Abstract
BACKGROUND
BRAF mutation has been recognized as a negative prognostic marker for metastatic colorectal cancer (mCRC), but these data are from common BRAF V600E-mutated mCRC. Combination therapy of BRAF inhibitor and anti-epidermal growth factor receptor (EGFR) antibody has been approved for BRAF V600E-mutated mCRC. However, BRAF non-V600 mutations are rare mutations, and their clinical behavior is not understood. Moreover, the BRAF K601E mutation is extremely rare in mCRC, and there have been no reports on its specific treatment.
CASE SUMMARY
Herein, we report the case of a 59-year-old female with super aggressive mCRC with multiple metastases, which extended to whole body including mediastinal to abdominal lymph nodes, bones, pleura, and peritoneum. The companion diagnostics of tumor tissues showed RAS/BRAF wild-type without microsatellite instability. She received chemotherapy with mFOLFOX6 (oxaliplatin plus infusional 5-fluorouracil [5-FU] and leucovorin) plus panitumumab, following FOLFIRI (irinotecan plus infusional 5-FU and leucovorin) plus ramucirumab. For the next regimen selection, a comprehensive genomic profiling panel was performed and revealed a BRAF K601E mutation, which was not covered in the initial companion diagnostics. After disease progression, a combination of encorafenib, binimetinib, and cetuximab was selected as third-line chemotherapy. The serum levels of tumor markers were immediately decreased accompanied by improvements in pleural effusion and ascites. However, the disease progressed again, and best supportive care was done instead.
CONCLUSION
This case offers novel insights into the clinical behaviors of BRAF non-V600E-mCRC, potentially advancing personalized therapy for rare and aggressive cases.
Keywords: BRAF K601E mutation, Metastatic colorectal cancer, Encorafenib, Binimetinib, Cetuximab, Case report
Core Tip: With the development and standardization of next-generation sequencing, it is anticipated that there will be increased discovery of previously unidentified genetic mutations. BRAF non-V600 mutations including the K601E mutation are rare, and the clinical behavior is not understood. In this case, a comprehensive genomic profiling panel revealed a BRAF K601E mutation, which was not covered in the initial companion diagnostics. A combination of encorafenib, binimetinib, and cetuximab had great effects. It is important to consider the possibility of discovering rare mutations, which could expand treatment options.
INTRODUCTION
Among the different types of cancer worldwide, colorectal cancer (CRC) has the third highest incidence and the second highest mortality[1], with approximately 20% of patient having metastases at the time of diagnosis and up to 50% presenting with initially localized disease and eventually developing metastases during treatment[2]. Fortunately, new systemic therapies and biomarker-based selection, particularly RAS and BRAF testing, have improved the prognosis of metastatic CRC (mCRC)[3]. RAS mutational status is predictive of the efficacy of anti-epidermal growth factor receptor (EGFR) monoclonal antibodies. On the other hand, BRAF mutations are rare driver oncogenes seen in 5%-21% of patients with mCRC[2,3], and are considered a negative prognostic marker for mCRC patients. BRAF encodes a serine and threonine protein kinase that promotes cell survival and proliferation via the mitogen-activated protein kinase pathway. Many BRAF mutation alleles have been identified, with the most common mutation being BRAF V600E. Regarding its treatment, a combination of encorafenib (BRAF inhibitor), binimetinib (MEK inhibitor), and cetuximab (anti-EGFR monoclonal antibody) was found to have significantly longer overall survival and a higher response rate than standard chemotherapy in patients with BRAF V600E-mutated mCRC[4]. Thus, the combination of encorafenib and cetuximab has been approved as a standard treatment for mCRC with BRAF V600E mutation worldwide, whereas the triple combination of encorafenib, cetuximab, and binimetinib has been approved in Japan. Meanwhile, less common BRAF non-V600E mutations may also have clinical relevance, as there is preliminary evidence of sensitivity to targeted therapies; however, there are very limited published data compared to the more common BRAF V600E mutations. Among them, the BRAF K601E mutation is extremely rare in mCRC[5], and its clinical significance remains unknown, with no reports on its specific treatment.
