Abstract
Background
In the Netherlands, the number of mpox cases started declining before mpox vaccination was initiated. Most cases were men who have sex with men (MSM). We investigated whether the decline in mpox could be attributed to infection-induced immunity or behavioral adaptations.
Methods
We developed a transmission model and accounted for possible behavioral adaptations: fewer casual partners and shorter time until MSM with mpox refrain from sexual contacts.
Results
Without behavioral adaptations, the peak in modelled cases matched observations, but the decline was less steep than observed. With behavioral adaptations in the model, we found a decline of 16%–18% in numbers of casual partners in June and 13%–22% in July 2022. Model results showed a halving of the time before refraining from sex. When mpox vaccination started, 57% of MSM with very high sexual activity in the model had been infected. Model scenarios revealed that the outbreak could have waned by November 2022 even without vaccination.
Conclusions
The limited duration of the mpox outbreak in the Netherlands can be ascribed primarily to infection-induced immunity among MSM with high sexual activity levels. The decline was accelerated by behavioral adaptations. Immunity among those most sexually active is essential to impede mpox resurgence.
Keywords: monkeypox, mpox, men who have sex with men, MSM, mathematical model, immunity, vaccination, sexual behavior
Modelling study shows that the decline in mpox cases among MSM in the Netherlands was primarily due to infection-induced immunity among those with high sexual activity levels and accelerated by behavioral adaptions. The outbreak could have faded even without vaccination.
Mpox (formerly known as monkeypox) is a zoonotic disease caused by the mpox virus (MPXV) [1]. In May-July 2022, mpox outbreaks were reported, mostly among men who have sex with men (MSM) [2, 3]. The number of cases peaked within 2–3 months in most countries and then declined [2]. Several factors may have contributed to the abrupt decline, including increased immunity due to MPXV infection, behavioral adaptations, and public health interventions [3–5].
In the Netherlands, the number of mpox cases increased sharply in June 2022, but started declining in the second week of July 2022 [6], about 2 weeks before the national mpox vaccination program started on 25 July 2022 [7]. In May and June 2022, a large media coverage of the mpox outbreak contributed to raise awareness of a new threat among MSM. In June 2022, health communication messages about mpox were disseminated in the media [7, 8]. Also, it was recommended to refrain from sexual or physical contacts when mpox was suspected or diagnosed [9]. In an online survey carried out in August 2022, most participants reported to be willing to refrain from close physical and sexual contacts in case of MPXV infection and some had reduced their number of sexual partners since the start of the outbreak [9]. Sex venues and parties in Amsterdam experienced a considerable drop in numbers of visitors in July 2022 [10].
Our study aimed to assess the factors that may have led to the decline in the number of mpox cases observed in the Netherlands before the start of the mpox vaccination program. We developed a mathematical model that describes the transmission of MPXV via sexual or intimate contacts among MSM and fitted the model to numbers of mpox cases in the Netherlands until 25 July 2022, when the mpox vaccination program started. To disentangle the impact of immunity due to infection and the impact of behavioral adaptations from the impact of vaccination in shaping the course of the outbreak, we examined also model scenarios without the vaccination program until the end of 2022.
METHODS
The Transmission Model
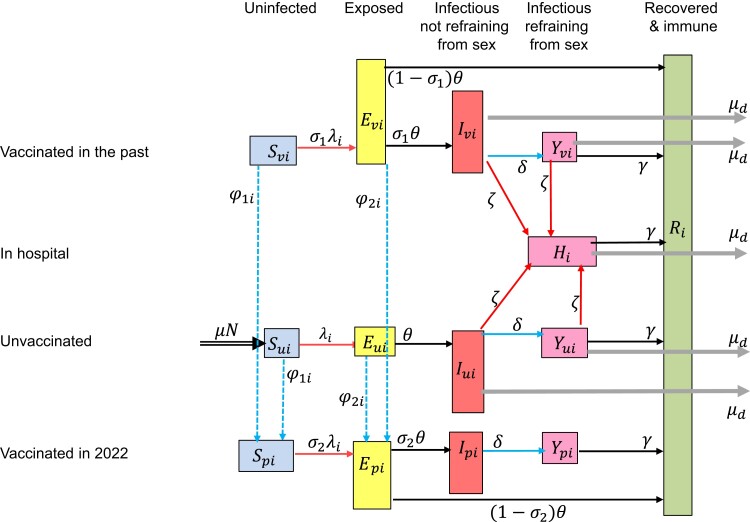
We developed a deterministic compartmental model that describes MPXV transmission among MSM (Figure 1). The model parameters are defined in Table 1, Table 2, and Table 3. We accounted only for transmission via sexual or intimate contacts with either main regular sex partners or casual sex partners, a distinction that follows earlier modelling of sexual relationships [11, 19, 28]. In this paper, the notion of “main regular sex partner” refers to a person with whom an individual engages in regular or ongoing sexual activity, typically (but not exclusively) within a committed relationship. Other relationships involving sexual contacts are referred to as casual partnerships in this study. Considering sexual or intimate contacts sufficient for MPXV transmission, for simplicity, we assumed 1 such contact per casual partner. In the model, MSM were divided into 4 groups based on level of sexual activity, which was measured in terms of the total number of sex partners men had: very low, fairly low, fairly high, and very high sexual activity (Table 2).
Figure 1.
