Abstract
Large numbers of polymorphonuclear leukocytes (PMNs) infiltrated the murine vaginal mucosa within 24 h after intravaginal inoculation with an attenuated strain of herpes simplex virus type 2 (HSV-2). The role of these cells in resolution of a primary genital infection and in protection of HSV-immune animals against challenge with a fully virulent HSV-2 strain was investigated. Depletion of greater than 95% of the PMNs at the vaginal mucosal surface prior to intravaginal inoculation with an attenuated HSV-2 strain resulted in significantly higher virus titers on days 3 to 7 but only slightly delayed resolution of the primary genital infection. These results suggest that neutrophils helped control the infection but that other immune mechanisms ultimately cleared the virus. Interestingly, depletion of PMNs from HSV-immune mice prior to challenge with a fully virulent HSV-2 strain resulted in a rise in virus titers to levels comparable to those of nonimmune mice and a more pronounced diminution of virus clearance from the vaginal mucosa despite the presence of HSV-specific B and T cells. Levels of gamma interferon (IFN-γ) and HSV-specific antibody were comparable in neutrophil-depleted and control-treated immune mice following HSV-2 challenge, suggesting that RB6-8C5 treatment did not impair T- and B-cell function. Therefore, these results suggest that neutrophils play a role in limiting and clearing HSV-2 vaginal infections and that they are, in association with HSV-specific B and T cells, an important component in immune protection of the vaginal mucosa.
Herpes simplex virus type 2 (HSV-2) typically initiates infection of humans at mucosal membranes. The virus replicates within epithelial cells, ascends sensory neurons, and establishes a latent infection within the sensory ganglia, thereby ensuring a lifelong infection of its host (10, 33). Periodic reactivation of latent HSV-2 may result in clinical disease with the formation of recurrent lesions at the epithelial surface or asymptomatic shedding, which increases the chances of spread to new individuals (34). The lesions which develop following symptomatic genital HSV-2 infection are not only painful but can also serve as portals of entry for other sexually transmitted pathogens, such as human immunodeficiency virus (11, 40). Effective vaccines are clearly needed to protect the genital mucosa and sensory ganglia from infection in order to prevent the establishment of latent HSV-2 infections and spread of HSV disease. However, much remains to be learned about the immune mechanisms which protect these sites.
In experimental animals, genital inoculation with attenuated strains of HSV-2 results in immune protection against subsequent HSV-2 exposure and serves as a useful model for examining the immune mechanisms protecting the vaginal mucosa and sensory ganglia (16, 20, 24, 31). Using a mouse model of genital inoculation with a thymidine kinase-deficient strain of HSV-2 (HSV-2 TK−) as a paradigm for an effective vaccine, we have previously shown that clearance of HSV-2 from the vaginal mucosae of normal mice is T cell dependent and is mediated primarily by mechanisms involving CD4+ T cells (18, 19). Although virus clearance is likely influenced by several cytokines, including gamma interferon (IFN-γ) (19, 20), the exact mechanisms responsible for resolution of HSV-2 genital infections are not well understood.
Polymorphonuclear leukocytes (PMNs) have long been recognized as a first line of defense in protection against pyogenic bacteria and fungi. However, their role in the resolution of infections involving facultative intracellular bacteria (6, 38) and viruses (35, 36) is also increasingly appreciated. Neutrophils represent the predominant leukocyte population in the vaginal epithelium (21), and they have been suggested to play a role in protection against genital infection with sexually transmitted pathogens, such as Chlamydia trachomatis (1). In this study, we demonstrated that large numbers of PMNs (primarily neutrophils) infiltrated the vaginal mucosa by 24 h after HSV-2 inoculation. Depletion of neutrophils prior to primary genital HSV-2 inoculation resulted in significantly higher virus titers over a period of 4 days but only slightly delayed resolution of the infection. In contrast, depletion of neutrophils from HSV-immune mice prior to challenge resulted in a more dramatic decrease in the ability to clear HSV-2 from the vagina, despite the presence of HSV-specific T and B cells. These results provide evidence that neutrophils play a role in clearance of HSV-2 from the genital mucosa. Further, the surprising dependence of HSV-immune mice on neutrophil-mediated protection during the first few days after challenge highlights the interactions among many cell types, both adaptive and innate, in immune protection of the genital tract against viral pathogens.
MATERIALS AND METHODS
Virus.
The thymidine kinase-deficient HSV-2 strain 333 (HSV-2 TK−) (30) and the fully virulent HSV-2 strain 186 were obtained originally from Lawrence Stanberry (Children’s Hospital Medical Center, Cincinnati, Ohio). Working stocks of both strains were prepared by infection of Vero cell monolayers at a multiplicity of infection of 0.01, release of virus by three cycles of freeze-thaw, and storage of the clarified virus preparation at −70°C as described previously (18).
Mice.
Six- to 8-week-old outbred Swiss Webster mice were obtained from Harlan Sprague-Dawley (Indianapolis, Ind.) and housed in sterile microisolator cages. The Children’s Hospital Research Foundation animal facility is approved by the American Association for the Accreditation of Laboratory Animal Care.
Intravaginal inoculation of mice.
Mice were immunized by intravaginal inoculation with 5 × 105 PFU of HSV-2 TK− or challenged intravaginally with 5 × 104 PFU of HSV-2 186 by a modification of the procedure described previously (20). The vaginal epithelium was prepared for inoculation by injecting the mice subcutaneously twice in a 1-week period with 3.0 mg of medoxyprogesterone acetate (The Upjohn Company, Kalamazoo, Mich.). Mice under sodium pentobarbital anesthesia were inoculated by swabbing with a calcium alginate swab followed by instillation of 20 μl of medium containing the desired HSV-2 inoculum into the vaginal lumen.
