Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected SCID-hu thymic implants depleted of CD4+ cells can support renewed thymopoiesis derived from both endogenous and exogenous T-cell progenitors after combination antiretroviral therapy. However, successful production of new thymocytes occurs transiently. Possible explanations for the temporary nature of this thymic reconstitution include cessation of the thymic stromal support function, exhaustion of T-cell progenitors, and viral resurgence. Distinguishing between these processes is important for the development of therapeutic strategies aimed at reconstituting the CD4+ T-cell compartment in HIV-1 infection. Using an HIV-1 strain engineered to express the murine HSA heat-stable antigen surface marker, we explored the relationship between HIV-1 expression and CD4+ cell resurgence kinetics in HIV-1-depleted SCID-hu implants following drug therapy. Antiviral therapy significantly suppressed HIV-1 expression in double-positive (DP) CD4/CD8 thymocytes, and the eventual secondary decline of DP thymocytes following therapy was associated with renewed viral expression in this cell subset. Thymocytes derived from exogenous T-cell progenitors induced to differentiate in HIV-1-depleted, drug-treated thymic implants also became infected. These results indicate that in this model, suppression of viral replication occurs transiently and that, in spite of drug therapy, virus resurgence contributes to the transient nature of the renewed thymic function.
An important requisite for full immune reconstitution following drug or hematopoietic stem cell gene therapy treatments of human immunodeficiency virus type 1 (HIV-1) infection is that precursor cells must be able to differentiate via the T-lymphoid pathway and give rise to naive T cells capable of responding to antigens. Although most adult patients treated with highly active antiretroviral therapy (HAART) experience an increase in CD4+ T-cell counts associated with a decrease in viral load in the peripheral blood (4, 13, 27–29, 36), much of this rise is secondary to an expansion of cells in the peripheral lymphoid compartment rather than de novo thymic output (4, 27). HIV-1 infection gives rise to pathology in the thymus, which is manifested in part by a severe depletion of double-positive (DP) CD4/CD8 cortical thymocytes (31). The degree of thymic dysfunction in pediatric HIV-1-infected patients correlates with progression to AIDS and survival (19). The thymus is known to involute in healthy adults (32, 34). However, ongoing thymopoiesis can be demonstrated in the adult thymus (5) and de novo T-cell development can occur in adults following T-cell depletion states, such as myelosuppression (11, 22) and HIV-1 infection following 4 to 6 months of HAART (4, 27). Radiographically measured increases in thymic tissue size have been correlated with high total and naive circulating CD4+ lymphocyte counts in HIV-1-infected adults (23). New findings indicate that the levels of detectable thymic emigrants increase rapidly in the periphery following HAART (9). Together, these data seem to indicate that a degree of immune reconstitution that is associated with de novo T-cell development occurs even in adult HIV-1-infected patients after viral replication is suppressed and that the thymus may play an important role in this process. However, it is unclear whether this partial immune restoration and de novo T-cell development observed after prolonged HAART can be sustained for a long period.
The severe combined immunodeficient (SCID) mouse implanted with human fetal liver and thymus (SCID-hu) has been employed as an in vivo system to study T-cell development and HIV-1 pathogenesis (1, 6, 14, 24–26). Using this model, we demonstrated that following antiretroviral therapy of animals bearing thymic implants severely depleted of human thymocytes by HIV-1 infection, renewed thymopoiesis occurs from both endogenous and exogenous precursors, indicating that both thymic support and stem cell functions are preserved after exposure to a high viral burden (37). However, despite continuing antiretroviral therapy, eventual thymocyte depletion ensues in this system. Our preliminary assessment of this eventual decline included measurements of proviral burden in thymocytes and viremia of treated and untreated animals (37). These experiments suggested that complete control of viral replication was not achieved and that viral breakthrough may have contributed, at least partially, to the eventual thymic failure following therapy. If viral replication is proven to remain in check during the period of eventual T-cell decline, other reasons such as cessation of thymic stromal support function or direct or indirect compromise of thymic progenitors would need to be invoked to explain the short-lived nature of T-cell renewal. Such scenarios would have important implications for the application of pharmacology-, stem cell-, and gene-based treatment approaches to HIV-1 infection.
To study the kinetics of virus expression in thymocytes in the context of antiretroviral therapy, we used an HIV-1 reporter construct that is pathogenic in vivo and induces expression of the murine CD24 surface marker on infected cells. Thus, the kinetics of virus expression and thymocyte resurgence and decline can be studied in this system by flow cytometry (15). Despite combination therapy, we found an increase in the percentage of cells expressing virus during the late stages of infection, coincident with a second decline in thymocytes. These data indicate that viral breakthrough is associated with late thymocyte decline and that renewed virus replication may be responsible for the eventual thymic failure observed in this system.
MATERIALS AND METHODS
Preparation of virus stocks.
Stock virus was made by electroporation of 20 μg of cloned infectious DNA of the HIV-1 reporter virus NL-r-HSAL (15) into 107 CEM cells. Virus production was quantitated serially by measuring p24 in the culture supernatant. CEM cells were added to the culture daily to maintain a concentration of 106 CEM cells/ml. Heat-stable antigen (HSA) expression in CEM cells was confirmed at days 8 and 13 of culture by flow cytometry. For SCID-hu mouse implant infection, virus supernatant was collected at days 6 and 16 postelectroporation. Similarly, HIV-1 NL4-3 viral supernatant was produced as previously described (14). Mock-infected implants were injected with supernatant from mock-electroporated CEM cells.