Flow diagram of the model for monkeypox virus (MPXV) infections. Individuals in the model are divided into classes according to stage of mpox infection, vaccination status, and whether they are refraining from sexual contacts. Unvaccinated individuals can be susceptible to MPXV (), exposed to MPXV (), infectious not refraining from sexual contacts (), or infectious but refraining from sexual contacts (). Individuals vaccinated in the past can be susceptible (), exposed (), infectious not refraining or refraining from sexual contacts (, ). Individuals vaccinated in 2022 can be susceptible (), exposed (), infectious not refraining or refraining from sexual contacts (). Infectious cases may need hospitalization (). After recovery, they are immune (). These classes are further divided into 4 subclasses according to level of sexual activity. In the model, we accounted for exit out of the population at a per capita rate μ that is not shown in the diagram.
Table 1.
Model Parameters a
| Parameter | Value | Source | |
|---|---|---|---|
| Duration latent period, d | 5–8 | [11–16] | |
| Days infectious totalb | 14–28 | [11, 13–15, 17, 18] | |
| Days infectious not refraining from sex | 2–8 | [11, 14, 19, 20] | |
| Days infectious refraining from sex | … | … | |
| Probability of transmission per sexual contact | 0.1–0.9 | [12, 18, 19] | |
| Vaccine efficacy for vaccines administered in the past | 50%–65% | Datad | |
| Vaccine efficacy for vaccines administered in 2022 | 80%–90% | [1, 13, 14] | |
| Mpox-related death rate | 0.0004 | [21, 22] | |
| Factor reducing infectiousness of those vaccinated in the past | … | ||
| Factor reducing infectiousness of those vaccinated in 2022 | … | ||
| Hospitalization rate | ζ | 0.01–0.02 | Datad [22] |
| % of MSM who were in the past vaccinated against smallpox | … | 25% | [23] |
| Rate of entry into and exit out of the populationc | μ | 0.02/y | … |
| Mixing parameter for main regular partnerships | 0.1–0.9 | … | |
| Mixing parameter for casual partnerships | 0.1–0.9 | … | |
| Vaccination rate, per de | 0.0003–0.0005 | … | |
| Factor reducing transmission from infectious in abstinence | w | 0.15–0.7 | [14] |
| Number of MSM in the Netherlands | 250 000 | [23, 24] | |
| Number casual partners/d, MSM very high sexual activity group | 0.7–1.0 | Table 2 |
Abbreviation: MSM, men who have sex with men.
aParameters with a range were included in the Latin hypercube sampling.
bThe total number of days a mpox case is infectious is the sum of the days being infectious not in sexual abstinence and the days being infectious in abstinence.
cBased on assumption that MSM are sexually active approximately for 50 y.
dData from the national database of notifiable infectious diseases of the Netherlands [7].
eThe vaccination rate is for uninfected and for exposed individuals of activity group i. Range of values shown is for fairly high and very high sexual activity groups, from 7 June 2022, chosen such that the weekly number of vaccinations was 0.5-1.5 times the weekly number of cases, assuming that in this period approximately 1 contact of each diagnosed case was vaccinated. The vaccination rate for the low activity groups was zero throughout the calculations.
Table 2.
Parameters that Depend on the Sexual Activity Group of Individuals
| Parameter For Activity Group | Activity Group | |||
|---|---|---|---|---|
| Very Low | Fairly Low | Fairly High | Very High | |
| % in specific activity groupa | 45.1 | 36.4 | 17.7 | 0.7 |
| Rate of forming main regular partnerships per ya ( | 0.1 | 0.2 | 0.7 | 0.7 |
| Number of sex contacts per main regular partner per d () | 0.03 | 0.06 | 0.14 | 0.14 |
| % with main regular partnera () | 60 | 51 | 54 | 42 |
| Number of casual partners per da ( | 0.006 | 0.044 | 0.139 | 0.7–1.0 |
aParameters estimated from data from the first round of the “COVID-19, Sex, and Intimacy Survey” [25]. We used only data from the questions relating to sexual activity in the second half of 2019 (before the start of the pandemic in the Netherlands). Men in the very high sexual activity group reported more than 100 partners in the second half of 2019.
Table 3.
Prior Distributions of Uncertain Parameters Relating to the Adaptations in Behavior of MSM That May Have Occurred in June/July 2022
| Days Infectious Not Refraining From Sexual Contacts | Reductiona in Number Casual Partners of Group, % | |||
|---|---|---|---|---|
| Lowb | Fairly High | Very High | ||
| Scenario with adaptations at 1 time point | ||||
| After | 2–8 | 0–30 | 0–30 | 0–30 |
| Scenario with adaptations at 2 time points | ||||
| Between and | 2–8 | 0–30 | 0–30 | 0–30 |
| After | 2–8 | 0–30 | 0–30 | 0–30 |
| Dates when adaptations occurred | ||||
| 17–27 June 2022 | ||||
| 5–15 July 2022 | ||||
Values were sampled from the uniform distribution with the ranges shown in this table.
aIt was assumed that the number of casual partners of activity group i was at the beginning of the outbreak (as estimated from the data; see Table 2) and was reduced to after day , with sampled from the uniform distribution between 0 and 0.3. The maximum of 30% reduction was based on preliminary results from the survey “Monkeypox: a new challenge for your sex life” [9, 26, 27]and reports from club owners in Amsterdam who experienced reduced numbers of visitors in July 2022, with the reduction reaching its maximum at the end of July 2022 with approximately 30% fewer visitors (P. Zantkuijl, personal communication).
bChange applied to both very low and fairly low activity groups.