In vivo depletion of neutrophils.
Mice were depleted of neutrophils by intraperitoneal injection of 0.5 mg of the granulocyte-specific monoclonal antibody RB6-8C5 (9) (obtained from Robert Coffman, DNAX Research Institute, Palo Alto, Calif.). The antibody was partially purified as described previously (19, 20) by ammonium sulfate precipitation of serum-free hybridoma culture supernatants. For neutrophil depletion during primary vaginal infection, antibody treatments began either the day prior to (day −1) or 2 days after (day +2) virus inoculation and continued every other day through day 8 postinoculation. For neutrophil depletion in HSV-immune mice, antibody treatments began 2 days prior to virus challenge and continued every other day through day 8 postchallenge. Control mice received 0.5 mg/dose of the isotype-matched rat-immunoglobulin G (IgG) monoclonal antibody SFR8-B6 (anti-HLA-Bw6). Neutrophil depletion at the vaginal mucosal surface was assessed by determining viable and differential cell counts of leukocytes obtained by vaginal lavage. Briefly, the vaginal vault was washed three times with 60 μl of Hank’s balanced salt solution with 5% newborn calf serum. Viable leukocyte numbers were obtained from hemocytometer counts of lavage cells which excluded the viable strain trypan blue. To obtain differential cell counts, an aliquot of the lavage fluid was spun onto glass slides and stained with a differential stain kit (Hema 3; Fisher Scientific Co., Pittsburgh, Pa.). A minimum of 100 cells/slide were counted to obtain the percentages of neutrophils, monocytes, and lymphocytes in the vaginal-lavage sample. Total numbers of viable neutrophils were estimated by the following formula: total number of viable lavage cells × percentage of lavage cells comprised of neutrophils = total viable neutrophils.
Quantification of HSV-specific IgG antibody.
Mice under methoxyflurane anesthesia were bled via the retroorbital plexus to obtain serum for antibody analysis. Vaginal secretions were collected by three successive washes of the vaginal vault with 60 μl of phosphate-buffered saline. Samples were stored frozen at −20°C and clarified by centrifugation prior to antibody quantification. For antibody quantification, a standard curve was prepared on each plate by plating a series of twofold dilutions of purified mouse IgG (Sigma, St. Louis, Mo.) in wells coated previously with anti-mouse immunoglobulin (Caltag, San Francisco, Calif.). A series of fivefold dilutions of serum samples beginning at 1:50 for immune sera or 1:20 for nonimmune sera were plated on wells coated previously with glycoprotein preparations from HSV-2-infected or uninfected cells (20). For quantification of vaginal antibody, a series of threefold dilutions of vaginal wash were plated on glycoprotein-coated wells. The plates were incubated at ambient temperature for 1 h, washed, and then developed by sequential additions of biotinylated anti-mouse IgG antibody (Southern Biotechnology, Birmingham, Ala.), peroxidase-conjugated goat anti-biotin antibody (Vector Laboratories, Birlingame, Calif.) and o-phenylenediamine dihydrochloride–hydrogen peroxide (Sigma). The optical density at 490 nm (OD490) was determined on a Thermo Max microplate reader (Molecular Devices, Sunnyvale, Calif.). Standard curves were generated, and antibody levels in unknown samples were calculated with the Softmax software program (Molecular Devices).
Quantification of IFN-γ in vaginal secretions.
Vaginal secretions were collected by vaginal lavage and the IFN-γ present was quantified by specific enzyme-linked immunosorbent assay as described previously (19, 20). Briefly, 96-well plates were coated with 50 μl of purified anti-IFN-γ (R4-6A2) at 5 μg/ml in carbonate buffer (pH 8.8) and incubated overnight at 4°C. After the plates were blocked with phosphate-buffered saline plus 5% bovine serum albumin, a series of twofold dilutions of recombinant IFN-γ (Sigma) or undiluted vaginal lavage samples were plated in duplicate and incubated overnight at 4°C. The plates were washed and incubated with rabbit anti-murine IFN-γ antibody (Biosource International, Camarillo, Calif.) followed by peroxidase-conjugated goat anti-rabbit IgG (United States Biochemical, Cleveland, Ohio). The plates were washed and developed with o-phenylenediamine dihydrochloride–peroxide in citrate buffer, followed by determination of the OD490. The limit of detection of the assay was considered to be the last concentration of recombinant IFN-γ standard which gave an OD490 value greater than the mean OD490 plus 3 standard deviations of at least 12 wells receiving only diluent and was less than 0.5 U/ml.
Statistical analysis.
The data were analyzed by one-way analysis of variance with the Bonferroni correction for multiple groups.
RESULTS
We previously used a murine model of intravaginal inoculation with a TK− strain of HSV-2 to examine the immune mechanisms which protect the genital mucosa. Although intravaginal inoculation with fully virulent HSV-2 normally results in death due to encephalitis, HSV-2 TK− does not replicate well in neurons (30) and is cleared from the vaginae of nonimmune mice within 6 to 7 days of inoculation (19). Mice immunized intravaginally with HSV-2 TK− develop immune responses which do not prevent reinfection but do result in rapid clearance of fully virulent HSV-2 strains from the vagina (16, 20, 24). Virus clearance is T cell dependent and is primarily mediated by Th1-type CD4+ T cells. IFN-γ is important in rapid clearance of HSV-2 TK− from the vaginae of normal mice as well as in the protection of the vaginal mucosae of HSV-immune mice (19, 20), although the exact mechanism responsible for this protection is not understood.
