Abstract
Background
Interventions introduced to reduce the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to a widespread reduction in childhood infections. However, from spring 2021 onwards the United Kingdom and Ireland experienced an unusual out-of-season epidemic of respiratory disease.
Methods
We conducted a prospective observational study (BronchStart), enrolling children 0–23 months of age presenting with bronchiolitis, lower respiratory tract infection, or first episode of wheeze to 59 emergency departments across England, Scotland, and Ireland from May 2021 to April 2022. We combined testing data with national admissions datasets to infer the impact of respiratory syncytial virus (RSV) disease.
Results
The BronchStart study collected data on 17 899 presentations for 17 164 children. Risk factors for admission and escalation of care included prematurity and congenital heart disease, but most admissions were for previously healthy term-born children. Of those aged 0–11 months who were admitted and tested for RSV, 1907 of 3912 (48.7%) tested positive. We estimate that every year in England and Scotland 28 561 (95% confidence interval, 27 637–29 486) infants are admitted with RSV infection.
Conclusions
RSV infection was the main cause of hospitalizations in this cohort, but 51.3% of admissions in infants were not associated with the virus. The majority of admissions were in previously healthy term-born infants.
Keywords: respiratory syncytial virus, respiratory infections, immunization
The BronchStart study collected data on 17 899 presentations in children aged <2 years with serious respiratory disease following the lifting of lockdown measures. For infant admissions, 51.3% were not associated with RSV; the majority were in previously healthy term infants.
Respiratory syncytial virus (RSV) is an RNA virus that prior to the coronavirus disease 2019 (COVID-19) pandemic caused annual epidemics of respiratory infections, most prominently bronchiolitis. The main burden of bronchiolitis disease is in those under 1 year of age [1]. In temperate climates RSV epidemics, similar to influenza, normally occur in the autumn and winter [2]. Although RSV is the main driver of winter seasonal bronchiolitis epidemics, other viruses such as rhinovirus and human metapneumovirus are also important contributors. Globally, viral bronchiolitis is one of the largest contributors to hospital admission for children under the age of 5 years [3–5].
Nonpharmaceutical interventions, introduced in early 2020 to limit the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led initially to a reduction in the burden of all childhood infectious disease [6, 7] and subsequently to a disruption in the timing of annual RSV epidemics. The autumn and winter of 2020–2021 saw very few RSV infections in children in the northern hemisphere countries of Europe, the United States, and China [8]. There was then an out-of-season increase in RSV in most of these countries the following spring or summer (of 2021) [9], reflecting what had already been observed in the southern hemisphere [10].
Despite significant investment, attempts to develop an effective RSV vaccine have proven unsuccessful until recently [11, 12]. Nirsevimab, a long-acting anti-RSV monoclonal antibody targeting site 0 of the RSV fusion protein, given as a single injection to infants at the start of their first RSV season, has recently shown significant benefit in a multinational randomized controlled trial [13], with recent Medicines and Healthcare Products Regulatory Agency [14] and European Medicines Agency [15] licensing approval. There are similar promising results from a phase 3 maternal immunization study for RSV [16]. It is therefore likely that monoclonal antibodies and/or maternal immunization will be introduced to routine clinical care soon, either for high-risk, or all, infants. Up-to-date information on the burden of disease is needed to inform the introduction of such immunizations.
The BronchStart study was initiated in the spring of 2021 to prospectively examine features of an anticipated unusual RSV summer season. At this point there were concerns that lack of exposure to viral infection in mothers and in the 0–23-month age group might lead to more severe disease in young children, and a shift in age distribution of disease in this group, and we therefore focused the study on this population. We have previously reported our interim results, showing transposed seasonality but no evidence for increased severity of disease in this cohort [17].
Here, we present full data from the study, with a focus on the hospital treatment burden of serious viral respiratory tract infections. We examine the risk of more severe disease in at-risk groups, and present data on the proportion of cases caused by RSV. Using the BronchStart cohort, we utilized national admissions data from England and Scotland from years before the COVID-19 pandemic to infer the total RSV-specific annual admissions and treatment burden for an average nonpandemic year, with the aim of informing decisions regarding the roll-out of any RSV preventative measure.