The Course of Mpox Infection
After infection, individuals are initially exposed but not yet infectious; later, they become infectious, but may not (yet) have symptoms [12, 13]. In the model, we assumed that infectious individuals may start refraining from sexual or intimate contacts a few days after becoming infectious, when they have symptoms or test positive for MPXV [13, 14]. MSM refraining from sexual/intimate contacts can still transmit MPXV (due to imperfect adherence or because they refrain only from specific types of contacts), but with a lower probability compared to those not refraining from sexual/intimate contacts [14]. Most mpox cases recover after a few weeks and become immune [15].
Vaccinated MSM
Smallpox vaccination was ended in the Netherlands in 1975. Based on the fraction of the male population 17–66 years old in the Netherlands being born before 1975, in the model we assumed that 25% of MSM in 2022 had been vaccinated in the past and that still offers some protection against mpox [7]. As we had no data to establish the percentage vaccinated in the past in each sexual activity group separately, we assumed the same percentage (25%) in all activity groups. The importance of this assumption was examined in sensitivity analyses (see below). From the second week of June 2022, close contacts of individuals diagnosed with mpox were offered vaccination with a single dose of Imvanex. To account for these vaccinations, we included in the model a small vaccination rate from 8 June 2022 onwards among those with the highest level of sexual activity.
Data
We used data on the daily numbers of mpox cases from the national surveillance system for notifiable infectious diseases [7]. The numbers of confirmed mpox cases were ordered according to date of symptom onset throughout the analyses presented in this study. The earliest date of symptom onset among confirmed mpox cases in the Netherlands was 27 April 2022.
Possible Behavioral Adaptations
We examined 2 possible adaptations: (1) MSM starting earlier to refrain from sexual contacts, thus resulting in shorter duration of the infectious period while not refraining from sex; and (2) a decline in the number of casual partners [7]. We investigated scenarios with these behavioral adaptations taking place (1) only in July 2022 or (2) in June and in July 2022 [9, 10]. The magnitude and timing of the adaptations were obtained from the fitting process.
Model Fitting
The model was calibrated to the number of mpox cases using a Bayesian approach. We defined uniform prior distributions for the uncertain parameters (Table 1) and sampled 10 000 combinations of parameter values. In a first step, we started the model calculations with 3 infectious unvaccinated cases in the subgroup with very high sexual activity level. With each combination of parameter values, we computed the daily number of mpox cases and its Poisson likelihood up to 5 July 2022 for the scenarios with adaptations only in July 2022 (and up to17 June 2022 for the scenarios with adaptations in June and July 2022). Table 3 shows parameters relating to the behavioral adaptations. This provided us with the posterior distributions of all uncertain parameters except those relating to the behavioral adaptations that occurred in June and July 2022. In a second step, we used the posterior distributions obtained from the first step and fitted the model to data for the remaining days until 25 July 2022. The prior distributions of the parameters in the first and the second step are shown in Table 1 and Table 3, respectively. The posterior distributions are shown in Supplementary Tables 1–3.
Sensitivity Analyses
Based on the size of the male population 17–66 years old [23] and available data on the prevalence of same-sex behavior [24], we assumed that the number of MSM in 2022 was 250 000. Due to uncertainty in those estimates, we repeated the analyses with a population size of 200 000 or 300 000 MSM. Also, we carried out sensitivity analyses for the fraction of the population that had been vaccinated before 1975 via the discontinued smallpox vaccination program. In our main results, we assumed that 25% of the current MSM population had been vaccinated against smallpox in the past. We examined also 2 scenarios where (1) the percentage vaccinated in the past was higher among those with higher levels of sexual activity: 23%, 25%, 30%, and 40% vaccinated in the group with very low, fairly low, fairly high, or very high level of sexual activity, respectively; and (2) the percentage vaccinated in the past was higher among those with lower levels of sexual activity: 29%, 25%, 15%, and 10% vaccinated in the group with very low, fairly low, fairly high, or very high level of sexual activity, respectively. These percentages were chosen in such a way that in the overall MSM population 25% had obtained smallpox vaccination in the past.
Hypothetical Scenarios for the Course of the Outbreak Without Vaccination
Earlier work has indicated that the mpox outbreaks could fade out within a few months even without vaccination or other major interventions [4, 5, 17, 19]. To investigate this possibility, we examined hypothetical scenarios for the course of the mpox outbreak until the end of 2022, without the mpox vaccination program.
We undertook model calculations until 31 December 2022 for the following scenarios: (1) without behavioral adaptations, and (2) with behavioral adaptations in July 2022. For both scenarios, the parameter values were those obtained via the fitting process using data up to 25 July 2022. Also, we investigated whether new introductions of mpox cases could elicit a resurgence in mpox. For the scenario with behavioral adaptations in July 2022, we examined 2 scenarios with import of unvaccinated infectious mpox cases into the group with very high sexual activity level: (1) import of 20 cases on 1 November 2022, or (2) import of 1 case per day from 1 November until 31 December 2022.
