Abstract
BACKGROUND
The outflow of pancreatic juice into the duodenum is often impaired in pancreatic inflammatory diseases. The basis of interventional treatment in these cases is anatomical transpapillary access of the main pancreatic duct during endoscopic retrograde cholangiopancreatography (ERCP), which ensures the physiological outflow of pancreatic juice into the lumen of the digestive tract. However, in some patients, anatomical changes prevent transpapillary drainage of the main pancreatic duct. Surgery is the treatment of choice in such cases.
AIM
To evaluate the effectiveness and safety of endoscopic pancreaticogastrostomy under endoscopic ultrasound (EUS) guidance.
METHODS
Retrospective analysis of treatment outcomes of all patients with acute or chronic pancreatitis who underwent endoscopic pancreatogastric anastomosis under EUS guidance in 2018-2023 at the Department of General, Gastroenterological and Oncological Surgery, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, Poland.
RESULTS
In 9 patients [7 men, 2 women; mean age 53.45 (36-66) years], endoscopic pancreatogastric anastomosis under EUS guidance was performed because of the lack of transpapillary access during ERCP. Narrowing of the main pancreatic duct at the head of the pancreas was observed in 4/9 patients (44.44%). Pancreatic fragmentation (disconnected pancreatic duct syndrome) was diagnosed in 3/9 patients (33.33%). In 2/9 patients (22.22%), narrowing of the pancreatoenteric anastomosis was observed after pancreaticoduodenectomy. Technical success of endoscopic pancreaticogastrostomy was observed in 8/9 patients (88.89%). Endotherapeutic complications were observed in 2/9 patients (22.22%). Clinical success was achieved in 8/9 patients (88.89%). The mean follow-up period was 451 (42-988) d. Long-term success of endoscopic pancreatogastric anastomosis was achieved in 7/9 patients (77.78%).
CONCLUSION
Endoscopic pancreaticogastrostomy under EUS guidance is an effective and safe treatment method, especially in the absence of transpapillary access to the main pancreatic duct.
Keywords: Pancreaticogastrostomy, Chronic pancreatitis, Acute pancreatitis, Disconnected pancreatic duct syndrome, Endotherapy
Core Tip: This study evaluated the effectiveness of an endoscopically created anastomosis between the stomach and main pancreatic duct for the treatment of chronic pain in the course of inflammatory diseases of the pancreas. If basic endoscopic treatment in the form of endoscopic retrograde cholangiopancreatography is ineffective or impossible to perform, creation of an anastomosis between the digestive tract and the pancreatic duct enables the effective treatment of chronic pain without the need for surgical intervention.
INTRODUCTION
Chronic pancreatitis (CP) is a serious condition that significantly affects patient quality of life. This recurrent inflammatory process causes irreversible reconstruction of the pancreatic parenchyma into fibrous connective tissue, leading to progressive exocrine and endocrine organ failure[1]. In addition to parenchymal remodeling, narrowing and widening of the main pancreatic duct (MPD) occurs, inside which deposits may form[2]. The resulting strictures cause difficulties in the outflow of pancreatic juice, resulting in an increase in the hydrostatic pressure at the MPD[1]. owing to the different etiologies of CP, morphological changes in the pancreas may be expressed differently. As a result, complaints of pain associated with this disease are characterized by considerable diversity[3]. Pain may be transient or permanent, and its severity may be mild, moderate, or severe. The mechanism of pain in CP is complex and not fully understood[2]. One of the main causes is the abovementioned increase in hydrostatic pressure in the MPD[4,5]. The treatment of choice at the beginning of CP therapy should be conservative treatment in the form of analgesics and pancreatic enzyme supplementation[6]. When conservative treatment is insufficient and does not achieve the intended results, interventional treatment should be considered, especially in patients with ductal hypertension.
The basic treatment method for stenosis of the MPD during CP is drainage, which involves ensuring the physiological outflow of pancreatic juice into the lumen of the digestive tract[6,7]. Surgical drainage of the MPD should be considered in patients with pain resistant to treatment and dilatation of the MPD (> 6 mm) above the stenosis site. The duct can be decompressed using the lateral pancreaticojejunostomy method proposed by Puestow and Gillesby[8]. However, this method is no longer used in modern CP therapy. Another popular technique is the modified Puestow method proposed by Partington and Rochelle, which involves the longitudinal cutting of almost the entire pancreatic duct, removing the deposits, and covering the duct incision with the arm of the Roux loop formed from the retrolaterally displaced jejunum[8]. A surgical method proposed by Izbicki involves longitudinal V-shaped excision of a fragment of the pancreatic parenchyma above the Wirsung duct. This technique allows for more effective drainage of the ducts and extends the indication for drainage surgery to cases in which the pancreatic duct is not dilated along its entire length[8].