Construction and HIV-1 infection of SCID-hu mice.
SCID-hu mice were constructed as previously described (1, 25). Two series of animals were used for these experiments. Briefly, human fetal thymus and liver were implanted under the left kidney capsule of 8.5- or 10.5-week-old SCID mice (7). Fetal tissue was obtained from donors at 16 to 24 weeks of gestation. Four and one-half or 5.5 months after engraftment, thymus-liver implants were inoculated with 50 or 85 ng of p24 of the reporter virus, NL-r-HSAL, by direct intrathymic injection of 70 μl of viral supernatant, as previously described (14). NL4-3 was injected into control implants at a dose of 5 ng of p24 in 50 μl of viral supernatant. Sequential implant biopsies were obtained at the time points indicated in the figures. All animal manipulations were performed under the guidelines and with the approval of the University of California at Los Angeles Animal Research Committee.
Antiretroviral therapy.
Antiretroviral drugs were administered as previously described (37). All drug treatments were carried out with a combination of zidovudine (AZT), didanosine (ddI), and indinavir sulfate. The approximate administered daily doses were 60 mg/kg of body weight for AZT, 225 mg/kg for indinavir, and 50 mg/kg for ddI. AZT (Aldrich Chemical Co., Milwaukee, Wis.) and indinavir (a gift from Merck Research Laboratories, Rahway, Wis.) were added to the drinking water (pH 3) at concentrations of 0.4 and 1.5 mg/ml, respectively. Based on a previous estimate of consumption of 3 ml of water daily, mice received an estimated dose of 1.2 mg of AZT and 4.5 mg of indinavir. ddI (Bristol-Myers Squibb, Princeton, N.J.) was administered by intraperitoneal injection at a dose of 1 mg/day. The drug was dissolved in 5 mM NaOH and adjusted to physiological osmolarity by addition of 1/10 of a volume of 10× phosphate-buffered saline to give a final concentration of 8 mg/ml. Drug stock solutions were prepared aseptically and filtered through a 0.2-μm-pore-size filter.
CD34+ cell preparation.
Donor CD34+ cells were purified from human fetal liver with the magnetic-activated cell sorting system (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer’s instructions. Flow cytometry on CD34+ cells was performed with a monoclonal antibody (MAb) to human CD34 (Becton Dickinson, Mountain View, Calif.) conjugated with phycoerythrin (PE). CD34+ cells from HLA-A2+ donors were cultured in the presence of stem cell factor and megakaryocyte growth and development factor (2) for subsequent injection into SCID-hu implants derived from HLA-A2− donors. Each implant received 2.5 × 105 CD34+ cells purified to greater than 80%.
Flow-cytometric analysis.
Single-cell suspensions were obtained from implant biopsies and washed with phosphate-buffered saline, and 106 cells were stained for flow cytometry with MAbs to CD4, CD8, CD45 (Becton Dickinson), and murine CD24 (HSA) (Pharmingen, San Diego, Calif.). These antibodies were directly conjugated to PE or allophycocyanin (CD4), to fluorescein isothiocyanate (FITC) (CD8 and CD45), and to biotin (HSA). Streptavidin red-613 or streptavidin tricolor (TC) was used as a second-step reagent for HSA staining. To distinguish the resurgence of thymopoiesis from endogenous versus exogenous progenitor cells, CD34+ cells derived from HLA-A2+ fetal liver tissue were injected into HLA-A2− implants. HLA-A2+ cells were detected with an immunoglobulin G1 MAb with specificity for HLA-A2 and B17. This antibody was derived from the hybridoma cell line MA2.1 (ATCC HB-54) and then purified and biotin conjugated (2). Streptavidin red-613 or streptavidin-TC was used as a second step for HLA-A2 staining. Nonspecific isotype control MAbs were included in all experiments and were used to set quadrants during data analysis. Data were acquired with a FACScan flow cytometer and analyzed with CellQuest software (Becton Dickinson). Five thousand to 10,000 events were acquired for each analysis. Forward–versus–side-scatter analysis was used to gate on the live thymocyte population.
Statistical methods.
We assessed the statistical significance of differences in outcomes at given time points by Wilcoxon’s rank sum test (because distributions contained outliers) and by analysis of variance (ANOVA) (assuming normal distributions). Significance of change over time in HSA-expressing thymocytes was evaluated by the sign test (with normal distribution not assumed [33]). To determine whether drug treatment altered the profile of outcomes over time, we evaluated the drug × time interaction term in repeated-measure ANOVA (drug × time design, assuming normal distribution). Animal mortality significantly diminished the number of observations available at late time points (see Table 1), so in ANOVA we used a program that tolerates missing data (BMDP version 5).
TABLE 1.