RESULTS
The Decline in the Mpox Outbreak With and Without Behavioral Adaptations
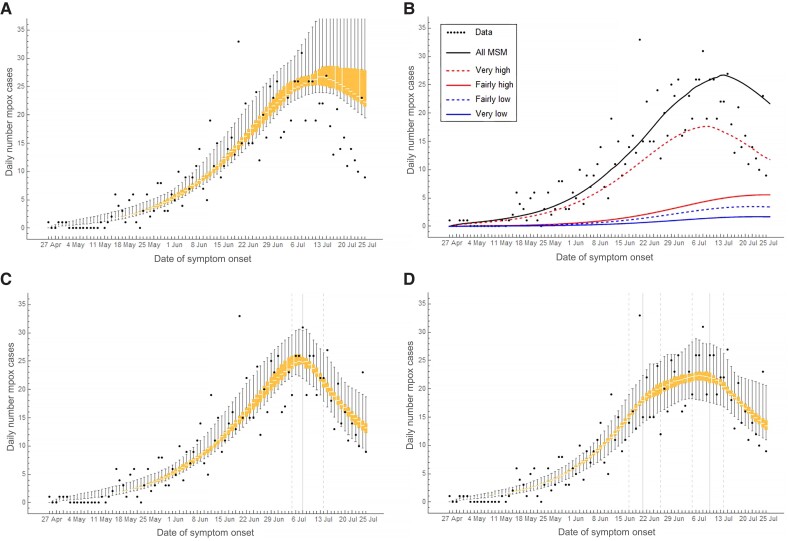
Without behavioral adaptations in the model, the daily number of mpox cases among MSM started declining around 8 July 2022, but the decline was less steep than observed (Figure 2A). The epidemic curve in the overall MSM population paralleled the epidemic curve in the group with very high sexual activity level (Figure 2B). Allowing for behavioral adaptations in July 2022, we found a decline of 13% (95% credible interval [CrI], 1%–28%), 15% (95% CrI, 1%–29%), and 24% (95% CrI, 1%–30%) in numbers of casual partners of MSM with low, fairly high, and very high sexual activity, respectively, compared to before the mpox outbreak (Supplementary Table 1). The infectious period while not refraining from sexual contacts was reduced from 6.0 days (95% CrI, 4.4–7.8 days) in May-June 2022 to 2.6 days (95% CrI, 2.0–4.3 days) in July 2022. The timing of these behavioral adaptations was calculated from the model at 7 July 2022 and there was a rather sharp decline in the number of cases thereafter (Supplementary Table 1 and Figure 2C). With behavioral adaptations in June and July 2022, we found that the numbers of casual partners of MSM with low, fairly high, and very high sexual activity were reduced by 17%, 16%, and 18%, respectively, in June, and by 13%, 18%, and 22%, respectively, in July (Supplementary Table 1). The infectious period while not refraining from sexual contacts was 6.9 days in May, 5.6 days in June, and 2.4 days in July 2022 (Supplementary Table 1). The adaptations occurred as of 21 June and 10 July 2022 (Supplementary Table 1 and Figure 2D).
Figure 2.
The daily number of mpox cases among men who have sex with men (MSM) in the Netherlands from 27 April to 25 July 2022. Bullets show data from the national database of notifiable diseases of the Netherlands. All other results were calculated from the model. Mpox cases are shown according to date of symptom onset. The box-plots show interquartile range and min-max range of the daily numbers of mpox cases in the overall MSM population: (A) without behavioral adaptations; (C) with behavioral adaptations only in July 2022; and (D) with behavioral adaptations in June and July 2022. B, Median daily number of mpox cases in the overall MSM population (thick line) and separately in the four groups with different sexual activity level. C and D, Vertical grey lines show medians (solid lines) and 95% credible intervals (dashed lines) of the day at which the behavioral adaptations occurred, as obtained from the model fitting. Model results were calculated in a population of 250 000 MSM.
Distribution of Mpox Cases According to Level of Sexual Activity
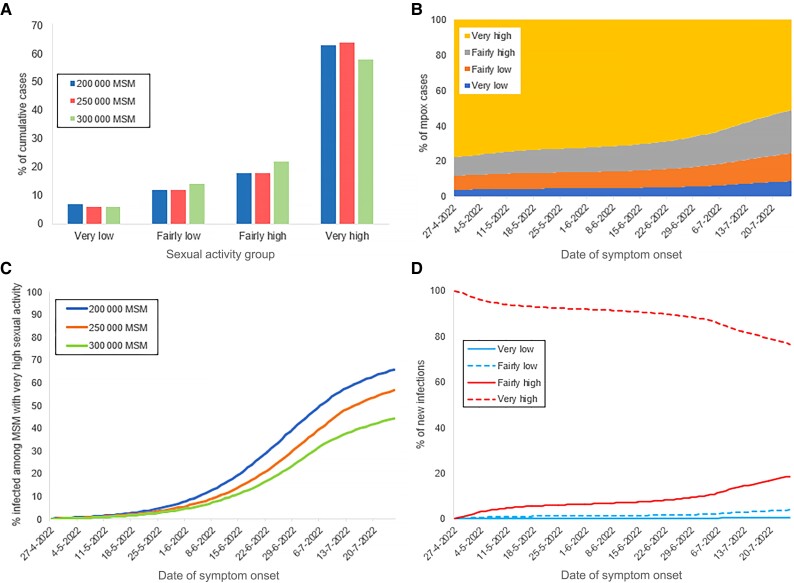
By 25 July 2022, 64% of mpox cases were MSM with very high and 18% with fairly high levels of sexual activity (Figure 3A and 3B). At that point, 57% (95% CrI, 33%–66%) of the group with a very high sexual activity level had been infected with MPXV (Figure 3C), but only 1.1% (95% CrI, 0.9%–1.3%) of the overall MSM population. Of all MPXV infections in the model until 25 July 2022, the infecting partner was a man with very high, fairly high, fairly low, or very low sexual activity in 76.7%, 18.7%, 3.9%, and 0.7% of infections, respectively (Figure 3D).