Herein, we describe a rare case of BRAF K601E-mutated mCRC, which responded to combination therapy with encorafenib, binimetinib, and cetuximab. Other possible therapeutic options are also discussed.
CASE PRESENTATION
Chief complaints
A 59-year-old Japanese female sought consult for a 1-mo history of right-sided abdominal pain.
History of present illness
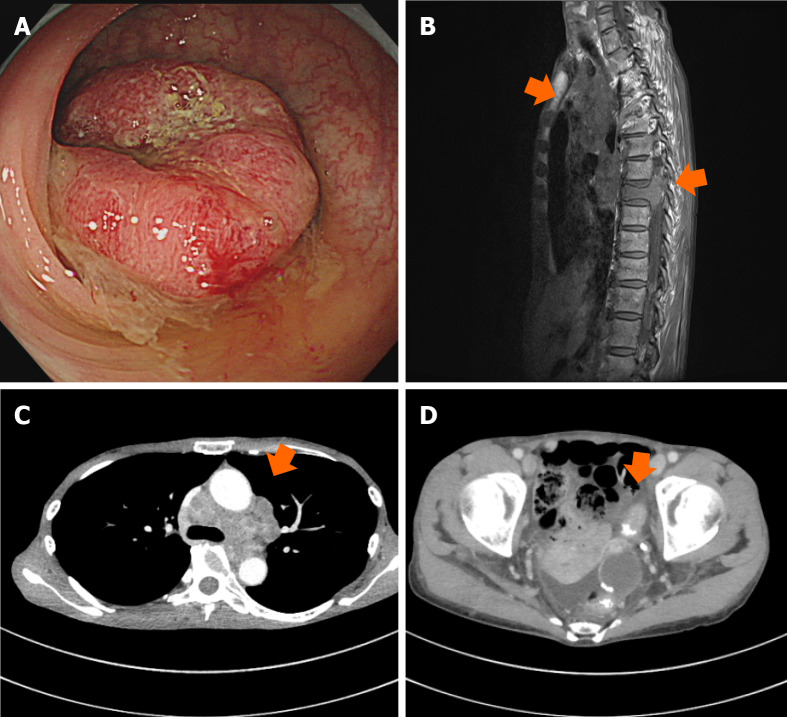
She was referred to our clinic by her family doctor. Computed tomography (CT) revealed wall thickness of the sigmoid colon, swelling of multiple lymph nodes, and spinal osteolytic tumors. The bone metastasis was advanced and extended into the spinal canal, compressing and draining the spinal cord, but with no clinically evident paralysis. Serum tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9), were markedly elevated. The colonoscopy performed before chemotherapy revealed advanced sigmoid colon cancer, and pathological tissue specimens contained differentiated adenocarcinoma (Figure 1). The companion diagnostics of tumor tissues showed RAS/BRAF wild-type without microsatellite instability. The clinical stage was cT3N3M1 based on the tumor-node-metastasis classification of malignant tumors, 8th edition. After radiotherapy (3 Gy × 10 Fr) for pain control and prevention of neurological disorders of bone metastases, we initiated first-line chemotherapy with mFOLFOX6 (oxaliplatin plus infusional 5-fluorouracil [5-FU] and leucovorin) plus panitumumab. The metastatic lymph nodes were markedly smaller, but regrew after 14 cycles of chemotherapy. Therefore, the treatment was switched to FOLFIRI (irinotecan plus infusional 5-FU and leucovorin) plus ramucirumab as second-line chemotherapy. However, the patient experienced vision loss after three cycles. CT scan revealed sphenoid bone metastasis with optic nerve compression. Radiotherapy (3 Gy × 12 Fr) was undertaken for neuroprotection of bone metastases.