Frey further radicalized the drainage procedure described above. In addition to cutting the ducts of Wirsung and Santorini, a fragment of the anterior surface of the pancreatic head should also be cut to create a cone-shaped cavity[8]. Despite the reported effectiveness of surgical methods in long-term follow-up, these procedures are associated with high perioperative risk. Owing to continuing medical developments, it is now possible to use minimally invasive methods to treat pain in patients with CP.
Extracorporeal shock wave lithotripsy (ESWL) is a minimally invasive treatment method that can be used to treat large deposits that cause obstruction of the MPD. After ESWL, only 9%-30% of patients require endoscopic retrograde cholangiopancreatography (ERCP)[9]. In patients with deposits located in the head or body of the pancreas and secondary dilatation of the pancreas, ESWL alone reduced pain to a similar extent as ESWL and ERCP combined, with significantly lower therapy costs. Before deciding on radical surgical treatment, endoscopic treatment of CP should be considered if conservative treatment is ineffective.
MPD obstruction may result from the presence of various lesions, which often require treatment with a combination of endoscopic methods such as sphincterotomy, dilation of strictures, extraction of deposits, or prosthetics. Several studies have indicated the effectiveness of endoscopic therapy for the treatment of pain and was associated with fewer hospitalizations and a reduced need for analgesics. ERCP is a test combining radiological and endoscopic methods that enables imaging of the bile and pancreatic ducts. An important reason for using endoscopic treatment is the possibility of repeating the procedure if symptoms recur. Endoscopic therapy is the first-line treatment in cases with contraindications or lack of surgical conditions, as well as a bridging procedure before surgical treatment[10].
However, in some patients with CP and inflammatory infiltration of the head of the pancreas covering the descending part of the duodenum and the peripapillary area, it is impossible to perform ERCP with a prosthesis of the MPD. For this group of patients, surgical treatment is available in most medical centers. The development of interventional treatment techniques using endoscopic ultrasound (EUS) has made it possible to perform extra-anatomical anastomoses of the MPD with the gastrointestinal tract[11]. Endoscopic pancreaticogastrostomy under EUS guidance involves puncturing the MPD through the duodenal wall, creating a fistula between the duct and the lumen of the gastrointestinal tract, and introducing a plastic "pigtail" stent through the fistula canal to effectively drain the MPD.
This study presents the results of the treatment of patients with CP who were not qualified for surgical treatment. Endoscopic pancreaticogastrostomy under EUS guidance was performed because of the inability to undergo ERCP due to peripapillary infiltration of the descending duodenum.
MATERIALS AND METHODS
Patients
This was a retrospective study conducted at the Department of General, Gastroenterological and Oncological Surgery, Collegium Medicum of the Nicolaus Copernicus University in Toruń, Poland. It included patients treated for complications of acute or CP between 2018 and 2023. All patients gave informed consent for the proposed treatment and agreed to participate in the study. The inclusion criterion for this study was the need to perform an endoscopic extra-anatomical pancreatogastric anastomosis under EUS guidance to achieve effective pancreatic duct drainage. The creation of an anastomosis was proposed to patients in whom transpapillary drainage could not be performed during ERCP because of significant inflammatory infiltration of the duodenum. The study also included patients who underwent the abovementioned prepapillary drainage, but in which it was ineffective, meaning that the hypertension in the canal continued and the pain it caused did not subside. Clinical data were obtained retrospectively from the patients' electronic medical records, including any previous gastrointestinal surgery, indication for pancreatic drainage surgery, previous ERCP- or EUS-guided interventions (if any), and reasons for their failure. Technical information about the procedure was also obtained (including the type and diameter of the needle used to gain access to the MPD, type of cystotome used to perform the anastomosis, and the length and diameter of the plastic stent placed transgastrically in the MPD). Attention was also paid to complications that occurred immediately after the procedure related to the technique of the procedure itself. Long-term complications were monitored by direct observation and symptoms reported by the patients. The observation period lasted until the last contact with the patient during clinic visits.
