Abstract
The nexus between eosinophils and microbes is attracting increasing attention. We previously showed that airway administration of sterile microbial products contained in dust collected from traditional dairy farms virtually abrogated bronchoalveolar lavage (BAL) eosinophilia and other cardinal asthma phenotypes in allergen-sensitized specific pathogen–free (SPF) mice. Interestingly, comparable inhibition of allergen-induced BAL eosinophilia and promotion of airway barrier integrity were found upon administration of a sterile, pharmacological-grade bacterial lysate, OM-85, to the airway compartment of allergen-sensitized SPF mice. Here, we asked whether intrinsic properties of airway-delivered microbial products were sufficient to inhibit allergic lung inflammation or whether these effects were mediated by reprogramming of the host microbiota. We compared germ-free (GF) mice and offspring of GF mice associated with healthy mouse gut microbiota and maintained under SPF conditions for multiple generations (Ex-GF mice). These mice were treated intranasally with OM-85 and evaluated in the ovalbumin and Alternaria models of allergic asthma focusing primarily on BAL eosinophilia. Levels of allergen-induced BAL eosinophilia were comparable in GF and conventionalized Ex-GF mice. Airway administration of the OM-85 bacterial lysate was sufficient to inhibit allergen-induced lung eosinophilia in both Ex-GF and GF mice, suggesting that host microbiota are not required for the protective effects of bacterial products in these models and local airway exposure to microbial products is an effective source of protection. OM-85–dependent inhibition of BAL eosinophilia in GF mice was accompanied by suppression of lung type 2 cytokines and eosinophil-attracting chemokines, suggesting that OM-85 may work at least by decreasing eosinophil lung recruitment.
Keywords: eosinophilia, germ-free mice, lung, microbes
Airway-delivered microbial signals inhibit allergen-induced lung eosinophilia.
1. Introduction
The functions of eosinophils are multiple and complex. Airway eosinophilia is a cardinal signature of allergic asthma in both mice1 and humans,2 but eosinophils also play homeostatic and regulatory roles at mucosal barriers and contribute to tissue repair and protection against parasitic infections.3 Additional complexities of eosinophil biology are highlighted by the transcriptional and functional heterogeneity of these multifaceted cells in tissues.4 The nexus between eosinophils and microbes is also attracting increasing attention. While eosinophils are found in germ-free (GF) mice, implying that microbial cues are dispensable for the development of this lineage, commensal microbes regulate the frequency and function of intestinal eosinophils, thereby influencing tissue repair, allergic sensitization to food antigens,5 and protection against pathogen intrusion.3
We became interested in eosinophils as targets of microbial signals during our studies of asthma protection in microbe-rich environments. Airway administration of microbial products contained in sterile, autoclaved extracts of dust collected from Amish or European traditional dairy farms virtually abrogated bronchoalveolar lavage (BAL) eosinophilia and other cardinal asthma phenotypes in allergen-sensitized specific pathogen–free (SPF) mice.6,7 In line with the microbial nature of the exposure, suppression of BAL eosinophilia depended on Myd88/Trif innate immune signaling6 and was accompanied by increased airway epithelial barrier function7 and profound reprogramming of the lung innate immune transcriptome (Van Linden, DeVries, and Vercelli, unpublished data). Interestingly, comparable inhibition of allergen-induced BAL eosinophilia and promotion of airway barrier integrity were found upon intranasal (i.n.) administration of a sterile, pharmacological grade bacterial lysate, OM-85, to the airway compartment of SPF mice sensitized to allergen systemically (with ovalbumin [OVA]) or through the airway mucosa (with Alternaria alternata).8
Although they pointed to eosinophils as targets of microbial exposures, these experiments did not reveal whether intrinsic properties of airway-delivered microbial products were sufficient to inhibit allergic lung inflammation or whether these effects were mediated by reprogramming of the host microbiota. To address this question, we associated GF mice from birth with healthy mouse gut microbiota or treated these mice i.n. with the OM-85 bacterial lysate. Allergen-induced responses were evaluated in the OVA and Alternaria asthma models, focusing primarily on BAL eosinophilia.