Neutrophil infiltration into the vaginal mucosae of nonimmune mice following intravaginal inoculation with HSV-2 TK−.
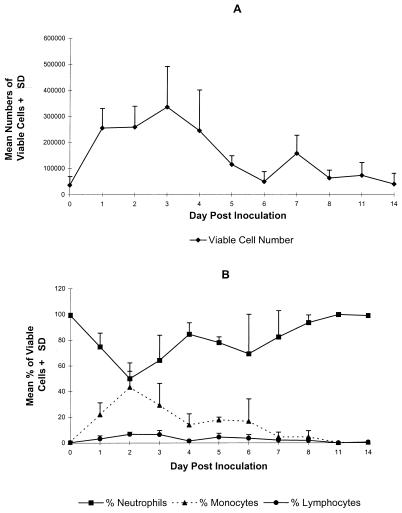
We previously showed that HSV-specific T cells infiltrated the vaginal mucosae of nonimmune mice by day 5 after inoculation (18). In the present studies we examined the vaginal mucosa at earlier times after HSV-2 TK− inoculation to identify other cell types which may be involved in the immune protection of the vaginal mucosa. A small, naturally occurring population of leukocytes was detected at the vaginal surfaces of normal mice prior to virus inoculation (day 0). However, within 24 h after inoculation, a large population of leukocytes had migrated to the vaginal surface (Fig. 1A). This cellular response was maintained through day 4 and then decreased to preinoculation levels by day 6. The majority (>95%) of leukocytes at the vaginal surfaces of uninfected mice were identified as neutrophils (Fig. 1B). The initial influx of cells at 24 h after HSV-2 inoculation was also predominantly neutrophils. However, by 48 h after inoculation the number of monocytes had increased such that approximately equal numbers of monocytes and neutrophils were present at the vaginal surface. Neutrophils predominated in the cellular response thereafter as the number of monocytes diminished and the vaginal mucosa returned to a preinoculation state. Lymphocytes were detected in the vaginal lavage by day 2 after inoculation but never constituted more than 10% of the leukocytes present in vaginal-lavage cells.
FIG. 1.
Composition of vaginal lavage cells following primary inoculation with HSV-2 TK−. Leukocytes were collected by vaginal lavage from six Swiss Webster mice on the indicated day relative to intravaginal inoculation with HSV-2 TK−. Viable cell counts (A) were obtained by trypan blue exclusion, and the percentages of neutrophils, monocytes, and lymphocytes were determined by differential staining (B). The results from one experiment of two performed are shown.
Role of neutrophils in resolution of a primary genital HSV-2 TK− infection.
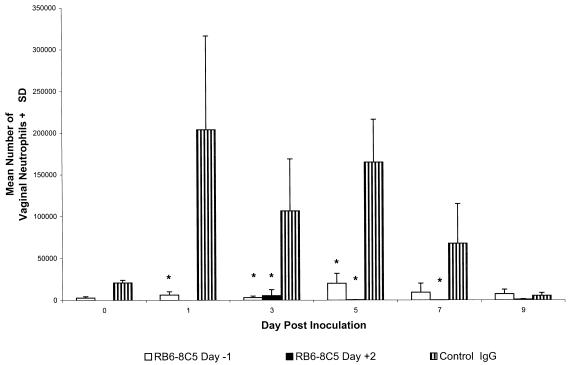
The role of neutrophils in resolution of a primary genital HSV-2 infection was examined by in vivo depletion with the granulocyte-specific monoclonal antibody RB6-8C5 (9). Outbred Swiss Webster mice were injected intraperitoneally with RB6-8C5 or control rat IgG beginning either the day before (day −1) or 2 days after (day +2) intravaginal HSV-2 TK− inoculation. The number of neutrophils at the vaginal surface rapidly increased in control IgG-treated mice following HSV-2 inoculation, remained high through day 5, and fell to preinoculation levels after day 7 as the infection was resolved (see Fig. 3). In contrast, vaginal neutrophil numbers in RB6-8C5-treated mice remained extremely low through day 9 (Fig. 2). Vaginal neutrophils in mice treated beginning day −1 were significantly reduced compared to those in control-treated mice on days 1 (P < 0.02), 3 (P < 0.01), and 5 (P < 0.001). Similarly, a significant reduction was observed on days 3 (P < 0.05), 5 (P < 0.001), and 7 (P < 0.05) in mice treated beginning day +2.
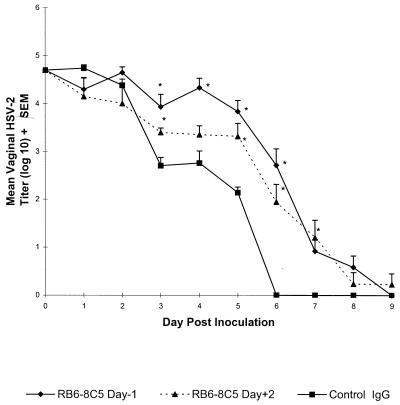
FIG. 3.