Endoscopic techniques
All endoscopic and interventional procedures were performed by the author of this study (Jagielski M) under fluoroscopic control and general anesthesia. A linear echoendoscope (EG3870UTK; Pentax Medical, Tokyo, Japan) was used to identify the MPD and color Doppler was used to ensure that there were no vascular structures between the MPD and the gastrointestinal tract. All patients underwent the procedure using the same endoscopic equipment. Ductal puncture was performed with a 19 G single-use aspiration needle (Olympus Corporation, Tokyo, Japan). Contrast-enhanced pancreatography was performed, and a 0.035-inch guidewire (Boston Scientific Corporation, Marlborough, MA, United States) was inserted through the needle into the conduit. After removing the needle from the guidewire, a 10 Fr cytostome (Cystotome CST-10; Cook Medical Inc., Bloomington, IN, United States) was passed over the guidewire through the pancreatogastric anastomosis to the MPD. Subsequently, a plastic stent with a single 7 Fr pigtail, 9 cm long, was introduced into the MPD (Cook Medical Inc.) through the anastomosis. The pigtail was inserted into the lumen of the MPD and the straight end of the prosthesis remained in the gastric lumen. The patients were observed after the procedure to assess early postprocedural complications. Laboratory blood tests (pancreatic enzyme levels and blood count) were monitored on the first day after the procedure, which ended when pancreatic parameters stabilized and the pain resulting from the procedure subsided. The mean hospital stay for the endoscopic procedure was 3 to 5 d. In patients with pancreatic juice leakage and consequent formation of a pancreatic pseudocyst, hospitalization lasted 16 d. The aim of extending hospitalization was to implement conservative treatment and assess the potential need for repeated endoscopic intervention. Patients were routinely referred for follow-up hospitalization at the clinic to assess the effectiveness of the anastomosis within 3-12 wk after discharge from the clinic. Plastic stents were left permanently in the lumen of the anastomosis to maintain the anastomosis and passive drainage of the MPD. To provide long-term care to patients after completing treatment at our clinic, they were referred for outpatient care at the general surgery clinic.
Statistical analysis
All statistical calculations were conducted using Statistica version 13 (TIBCO Software Inc., Palo Alto, CA, United States). Quantitative variables were reported as arithmetic means, standard deviation, medians, and minimum and maximum values (range). Qualitative variables were reported as numbers and percentage. The statistical methods of this study were reviewed by a biomedical statistician.
RESULTS
Patient characteristics
This study retrospectively included 9 patients treated at our clinic between 2018 and 2023. Seven were men and two were women, and the average age was 53.45 (36-66) years. All patients underwent endoscopic pancreatogastric anastomosis under EUS guidance owing to the lack of transpapillary access during ERCP. The indications for the procedure were narrowing of the MPD in the head of the pancreas in the course of CP in 4/9 patients (44.44%), fragmented pancreatic duct syndrome in 3/9 patients (33.33%), and narrowing of the pancreatoenteric anastomosis after pancreaticoduodenectomy in 2/9 patients (22.22%). In all patients, the dominant symptom that led to interventional treatment was abdominal pain that did not respond to conservative treatment with opioids. Moreover, 3 patients had uncontrolled insulin-dependent diabetes. All procedures were performed as planned, and none of the patients required anastomosis following exacerbation of the underlying disease (Table 1).
Table 1.
Characteristic of the patients in the study group
|
Parameter
|
Value
|
| Sex | |
| Female | 2 (22.22) |
| Male | 7 (77.78) |
| Age in years | 53.45 (36-66) |
| Diagnosis of chronic pancreatitis | 4 (44.44) |
| Diameter of MPD in mm | 8 (6-16) |
| Indication for endotherapy | |
| Stricture of the MPD | 4 (44.44) |
| Disconnected duct syndrome | 3 (33.33) |
| Stricture of pancreatoenteric anastomosis | 2 (22.22) |
| Size of pancreatic stent | |
| Diameter 7 Fr | 9 (100) |
| Length 9 cm | 9 (100) |
| Technical success | 8 (88.89) |
| Clinical success | 8 (88.89) |
| Long-term success | 7 (77.78) |
Data are n (%) or mean (range). MPD: Main pancreatic duct.