Kinetics of all and virus-expressing thymocyte precursors after infection with the HIV reporter virusa
| No. of wks postinfection | No. of mice
|
Mean % of CD4/CD8 cells +/− SE in:
|
P value
|
Mean % of CD4/CD8/HSA cells +/− SE in:
|
P value
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | Treated | Untreated mice | Treated mice | Rank sum test | ANOVA | Untreated mice | Treated mice | Rank sum test | ANOVA | |
| 7 | 12 | 15 | 9.7 ± 6.9 | 7.2 ± 3.7 | 13.5 ± 3.7 | 16.5 ± 2.7 | ||||
| 10 | 12 | 15 | 11.4 ± 5.6 | 41.8 ± 7.4 | 0.002 | 0.02 | 18.3 ± 3.9 | 4.6 ± 1.8 | 0.008 | 0.09 |
| 15 | 9 | 10 | 1.2 ± 0.8 | 21.5 ± 9.9 | 0.003 | 0 | 6.2 ± 3.9 | 0.003 | ||
| 18 | 5 | 6 | 7.2 ± 6.1 | 8.9 ± 2.8 | 0.796 | 17.3 ± 16.3 | 27.6 ± 12.3 | 0.195 | ||
Animals were infected at day 0, and triple-drug therapy was begun at week 8. Flow cytometry was done at week 7 for both cohorts, which were separated by intention to treat. Analysis of HSA expression in DP CD4/CD8 thymocytes for the untreated group at week 18 included three animals, as two of the five implants were completely devoid of DP thymocytes at that time point. Significance values are provided for each time point after the initiation of drug treatment (rank sum test) and for the overall drug × time interaction effect on differences in the profile of results over all time points (ANOVA).
RESULTS
Effects of antiretroviral therapy on thymic depletion.
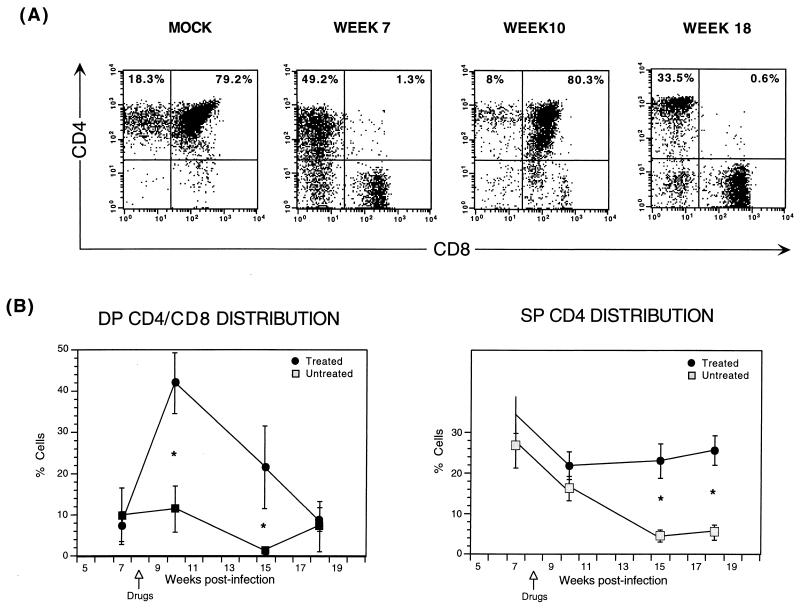
The effects of combination antiretroviral therapy following HIV-1 depletion of thymic implants have been described previously (17, 37). In summary, HIV-1 infection causes a profound loss of CD4+ thymocytes, most notably in the DP CD4/CD8 subset, which is associated with a relative increase in the percentage of single-positive (SP) CD4 cells. Antiretroviral therapy initiated following CD4 cell depletion results in a renewed burst of thymopoiesis that is sustained only for several weeks. Figure 1A illustrates this transient thymic resurgence.
FIG. 1.
Transient renewal of thymopoiesis in animals receiving antiretroviral therapy following infection with the reporter virus, NL-r-HSAL. Animals were infected with the reporter virus NL-r-HSAL, and biopsies were obtained at weeks 7, 10, 15, and 18 postinfection. Drug therapy was initiated at week 8 (arrows shown in panel B). Thymocytes were costained with CD4-PE and CD8-FITC. (A) Results of flow-cytometric analysis of CD4 and CD8 expression are shown for a representative animal that received antiretroviral therapy after DP CD4/CD8 thymocyte depletion, which was documented 7 weeks postinfection. In spite of continuing antiretroviral treatment, a secondary decline of DP CD/CD8 thymocytes occurred by week 18 postinfection. Results for a mock-infected animal are shown in the far-left plot to illustrate CD4/CD8 thymocyte distribution of an uninfected implant. The percentages of the subsets are indicated in the SP CD4 and DP CD4/CD8 quadrants. (B) Comparison of DP CD4/CD8 and SP CD4 thymocyte subset distributions of untreated and treated implants. Mean percentages of DP CD4/CD8 and SP CD4 cells and standard error bars are shown in each graph. An ∗ indicates a significant P value (see the text and Table 1). The numbers of animals analyzed at all time point are outlined in Table 1.
To correlate thymocyte depletion with viral expression, we performed kinetic experiments with thymic implants depleted by an HIV-1 reporter virus termed NL-r-HSAL, which contains the murine CD24 (HSA) gene in the deleted vpr region of the laboratory HIV-1 isolate NL4-3. Cells infected by this reporter virus express HSA on their surfaces and can be detected by flow cytometry. The construction and in vivo replication kinetics of this reporter virus have been described previously (15). Infection of thymus-liver implants with 100 infectious units of HIV-1 NL4-3 results in nearly complete CD4+ thymocyte depletion by day 30 postinfection (14). Compared to mock-infected animals, animals infected with NL-r-HSAL showed significant depletion of DP CD4/CD8 thymocytes at 28 days postinfection (mean percentages of DP CD4/CD8 thymocytes, 80% ± 1.6% [mock infected animals] and 8.4% ± 5.3% [NL-r-HSAL infected animals]). However, significant depletion of SP CD4 thymocytes compared to levels in mock-infected animals was not observed until the third biopsy time point 105 days postinfection (mock-infected animals, 25.6% ± 6.9%; NL-r-HSAL-infected animals, 4.5% ± 1.5%).