Resolution of primary genital HSV-2 infection in neutrophil-depleted Swiss Webster mice. Groups of eight Swiss Webster mice were treated with a control rat IgG monoclonal antibody or RB6-8C5 beginning 1 day before or 2 days after intravaginal HSV-2 TK− inoculation. Vaginal swabs were taken daily, and mean HSV-2 titers from each group were determined by titration on Vero cell monolayers. The values marked with asterisks differ significantly from control IgG group values (P < 0.05). The results shown are from a representative experiment of three performed. SEM, standard error of the mean.
FIG. 2.
Depletion of neutrophils from the vaginal mucosa by injection of RB6-8C5 antibody. Groups of eight Swiss Webster mice were treated with monoclonal antibody RB6-8C5 or control rat IgG (SFR8-B6; anti-HLA-Bw6) beginning the day prior to (day −1) intravaginal inoculation with HSV-2 TK− or with RB6-8C5 beginning 2 days after intravaginal inoculation (day +2). Vaginal leukocytes were collected by lavage on the days indicated relative to intravaginal HSV-2 TK− inoculation, and the number of neutrophils was determined from viable and differential cell counts as described in Materials and Methods. Neutrophil counts from mice treated with RB6-8C5 on day +2 were obtained only on days 3 to 9. Values marked with an asterisk are significantly different than values from control IgG-treated mice (P < 0.05). SD, standard deviation.
Vaginal swabs were taken daily from these mice after inoculation to assess the effect neutrophil depletion had on resolution of the primary genital infection. Results of earlier studies (20, 23) have shown that nonimmune mice pretreated with progesterone are susceptible to vaginal HSV-2 infection as detected by the presence of HSV-2 in the vagina through at least day 6 postinoculation. In contrast, no virus is detected at times greater than 24 h in mice inoculated during the estrous phase of the reproductive cycle, indicating that the infection does not take and the original inoculum does not remain viable in the mouse vagina (17). Therefore, virus titers at 24 h represent replicating virus and not the original inoculum. Vaginal HSV-2 titers in mice treated with RB6-8C5 beginning day −1 were comparable to those in control IgG-treated mice on the first 2 days after inoculation but were significantly higher than those in controls on days 3 to 6 (P < 0.001) (Fig. 3). In fact, the titers were approximately 100-fold higher on days 4 and 5 compared to those in control mice. However, resolution of the infection was delayed by only 3 days. RB6-8C5 treatment could be delayed until day 2 after inoculation and still delay the resolution of the infection. Virus titers in mice depleted of neutrophils beginning day +2 were significantly higher than those in control IgG-treated mice on days 3 and 5 to 7. Although neutrophils remained depleted in these mice through day 9 (Fig. 2), the virus was ultimately cleared in 7 of 8 RB6-8C5-treated mice by day 9.
Role of neutrophils in protection of the vaginal mucosae of HSV-immune mice.
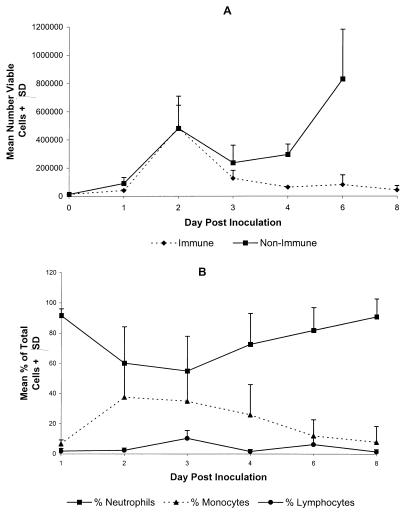
Mice immunized by intravaginal inoculation of HSV-2 TK− exhibit rapid virus clearance upon challenge with fully virulent strains of HSV-2 (16, 20, 24). The involvement of neutrophils in this protection of the vaginal mucosae of immune mice was examined. A rapid influx of leukocytes into the vaginal tract was detected following intravaginal challenge of HSV-immune mice and was similar in magnitude and cellular composition to that observed following primary inoculation of nonimmune mice (Fig. 4A). As shown previously for uninoculated mice, low numbers of leukocytes were present at the vaginal surfaces of HSV-immune mice prior to rechallenge. The number of viable leukocytes in challenged HSV-immune and nonimmune mice began increasing by 24 h postinoculation and then rose sharply by day 2. After a 2-day plateau, cell numbers rose again after day 4 in nonimmune mice. In contrast, vaginal leukocytes decreased to prechallenge levels in HSV-immune mice after day 3 as virus was cleared from the vaginal tissue (Fig. 5, experiment 1). As demonstrated previously for nonimmune mice (Fig. 1B), the cellular infiltrate in HSV-immune mice was composed primarily of neutrophils on day 1 (Fig. 4B). An influx of monocytes was detected on days 2 to 3 in HSV-immune mice, which declined through day 8 as the infection was cleared. Few lymphocytes were detected in the vaginal lumen on any day after challenge.
FIG. 4.
Magnitude and composition of the leukocyte infiltrate in the vaginae of HSV-immune mice after rechallenge with HSV-2. Vaginal washes were taken from groups of four HSV-immune or nonimmune Swiss Webster mice on the days indicated relative to HSV-2 challenge. The number of viable vaginal leukocytes (A) was obtained by trypan blue exclusion, and the percentage of vaginal leukocytes composed of neutrophils, monocytes, or lymphocytes in HSV-2-rechallenged immune mice (B) was determined by differential staining. Swelling of vaginal tissue in nonimmune mice challenged with HSV-2 strain 186 prevented taking accurate samples after day 6. The results from one experiment of two performed are shown. SD, standard deviation.