2. Methods
2.1. Mice
BALB/c GF mice (Taconic) were bred and housed in sterile isolators at the University of Arizona Gnotobiotic Animal Facility with unlimited access to autoclaved food (2019S; Inotiv) and water. GF status of the colony was monitored routinely by anaerobic and aerobic culture of stool samples. A colony of Ex-GF BALB/c mice was established by transferring GF mice to the SPF rooms of the University of Arizona Animal Facility and exposing them for 2 to 3 wk to soiled bedding from a sentinel cage. Ex-GF mice were bred and their offspring were maintained under SPF conditions for multiple generations (>1.5 yr). These mice were SPF for murine norovirus, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler's murine encephalomyelitis virus, rotavirus, Sendai virus, pneumonia virus of mice, reovirus type 3, lymphocytic choriomeningitis virus, Ectromelia, mouse adenovirus-1 and -2, polyoma virus, Helicobacter, Rodentibacter, Mycoplasma pulmonis, cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Salmonella, fur mites, lice, nematodes, cestodes, protozoa, and microsporidia. All experimental procedures were approved by the University of Arizona Institutional Animal Care and Use Committee.
2.2. Bacterial 16S rRNA gene quantitative polymerase chain reaction
One week before each experiment, GF mice were transferred into ISOcage P cages (Tecniplast), where all experimental procedures were performed. Fecal samples were collected immediately after transfer, weekly during the experiment, and immediately before mice were sacrificed. A bacterial 16S rRNA gene quantitative polymerase chain reaction (qPCR)9 was performed on these fecal samples. DNA was extracted using the DNeasy PowerSoil Pro kit (Qiagen) and quantified with a Qubit 2.0 Fluorometer (Life Technologies). qPCR reactions were performed using the SYBR green Perfecta FastMix (QuantaBio) and run in triplicate on an Applied Biosystems 7900 instrument. The bacterial 16S rRNA gene was amplified using eubacterial universal primers 926F (5′-AAACTCAAAKGAATTGACGG-3′) and 1062R (5′-CTCACRRCACGAGCTGAC-3′).9 A standard curve was generated using dilutions of 16S qPCR standards (Zymo Research) covering a 32 to 3.2207 copy range. Ct values were converted to copies/reaction.10 16S rRNA gene copy numbers were presented as copy numbers/ng of DNA. No template mix and fecal DNA from SPF mice were included as controls.
2.3. Allergic asthma models
Experiments in the OVA model were performed as previously described,7 with minor modifications. Briefly, to compare OVA-induced responses in GF and Ex-GF mice, 7 to 8 wk old GF and Ex-GF BALB/c mice were sensitized intraperitoneally (i.p.) with OVA (chicken egg white albumin [Sigma-Aldrich]: 50 μg) + Alum (Imject Alum [Thermo Fisher Scientific]: 150 μL) at day 0 and 7, challenged i.n. with OVA (100 μg) at days 14 to 16, and sacrificed at day 21. To assess the effects of OM-85 on OVA-induced BAL eosinophilia in GF BALB/c mice, 7- to 8-wk-old animals were sensitized with OVA (50 μg) + Alum (150 μL) i.p. at day 0 and 9, challenged i.n. with OVA (100 μg) at day 22 to 24, and sacrificed at day 29. OM-85 (1 mg in 0.9% sterile saline/treatment × 9 treatments) was administered i.n. (25 μL/nostril) under 1% to 5% isoflurane anesthesia every 2 to 3 d from days 0 to +17.