To ascertain whether the transient nature of thymopoiesis renewal observed with the wild-type NL4-3 strain (37) was also reproduced with the NL-r-HSAL reporter virus, 37 SCID-hu implants were infected with either NL-r-HSAL or mock virus by direct intraimplant injection and phenotypic analysis of thymocytes obtained from implant biopsies was performed at four time points, up to 126 days postinfection (weeks 7, 10, 15, and 18). At week 8, 15 infected animals were started on antiretroviral therapy. Thymocytes obtained from biopsy samples were costained for the human surface markers CD4 and CD8. Figure 1B shows the distribution of DP CD4/CD8 and SP CD4 cells in both treated and untreated cohorts. Table 1 shows the number of animals analyzed, the percentages of DP CD4/CD8 cells at each time point, and significance values for each time point comparison of treated and untreated groups (rank test) and for the overall drug × time interaction effect (ANOVA of drug effects over all time points). The differences in the levels of DP CD4/CD8 thymocytes at weeks 10 and 15 after infection and the overall drug × time effect were statistically significant (P values = 0.002, 0.003, and 0.02, respectively). Consistent with the precursor nature of these DP CD4/CD8 thymocytes, the difference between values for treated and untreated groups of DP thymocytes is significant at an earlier time point than that of SP CD4 thymocytes. This difference is lost at week 18 as a result of both a decrease in DP CD4/CD8 thymocytes in the treated group and an increase in DP CD4/CD8 thymocytes in the untreated group (Table 1). For SP CD4 thymocytes, the differences in the percentages of thymocytes at weeks 15 and 18 postinfection were statistically significant (P values = 0.001 and 0.02, respectively). Mean percentages of SP CD4 thymocytes ± standard errors are as follows: 4.5% ± 1.5% for untreated mice and 23% ± 4.3% for treated mice at week 15 and 5.5% ± 2.0% for untreated mice and 25.6% ± 3.7% for treated mice at week 18. The depletion nadir in the untreated group was observed at week 15, when the percentage of SP CD4 thymocytes had declined by 83% from the 7-week-postinfection level. Both treated and untreated mock-infected animals retained normal numbers and distributions of thymocytes throughout the duration of the experiment (data not shown and reference 37). Therefore, in spite of slower replication kinetics compared to those of the wild-type virus (15), a second round of depletion of DP CD4/CD8 thymocytes eventually occurs after infection with the NL-r-HSAL HIV-1 reporter virus in drug-treated animals. These data parallel our prior observations obtained with the NL4-3 viral strain (37).
Correlation between kinetics of thymocyte reconstitution and viral expression.
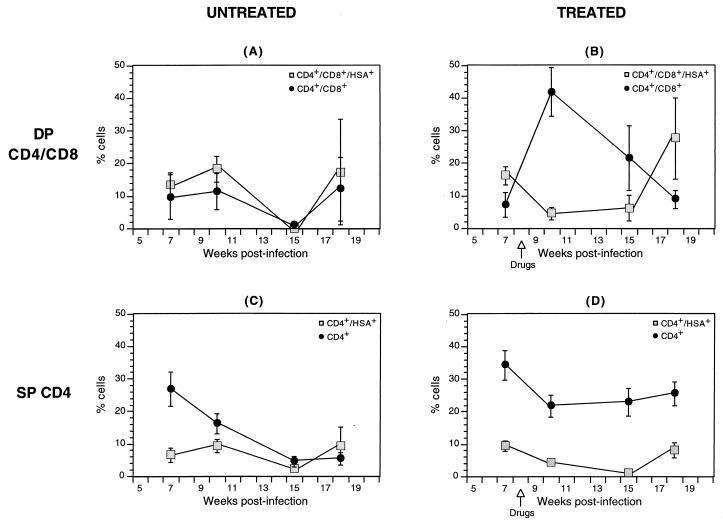
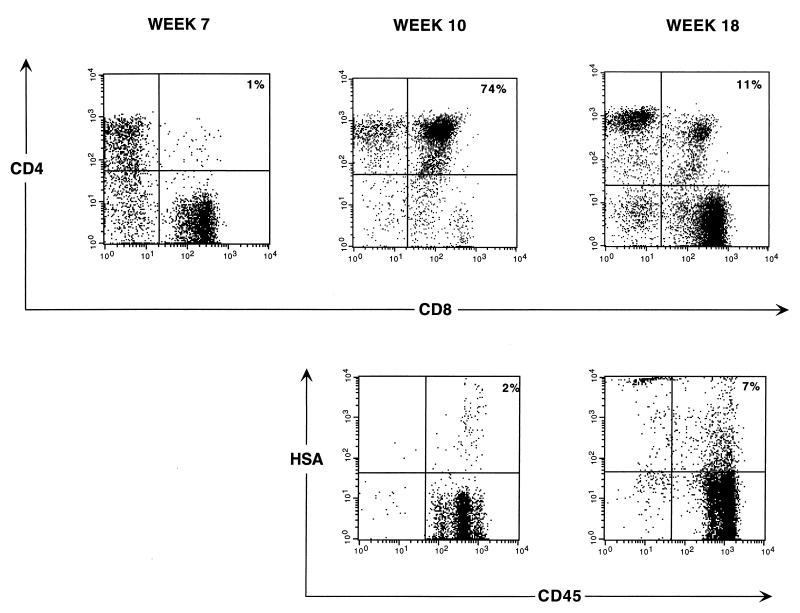
As shown above, the initially observed renewal of thymopoiesis following antiretroviral therapy in this system is not maintained for a long period. To ascertain whether this eventual thymic failure is associated with virus resurgence, we studied the patterns of virus expression in thymocytes of the above-described groups of untreated and treated animals. As indicated in Table 1, a significant difference in the percentages of DP CD4/CD8 thymocytes that expressed the reporter virus was evident at week 10 between the untreated and treated groups and was maintained at week 15 (rank sum test P values = 0.08 and 0.003, respectively). The overall drug × time effect on virus expressing DP CD4/CD8 thymocytes was also significant (ANOVA P value = 0.009). Figure 2 shows the percentages of DP CD4/CD8 and SP CD4 thymocytes in untreated and treated animals over time. To illustrate the kinetics of viral expression