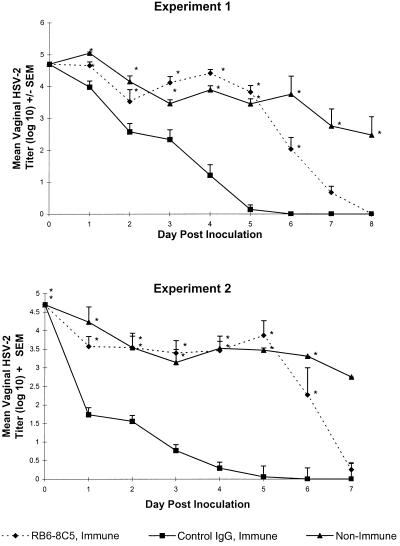
FIG. 5.
Effect of neutrophil depletion on protection of the vaginal mucosae of HSV-immune mice against HSV-2 reinfection. Swiss Webster mice were immunized by intravaginal inoculation with HSV-2 TK−. Four weeks later, groups of eight immune mice were treated with RB6-8C5 or control rat IgG monoclonal antibody. These mice and age-matched nonimmune mice were challenged intravaginally 2 days later with 5 × 104 PFU of HSV-2 186. Daily vaginal swabs were taken to quantitate HSV-2. Values marked with asterisks differ significantly from those for control IgG-treated HSV-immune mice (P < 0.001). Results from two of four experiments performed are shown. SEM, standard error of the mean.
We have shown that T lymphocytes play a very important role in the resistance of HSV-immune mice within 24 h after HSV-2 challenge (20). HSV-immune mice were depleted of neutrophils to determine if these cells were involved in this early T-cell-orchestrated protection of the vaginal mucosa. The mice were immunized by intravaginal inoculation with HSV-2 TK−. Four weeks later, immune mice were depleted of neutrophils or control treated prior to challenge with fully virulent HSV-2 strain 186. Neutrophil depletion was consistently less complete over time in HSV-immune mice. Although treatment of immune mice with RB6-8C5 resulted in depletion of greater than 95% of vaginal neutrophils on the day of virus challenge, only a 68% depletion of vaginal neutrophils was observed on day 8. As shown in Fig. 5, HSV-2 titers in the vaginae of immune mice were reduced greater than 90% on the first day after challenge compared to those in nonimmune mice, and virus was cleared from the genital mucosae by day 6. In contrast to the delay in neutrophil participation in HSV-2 clearance observed in nonimmune mice (Fig. 3), neutrophils from HSV-immune mice apparently played a role in virus clearance as soon as 24 h after HSV-2 challenge, as virus titers in neutrophil-depleted mice were significantly higher (P < 0.01) at this time than those in control-treated mice. Although HSV-2 was cleared from the vaginal mucosae of neutrophil-depleted HSV-immune mice by day 8, virus titers remained high through day 6 after challenge and exceeded those of nonimmune mice on days 3 to 5.
Effect of neutrophil depletion on HSV-specific B- and T-cell responses.
To test if RB6-8C5 treatment might have negatively affected the antigen-specific immune mechanisms necessary for rapid virus clearance in HSV-immune mice, we quantified vaginal HSV-specific antibody and IFN-γ levels in neutrophil-depleted and control-treated immune mice as a measure of B- and T-cell function. HSV-specific serum and vaginal IgG levels were comparable in neutrophil-depleted and control-treated HSV-immune mice on the day of HSV-2 challenge (P > 0.05) and titers in both groups increased through day 8 (Table 1). RB6-8C5 treatment did not diminish the local antibody response during the infection, as HSV-specific vaginal IgG levels were higher in neutrophil-depleted mice than in control-treated mice on day 8 after challenge (Table 1).
TABLE 1.
HSV-specific IgG levels in neutrophil-depleted immune mice
| Treatmenta | IgG level in:
|
||
|---|---|---|---|
| Serumb (μg/ml) on day 0 | Vaginal secretionsc (ng/ml) on:
|
||
| Day 0 | Day +8 | ||
| RB6-8C5 (HSV immune) | 35.1 ± 7.5 | 6.6 ± 2.3 | 855.9 ± 364.9 |
| Control-IgG (HSV immune) | 42.1 ± 7.1 | 30.6 ± 21.0 | 246.9 ± 59.4 |
| None (nonimmune) | 0.005 ± 0.001 | 0.05 ± 0.05 | NDd |
Swiss Webster Mice were immunized by intravaginal inoculation of 5 × 105 PFU of HSV-2 TK−. Four weeks later groups of eight mice were treated with RB6-8C5 or control IgG. Age-matched nonimmune mice were included as controls.
Serum was collected on the day of virus challenge.
Vaginal secretions were collected by vaginal lavage on the day of virus challenge and on day 8 postchallenge.
ND, not determined. Five of eight nonimmune mice died prior to day 8, and vaginal swelling in survivors precluded taking accurate samples.