In the Alternaria model, 7- to 8-wk-old GF and Ex-GF BALB/c mice were sensitized i.n. with A. alternata extracts (Greer Laboratories; 50 μg of dry weight in 50 μL of saline) at days 0 and 1, challenged i.n. with Alternaria extracts (25 μg of dry weight in 50 μL) at days 21 to 23, and sacrificed at day 26. OM-85 (1 mg in 0.9% sterile saline/treatment × 14 treatments) was administered i.n. (25 μL/nostril) under 1% to 5% isoflurane anesthesia every 2 to 3 days from days −14 to day +16.8 In all models, terminal assessments included invasively measured lung function (airway hyperresponsiveness [AHR]), BAL cellularity with differentials, and levels of lung cytokine and chemokine messenger RNA (mRNA) measured by reverse-transcription qPCR.7,8
2.4. Statistical analyses
Experiments were designed to test the hypothesis, based on prior knowledge, that early association with healthy mouse gut microbiota or i.n. administration of microbial products affects asthma-related phenotypes. Therefore, statistical analyses focused on the comparison between mice treated with allergen + microbes or microbial products and mice treated with allergen alone. Saline-treated groups served as experimental controls. Intergroup differences were assessed by an unpaired, 2-tailed t test after determining the normality of sample value distribution with the Shapiro-Wilk test. Unless otherwise stated, P values <0.05 were considered statistically significant. AHR dose-response curves were compared using linear mixed-effects models, with treatment group and dosage of bronchoconstrictor as independent variables (fixed effects) and using each mouse identifier to account for repeated measures within each subject (random effects).7,8
3. Results
3.1. Association with healthy gut microbiota from birth inhibits allergen-induced AHR in GF mice
To investigate whether host microbiota mediate the asthma-protective effects of airway-delivered microbial products, we used BALB/c GF mice as well as offspring of GF mice initially exposed for 2 to 3 wk to microbiota from healthy SPF mice of the same strain and then maintained under SPF conditions for multiple generations (>1.5 yr) (Ex-GF mice). Thus, all Ex-GF mice discussed herein were genetically identical to the GF ones and acquired their microbiota from birth, i.e. during the critical age window that allows the transferred microbiota to fully mature and exert their immunomodulatory effects.11
We then compared GF and Ex-GF mice in 2 distinct models of allergic asthma, monitoring lung function (AHR) and BAL eosinophilia. Figure 1A shows that AHR elicited by i.p. sensitization with OVA + Alum followed by i.n. challenge with OVA alone was significantly (P = 0.0003) reduced in Ex-GF relative to GF mice. Similarly, AHR was significantly (P = 0.03) less intense in Ex-GF than GF mice sensitized and challenged with the aeroallergen A. alternata (Fig. 1B). The latter data were especially significant because Alternaria, a protease-rich fungus, is strongly associated with the development of allergic asthma in children and adults.12 In mice, i.n. exposure to Alternaria extracts in the absence of adjuvants induces allergic lung inflammation that closely recapitulates the human phenotype and reflects the activation of innate and adaptive immune pathways triggered by damage to the airway epithelial barrier.8,13,14 Our current results demonstrated that microbial conventionalization from birth protected mice from alterations in lung function linked to the lack of microbial colonization.
Fig. 1.
GF mice associated with healthy gut microbiota from birth (Ex-GF mice) exhibit attenuated AHR in 2 allergic asthma models. GF (black symbols) and Ex-GF (gray symbols) BALB/c mice were sensitized and challenged with OVA (A) or Alternaria alternata extracts (B). Shown are invasive measurements of total airway resistance (Rrs) from 2 independent experiments for each model. Numbers of mice/group are indicated in the figure captions. Symbols and bars denote mean and SEM, respectively. Statistical differences between dose-response curves of total airway resistance in GF and Ex-GF mice were assessed by linear mixed-effects models. ALT = Alternaria.