in each cohort, the fractions of thymocytes in each phenotypic group expressing the reporter virus are represented in parallel in the same graphs. The percentages of DP CD4/CD8 thymocytes expressing virus at each time point in untreated and treated groups are also shown in Table 1. In the DP CD4/CD8 untreated thymocytes, the percentage of cells expressing the reporter virus parallels the relative number of DP cells. A slight resurgence of DP CD4/CD8 thymocytes is observed at week 18, likely reflecting resumed thymopoiesis following the decline in virus replication associated with the loss of target cells (Fig. 2A). This resurgence is similar to what occurs very late in infection with wild-type virus (37). The profile of the DP CD4/CD8 thymocytes in the treated cohort, along with that of the fraction of these thymocytes that express the reporter virus, is depicted in Fig. 2B. At the peak of DP thymocyte resurgence (week 10), the percentage of cells that express the reporter virus reaches a nadir. However, coincidental with the subsequent decline in DP CD4/CD8 thymocytes is an increase in the proportion of these cells that express the reporter virus, with the proportion of human cells expressing HSA peaking at week 18, when the percentage of DP cells is at its lowest. This increase in DP CD4/CD8 thymocytes that express the reporter virus between weeks 15 and 18 is statistically significant (P = 0.002). Figure 3 further illustrates this resurgence of viral expression in a representative animal that displays an increase in viral expression in total CD45+ (human) cells that coincides with DP CD4/CD8 thymocyte decline. For the SP CD4 thymocyte subset, between 2.5 and 9.4% of thymocytes derived from untreated implants were found to express the HIV-1 reporter virus in this declining thymocyte group (Fig. 2C). In the treated animals, levels of SP CD4 thymocytes were comparable to those of mock-infected animals, with 8.2% ± 2.5% of these SP CD4 thymocytes expressing the reporter virus at week 18 (Fig. 2D). Taken together, these results indicate that drug-mediated resurgence of thymopoiesis is associated with inhibition of virus expression and that the eventual thymic failure observed in this model during combination antiretroviral therapy is associated with renewed viral expression, reflecting treatment failure.
FIG. 2.
Distribution of total and HSA-expressing thymocyte subsets in treated and untreated implants. The distributions of DP CD4/CD8 (A and B) and SP CD4 (C and D) thymocytes are displayed in parallel with the percentages of the subsets that express the reporter virus (shaded squares) to contrast the trend of thymocyte kinetics with that of virus expression. Implants were infected with the reporter virus NL-r-HSAL, and biopsies were obtained at weeks 7, 10, 15, and 18 postinfection. The time of initiation of drug therapy is indicated in panels B and D. Thymocytes were costained with CD4-PE, CD8-FITC, and HSA-TC. Relevant isotype control antibodies were used to set quadrants. HSA-expressing subsets were determined by gating on DP CD4/CD8 or on SP CD4 thymocytes and by analyzing HSA expression. The numbers of animals analyzed at all time points are outlined in Table 1. Significance values are provided in the text and in Table 1.
FIG. 3.
Thymocyte subset distribution and HSA expression. Thymocytes obtained from biopsy samples of a representative animal at the indicated time points postinfection were costained for CD4-PE and CD8-FITC (top panels) and for CD45-FITC (human cells) and HSA-streptavidin-TC (lower panels). The percentage of each subset is indicated in each DP quadrant. Drugs were administered at week 8 postinfection.
Analysis of viral expression after transplantation with exogenous thymocyte progenitors.