Figure 3.
The role of subgroups of men who have sex with men (MSM) with different levels of sexual activity, as calculated from the model, for the scenarios with behavioral adaptations in July 2022. A, Distribution of cumulative mpox cases until 25 July 2022. B, Distribution of daily mpox cases. C, Percent of MSM with very high sexual activity being susceptible to monkeypox virus. D, Distribution of new monkeypox virus infections among MSM according to the sexual activity group of the infecting individual. A and C, Results with population of 200 000, 250 000, or 300 000 MSM. B and D, results with population of 250 000 MSM.
Sensitivity Analyses for the Size of the MSM Population
With a smaller population of 200 000 MSM (Supplementary Figure 2 and Supplementary Table 2), the modelled decline in the daily number of cases started earlier and the number of cases in the very high sexual activity group was lower than in the scenario with 250 000 MSM. In a larger population of 300 000 MSM, the outbreak without behavioral adaptations would have been more severe than what we observed in the data (Supplementary Figure 3 and Supplementary Table 2). The number of mpox cases in the model started declining around 20 July 2022, about 10 days later than observed (Supplementary Figure 3A). The fraction of the group with very high sexual activity level that had been infected with MPXV by 25 July 2022 was 66% in a population of 200 000 MSM and 45% in a population of 300 000 MSM (Figure 3C).
With different percentages of MSM vaccinated against smallpox in the past across the different sexual activity groups, the results were similar (Supplementary Figure 4 and Supplementary Table 3). The transmission probability per sexual act was estimated at 0.60 (95% CrI, 0.47–0.80) with a higher percentage vaccinated among those with higher numbers of sex partners (Supplementary Table 3); 0.42 (95% CrI, 0.32–0.68) with a higher percentage vaccinated among those with lower numbers of sex partners (Supplementary Table 3); and 0.48 (95% CrI, 0.39–0.81) in the scenario with the same percentage vaccinated in all activity groups (Supplementary Table 1).
Hypothetical Scenarios for the Course of the Outbreak Without Vaccination
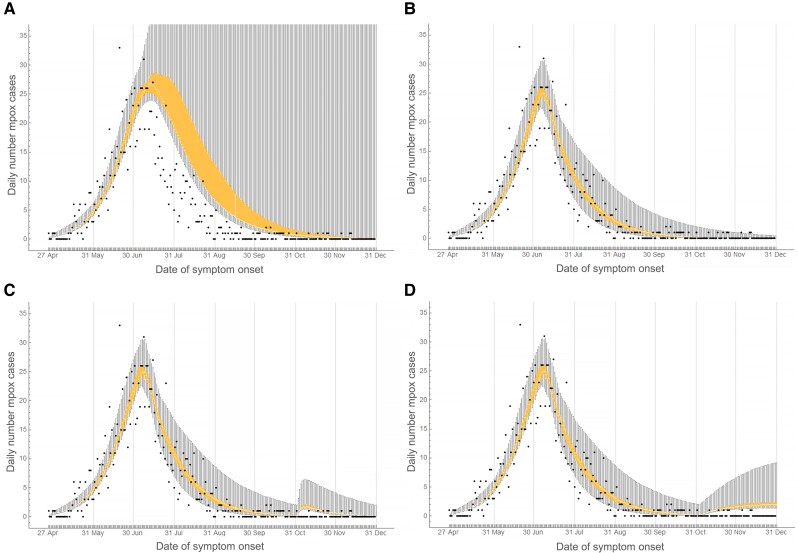
Figure 4 shows the daily numbers of mpox cases from the model and from data until 31 December 2022. Without behavioral adaptations in 2022, the outbreak faded out by December 2022 with most of the parameter combinations (Figure 4A and Supplementary 5). With the remaining parameter combinations, the modelled number of mpox cases reached its peak after July 2022, but it rapidly declined by the end of 2022. In 4.6% of the parameter combinations, we found more than 3 mpox cases by 31 December 2022 (Supplementary Figure 5). These parameter combinations included high transmission probabilities combined with long infectious period without refraining from sexual contacts and low level of assortativeness in sexual mixing with casual partners. With behavioral adaptations in July 2022 in the model, the outbreak faded out by November 2022 (Figure 4B).
Figure 4.
Daily number of mpox cases among men who have sex with men (MSM) in the Netherlands from 27 April to 31 December 2022. Bullets show data obtained from the national database of notifiable infectious diseases of the Netherlands. Box-plots show medians, interquartile ranges, and min-max ranges calculated from the model in a population of 250 000 MSM. Vertical solid grey lines show the last day of each calendar month. A, Without behavioral adaptations. B, With behavioral adaptations in July 2022. C, With behavioral adaptations in July 2022 and import of 20 unvaccinated infectious mpox cases into the group with very high level of sexual activity on 1 November 2022. D, With behavioral adaptations in July 2022 and import of 1 infectious case per day (in the group with very high level of sexual activity) from 1 November to 31 December 2022.