Procedure outcomes and complications
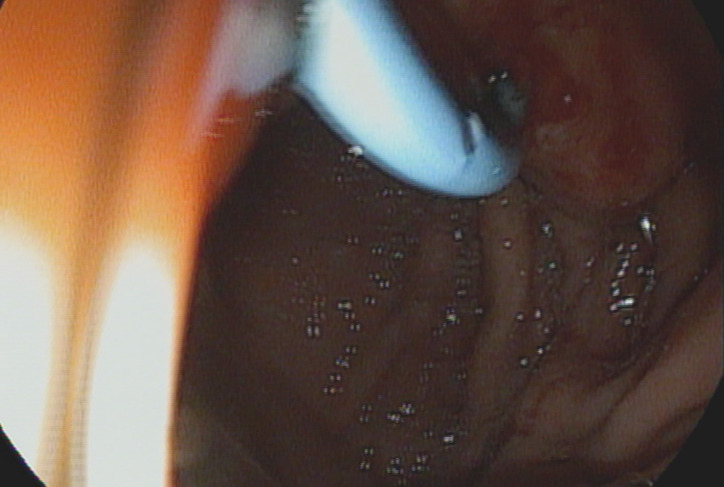
Technical success of endoscopic pancreaticogastrostomy was observed in 8/9 patients (88.89%). The lack of technical success in 1 patient was resulted from inability to perform pancreatogastric anastomosis with cystotomy after puncture of the MPD with the needle. As a result, it was impossible to perform the entire procedure and introduce a single pigtail pancreatic stent. Of the remaining patients, only 2 experienced complications. In 1 patient, there was a leak in the endoscopic anastomosis, which resulted in the leakage of pancreatic juice and the development of a pancreatic pseudocyst. The patient was treated conservatively, and the cyst regressed spontaneously within 6 wk. Regression was confirmed by a follow-up imaging examination and contrast-enhanced computed tomography of the abdominal cavity and pelvis. Because of this complication, the patient did not require a secondary endoscopic or surgical intervention. The complication that occurred in the second patient was bleeding into the lumen of the upper gastrointestinal tract at the anastomosis site. Bleeding was treated endoscopically by injecting an adrenaline solution into the bleeding site. Endoscopic intervention was performed on the first day after the anastomosis. The patient did not require transfusion of blood products (Figure 1).
Figure 1.
Endoscopic image obtained during endoscopic pancreaticogastrostomy in a patient with chronic pancreatitis.
Clinical success and long-term success
Clinical success was achieved in 8/9 patients (88.89%). The lack of clinical success in 1 patient resulted from a technically unsuccessful procedure. The patient did not require repeated endoscopic interventions. No complications related to the presence of plastic stents were observed in patients who successfully underwent pancreatogastric anastomosis. The mean follow-up period was 451 (42-988) d. Long-term success of endoscopic pancreatogastric anastomosis was achieved in 7/9 patients (77.78%). In 1 patient, long-term success was not achieved due to technical failure of the endoscopic procedure. In another patient, despite technical success and initial relief of symptoms during the follow-up period after the procedure, there was no long-term relief from abdominal pain. The probable reason for the lack of long-term effectiveness is the complexity of the etiology of pain in CP, and ductal hypertension is one of the components of CP, as mentioned earlier.
DISCUSSION
Therapeutic EUS for decompression of the MPD continues to evolve and is currently used as a salvage treatment after failed endoscopic access to the MPD. Access of the pancreatic duct under EUS guidance was described in 1995 by Harada et al[12] as pancreatography under EUS guidance after a failed ERCP. EUS-guided pancreatic duct drainage (EUS-PD) was first described in 2002 as a rendezvous technique and for transmural drainage[11], and has been used to decompress the MPD in patients with native or surgically altered anatomy. Previous reports have been limited to patients with relatively dilated pancreatic ducts and the use of invasive methods to gain access to the duct, including electrocautery, bougie dilation, and screw dilation. Since then, multiple studies of EUS-PD have been published, but most are case reports or case series as this is one of the most technically challenging EUS interventional procedures. In this study, we investigated a less aggressive method of access, dilation, and stenting of the pancreatic ducts to eliminate chronic abdominal pain during the course of the underlying disease. It is also worth noting that the patients described in this study were included regardless of the diameter of the MPD before the procedure.
