.Y., H.b.L., X.Y.D., H.B.L., H.W., Q.W., C.W., L.G.W., H.Y.Y., S.B., X.B.X., and J.T.Y. contributed reagents, materials, or analysis tools. S.F.Q., Y.X., K.P.Z., Y.W.Z., and K.Y.M. wrote the paper. All authors reviewed the manuscript critically for content and approved the decision to submit it for publication.
Peer review
Peer review information
Nature Communications thanks Aaron White, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The publicly available sequences used in this study are available in GenBank (https://www.ncbi.nlm.nih.gov/datasets/genome/), with accession numbers listed in Supplementary Data 1. The sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive under the BioProject number PRJNA981225. The annotated plasmid sequences have been deposited in GenBank under accession numbers PP852730 and PP852731. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD047483. Source data are provided with this paper.
Code availability
No custom software or code was developed for this study. All analyses relied on standard statistical and bioinformatics methods and were performed using publicly available tools and packages, which have been appropriately cited in the Methods section.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ying Xiang, Kunpeng Zhu, Kaiyuan Min, Yaowen Zhang, Jiangfeng Liu.
Contributor Information
Xuebin Xu, Email: xxb72@sina.com.
Juntao Yang, Email: yangjt@pumc.edu.cn.
Hongbin Song, Email: hongbinsong@263.net.
Shaofu Qiu, Email: qiushf0613@hotmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-50331-y.
References
- 1.Kirk, M. D. et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med.12, e1001921 (2015). 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okoro, C. K. et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet.44, 1215–1221 (2012). 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrovska, L. et al. Microevolution of monophasic Salmonella Typhimurium during epidemic, United Kingdom, 2005-2010. Emerg. Infect. Dis.22, 617–624 (2016). 10.3201/eid2204.150531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden, H. S. et al. Genomic analysis of Salmonella enterica serovar Typhimurium characterizes strain diversity for recent U.S. salmonellosis cases and identifies mutations linked to loss of fitness under nitrosative and oxidative stress. MBio7, e00154 (2016). 10.1128/mBio.00154-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanis, E. et al. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerg. Infect. Dis.12, 381–388 (2006). 10.3201/eid1205.050854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ran, L. et al. Laboratory-based surveillance of nontyphoidal Salmonella infections in China. Foodborne Pathog. Dis.8, 921–927 (2011). 10.1089/fpd.2010.0827 [DOI] [PubMed] [Google Scholar]
- 7.Sun, J. et al. The molecular epidemiological characteristics and genetic diversity of Salmonella Typhimurium in Guangdong, China, 2007-2011. PLoS ONE9, e113145 (2014). 10.1371/journal.pone.0113145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris, T. L. & Baumler, A. J. Phase variation of the lpf operon is a mechanism to evade cross-immunity between Salmonella serotypes. Proc. Natl Acad. Sci. USA96, 13393–13398 (1999). 10.1073/pnas.96.23.13393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellington, M. J. et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: report from the EUCAST Subcommittee. Clin. Microbiol. Infect.23, 2–22 (2017). 10.1016/j.cmi.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 10.Diep, B. et al. Salmonella serotyping; comparison of the traditional method to a microarray-based method and an in silico platform using whole genome sequencing data. Front. Microbiol.10, 2554 (2019). 10.3389/fmicb.2019.02554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingle, D. J. et al. Evolutionary dynamics of multidrug resistant Salmonella enterica serovar 4,[5],12:i:- in Australia. Nat. Commun.12, 4786 (2021). 10.1038/s41467-021-25073-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingsley, R. A. et al. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. mBio4, e00565–00513 (2013). 10.1128/mBio.00565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather, A. E. et al. New variant of multidrug-resistant Salmonella enterica serovar Typhimurium Associated with invasive disease in immunocompromised patients in Vietnam. mBio9, 10.1128/mBio.01056-18 (2018). [DOI] [PMC free article] [PubMed]
- 14.Mather, A. E. et al. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science341, 1514–1517 (2013). 10.1126/science.1240578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai, N. et al. Phylogenetic characterization of Salmonella enterica serovar Typhimurium and its monophasic variant isolated from food animals in japan revealed replacement of major epidemic clones in the last 4 decades. J. Clin. Microbiol.56, 10.1128/jcm.01758-17 (2018). [DOI] [PMC free article] [PubMed]