Import of unvaccinated infectious MSM with very high level of sexual activity resulted in a small increase in mpox cases. With import of 20 cases on 1 November 2022, we found 41 (95% CrI, 30–106) additional mpox cases (Figure 4C); with import of 1 case per day from 1 November to 31 December 2022, we found 81 (95% CrI, 63–211) additional cases (Figure 4D) compared to the scenario without import.
DISCUSSION
We developed a transmission model of the mpox outbreak among MSM and fitted this model to the daily numbers of mpox notifications in the Netherlands. We demonstrated that reduction in the number of susceptibles among MSM with very high levels of sexual activity may have limited further growth of the mpox epidemic, but could not solely account for the rapid decline after the peak. This suggests that behavioral adaptations may have accelerated the decrease in mpox cases. Furthermore, our findings suggest that, even without the mpox vaccination program, the level of immunity due to infection among MSM with a very high level of sexual activity was sufficiently high to lead to the fading out of the outbreak by November 2022.
The major strength of our study is that we quantified the contribution of infection-induced immunity and the contribution of behavioral adaptations in shaping the outbreak. The model, informed by the daily numbers of registered mpox cases during the rise and the decline of the mpox outbreak, allowed us to explore counterfactual situations and to show that the outbreak could have waned by the end of 2022 even without mpox vaccination or behavioral adaptations.
We incorporated heterogeneity in sexual activity by dividing the population into groups with different levels of sexual activity and captured the assortative mixing of highly active individuals that is characteristic of sexual contact networks. This modelling approach has the advantage of being well established, validated, and extensively studied in the past 4 decades in the context of several sexually transmitted infections [25, 29–31]. Heterogeneity in the number of sexual partners and assortative mixing may enhance the selective spread of infection among individuals with higher sexual activity (see [29, 30] and references herein). Our findings are consistent with previous studies on MPXV transmission showing that a very small group of MSM with high level of sexual activity can be sufficient to cause large outbreaks of short duration [4, 17, 19]. Our results correspond well also with a study that showed how the observed decline in mpox cases could be primarily attributed to infection-induced immunity [5]. The present study extends earlier findings as our results quantify the contribution of infection-induced immunity and the contribution of behavioral adaptations and reveal how these factors shaped the outbreak. In contrast with other studies projecting smaller outbreaks [32] or more severe and longer-lasting outbreaks [4, 11, 19], we found that the size of the 2022 mpox outbreak was large but self-limited and could have died out within a few months even without behavioral adaptations or vaccination.
We showed that, without behavioral adaptations, the decline in mpox cases in a larger population of 300 000 MSM started later than what we observed in the data. This suggests that the observed decline could have been possible only with behavioral adaptations. When extending the model results without behavioral adaptations to the end of 2022, we found that in a small fraction of the fitted parameter combinations, the number of mpox cases peaked after July 2022 and the outbreaks faded out after the end of 2022. In this situation, behavioral adaptations and vaccination could have prevented a large number of cases. Our results suggest that a reduction in numbers of sexual partners considerably expedited the decline in mpox cases. MSM may have started changing their behavior with the first news of mpox in the general media. This effect may have been strengthened by the mpox awareness campaigns that were conducted in gay sex venues and media [10, 33]. Accurate and timely communication with populations most at risk appears essential for public health response, as similar outbreaks could occur with other pathogens when introduced into subpopulations with sufficiently high contact levels. The mpox vaccination program possibly accelerated the impact of awareness and behavioral adaptations and offered protection against mpox at the individual level. Vaccination also reduced the risk of resurgence of mpox because it enhanced immunity at the population level. Our model can be used to investigate the impact of vaccination for specific subgroups of MSM. Furthermore, our model could be extended to account for transitioning across sexual activity groups.
Our study has important implications for the prevention of mpox outbreaks in the future. Prevention can be achieved by maintaining a high level of immunity among those with the highest sexual activity level. First, it should be taken into account that the group of MSM with the highest sexual activity level may not be a closed and fixed group over time: it is likely that over long time periods, some turnover occurs among this group and the rest of the MSM population. For example, MSM with low levels of sexual activity who did not receive vaccination in 2022 might become more sexually active and replenish the pool of susceptible MSM with a high level of sexual activity [30], thus making a new mpox outbreak possible. Second, in assessing the effectiveness of public health messages and interventions, it is important to consider the venues that the target group might visit. Having sex in sex clubs and parties has been identified as an independent predictor of MPXV infection [9]. Third, in the current study, we assumed that the percentage of MSM that had received smallpox vaccination in the past was the same in each sexual activity group. Our sensitivity analysis indicates that this assumption had a limited impact on the results. However, the percentages immune due to the old smallpox vaccination programs may not be exactly the same across the MSM population, depending on the age distribution of men in each sexual activity group. For future prevention of mpox outbreaks it is important to include the changing distribution of immunity with age. Furthermore, caution is needed when extrapolating our results to other settings. We allowed for only small behavioral adaptations. With the high transmission potential of MPXV, it is possible that with less assortative sexual mixing, the virus could have spread faster among the groups of MSM with low sexual activity levels. In that case, infection-induced immunity within the group with very high sexual activity level might have increased slower than in our analyses, making persistence more likely. Then fading of the outbreak would not have been possible without large reductions in numbers of sex partners. However, this is unlikely for the mpox outbreak in the Netherlands, because monthly numbers of sexually transmitted infection diagnoses, tests, and consultations were more or less stable throughout 2022 [34], therefore, we have no evidence of a large decline in numbers of sexual partners of MSM. Finally, recent reports point to the possibility of reinfections [35], but this was not accounted for in this study.