3.2. Allergen-induced BAL eosinophilia in GF mice is unaffected by early association with healthy mouse gut microbiota but is inhibited by airway administration of a bacterial lysate
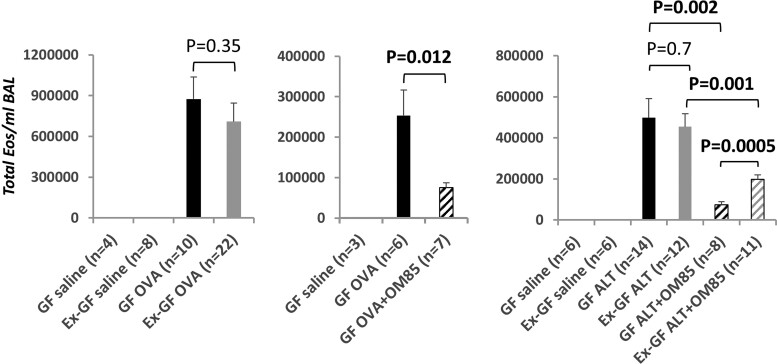
The next set of experiments compared allergen-induced BAL eosinophilia in GF and Ex-GF mice. Intense eosinophilia was detected in OVA-sensitized mice, but to our surprise no significant differences were found between GF and Ex-GF animals (P = 0.35) (Fig. 2, left). Similarly, GF and Ex-GF mice developed robust but comparable Alternaria-dependent BAL eosinophilia (P = 0.7) (Fig. 2, right). Alternaria-sensitized GF and Ex-GF mice also harbored closely comparable proportions (13.6 ± 4.1% in GF mice vs 15.9 ± 4.6% in Ex-GF mice) and numbers (not shown) of BAL eosinophils with ring-shaped nuclei, which are thought to represent less mature, potentially homeostatic cells.15
Fig. 2.
Airway administration of a bacterial lysate but not early association with healthy mouse gut microbiota inhibits allergen-induced BAL eosinophilia in GF mice BAL cellularity was measured in GF (black bars) and Ex-GF (gray bars) BALB/c mice sensitized and challenged with OVA (left and center) or Alternaria alternata (right). Additional groups of mice (hatched bars) received allergen + 9 (OVA model) or 14 (Alternaria [ALT] model) i.n. OM-85 treatments (1 mg/mouse/treatment). Shown are total numbers of BAL eosinophils (Eos)/mouse (mean and SEM) from 2 independent experiments for each model. Numbers of mice/group are indicated in the figure captions. Statistical differences between groups were assessed by an unpaired, 2-tailed t test after evaluating the normality of sample value distributions. Bolded P values remained significant after Bonferroni correction (P < 0.016).
Interestingly, marked differences in response patterns were observed when GF and Ex-GF mice received a sterile bacterial lysate, OM-85, directly through the airways. Indeed, i.n. administration of OM-85 was sufficient to strongly and significantly reduce Alternaria-dependent BAL eosinophilia not only, as expected,8 in Ex-GF mice (P = 0.001) but also in GF animals (P = 0.002) (Fig. 2, right). OVA-induced BAL eosinophilia was also significantly (P = 0.012) suppressed in OM-85–treated GF mice (Fig. 2, center), demonstrating that the effects of the bacterial lysate are not allergen or model specific.
3.3. Airway administration of a bacterial lysate inhibits Alternaria-induced expression of type 2 cytokines and eosinophil chemoattractants in the lung
To begin investigating the mechanisms through which airway delivery of a bacterial lysate inhibits allergen-induced BAL eosinophilia in mice lacking microbiota, we compared cytokine and chemokine expression in the lungs of Alternaria-immunized GF and Ex-GF mice that did or did not receive OM-85 intranasally. The type 2 cytokines Il5 and Il13 have powerful effects on eosinophil biology: interleukin (IL)-5 promotes eosinophil production, priming, and survival, and IL-13 induces lung cells to produce eotaxins (Ccl11 and Ccl24), which attract circulating eosinophils to the tissue.16 Lung levels of Alternaria-induced Il5 and Il13 mRNA were closely comparable in GF and Ex-GF mice (P = 0.5 and P = 0.9, respectively). In contrast, expression of both Il5 and Il13 was markedly reduced in the lungs of GF mice treated i.n. with OM-85 (P = 0.00001 and P = 0.00009, respectively). Consistent with our previous results in SPF mice,8 a strong reduction in type 2 cytokines was detected in the lungs of OM-85–treated Ex-GF mice (P = 0.00004 for Il5 and P = 0.0003 for Il13) (Fig. 3A and B).