- 16.Lu, X. et al. Epidemiologic and genomic insights on mcr-1-harbouring Salmonella from diarrhoeal outpatients in Shanghai, China, 2006-2016. EBioMedicine42, 133–144 (2019). 10.1016/j.ebiom.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Puyvelde, S. et al. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat. Commun.10, 4280 (2019). 10.1038/s41467-019-11844-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammarlöf, D. L. et al. Role of a single noncoding nucleotide in the evolution of an epidemic African clade of Salmonella. Proc. Natl Acad. Sci. USA115, E2614–e2623 (2018). 10.1073/pnas.1714718115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulford, C. V. et al. Stepwise evolution of Salmonella Typhimurium ST313 causing bloodstream infection in Africa. Nat. Microbiol6, 327–338 (2021). 10.1038/s41564-020-00836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm, E. J. et al. Emergence of host-adapted Salmonella Enteritidis through rapid evolution in an immunocompromised host. Nat. Microbiol1, 15023 (2016). 10.1038/nmicrobiol.2015.23 [DOI] [PubMed] [Google Scholar]
- 21.Tassinari, E. et al. Whole-genome epidemiology links phage-mediated acquisition of a virulence gene to the clonal expansion of a pandemic Salmonella enterica serovar Typhimurium clone. Microbial Genom.6 e000456 (2020). [DOI] [PMC free article] [PubMed]
- 22.Baker, K. S. et al. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nat. Commun.9, 1462 (2018). 10.1038/s41467-018-03949-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner, A. K., Nair, S. & Wain, J. The acquisition of full fluoroquinolone resistance in Salmonella Typhi by accumulation of point mutations in the topoisomerase targets. J. Antimicrob. Chemother.58, 733–740 (2006). 10.1093/jac/dkl333 [DOI] [PubMed] [Google Scholar]
- 24.Morgan-Linnell, S. K. & Zechiedrich, L. Contributions of the combined effects of topoisomerase mutations toward fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother.51, 4205–4208 (2007). 10.1128/AAC.00647-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland, M. et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature413, 852–856 (2001). 10.1038/35101614 [DOI] [PubMed] [Google Scholar]
- 26.Liebert, C. A., Hall, R. M. & Summers, A. O. Transposon Tn21, flagship of the floating genome. Microbiol Mol. Biol. Rev.63, 507–522 (1999). 10.1128/MMBR.63.3.507-522.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Römling, U., Sierralta, W. D., Eriksson, K. & Normark, S. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol28, 249–264 (1998). 10.1046/j.1365-2958.1998.00791.x [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie, K. D. et al. Parallel evolution leading to impaired biofilm formation in invasive Salmonella strains. PLoS Genet.15, e1008233 (2019). 10.1371/journal.pgen.1008233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singletary, L. A. et al. Loss of multicellular behavior in epidemic African nontyphoidal Salmonella enterica serovar Typhimurium ST313 strain D23580. mBio7, e02265 (2016). 10.1128/mBio.02265-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Z., Niu, H., Wu, S. & Huang, R. CsgD regulatory network in a bacterial trait-altering biofilm formation. Emerg. Microbes Infect.3, e1 (2014). 10.1038/emi.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simm, R., Ahmad, I., Rhen, M., Le Guyon, S. & Romling, U. Regulation of biofilm formation in Salmonella enterica serovar Typhimurium. Future Microbiol.9, 1261–1282 (2014). 10.2217/fmb.14.88 [DOI] [PubMed] [Google Scholar]
- 32.Threlfall, E. J. Epidemic Salmonella Typhimurium DT 104-a truly international multiresistant clone. J. Antimicrob. Chemother.46, 7–10 (2000). 10.1093/jac/46.1.7 [DOI] [PubMed] [Google Scholar]
- 33.Ciofu, O., Moser, C., Jensen, P. & Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol.20, 621–635 (2022). 10.1038/s41579-022-00682-4 [DOI] [PubMed] [Google Scholar]
- 34.Anriany, Y., Sahu, S. N., Wessels, K. R., McCann, L. M. & Joseph, S. W. Alteration of the rugose phenotype in waaG and ddhC mutants of Salmonella enterica serovar Typhimurium DT104 is associated with inverse production of curli and cellulose. Appl. Environ. Microbiol.72, 5002–5012 (2006). 10.1128/AEM.02868-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, H. et al. The exopolysaccharide gene cluster pea is transcriptionally controlled by RpoS and repressed by AmrZ in Pseudomonas putida KT2440. Microbiol. Res.218, 1–11 (2019). 10.1016/j.micres.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Gómez, P., López, M., Prieto, M., González-Raurich, M. & Alvarez-Ordóñez, A. The role of the general stress response regulator RpoS in Cronobacter sakazakii biofilm formation. Food Res. Int.136, 109508 (2020). 10.1016/j.foodres.2020.109508 [DOI] [PubMed] [Google Scholar]
- 37.Wolska, K. I., Grudniak, A. M., Rudnicka, Z. & Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet.57, 225–238 (2016). 10.1007/s13353-015-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaron, S. & Romling, U. Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb. Biotechnol.7, 496–516 (2014). 10.1111/1751-7915.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, F. et al. Flagellar motility is critical for Salmonella enterica serovar Typhimurium biofilm development. Front. Microbiol.11, 1695 (2020). 10.3389/fmicb.2020.01695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colin, R., Ni, B., Laganenka, L. & Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev.45, 10.1093/femsre/fuab038 (2021). [DOI] [PMC free article] [PubMed]