Figure 1.
Imaging findings before treatment. A: Colonoscopy image of sigmoid colon; B: Magnetic resonance image of mediastinal lymph nodes and spinal bone; C and D: Computed tomographic image of mediastinal lymph nodes and sigmoid colon.
History of past illness
Her past medical history was unremarkable.
Personal and family history
Her father died of pancreatic cancer.
Physical examination
On physical examination, the vital signs were as follows: body temperature, 37.3 °C; blood pressure, 124/84 mmHg; heart rate, 70 beats per min; respiratory rate, 19 breaths per min.
Laboratory examinations
Serum tumor markers, including CEA and CA 19-9, and lactate dehydrogenase (LDH) were markedly elevated (CEA 1375.9 ng/mL, CA19-9 2672.5 U/mL, and LDH 688 U/L).
Imaging examinations
CT scan revealed hyperprogressive pleural effusion and ascites, suggesting failure on second-line chemotherapy.
Further diagnostic work-up
A comprehensive genomic profiling panel, FoundationOne® CDx (Foundation Medicine, Inc., San Diego, CA, United States), was used to explore the clinically relevant biomarkers and genomic alterations to transfer approved targeted therapies and clinical trial options. The tumor tissue sample before chemotherapy was also used for the genomic panel, which was same as the initial companion diagnostics. Molecular analysis with the genomic panel revealed the BRAF K601E mutation, which was not covered in the initial companion diagnostics.
FINAL DIAGNOSIS
BRAF K601E-mutated CRC with systemic metastasis including bone metastasis.
TREATMENT
A combination of encorafenib, binimetinib, and cetuximab was selected as third-line chemotherapy.
OUTCOME AND FOLLOW-UP
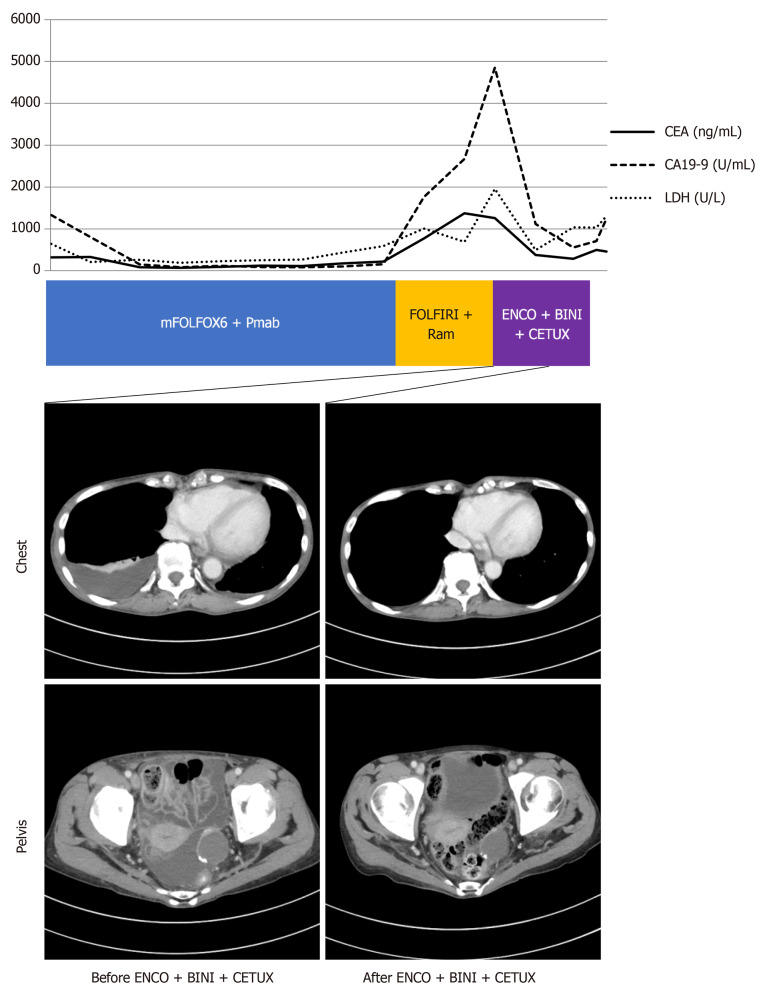
The serum levels of CEA, CA 19-9, and LDH were immediately decreased after third-line treatment (CEA 285.5 ng/mL, CA19-9 556.1 U/mL, and LDH 491 U/L), accompanied by improvement in pleural effusion and ascites (Figure 2). After 2 mo, the disease progressed again, and best supportive care was selected. The patient died 1 mo after discontinuing medical treatment.