Fig. 3.
Airway administration of a bacterial lysate but not early association with healthy mouse gut microbiota inhibits Alternaria (ALT)-induced expression of type 2 cytokines and eosinophil-attracting chemokines in the lung. mRNA levels of type 2 cytokines (Il5 [A], Il13 [B]) and eosinophil chemoattractants (Ccl11 [C], Ccl24 [D]) were measured by reverse-transcription qPCR in the lungs of GF (black bars) and Ex-GF (gray bars) BALB/c mice sensitized and challenged with ALT. Additional groups of mice (hatched bars) received ALT + 14 i.n. administrations of OM-85 (1 mg/mouse/treatment). Shown are cytokine and chemokine levels (mean and SEM) from 2 independent experiments. Numbers of mice/group are indicated in the figure captions. Statistical differences between groups were assessed by an unpaired, 2-tailed t test after evaluating the normality of sample value distributions. Bolded P values remained significant after Bonferroni correction (P < 0.016).
We next focused on the eosinophil chemoattractants Ccl11 and Ccl24 because they are both regulated by IL-5 and IL-13, bind CCR3 and promote eosinophil migration to tissues, albeit with distinct temporal patterns.17 While GF and Ex-GF mice sensitized and challenged with Alternaria alone exhibited no significant differences in Ccl11 and Ccl24 mRNA levels (P = 0.17 and P = 0.15, respectively), GF mice treated intranasally with OM-85 had markedly lower lung expression of both chemokines relative to Alternaria-sensitized animals (P = 0.00009 and P = 0.00000007, respectively). Like type 2 cytokines, Ccl11 expression was significantly reduced in the lungs of Ex-GF mice treated with Alternaria + OM-85 compared with Ex-GF mice that had received Alternaria alone (P = 0.003); the OM-85–induced decrease in Ccl24 closely approached statistical significance (P = 0.017) (Fig. 3C and D).
4. Discussion
Our data demonstrate that administration of a bacterial lysate to the airway compartment is sufficient to inhibit OVA- and Alternaria-induced lung eosinophilia in GF mice, implying that host microbiota are not required for the protective effects of bacterial products in these models of allergic airway inflammation. Our results also suggest that local airway exposure to microbial products is especially effective in conferring protection. Mechanistically, suppression of allergen-induced eosinophil recruitment to the lung may be important for the OM-85–dependent inhibition of BAL eosinophilia in GF animals.
GF mice associated with human gut microbiota are often used to study causal relationships between altered microbiomes and host pathology. Insightful results have been provided by studies in intestinal inflammatory disease models (reviewed in Walter et al.).18 In contrast, work on lung disease, and asthma in particular, has produced mixed results. For instance, GF mice associated with potentially protective human bacterial taxa exhibited only modest attenuation of allergic airway inflammation and no significant inhibition of allergen-induced BAL eosinophilia. Effects on AHR were not tested.19 A concern raised by interspecies microbial association is that it may lead to incomplete and/or selective colonization and thus may not adequately recapitulate the compositional and functional features of the transferred inoculum and the phenotypic characteristics of its donor.18 To address this concern and provide proof-of-concept evidence about a link between microbes and lung eosinophilia, we associated GF mice with gut microbiota from healthy SPF mice or treated GF mice i.n. with a sterile pharmacological-grade bacterial lysate, OM-85. Surprisingly, the short-term airway exposure of adult GF mice to the bacterial lysate succeeded in suppressing airway eosinophilia, type 2 cytokines, and eosinophil chemoattractants in the lung, but these effects were not observed in Ex-GF mice. The reasons behind these findings are unclear at this time. The Ex-GF mice used in our experiments were colonized with healthy mouse gut microbiota from birth, and the colony was maintained under SPF conditions for over a year. Changes in microbial composition over this time and/or incomplete reconstitution of the airway microbiome might have led to the emergence of communities with metabolic profiles unable to fully support protective lung immune responses to asthma-promoting stimuli. However, the finding that AHR was attenuated in Ex-GF mice in 2 distinct allergic asthma models militates against this possibility. It is also notable that throughout multiple experiments performed in parallel over more than 1 year, i.n. administration of OM-85 induced a robust and comparable average reduction of Alternaria-dependent BAL eosinophilia in Ex-GF and SPF mice (2.3- and 3.3-fold, respectively). However, the average reduction of eosinophilia observed in GF mice treated with OM-85 during the same period was 6.7-fold—i.e. even stronger.