- 41.Jennings, M. E. et al. Characterization of Salmonella type III secretion hyper-activity which results in biofilm-like cell aggregation. PLoS ONE7, e33080 (2012). 10.1371/journal.pone.0033080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteiro, C. et al. Hfq and Hfq-dependent small RNAs are major contributors to multicellular development in Salmonella enterica serovar Typhimurium. RNA Biol.9, 489–502 (2012). 10.4161/rna.19682 [DOI] [PubMed] [Google Scholar]
- 43.Sittka, A., Pfeiffer, V., Tedin, K. & Vogel, J. The RNA chaperone Hfq is essential for the virulence of Salmonella Typhimurium. Mol. Microbiol63, 193–217 (2007). 10.1111/j.1365-2958.2006.05489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bawn, M. et al. Evolution of Salmonella enterica serotype Typhimurium driven by anthropogenic selection and niche adaptation. PLoS Genet.16, e1008850 (2020). 10.1371/journal.pgen.1008850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Germain, E., Castro-Roa, D., Zenkin, N. & Gerdes, K. Molecular mechanism of bacterial persistence by HipA. Mol. Cell52, 248–254 (2013). 10.1016/j.molcel.2013.08.045 [DOI] [PubMed] [Google Scholar]
- 46.Kaspy, I. et al. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat. Commun.4, 3001 (2013). 10.1038/ncomms4001 [DOI] [PubMed] [Google Scholar]
- 47.Schumacher, M. A. et al. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science323, 396–401 (2009). 10.1126/science.1163806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu, Y. Y. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis.16, 161–168 (2016). 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 49.Tyson, G. H. et al. The mcr-9 gene of Salmonella and Escherichia coli is not associated with colistin resistance in the United States. Antimicrob. Agents Chemother.64, 10.1128/aac.00573-20 (2020). [DOI] [PMC free article] [PubMed]
- 50.Zhou, Y. et al. Exploiting a conjugative endogenous CRISPR-Cas3 system to tackle multidrug-resistant Klebsiella pneumoniae. EBioMedicine88, 104445 (2023). 10.1016/j.ebiom.2023.104445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourouis, A., Ben Moussa, M. & Belhadj, O. Multidrug-resistant phenotype and isolation of a novel SHV- beta-Lactamase variant in a clinical isolate of Enterobacter cloacae. J. Biomed. Sci.22, 27 (2015). 10.1186/s12929-015-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao, R., Zhao, R., Qiu, S., Wang, L. & Song, H. Antibiotics crisis in China. Science348, 1100–1101 (2015). 10.1126/science.348.6239.1100-d [DOI] [PubMed] [Google Scholar]
- 53.Qiao, M., Ying, G. G., Singer, A. C. & Zhu, Y. G. Review of antibiotic resistance in China and its environment. Environ. Int.110, 160–172 (2018). 10.1016/j.envint.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 54.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility TestingCLSI supplement M100 31st edn (Clinical & Laboratory Standards Institute, 2021) [DOI] [PMC free article] [PubMed]
- 55.Croucher, N. J. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res.43, e15 (2015). 10.1093/nar/gku1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng, L., Connor, T. R., Siren, J., Aanensen, D. M. & Corander, J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol. Biol. Evol.30, 1224–1228 (2013). 10.1093/molbev/mst028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva, M. et al. chewBBACA: a complete suite for gene-by-gene schema creation and strain identification. Microb. Genom.4, 10.1099/mgen.0.000166 (2018). [DOI] [PMC free article] [PubMed]
- 58.Nascimento, M. et al. PHYLOViZ 2.0: providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics33, 128–129 (2017). 10.1093/bioinformatics/btw582 [DOI] [PubMed] [Google Scholar]
- 59.Francisco, A. P., Bugalho, M., Ramirez, M. & Carriço, J. A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinform.10, 152 (2009). 10.1186/1471-2105-10-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McArthur, A. G. et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother.57, 3348–3357 (2013). 10.1128/AAC.00419-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen, L. et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res.33, D325–D328 (2005). 10.1093/nar/gki008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinform.10, 421 (2009). 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics30, 2068–2069 (2014). 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 64.Page, A. J. et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics31, 3691–3693 (2015). 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The publicly available sequences used in this study are available in GenBank (https://www.ncbi.nlm.nih.gov/datasets/genome/), with accession numbers listed in Supplementary Data 1. The sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive under the BioProject number PRJNA981225. The annotated plasmid sequences have been deposited in GenBank under accession numbers PP852730 and PP852731. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD047483. Source data are provided with this paper.
No custom software or code was developed for this study. All analyses relied on standard statistical and bioinformatics methods and were performed using publicly available tools and packages, which have been appropriately cited in the Methods section.