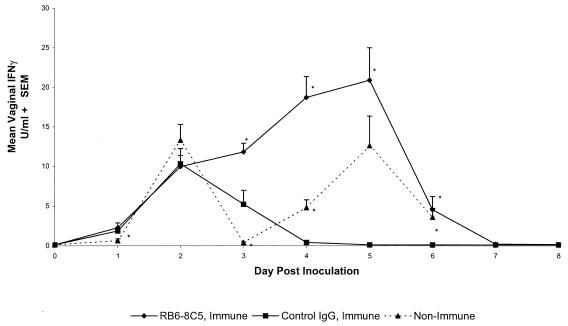
We have previously shown that T-cell-secreted IFN-γ could be detected in the vaginal secretions of HSV-immune mice by 24 h after HSV-2 challenge and that this IFN-γ was important for rapid clearance of virus (20). In the present experiments, IFN-γ was not detected in vaginal secretions of immune mice prior to HSV-2 challenge (day 0) whereas comparable levels of IFN-γ were detected in vaginal secretions of neutrophil-depleted and control-treated HSV-immune mice on days 1 and 2 after challenge (Fig. 6). IFN-γ levels in the control-treated group fell thereafter as the virus was cleared (Fig. 5, experiment 1). However, IFN-γ levels continued to rise through day 5 in RB6-8C5-treated mice, suggesting that antibody treatment did not interfere with T-cell-mediated IFN-γ production during the infection. Interestingly, HSV-2 titers remained high in the vaginal tissue of neutrophil-depleted immune mice (Fig. 5) despite the presence of high levels of IFN-γ in vaginal secretions (Fig. 6).
FIG. 6.
Production of IFN-γ in vaginal tracts of HSV-immune mice following HSV-2 rechallenge is unaffected by depletion of neutrophils. Swiss Webster mice were immunized by intravaginal inoculation with HSV-2 TK−. After 4 weeks, groups of eight mice were treated with RB6-8C5 or control rat IgG. Two days later, HSV-immune groups and age-matched nonimmune mice were challenged intravaginally with 5 × 104 PFU of HSV-2 186. Vaginal washes were taken on the indicated days relative to HSV-2 challenge, and the concentration of IFN-γ in vaginal secretions was determined by enzyme-linked immunosorbent assay. Swelling of vaginal tissue in nonimmune mice challenged with HSV-2 186 prevented taking accurate samples after day 6. The results marked with asterisks are significantly different from those of control IgG-treated mice (P < 0.05). The results of a representative experiment of three performed are shown. SEM, standard error of the mean.
DISCUSSION
Neutrophils are the most common leukocytes present in the vaginal epithelia of normal mice (5, 21). Sonoda et al. (29) demonstrated neutrophil migration into the vaginal epithelia during the metestrus-2 phase of the murine reproductive cycle, resulting from local production of the murine interleukin-8 homologue protein, macrophage inflammatory protein 2. In agreement with these findings, we detected a leukocyte population consisting predominantly of neutrophils in the vaginal lavage of progesterone-treated, uninfected mice. Additionally, large numbers of neutrophils infiltrated the vaginal mucosa within 24 h of intravaginal HSV-2 inoculation and were maintained until virus was cleared from the mucosa.
Treatment of Swiss Webster mice with RB6-8C5 antibody prior to intravaginal inoculation with an attenuated HSV-2 strain severely depleted the number of neutrophils present at the vaginal mucosal surface and resulted in significantly higher HSV-2 titers over a 4-day period compared to those in control-treated animals. Our results suggest that neutrophils contributed to the resolution of a primary HSV-2 genital infection in normal mice only after the second day postinoculation (Fig. 3). It seems unlikely that this reflects insufficient neutrophils at the site of infection during this time, since large numbers of vaginal neutrophils were detected in control-treated animals during the first 48 h of infection (Fig. 1 and 2). It is possible that this delay reflects a requirement for optimal neutrophil activation by cytokines produced by the macrophages and lymphocytes which infiltrate the vaginal mucosa later after HSV-2 inoculation (18). Interestingly, the infection was eventually cleared even though vaginal neutrophil numbers remained extremely low, suggesting that neutrophils were not strictly required for virus clearance and that other immune mechanisms resolved the infection. These results are consistent with a model in which HSV-2 infection of the vaginal epithelia initiates the early infiltration of neutrophils and macrophages into vaginal tissue followed later by antigen-specific T cells (18). Optimal neutrophil activation may require local production of cytokines, such as IFN-γ, tumor necrosis factor alpha, and granulocyte-monocyte colony-stimulating factor by infiltrating T cells and macrophages. Virus clearance and resolution of the primary infection may then be mediated, at least in part, by activated neutrophils. Although such a mechanism may be important for quick resolution of the infection, alternative immune mechanisms mediated by macrophages or HSV-specific CD4+ and CD8+ T lymphocytes ultimately eliminate the infection.
The delay in clearance of HSV-2 from the vaginal mucosae of neutrophil-depleted mice is similar to the results of Tumpey et al. (36) and Thomas et al. (35), in which replication of HSV-1 was prolonged in the corneas of neutrophil-depleted BALB/c mice. The rapid neutrophil infiltration documented in this study extends their results to suggest that migration of neutrophils to HSV-infected tissue is a common event not dependent on the inoculation site. Interestingly, Thomas et al. (35) documented two distinct phases of neutrophil infiltration into the eye following HSV-1 inoculation. Although the first phase provided protection, the second influx of neutrophils was implicated along with CD4+ T cells in tissue destruction. While the results of the current study demonstrate the protective function of vaginal neutrophils against HSV-2 infection, the occurrence and extent of any coincidental genital-tissue damage due to the presence of large numbers of activated granulocytes was not determined. Perineal scarring is a relatively common event following resolution of primary HSV-2 infection in mice (39, 42). The extent to which neutrophils may be involved in this damage of perivaginal or other genital tissue is not known and will be the subject of future investigation.