We hypothesize that the route through which microbial signals were delivered, and the ability of these signals to target the airway epithelium may underlie the remarkable sensitivity of GF airways to microbial products. Indeed, we recently showed that the OM-85 bacterial lysate restores barrier function in airway epithelial cells exposed to Alternaria.8 Similarly, microbial farm dust products with asthma-protective activity boosted barrier function in serum-starved airway epithelium.7 We speculate that the barrier restorative properties of microbial products may be especially crucial in the airways, where epithelial cell turnover is approximately 10-fold lower than in other locations such as the gut,20 and even more so in the airways of GF mice, which lack the resident microbial communities normally contributing to epithelial barrier integrity.21 In this scenario, the concentrations of microbial products (including metabolites) required to jump-start eosinophilia-limiting responses might be achieved more readily and efficiently by directly treating GF airways with sterile bacterial lysates or products. While the primary cellular and molecular mechanisms underlying these effects require further investigation, our data already suggest that testing complex mixtures of microbial metabolites in GF animals might provide a powerful tool to characterize bioactive substances in the absence of confounding factors derived from the host microbiota.
Contributor Information
Ashley N Michael, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Oksana Pivniouk, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Peace C Ezeh, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Sunil Banskar, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Seongmin Hahn, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Avery DeVries, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States.
Kathryn O’Connell, University Animal Care, University of Arizona, BIO5 Institute, 1657 E Helen Street, Tucson, AZ 85721, United States.
Vadim Pivniouk, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States; Department of Cellular and Molecular Medicine, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245044, Tucson AZ 85724-5044, United States.
Donata Vercelli, Asthma and Airway Disease Research Center, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245030, Tucson, AZ 85724, United States; Department of Cellular and Molecular Medicine, University of Arizona, 1501 N. Campbell Avenue P.O. Box 245044, Tucson AZ 85724-5044, United States; BIO5 Institute, University of Arizona, 1657 E Helen Street, Tucson, AZ 85721, United States; Arizona Center for the Biology of Complex Diseases, University of Arizona, BIO5 Institute, University of Arizona, Tucson, AZ 85721, United States.
Funding
This work was supported by the National Institutes of Health (P01AI148104 and U19AI125357 to D.V.) and a research grant from OM Pharma SA (Meyrin, Switzerland) to the University of Arizona.