In conclusion, we demonstrated that depletion of susceptible MSM with high levels of sexual activity as well as behavioral adaptations accounted for the timing and speed of the decline in mpox cases among MSM in the Netherlands. The level of immunity due to infection within the subgroup of MSM with the highest level of sexual activity was build up so fast that the outbreak could have waned within a few months, even without interventions or behavioral adaptations. The risk of a resurgence of mpox in the short term seems very low, even with import of mpox infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Maria Xiridou, Centre for Infectious Disease Control, National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
Fuminari Miura, Centre for Infectious Disease Control, National Institute of Public Health and the Environment, Bilthoven, The Netherlands; Center for Marine Environmental Studies, Ehime University, Ehime, Japan.
Philippe Adam, Centre for Social Research in Health, University of New South Wales, Sydney, Australia; Institute for Prevention and Social Research, Utrecht, The Netherlands.
Eline Op de Coul, Centre for Infectious Disease Control, National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
John de Wit, Centre for Social Research in Health, University of New South Wales, Sydney, Australia; Interdisciplinary Social Science, Utrecht University, Utrecht, The Netherlands.
Jacco Wallinga, Centre for Infectious Disease Control, National Institute of Public Health and the Environment, Bilthoven, The Netherlands; Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands.
Notes
Acknowledgments. The authors thank Paul Zantkuijl (Soa Aids Nederland) for information on mpox messages in media and health promotion platforms for MSM in the Netherlands and for information about the reduction in visitors in clubs and sex venues in Amsterdam in July 2022. Anonymous club owners are also thanked for providing the above information. We express our gratitude to the participants, the research teams, and the organizations involved in the “COVID-19, Sex, and Intimacy Survey” and the survey “Monkeypox: a new challenge for your sex life” led by Philippe Adam and John de Wit. Both surveys were conducted in partnership between Utrecht University, the Institute for Prevention and Social Research, Utrecht, Soa Aids Nederland, and the National Institute of Public Health and Environment. We thank the anonymous reviewers for providing comments and suggestions that substantially improved the paper.
Author contributions. M. X., J. W., P. A., and F. M. contributed conceptualization and study design. P. A. and J. d. W. performed data curation and data collection. M. X., P. A., F. M., E. O. d. C., and J. d. W. performed data analyses. M. X. performed model development and analyses. M. X., F. M., P. A., and E. O. d. C. performed literature search. All authors contributed scenario investigation, interpretation, and writing.
Availability of data. The data for the numbers of daily mpox cases in the Netherlands described in this study can be freely and openly accessed on the website of the National Institute of Public Health and the Environment of the Netherlands (https://www.rivm.nl/en/mpox). The data from the “COVID-19, Sex, and Intimacy Survey” and the survey “Monkeypox: a New Challenge for Your Sex Life” are third-party data and are not freely available; enquiries for data access can be addressed to J. d. W. Description of data from the COVID-19, sex, and intimacy survey have been published [25]. The code for the model analyses is available at https://github.com/rivm-syso/fadingmpoxoutbreak.
Disclaimer. The funding source had no involvement in the analyses or interpretation of results.
Financial support. This work was supported by the Japan Society for the Promotion of Science (grant number 20J00793 to F. M.).Funding to pay the Open Access publication charges for this article was provided by the National Institute of Public Health and the Environment of The Netherlands.
References
- 1. Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 1988; 17:643–50. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . 2022 Mpox (monkeypox) outbreak: global trends, 13 December 2022. https://worldhealthorg.shinyapps.io/mpx_global/_w_77b7113a/#section-fns. Accessed 28 December 2022.
- 3. European Centre for Disease Prevention and Control . Monkeypox multi-country outbreak—second update 18 October 2022. https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-country-outbreak-second-update.pdf. Accessed 28 December 2022.
- 4. Brand SPC, Cavallaro M, Hilton Jet al. The role of vaccination and public awareness in forecasts of Mpox incidence in the United Kingdom. Nat Commun 2023; 14:4100. doi: 10.1038/s41467-023-38816-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murayama H, Pearson CAB, Abbott S, et al. Accumulation of immunity in heavy-tailed sexual contact networks shapes mpox outbreak sizes. J Infect Dis 2024; 229:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute of Public Health and the Environment (RIVM) . Monkeypox. https://www.rivm.nl/en/monkeypox. Accessed 3 November 2022.
- 7. van Ewijk C, Miura F, van Rijckevorsel G, et al. Monkeypox outbreak in the Netherlands in 2022: public health response, epidemiological and clinical characteristics of the first 1000 cases and protection of the first-generation smallpox vaccine. Euro Surveill 2023; 28:2200772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Man Tot Man . Do this if you have monkeypox. https://www.mantotman.nl/en/do-this-if-you-have-monkeypox. Accessed 19 July 2022.