Although neutrophil depletion diminished the ability of nonimmune mice to resolve a primary HSV-2 TK− infection, these cells appeared to be very important for protection of the vaginal mucosae of immune mice against challenge with a fully virulent HSV-2 strain. Importantly, virus in neutrophil-depleted immune mice quickly replicated to levels comparable to those in nonimmune mice despite the presence of HSV-specific antibody and IFN-γ in vaginal secretions at levels comparable to those in control-treated immune mice. These results strongly suggest that the diminished protection was directly due to a loss of neutrophil effector function rather than an unintentional alteration of antigen-specific B- or T-cell function. The exact mechanism by which neutrophils clear HSV-2 is currently unknown but may include phagocytosis of free virions or virus-infected cells (2, 37), release of antiviral cytokines (3) or defensins (7, 8), and antibody-dependent cell-mediated cytolysis of HSV-infected cells (22, 28). Additionally, given the ability of human neutrophils to secrete cytokines, including interleukin-12 (4) and IFN-γ (43), local release of these cytokines by tissue neutrophils may help bias immune responses towards the development of protective Th1 responses.
The depletion of vaginal neutrophils decreased over time in HSV-immune mice, ranging from 95% on the day of challenge to 68% on day 8. It is possible that the clearance of virus in RB6-8C5-treated immune mice was ultimately due to either the presence of increasing numbers of neutrophils or an influx of antigen-specific effector T cells. Therefore, a strict requirement for neutrophils to completely resolve the infection in HSV-immune mice remains speculative. Nonetheless, the presence of 100- to 1,000-fold-higher HSV-2 titers in neutrophil-depleted mice than in control-treated mice during the first few days after challenge demonstrates the importance of these cells in protection of the vaginal mucosa and underscores the importance of the innate arm of the immune response in protection against viral pathogens.
HSV-2 titers remained high in neutrophil-depleted, HSV-immune mice even in the prolonged presence of high concentrations of IFN-γ in the vaginal tract (Fig. 5 and 6). These results suggest that the main protective effect of IFN-γ in this model was most likely due to its ability to activate immune cells such as infiltrating neutrophils rather than to a direct antiviral effect (14). Other cytokines known to activate PMNs, such as tumor necrosis factor alpha and granulocyte-monocyte colony-stimulating factor (32), are most likely also involved in activation of neutrophils in this model. Release of these cytokines by HSV-specific memory T cells following recognition of HSV antigens may fully activate infiltrating neutrophils, resulting in increased oxygen metabolism and production of microbicidal enzymes (27, 41), increased phagocytosis (13, 15, 26), expression of high-affinity Fc receptors (25), and increased cytotoxicity (25). In this regard, we have shown that HSV-specific memory T cells reside in the vaginal mucosa following intravaginal inoculation with HSV-2 TK− (18). The release of activating cytokines by memory T cells soon after virus challenge may explain why neutrophils were active early after challenge of immune mice (Fig. 5) but not nonimmune mice (Fig. 3).
The significance of neutrophils in defense against human genital HSV-2 infection is not well understood. Neutrophils have been detected as part of the immune cell infiltrate into herpetic lesions (12). Further, degraded virions were detected by electron microscopy in the lysosomes of human neutrophils present within a recurrent lesion (2). Therefore, it seems possible that neutrophils play an active role in HSV-2 clearance or in limiting the spread of virus in humans. In the murine model of genital HSV-2 infection, it is possible that the neutrophil-dependent protection we observed is one manifestation of the protection orchestrated by HSV-specific T cells. Given the quick onset of neutrophil-dependent protection in immune mice following HSV-2 challenge (Fig. 5), these cells may help restrict virus spread and mediate virus clearance prior to the arrival of large numbers of effector T lymphocytes from the regional lymph nodes. As a result, less virus may gain access to the sensory neurons and therefore the number of latently infected neurons may be limited. In this regard, neutrophils have been suggested to restrict HSV access to the peripheral and central nervous systems after HSV-1 ocular inoculation (35, 36). Studies are under way to further elucidate the role of these cells in protection of the genital tract and the mechanisms by which they exert their antiviral activity.
ACKNOWLEDGMENTS
I thank Kristen Dudley for expert technical assistance and Nigel Bourne and Lawrence Stanberry for critical review of the manuscript.
This work was supported by the Gamble Center for Infectious Diseases and National Institutes of Health Grant AI 42815.
REFERENCES
- 1.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boddingius J, Dijkman H, Hendriksen E, Schift R, Stolz E. HSV-2 replication sites, monocyte and lymphocytic cell infection and virion phagocytosis by neutrophils, in vesicular lesions on penile skin. Electronoptical studies of a biopsy. J Cutan Pathol. 1987;14:165–175. doi: 10.1111/j.1600-0560.1987.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 3.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 4.Cassatella M A, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 5.Champlin A K, Dorr D L, Gates A H. Determining the stage of estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1974;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 6.Conlan J W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daher K A, Selsted M E, Lehrer R I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hestdal K, Ruscetti F W, Ihle J N, Jacobsen S E W, Dubois C M, Kopp W C, Longo D C, Keller J R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 10.Hill T J. Herpes simplex virus latency. In: Roizman B, editor. The herpesviruses. Vol. 3. New York, N.Y: Plenum Press; 1985. p. 175. [Google Scholar]
- 11.Holmberg S D, Stewart J A, Gerber A R. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA. 1988;259:1048–1050. [PubMed] [Google Scholar]
- 12.Huff J C, Krueger G G, Overall J C, Copeland J, Spruance S L. The histopathologic evolution of recurrent herpes simplex labialis. J Am Acad Dermatol. 1981;5:550–557. doi: 10.1016/s0190-9622(81)70115-4. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff S J, Vadas M A, Harlan J M, Sparks L H, Gamble J R, Agosti J M, Waltersdorph A M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–4225. [PubMed] [Google Scholar]
- 14.Klotzbucher A, Mittnacht S, Kirchner H, Jacobsen H. Different effects of IFN-γ and IFNα/β on “immediate early” gene expression of HSV-1. Virology. 1990;179:487–491. doi: 10.1016/0042-6822(90)90322-i. [DOI] [PubMed] [Google Scholar]
- 15.Lopez A D, Williamson D J, Gamble J R, Begley C G, Harlan J M, Klebanoff S J, Waltersdorph A, Wang G, Clark S C, Vadas M A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Investig. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott M R, Smiley J R, Leslie P, Brais J, Rudzroga H E, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan, G. N. Unpublished data.