Figure 2.
Response to combination therapy with encorafenib, binimetinib, and cetuximab. Computed tomography images before and after treatment with encorafenib, binimetinib, and cetuximab. The graph shows fluctuation of serum levels of carcinoembryonic antigen (CEA; normal range: 0-5 ng/mL), carbohydrate antigen 19-9 (CA 19-9; normal range: 0-37 U/mL) and lactate dehydrogenase (LDH; normal range: 124-222 U/L).
DISCUSSION
BRAF mutations have been reported in up to 60% of melanoma cases and in 40%-70% of thyroid carcinomas[6]. Most mutations are observed in BRAF V600, but next-generation sequencing (NGS), including gene panel testing, has revealed the presence of BRAF non-V600 mutations.
CRC is a heterogeneous disease with subtypes characterized by genetic alterations. BRAF mutations are seen in 5%-21% of patients with mCRC[4], the majority of which are V600E substitution. Conversely, BRAF mutations occurring outside codon 600 are identified in 1.6%-5.1% of patients with mCRC[7-10]. Currently, there are no treatment recommendations for BRAF non-V600E-mutated CRC. A previous multicenter study showed that BRAF non-V600 mutations occurred in approximately 2.2% (208/9643) of patients with verified mCRC, whereas the BRAF K601E mutation occurred in 0.09% (9/9643) as determined using NGS databases.
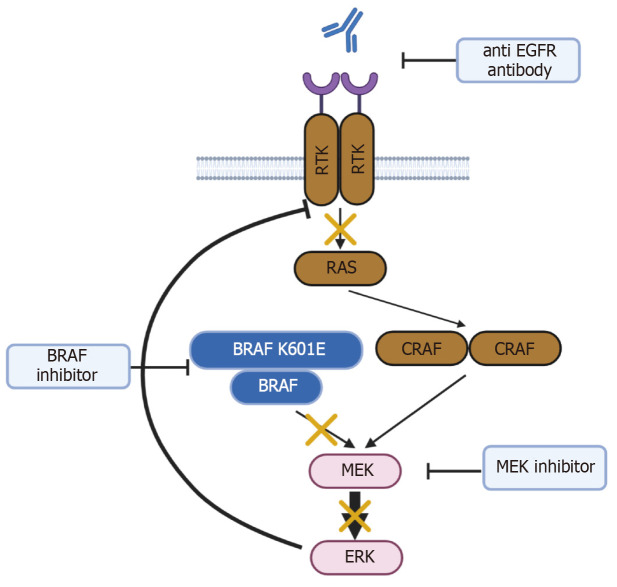
BRAF mutations are classified into three types according to the kinase activity of the mutant BRAF protein[11]. In class I, the kinase activity of the BRAF V600 mutant protein is approximately 500 times higher than that of wild-type BRAF, and the monomeric mutant BRAF directly activates downstream signals. In class II, the BRAF mutant forms a dimer with wild-type BRAF and activates downstream signaling. Meanwhile, class III mutant BRAF has reduced kinase activity, but forms dimers with wild-type BRAF or CRAF and activates downstream signaling through receptor tyrosine kinase (RTK)-RAS activation of the dimers. The BRAF K601E mutation is categorized as a class II BRAF mutation. Class II mutants exhibit impaired RTK and RAS signaling by negative feedback, but signal as dimers with BRAF and/or CRAF to activate mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling[11]. K601E-mutated BRAF is activated as a dimer, which interferes with the binding ability of BRAF inhibitors. Thus, MEK inhibitors can be useful in cancer patients with the BRAF K601E mutation (Figure 3). For example, binimetinib is a MEK inhibitor that works by blocking MEK molecules in the MEK signaling pathway that mediates cell growth and survival.