Our prior work assessing the engraftment potential of exogenous thymocyte progenitors in HIV-1-depleted thymic implants indicated that, as in the case of endogenous resurgence, engraftment was transient (37). To study how HAART influences viral expression in thymocytes derived from exogenous progenitors, we injected CD34+ cells derived from an HLA-A2+ fetal liver into HLA-A2− implants previously infected with NL-r-HSAL. HIV-1 infection in all implants was demonstrated by flow cytometry by analyzing HSA expression in thymocytes 5 weeks postinfection. CD34+ cells were injected at week 6, 2 days after half the animals had been started on antiretroviral treatment. Flow cytometry for HSA, HLA-A2, and CD4 expression was performed on thymocytes 4 weeks later (week 10 postinfection). Table 2 shows the percentages of DP CD4/CD8 thymocytes, the fractions of these thymocyte precursors expressing virus, and significance values for each time point comparison of treated and untreated (rank sum test) and for the overall drug × time interaction effect (ANOVA). A significant difference in the percentages of DP CD4/CD8 thymocyte precursors (of both donor and recipient origin) was again observed between treated and untreated groups after infection (P = 0.001). As was observed in our prior experiments, the difference between untreated and treated HSA expression in the DP CD4/CD8 thymocyte subsets at week 10 was also significant (P = 0.01). The overall drug × time effects on DP CD4/CD8 thymocytes and on virus-expressing DP CD4/CD8 thymocytes were also significant (ANOVA P values = 0.01 and 0.04, respectively).
TABLE 2.
Kinetics of all and virus-expressing thymocyte precursors after infection with the HIV reporter virus and transplantation of exogenous progenitor cellsa
| No. of wks postinfection | No. of mice
|
Mean % of CD4/CD8 thymocytes in all thymocytesb ± SE in:
|
P value
|
Mean % of CD4/CD8/HSA thymocytes in all thymocytesb ± SE in:
|
P value
|
Mean % of HLA-A2+ (donor) thymocytes +/− SE in:
|
P value (rank sum test) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | Treated | Untreated mice | Treated mice | Rank sum test | ANOVA | Untreated mice | Treated mice | Rank sum test | ANOVA | Untreated mice | Treated mice | ||
| 5 | 8 | 9 | 54.3 ± 8.7 | 55.6 ± 9.0 | 0.01 | 8.8 ± 2.1 | 12.1 ± 4.0 | 0.04 | |||||
| 10 | 7 | 9 | 3.8 ± 1.1 | 47.0 ± 7.8 | 0.001 | 12.7 ± 4.4 | 0.3 ± 0.1 | 0.01 | 53.6 ± 5.6 | 27.6 ± 9.4 | 0.03 | ||
Animals were infected at day 0, and HLA-A2-disparate CD34+ cells were injected at week 6, 2 days after initiation of triple-drug therapy. Flow cytometry was done at week 5 in both cohorts, which were separated by intention to treat. Values at week 10 are shown for all thymocytes (both donor and recipient DP CD4/CD8 and virus-expressing DP CD4/CD8) and for all thymocytes of donor origin (HLA-A2+, week 10 only). Significance values are provided for each time point after the initiation of drug treatment (rank sum test) and, from the analysis of all (donor and recipient) thymocytes, for the overall drug × time interaction effect on differences in the profile of results over all time points (ANOVA).
All thymocytes include HLA-A2+ (donor origin) and HLA-A2− (recipient origin) thymocytes.
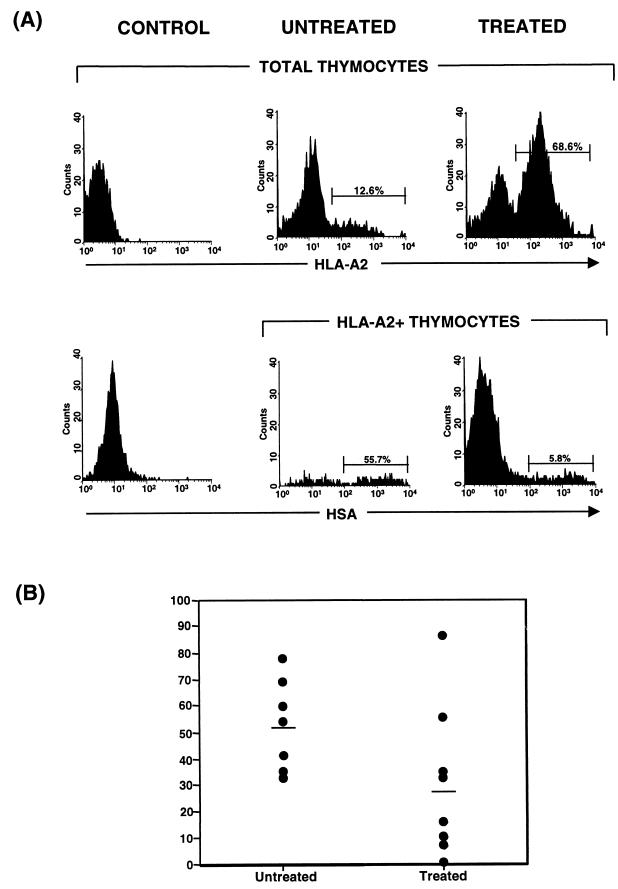
By gating in the HLA-A2+ thymocyte subset, levels of virus expression in the donor-derived thymocytes of untreated and treated cohorts were compared (Table 1). Figure 4A illustrates the results of this analysis of representative treated and untreated animals, and Fig. 4B shows the distribution of donor-derived thymocytes expressing virus for all animals evaluated (untreated, 53.6% ± 5.6%, and treated, 27.6% ± 9.4% [P = 0.03]). These results indicate that although HAART reduces the fraction of virus-expressing cells derived from both endogenous and exogenous progenitors, significant virus expression remains, even in the presence of antiretroviral therapy in this system. The fact that virus expression is present in donor-derived thymocytes is likely to account for the transient nature of progenitor engraftment observed in treated animals (37).