- 18.Milligan G N, Bernstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 19.Milligan G N, Bernstein D I. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 20.Milligan G N, Bernstein D, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 21.Nandi D, Allison J P. Characterization of neutrophils and T lymphocytes associated with the murine vaginal epithelium. Reg Immunol. 1994;5:332–338. [PubMed] [Google Scholar]
- 22.Oleske J M, Ashman R B, Kohl S, Shore S L, Starr S E, Wood P, Nahmias A J. Human polymorphonuclear leucocytes as mediators of antibody dependent cellular cytotoxicity to herpes simplex virus-infected cells. Clin Exp Immunol. 1977;27:446–453. [PMC free article] [PubMed] [Google Scholar]
- 23.Parr M B, Kepple L, McDermott M R, Drew M D, Bozzola J J, Parr E L. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Investig. 1994;70:369–380. [PubMed] [Google Scholar]
- 24.Parr M B, Parr E L. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998;72:2677–2685. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perussia B, Kobayaski M, Rossi M E, Anegon I, Trinchieri G. Immune interferon enhances properties of human granulocytes: role of Fc receptors and effects of lymphotoxin, tumor necrosis factor, and granulocyte macrophage colony-stimulating factor. J Immunol. 1987;138:765–774. [PubMed] [Google Scholar]
- 26.Shalaby M R, Aggarwal B B, Rinderknecht E, Sveersky L P, Findle B S, Palladino M A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985;135:2069–2073. [PubMed] [Google Scholar]
- 27.Shalaby M R, Palladino M A, Hirabayashi S E, Eassalu T E, Lewis G D, Shephard H M, Aggarwal B B. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J Leukoc Biol. 1987;41:196–204. doi: 10.1002/jlb.41.3.196. [DOI] [PubMed] [Google Scholar]
- 28.Siebens H, Tevethia S S, Babior B M. Neutrophil-mediated antibody-dependent killing of herpes simplex-virus infected cells. Blood. 1979;54:88–94. [PubMed] [Google Scholar]
- 29.Sonoda Y, Mukaida N, Wang J-B, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–6165. [PubMed] [Google Scholar]
- 30.Stanberry L R, Kit S, Myers M G. Thymidine kinase-deficient herpes simplex virus type 2 genital infection in guinea pigs. J Virol. 1985;55:322–328. doi: 10.1128/jvi.55.2.322-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanberry L R, Bernstein D I, Kit S, Myers M G. Genital reinfection after recovery from initial genital infection with herpes simplex virus type 2 in guinea pigs. J Infect Dis. 1986;153:1055–1061. doi: 10.1093/infdis/153.6.1055. [DOI] [PubMed] [Google Scholar]
- 32.Steinbeck M J, Roth J A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989;11:549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- 33.Stevens J G, Cook M L. Latent herpes simplex in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 34.Stevens J G. Human herpesvirus: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 36.Tumpey T M, Chen S H, Oakes J E, Lausch R N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Strijp J A, Van Kessel K P, van der Tol M E, Fluit A C, Snippe H, Verhoef J. Phagocytosis of herpes simplex virus by human granulocytes and monocytes. Arch Virol. 1989;104:287–298. doi: 10.1007/BF01315550. [DOI] [PubMed] [Google Scholar]
- 38.Vassiloyanakopoulos A B, Okamoto S, Fierer J. The crucial role of polymorphonuclear leukocytes in resistance to Salmonella dublin infections in genetically susceptible and resistant mice. Proc Natl Acad Sci USA. 1998;95:7676–7681. doi: 10.1073/pnas.95.13.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walz M A, Price R W, Hayashi K, Katz B J, Notkins A L. Effect of immunization on acute and latent infections of vaginouterine tissue with herpes simplex virus types 1 and 2. J Infect Dis. 1977;135:744–752. doi: 10.1093/infdis/135.5.744. [DOI] [PubMed] [Google Scholar]
- 40.Wasserheit J N. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 41.Weisbart R H, Gholde D W, Clark S C, Wang G G, Gasson J H C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314:361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- 42.Wrzos H, Rapp F. Experimental model for activation of genital herpes simplex virus. J Infect Dis. 1985;151:349–354. doi: 10.1093/infdis/151.2.349. [DOI] [PubMed] [Google Scholar]
- 43.Yeaman G R, Collins J E, Currie J K, Guyre P M, Wira C R, Fanger M W. IFN-γ is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]