In this study, technical success was achieved in 8/9 patients. The failure rate of the procedure was approximately 11%, which is lower than or comparable to that reported in previous studies (0%-40%)[6,13-15]. Unlike other described cases and groups of patients, we used a 10 Fr cystotome, which in our opinion ensured the patency and durability of the anastomosis. Premural drainage was attempted regardless of the pancreatic duct diameter. In 1 patient, an attempt to perform a pancreaticogastrostomy failed because of the inability to insert a cystotome after puncturing the MPD with a needle and inserting a guidewire. Despite this, the patient did not require repeated endoscopic interventions. Postprocedural complications occurred in 2 patients. One patient required repeated endoscopic intervention because of bleeding into the gastrointestinal tract from a previously created fistula. The other patient developed a pancreatic pseudocyst that resulted from leakage of pancreatic juice and spontaneously regressed within 6 wk after the procedure. Clinical success was seen in 88.89% of patients, which highlights the effectiveness of the procedure compared with previously described publications. The mean patient follow-up period was 451 (42-988) d, which is comparable to that reported in other studies[15-18]. Long-term effectiveness was achieved in almost 78% of patients (7/9). The lack of success in 1 patient resulted from failure to complete the procedure. In the other patient, abdominal pain recurred. The lack of response was probably due to the fact that pain in CP is multifactorial and only partially related to ductal hypertension[3].
The mortality rate in our study was 0%, which is consistent with the studies cited earlier. This information may support the hypothesis that endoscopic interventions are less invasive and better tolerated by patients than surgical procedures. A significantly lower number of complications and shorter hospitalization time significantly tips the balance of benefits in favor of endoscopic therapy[19,20]. Interestingly, stent dysfunction because of stent migration was a problem in previous pancreaticogastrostomy series and has been reported in 50%-55% of cases[6,21]. In our study, no instances of stent migration occurred. It can be hypothesized that unlike the larger 5-7 Fr stents most commonly used in other studies, which provide drainage but ultimately occlude the lumen, 3-Fr stents with long segments extending into the gastric lumen keep the pancreatic-gastric fistula-open. Previous studies used metal prostheses in some patients[16]. Based on previous reports and the results of the study described above, the use of plastic "single pigtail" prostheses to maintain the anastomotic lumen may carry a lower risk of postprocedural complications in the form of bleeding from the pancreaticogastrostomy site. Gastrointestinal perforation was described as a postprocedural complication in one study[16]. In our patients, there were no single perforations, which shows that the equipment (a 10 Fr cystotome) used in these patients was safe and more reliable alternative for performing the anastomosis.
It is worth mentioning that endoscopic pancreaticogastrostomy is not a radical procedure. Unlike surgical techniques, this method allows subsequent actions (either endoscopic or surgical) to be performed when clinical outcomes are not satisfactory. The low invasiveness of the procedure also encourages choosing to perform endotherapy first before deciding to escalate to surgery[22]. As described in this study and others that were cited[15-18], the performing pancreatogastrostomy under EUS guidance is a procedure that can be considered as a complement to surgical treatment, for example, in patients after the Whipple method. This feature indicates the great versatility of pancreaticobiliary endoscopy and emphasizes its development to offer patients further improved forms of endotherapy, which can be used in monotherapy or in combined treatment with surgical procedures. The limitations of this study include its retrospective nature and the fact that it was conducted at a single center.
CONCLUSION
The abovementioned results of endoscopic treatment by therapeutic EUS in patients with CP in an expert center are promising, but further multicenter randomized studies are needed to confirm the effectiveness and safety of this method. When transpapillary drainage during ERCP is ineffective, EUS-guided pancreaticogastrostomy is an effective and safe alternative in patients with CP-associated ductal hypertension. The proposed treatment method is particularly recommended in expert pancreaticobiliary endoscopy centers owing to the high level of technological advancement in the described procedure.
ACKNOWLEDGEMENTS
We thank all the medical staff from the General Gastroenterology and Oncology Surgery Department, the Endoscopic Team, and the Anesthetic Team who agreed to participate in this study.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of Collegium Medicum of Nicolaus Copernicus University (Toruń, Poland), No. KB 379/2023.
Informed consent statement: All patients gave informed consent for the proposed treatment and agreed to participate in the study.
Conflict-of-interest statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Liu C, China S-Editor: Li L L-Editor: Filipodia P-Editor: Zheng XM
Contributor Information
Mateusz Jagielski, Department of General, Gastroenterological and Oncological Surgery, Nicolaus Copernicus University, Toruń 87-100, Poland. matjagiel@gmail.com.
Eryk Bella, Department of General, Gastroenterological and Oncological Surgery, Nicolaus Copernicus University, Toruń 87-100, Poland.
Marek Jackowski, Department of General, Gastroenterological and Oncological Surgery, Nicolaus Copernicus University, Toruń 87-100, Poland.
Data sharing statement
No additional data are available.