FIG. 4.
Distributions of virus expression in thymocytes of donor origin in treated and untreated implants. Implants derived from HLA-A2− fetal tissue were injected with NL-r-HSAL virus. Infection was confirmed by measuring HSA expression at week 5. At week 6, nine animals were started on antiretroviral therapy and all implants were injected with 2.5 × 105 CD34+ cells purified from an HLA-A2+ fetal liver. Costaining with HSA-FITC and HLA-A2 streptavidin-TC was performed at week 10 postinfection. (A) In the upper graphs HLA-A2 staining reveals chimeric engraftment in untreated and treated representative animals. In the lower graphs HSA expression in thymocytes derived from exogenous progenitors is shown by analyzing the HSA expression profile in the HLA-A2+ population (human cells of donor origin). Cells from a control, mock-infected, nontransplanted animal were stained in parallel with the same antibodies to set the relevant gates. (B) Distributions of HSA expression in donor-derived thymocytes of untreated and treated implants. Numeric values indicate the means of all data points for treated and untreated implants. Significance values are provided in the text and in Table 2.
DISCUSSION
The ability of the immune system to regenerate in HIV-1-infected patients undergoing treatment with HAART is a subject of intense study. The available results to date indicate that following an increase in memory CD4+ cells and a reduction in T-cell activation parameters, HIV-1-positive patients on protease inhibitor-containing multidrug regimens experience an increase in naive CD4+ and CD8+ cells, with there being partial restoration of CD4+ T-cell in vitro reactivity and improvement in cutaneous reactivity to recall antigens in about 25% of patients after 48 weeks of therapy (3, 4, 21). The extent of this immune restoration is determined in part by the ability of T-cell progenitors to differentiate into mature naive T cells in a setting where hematopoietic stem cells, lymphoid precursors, and thymic stroma are exposed to high levels of HIV-1 replication. Our prior results indicated that de novo thymopoiesis occurred in thymic implants after viral replication was controlled with HAART; however, only partial, transient restoration was achieved. These previous studies further demonstrated that longer-term reconstitution was achieved when indinavir was used instead of saquinavir (30) or A77003 (18), suggesting that better viral control was associated with a more prolonged renewal. In addition, viral load measurements in the peripheral blood of animals receiving drug therapy demonstrated measurable virus in some of these animals, suggesting that incomplete control of virus replication had resulted in eventual thymocyte decline. In our present study, we have employed an HIV-1 reporter virus to demonstrate that, as viral load measurements had previously suggested, virus replication is ongoing at later times in this model, even during HAART, and that the inability of the drug combination to fully control virus replication is likely responsible for the transient nature of the immune reconstitution observed in this system. Therefore, although it is possible that other drug combinations would result in a more durable control of HIV replication, our findings suggest that thymic reconstitution in this model is limited by drug failure.
Delayed kinetics of replication (attributed to the larger genome and potential packaging constraints) of the recombinant NL-r-HSAL virus likely account for the lack of eventual SP CD4 depletion observed in the treated cohort. We have previously shown that productive infection of the SP CD4 population is generally low, likely due to low levels of coreceptor (CXCR4) and low metabolic activity (16). However, HSA-expressing SP CD4 cells were detected in the treated group, suggesting that an eventual decline of this subset might have been observed with longer follow-up. Our analysis of virus expression in DP CD4/CD8 thymocytes in treated animals demonstrates that, although the differences between the numbers of thymocytes expressing virus in untreated and treated implants are significant at the time of maximum thymocyte resurgence (week 10), the eventual decline of thymocytes is associated with a significant rise in the percentage of these cells that express the reporter virus. These results argue that failure of the drug regimen to control virus replication in the long term rather than failure of the stem cell or thymic support functions is probably responsible for the eventual thymocyte decline. The presence of recoverable virus from patients undergoing HAART has been demonstrated (8, 10, 20, 38). These viruses generally do not show mutations associated with resistance to the relevant antiretroviral drugs, reflecting the inability of drug therapy to completely suppress drug-sensitive virus. Although we cannot rule out the development of drug resistance during our present experiments, our prior sequencing results of virus recovered from treated and depleted implants showed no mutations associated with resistance to the relevant protease inhibitor (37). This situation is analogous to what has been observed in some patients with virus breakthrough in the presence of triple-drug therapy (10a). Drug failure in the absence of mutations affecting resistance to any of the drugs of a HAART regimen was demonstrated for 22% of patients in one study, and lack of compliance is likely to account for at least a fraction of these failures (12, 35). While lack of compliance is unlikely in the SCID-hu model, an explanation for the uniform development of drug failure observed in this system is that virus replication is not controlled by drug therapy once the number of permissive cells increases over a threshold. Our analysis of virus expression in donor-derived thymocytes after transplantation of infected implants with exogenous fetal liver progenitors revealed that although treated implants contain statistically significantly lower numbers of thymocytes expressing virus than untreated implants, substantial virus expression was detected in donor cells in the treated group, which probably accounts for the rapid decline of donor cells observed in this system.
Recently, measurements of excisional DNA products of T-cell receptor gene rearrangement have shown that treatment of HIV-infected adults with HAART is associated with a rapid and sustained increase in thymic output, which inversely correlates with viremia. Consistent with our findings, resurgence of viremia in infected adult subjects was associated with a secondary decline in recent thymic emigrants in the periphery (9). While these clinical findings do not establish whether this decline was due to peripheral destruction of thymic emigrants versus a decline in thymic output, our results suggest that the effects of HIV in the thymus may influence the kinetics of naive T-cell reconstitution.