Figure 3.
Schema of class II BRAF mutation and action of encorafenib, binimetinib, and cetuximab. This schema was created with BioRender.com.
BRAF mutations also occur in thyroid, colorectal, and lung cancers, albeit less frequently than in melanomas[12]. Advancements have been made in BRAF-targeting therapies for melanomas, and BRAF and MEK inhibitors have been approved as standard therapies. Because BRAF inhibitor monotherapy is effective in BRAF V600E-mutated melanoma patients, treatment may be given without MEK inhibitors depending on the patient’s condition. On the other hand, in BRAF V600E-mutated mCRC patients, combination therapy including encorafenib and cetuximab with or without binimetinib is the standard because BRAF inhibitor monotherapy has not shown a sufficient response in previous clinical trials[4,13]. Notably, a previous case report of BRAF K601E-mutated melanoma demonstrated the efficacy of BRAF inhibitor monotherapy, but the differences between CRC and melanoma need to be considered[14].
In a multicenter study in the United States, the BRAF non-V600-mutated mCRC group was characterized by younger patients, fewer females, fewer high-grade tumors, right-sided primary tumors, and longer overall survival than the BRAF V600-mutated group[5]. In contrast, a report from Japan showed that patients with non-BRAF V600-mutated mCRC had a low response rate and poor progression-free survival[15]. Race may play a role in these differences, but another plausible reason is that the various types of BRAF non-V600E mutations can have different characteristics depending on the location and subtypes of the mutation.
In the present case, anti-EGFR combination therapy with FOLFOX plus panitumumab showed a dramatic tumor reduction as the first-line chemotherapy, but this may be due to the effect of FOLFOX. This first-line chemotherapy regimen has a progression-free survival of 7 mo because of its aggressiveness. Nevertheless, this result is still a valuable finding since the response of non-BRAF V600-mutated mCRC to anti-EGFR antibody was previously unclear.
To the best of our knowledge, this is the first report to demonstrate the effectiveness of combination therapy with encorafenib, binimetinib, and cetuximab in BRAF K601E-mutated mCRC. This regimen was effective as third-line chemotherapy, as evidenced by the marked improvement in pleural effusion. However, the response duration was quite short, which could be attributed to the high tumor burden in our case. Since future advancements in NGS, including gene panel testing, allow for the detection of more mCRC patients with BRAF non-V600E mutations, further studies into its treatment options are expected.
CONCLUSION
We report a very rare case of BRAF K601E-mutated mCRC that responded to targeted combination therapy with encorafenib, binimetinib, and cetuximab. This case report provides novel insights into the clinical behaviors of BRAF non-V600E-mutated CRC. The further accumulation of similar cases will hopefully lead to the development of personalized therapy for this rare and aggressive disease.
Footnotes
Informed consent statement: Informed consent has been obtained from the patients’ guardians/next of kin to participate and to publish this paper.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: ASCO.
Specialty type: Oncology
Country of origin: Japan
Peer-review report’s classification
Scientific Quality: Grade C, Grade C, Grade C
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade C
P-Reviewer: Wani I, India; Zhang JW, China S-Editor: Qu XL L-Editor: Filipodia P-Editor: Che XX
Contributor Information
Makiko Sasaki, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Takaya Shimura, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan. tshimura@med.nagoya-cu.ac.jp.