References
- 1.Olakowski M, Wojtyczka A, Kabat J Chirurgiczne metody leczenia bólu w przewlekłym zapaleniu trzustki. Surgical methods of pain treatment in chronic pancreatitis. Gastroenterol Pol. 2005;12:439–444. [Google Scholar]
- 2.Dumonceau JM, Devière J, Le Moine O, Delhaye M, Vandermeeren A, Baize M, Van Gansbeke D, Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547–555. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 3.Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R, Hintze R, Adler A, Neuhaus H, Zavoral M, Zavada F, Schusdziarra V, Soehendra N European Society of Gastrointestinal Endoscopy Research Group. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765–771. doi: 10.1055/s-2002-34256. [DOI] [PubMed] [Google Scholar]
- 4.François E, Kahaleh M, Giovannini M, Matos C, Devière J. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128–133. doi: 10.1067/mge.2002.125547. [DOI] [PubMed] [Google Scholar]
- 5.James TW, Baron TH. Antegrade pancreatoscopy via EUS-guided pancreaticogastrostomy allows removal of obstructive pancreatic duct stones. Endosc Int Open. 2018;6:E735–E738. doi: 10.1055/a-0607-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada N, Kouzu T, Arima M, Asano T, Kikuchi T, Isono K. Endoscopic ultrasound-guided pancreatography: a case report. Endoscopy. 1995;27:612–615. doi: 10.1055/s-2007-1005769. [DOI] [PubMed] [Google Scholar]
- 7.Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–230. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Oh D, Park DH, Cho MK, Nam K, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: early and midterm outcomes (with video) Gastrointest Endosc. 2016;83:366–73.e2. doi: 10.1016/j.gie.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Hayat U, Freeman ML, Trikudanathan G, Azeem N, Amateau SK, Mallery J. Endoscopic ultrasound-guided pancreatic duct intervention and pancreaticogastrostomy using a novel cross-platform technique with small-caliber devices. Endosc Int Open. 2020;8:E196–E202. doi: 10.1055/a-1005-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Will U, Reichel A, Fueldner F, Meyer F. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol. 2015;21:13140–13151. doi: 10.3748/wjg.v21.i46.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puri R, Choudhary NS, Kotecha H, Rawat A, Sud R. Pancreatic duct leak in a case of post Whipple surgery: Managed by endoscopic ultrasound guided pancreatogastrostomy. Endosc Ultrasound. 2014;3:195–197. doi: 10.4103/2303-9027.138795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katanuma A, Maguchi H, Fukazawa M, Kurita A, Ichiya T, Kin T, Osanai M, Takahashi K. Endoscopic ultrasonography-guided pancreaticogastrostomy for a case of occlusion of gastro-pancreatic anastomosis after pancreaticoduodenectomy. Dig Endosc. 2009;21 Suppl 1:S87–S91. doi: 10.1111/j.1443-1661.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 14.Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, Garg PK, van Goor H, Halloran C, Isaji S, Neoptolemos JP, Olesen SS, Palermo T, Pasricha PJ, Sheel A, Shimosegawa T, Szigethy E, Whitcomb DC, Yadav D Working group for the International (IAP - APA - JPS - EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720–731. doi: 10.1016/j.pan.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K, Bruno MJ. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 16.Cahen DL, Gouma DJ, Laramée P, Nio Y, Rauws EA, Boermeester MA, Busch OR, Fockens P, Kuipers EJ, Pereira SP, Wonderling D, Dijkgraaf MG, Bruno MJ. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 2011;141:1690–1695. doi: 10.1053/j.gastro.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518–525. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 18.Ponchon T, Bory RM, Hedelius F, Roubein LD, Paliard P, Napoleon B, Chavaillon A. Endoscopic stenting for pain relief in chronic pancreatitis: results of a standardized protocol. Gastrointest Endosc. 1995;42:452–456. doi: 10.1016/s0016-5107(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 19.Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171–176. doi: 10.1055/s-2007-1010649. [DOI] [PubMed] [Google Scholar]
- 20.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–57; discussion 257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne RL, Gompertz RH, Venables CW. Surgery for chronic pancreatitis: a review of 12 years experience. Ann R Coll Surg Engl. 1997;79:405–409. [PMC free article] [PubMed] [Google Scholar]
- 22.Ebbehøj N, Borly L, Bülow J, Rasmussen SG, Madsen P, Matzen P, Owre A. Pancreatic tissue fluid pressure in chronic pancreatitis. Relation to pain, morphology, and function. Scand J Gastroenterol. 1990;25:1046–1051. doi: 10.3109/00365529008997633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.