References
- 1. Vercelli D. From Amish farm dust to bacterial lysates: the long and winding road to protection from allergic disease. Semin Immunol. 2023:68:101779. 10.1016/j.smim.2023.101779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez FD, Vercelli D. Asthma. Lancet. 2013:382(9901):1360–1372. 10.1016/S0140-6736(13)61536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gurtner A, Gonzalez-Perez I, Arnold IC. Intestinal eosinophils, homeostasis and response to bacterial intrusion. Semin Immunopathol. 2021:43(3):295–306. 10.1007/s00281-021-00856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gurtner A, Borrelli C, Gonzalez-Perez I, Bach K, Acar IE, Núñez NG, Crepaz D, Handler K, Vu VP, Lafzi A, et al. Active eosinophils regulate host defence and immune responses in colitis. Nature. 2023:615(7950):151–157. 10.1038/s41586-022-05628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiménez-Saiz R, Anipindi VC, Galipeau H, Ellenbogen Y, Chaudhary R, Koenig JF, Gordon ME, Walker TD, Mandur TS, Abed S, et al. Microbial regulation of enteric eosinophils and its impact on tissue remodeling and th2 immunity. Front Immunol. 2020:11:155. 10.3389/fimmu.2020.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques dos Santos M, Anderson RL, Metwali N, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016:375(5):411–421. 10.1056/NEJMoa1508749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marques Dos Santos M, Pivniouk V, Rankl B, Walker A, Pagani G, Hertkorn N, Schmitt-Kopplin P, Müller C, Bracher F, Merl-Pham J, et al. Asthma-protective agents in dust from traditional farm environments. J Allergy Clin Immunol. 2023:152(3):610–621. 10.1016/j.jaci.2023.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pivniouk V, Gimenes-Junior JA, Ezeh P, Michael A, Pivniouk O, Hahn S, VanLinden SR, Malone SP, Abidov A, Anderson D, et al. Airway administration of OM-85, a bacterial lysate, blocks experimental asthma by targeting dendritic cells and the epithelium/IL-33/ILC2 axis. J Allergy Clin Immunol. 2022:149(3):943–956. 10.1016/j.jaci.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bacchetti De Gregoris T, Aldred N. Clare AS and Burgess JG improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011:86(3):351–356. 10.1016/j.mimet.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 10. Barua VB, Juel MAI, Blackwood AD, Clerkin T, Ciesielski M, Sorinolu AJ, Holcomb DA, Young I, Kimble G, Sypolt S, et al. Tracking the temporal variation of COVID-19 surges through wastewater-based epidemiology during the peak of the pandemic: a six-month long study in Charlotte, North Carolina. Sci Total Environ. 2022:814:152503. 10.1016/j.scitotenv.2021.152503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borbet TC, Pawline MB, Zhang X, Wipperman MF, Reuter S, Maher T, Li J, Iizumi T, Gao Z, Daniele M, et al. Influence of the early-life gut microbiota on the immune responses to an inhaled allergen. Mucosal Immunol. 2022:15(5):1000–1011. 10.1038/s41385-022-00544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halonen M, Stern D, Wright AL, Taussig L, Martinez F. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Resp Crit Care Med. 1997:155(4):1356–1361. 10.1164/ajrccm.155.4.9105079 [DOI] [PubMed] [Google Scholar]
- 13. Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 regulates the development of eosinophilic versus neutrophilic asthma in response to Alternaria alternata. J Immunol. 2016:197(12):4541–4551. 10.4049/jimmunol.1600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doherty T, Khorram N, Chang J, Kim H, Rosenthal P, Croft M, Broide D. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012:303(7):L577–L588. 10.1152/ajplung.00174.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016:126(9):3279–3295. 10.1172/JCI85664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandikattu HK, Upparahalli Venkateshaiah S, Mishra A. Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019:47:83.–. 10.1016/j.cytogfr.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, Yang M, Kaiko GE, Hansbro PM, Kumar RK, et al. Modeling T(H) 2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017:278(1):20–40. 10.1111/imr.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020:180(2):221–232. 10.1016/j.cell.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 19. Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015:7(307):307ra152. 10.1126/scitranslmed.aab2271 [DOI] [PubMed] [Google Scholar]
- 20. Ruysseveldt E, Martens K, Steelant B. Airway basal cells, protectors of epithelial walls in health and respiratory diseases. Front Allergy. 2021:2:787128. 10.3389/falgy.2021.787128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. 2020:38(1):23–48. 10.1146/annurev-immunol-070119-115104 [DOI] [PubMed] [Google Scholar]