- 9. Adam PCG, de Coul ELM O, Bos H, et al. Monkeypox-related changes in behaviour among MSM in the Netherlands and their impact on the monkeypox outbreak: using behavioural research and theorising to inform the monkeypox response. Utrecht, the Netherlands: Utrecht University and Institute for Prevention and Social Research, 2022. [Google Scholar]
- 10. Soa Aids Nederland . Samenwerking zorg en horeca onmisbaar in aanpak monkeypox-uitbraak.https://www.soaaids.nl/nl/professionals/actueel/nieuwsbericht/samenwerking-zorg-horeca-onmisbaar-in-aanpak-monkeypox-uitbraak. Accessed 6 October 2022.
- 11. Van Dijck C, Hens N, Kenyon C, Tsoumanis A. The roles of unrecognized monkeypox cases, contact isolation and vaccination in determining epidemic size in Belgium. A modelling study. Clin Infect Dis 2023; 76:e1421–3. [DOI] [PubMed] [Google Scholar]
- 12. Beer E, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019; 13:e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Mpox (monkeypox) key facts, update 13 April 2023. https://www.who.int/news-room/fact-sheets/detail/monkeypox?gclid=EAIaIQobChMIwb_jz_DbgQMVrjsGAB2DgQ54EAAYASABEgJQJfD_BwE. Accessed 4 October 2023.
- 14. European Centre for Disease Prevention and Control . Considerations for contact tracing during the monkeypox outbreak in Europe, 2022. 28 June 2022. https://www.ecdc.europa.eu/en/publications-data/considerations-contact-tracing-during-monkeypox-outbreak-europe-2022. Accessed 4 October 2023.
- 15. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis 2004; 4:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raccagni AR, Canetti D, Mileto D, et al. Two individuals with potential monkeypox virus reinfection. Lancet Infect Dis 2023; 23:522–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endo A, Murayama H, Abbott S, et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak, 2022. Science 2022; 378:90–4. [DOI] [PubMed] [Google Scholar]
- 18. Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill 2022; 27:2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spicknall IH, Pollock ED, Clay PA, et al. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men—United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bankuru SV, Kossol S, Hou W, Mahmoudi P, Rychtář J, Taylor D. A game-theoretic model of monkeypox to assess vaccination strategies. PeerJ 2020; 8:e9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brosius I, Van Dijck C, Coppens Jet al. Presymtpomatic viral shedding in high-risk mpox contacts: a prospective cohort study. J Med Virol 2023; 95:e028769. doi: 10.1002/jmv.28769. [DOI] [PubMed] [Google Scholar]
- 22. Kozlov M. How deadly is monkeypox? What scientists know. 2022. https://www.nature.com/articles/d41586-022-02931-1. Accessed 26 September 2022.
- 23. Statistics Netherlands . The Netherlands in figures 2022. https://opendata.cbs.nl/#/CBS/en. Accessed 15 November 2022.
- 24. Rutgers NISSO Group . Sexual health in The Netherlands. Delft: Eburon, 2006. [Google Scholar]
- 25. Xiridou M, Heijne J, Adam P, et al. How the disruption in sexually transmitted infection care due to the COVID-19 pandemic could lead to increased sexually transmitted infection transmission among men who have sex with men in the Netherlands: a mathematical modeling study. Sex Transm Dis 2022; 49:145–53. [DOI] [PubMed] [Google Scholar]
- 26. Fink D, Callaby H, Luintel A, et al. Clinical features and management of individuals admitted to hospital with monkeypox and associated complications across the UK: a retrospective cohort study. Lancet Infect Dis 2022; 23:589–97. [DOI] [PubMed] [Google Scholar]
- 27. Adam PCG, Op de Coul ELM, Zantkuijl P. A survey-based assessment of rates and covariates of mpox diagnosis and vaccination provides evidence to refine eligibility criteria for mpox vaccination among gay, bisexual and other men who sex with men in the Netherlands. Frontiers Public Health 2023; In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenness SM, Le Guillou A, Chandra C, et al. Projected HIV and bacterial sexually transmitted infection incidence following COVID-19-related sexual distancing and clinical service interruption. J Infect Dis 2021; 223:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yorke J, Heathcote H, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis 1978; 5:51–6. [DOI] [PubMed] [Google Scholar]
- 30. Anderson RM, May RM. Infectious diseases of humans; dynamics and control. 1st ed. Oxford: Oxford University Press, 1992. [Google Scholar]
- 31. Hethcote HW, Yorke J. Gonorrhea transmission dynamics and control. Heidelberg: Springer-Verlag Berlin, 198456. [Google Scholar]
- 32. Bisanzio D, Reithinger R. Projected burden and duration of the 2022 monkeypox outbreaks in non-endemic countries. Lancet Microbe 2022; 3(9):e643. doi: 10.1016/S2666-5247(22)00183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soa Aids Nederland . Uit de praktijk: 2 plekjes op de eikel, dat is toch geen monkeypox?https://www.soaaids.nl/nl/professionals/themas/seksoa-magazine/uit-praktijk-2-plekjes-op-eikel-dat-is-toch-geen-monkeypox. Accessed 30 June 2022.
- 34. Miura F, Backer JA, van Rijckevorsel G, et al. Time scales of human mpox transmission in the Netherlands. J Infect Dis 2024; 229:800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Institute of Public Health and the Environment (RIVM) . Thermometer sexual health, April 2023. https://www.rivm.nl/documenten/thermometer-seksuele-gezondheid-april-2023. Accessed 4 October 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.