Hirotada Nishie, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Keita Kuroyanagi, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Takuya Kanno, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Shigeki Fukusada, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Naomi Sugimura, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Yusuke Mizuno, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Takayuki Nukui, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Konomu Uno, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Yuki Kojima, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Ruriko Nishigaki, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Mamoru Tanaka, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Keiji Ozeki, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Eiji Kubota, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
Hiromi Kataoka, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Science, Nagoya 467-8601, Japan.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72:372–401. doi: 10.3322/caac.21728. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 4.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 5.Jones JC, Renfro LA, Al-Shamsi HO, Schrock AB, Rankin A, Zhang BY, Kasi PM, Voss JS, Leal AD, Sun J, Ross J, Ali SM, Hubbard JM, Kipp BR, McWilliams RR, Kopetz S, Wolff RA, Grothey A. (Non-V600) BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J Clin Oncol. 2017;35:2624–2630. doi: 10.1200/JCO.2016.71.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One. 2012;7:e47054. doi: 10.1371/journal.pone.0047054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremolini C, Di Bartolomeo M, Amatu A, Antoniotti C, Moretto R, Berenato R, Perrone F, Tamborini E, Aprile G, Lonardi S, Sartore-Bianchi A, Fontanini G, Milione M, Lauricella C, Siena S, Falcone A, de Braud F, Loupakis F, Pietrantonio F. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann Oncol. 2015;26:2092–2097. doi: 10.1093/annonc/mdv290. [DOI] [PubMed] [Google Scholar]
- 8.Shimada Y, Tajima Y, Nagahashi M, Ichikawa H, Oyanagi H, Okuda S, Takabe K, Wakai T. Clinical Significance of BRAF Non-V600E Mutations in Colorectal Cancer: A Retrospective Study of Two Institutions. J Surg Res. 2018;232:72–81. doi: 10.1016/j.jss.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Wang J, Han X, Yang H, Wang S, Lin D, Shi Y. Effectors of epidermal growth factor receptor pathway: the genetic profiling ofKRAS, BRAF, PIK3CA, NRAS mutations in colorectal cancer characteristics and personalized medicine. PLoS One. 2013;8:e81628. doi: 10.1371/journal.pone.0081628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciardiello F, Normanno N, Maiello E, Martinelli E, Troiani T, Pisconti S, Giuliani F, Barone C, Cartenì G, Rachiglio AM, Montesarchio V, Tonini G, Rizzi D, Cinieri S, Bordonaro R, Febbraro A, De Vita F, Orditura M, Fenizia F, Lambiase M, Rinaldi A, Tatangelo F, Botti G, Colucci G. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25:1756–1761. doi: 10.1093/annonc/mdu230. [DOI] [PubMed] [Google Scholar]
- 11.Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O'Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marconcini R, Galli L, Antonuzzo A, Bursi S, Roncella C, Fontanini G, Sensi E, Falcone A. Metastatic BRAF K601E-mutated melanoma reaches complete response to MEK inhibitor trametinib administered for over 36 months. Exp Hematol Oncol. 2017;6:6. doi: 10.1186/s40164-017-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinozaki E, Yoshino T, Yamazaki K, Muro K, Yamaguchi K, Nishina T, Yuki S, Shitara K, Bando H, Mimaki S, Nakai C, Matsushima K, Suzuki Y, Akagi K, Yamanaka T, Nomura S, Fujii S, Esumi H, Sugiyama M, Nishida N, Mizokami M, Koh Y, Abe Y, Ohtsu A, Tsuchihara K. Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: the Biomarker Research for anti-EGFR monoclonal Antibodies by Comprehensive Cancer genomics (BREAC) study. Br J Cancer. 2017;117:1450–1458. doi: 10.1038/bjc.2017.308. [DOI] [PMC free article] [PubMed] [Google Scholar]