The SCID-hu mouse model infected with a reporter HIV strain represents a uniquely useful tool for in vivo study of drug susceptibility. While the results of our present experiments do not formally rule out all potential effects of HIV on thymopoiesis, such as depletion of progenitor cells or stromal dysfunction, consistent with clinical findings (9), our results demonstrate that virus resurgence contributes to the transient nature of naive T-cell reconstitution observed during antiretroviral therapy and argue that with better control of viral replication, more durable immune reconstitution may result. As virus is observed in thymocytes arising from exogenous progenitors, more sustained T-lymphoid engraftment may also be observed with better virus control. The use of stem cell gene therapeutic strategies against HIV-1 may render developing thymocytes resistant to virus replication. Hence, the use of strategies that combine pharmacological and gene therapies may be associated with longer-term donor-derived thymopoiesis.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI36554, 36059, and HL55205. J.A.Z. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. HIV-1 infection of the SCID-hu mouse: an animal model for virus pathogenesis. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 2.Amado R G, Symonds G, Jamieson B D, Zhao G, Rosenblatt J D, Zack J A. Effects of megakaryocyte growth and development factor on survival and retroviral transduction of T lymphoid progenitor cells. Hum Gene Ther. 1998;9:173–183. doi: 10.1089/hum.1998.9.2-173. [DOI] [PubMed] [Google Scholar]
- 3.Angel J B, Kumar A, Parato K, Filion L G, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard J M, Cameron D W. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 5.Bertho J M, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol. 1997;179:30–40. doi: 10.1006/cimm.1997.1148. [DOI] [PubMed] [Google Scholar]
- 6.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 7.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 8.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 10.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 10a.Havlir, D., and D. Richman. Personal communication.
- 11.Heitger A, Neu N, Kern H, Panzer-Grümayer E R, Greinix H, Nachbaur D, Niederwieser D, Fink F M. Essential role of the thymus to reconstitute naïve (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–857. [PubMed] [Google Scholar]
- 12.Hirsch M S, Conway B, D’Aquila R T, Johnson V A, Brun-Vézinet F, Clotet B, Demeter L M, Hammer S M, Jacobsen D M, Kuritzkes D R, Loveday C, Mellors J W, Vella S, Richman D D. Antiretroviral drug resistance testing in adults with HIV infection: implication for clinical management. International AIDS Society—USA Panel. JAMA. 1998;279:1984–1991. doi: 10.1001/jama.279.24.1984. [DOI] [PubMed] [Google Scholar]
- 13.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B, Gao L, Bloch L M, Chen I S Y, Zack J A. Requirement of HIV-1 nef for in vivo replication and pathogenesis. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson B D, Zack J A. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J Virol. 1998;72:6520–6526. doi: 10.1128/jvi.72.8.6520-6526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koka P S, Fraser J K, Bryson Y, Bristol G C, Aldrovandi G M, Daar E S, Zack J A. Human immunodeficiency virus inhibits multilineage hematopoiesis in vivo. J Virol. 1998;72:5121–5127. doi: 10.1128/jvi.72.6.5121-5127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kort J J, Bilello J A, Bauer G, Drusano G L. Preclinical evaluation of antiviral activity and toxicity of Abbott A77003, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1993;37:115–119. doi: 10.1128/aac.37.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 20.Lafeuillade A, Chollet L, Hittinger G, Profizi N, Costes O, Poggi C. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/mL. J Infect Dis. 1998;177:235–238. doi: 10.1086/517362. [DOI] [PubMed] [Google Scholar]
- 21.Lederman M M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 22.Mackall C L, Fleisher T A, Brown M R, Andrich M P, Chen C C, Feuerstein I M, Horowitz M E, Magrath I T, Shad A T, Steinberg S M, Wexler L H, Gress R E. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 23.McCune J M, Loftus R, Schmidt D K, Carroll P, Webster D, Swor-Yim L B, Francis I R, Gross B H, Grant R M. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Investig. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 25.Namikawa R, Kaneshima H, Lieberman M, Weissman I L, McCune J M. Infection of SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 26.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 28.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 29.Perelson A S, Essunger P, Ho D D. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS. 1997;11(Suppl. A):S17–S24. [PubMed] [Google Scholar]
- 30.Pettoello-Mantovani M, Kollmann T R, Raker C, Kim A, Yurasov S, Tudor R, Wiltshire H, Goldstein H. Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: an in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob Agents Chemother. 1997;41:1880–1887. doi: 10.1128/aac.41.9.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Simpson J G, Gray E S, Beck J S. Age involution in the normal human adult thymus. Clin Exp Immunol. 1975;19:261–265. [PMC free article] [PubMed] [Google Scholar]
- 33.Sprent P. Applied nonparametric statistical methods. New York, N.Y: Chapman and Hall; 1989. [Google Scholar]
- 34.von Gaudecker B. Ultrasound of the age-involuted adult human thymus. Cell Tissue Res. 1978;186:507–525. doi: 10.1007/BF00224939. [DOI] [PubMed] [Google Scholar]
- 35.Wainberg M A, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 36.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 37.Withers-Ward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I S Y, Zack J A. Transient renewal of thymopoiesis in HIV infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 38.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]