METHODS
Inferring the impact of respiratory syncytial virus disease in a typical year requires integration of findings from the BronchStart study with national admissions datasets from England and Scotland. The methods used to generate each dataset are therefore presented separately below.
BronchStart Study Methods
BronchStart Data Collection, Inclusion and Exclusion Criteria, Data Capture, and Outcomes
BronchStart was a multinational, multicentre prospective observational study conducted at Paediatric Emergency Research in the United Kingdom and Ireland (PERUKI) network sites [18] supported by the RESCEU Consortium (www.resc-eu.org). The BronchStart protocol was structured in keeping with the principles of the STROBE statement [19] and has been previously peer reviewed and published [20]. Further details can be found in the Supplementary Methods.
BronchStart Statistical Analyses
Data extraction and analysis was performed using RStudio and R version 4.2.2 [21]. Descriptive tables were generated using gtsummary [22]. Risk ratios and confidence intervals (CIs) for risk of different outcomes for infants born preterm (at <37 weeks) and/or with congenital heart disease were calculated. For each dichotomous outcome (level of care), the proportions of those in the at-risk group compared to those with no documented comorbidities were calculated using a log binomial regression model, which allowed the calculation of a relative risk (rather than an odds ratio) with confidence intervals [23]. A proportional odds model analysis was performed to calculate the odds ratio of admission in the 0–11-month age group, adjusting for gestation and age. The reference group were children born at term aged 12–23 months at the time of admission. Full details of the statistical analysis are available on the BronchStart GitLab page (https://git.ecdf.ed.ac.uk/twillia2/bronchstart-impact-of-rsv-analysis).
BronchStart Ethics, Funding, and Public and Patient Engagement
Full details for these can be found in the Supplementary Methods.
National Hospital Admissions Methods
Creating Hospital Admissions Datasets and Ethics
We used national datasets of admissions in England and Scotland for bronchiolitis, lower respiratory tract infection (LRTI) and wheeze for the years preceding the COVID-19 pandemic to estimate an annual national inpatient treatment burden. For England, we used Hospital Episode Statistics Admitted Patient Care (HES APC) data to identify admissions, as described previously [24]. For Scotland we used General Acute Inpatient and Day Case-Scottish Morbidity Record (SMR01) data to identify admissions. Full details on the use of these datasets can be found in the Supplementary Methods.
Statistical Analysis Linking BronchStart Testing Results to Hospital Admissions Dataset
We used BronchStart data to infer the RSV-specific hospital treatment burden for years preceding the COVID-19 pandemic. We first calculated the percentage of children testing positive for RSV in all patients who were tested for the virus in the BronchStart dataset, and applied these to the annual mean admissions numbers for all admissions in those aged 0–11 months in England (from the HES dataset) and Scotland (from the SMR01 dataset), focusing on the 0–11-month age group as this is the patient population most likely to benefit from the currently available anti-RSV interventions. For full details on statistical analyses see the Supplementary Methods. Analysis scripts are available at GitLab (https://git.ecdf.ed.ac.uk/twillia2/bronchstart-impact-of-rsv-analysis).
RESULTS
Case Recruitment to the BronchStart Study
From 1 May 2021 to 30 April 2022, 17 935 emergency department (ED) attendance episodes were recorded as part of the BronchStart study. We excluded 36 records with incomplete data, leaving a final cohort of 17 899 episodes by 17 164 individual children from 59 sites. Of the 17 164 children included, 15 933 (89%) were recruited at sites in England, 1490 (8.3%) in Scotland, and 476 (2.7%) in the Republic of Ireland (Supplementary Figure 1). Males predominated (10 357 of 17 164; 60.3%), and of 15 579 children with socioeconomic status available, 2588 (16.4%) were from the least deprived quintile, and 4378 (27.8%) from the most deprived quintile.
Diagnoses Assigned by Country
The most common diagnosis assigned to attendance episodes within the BronchStart cohort was bronchiolitis (14 480 of 17 899; 80.9%), followed by first episode of viral-induced wheeze (1898 of 17 899; 10.6%), and LRTI (1521 of 17 899; 8.5%). The proportion of bronchiolitis diagnoses assigned was higher in Scotland (1309 of 1490; 87.9%) than England (12 791 of 15 933; 80.3%) or Ireland (380 of 476; 79.8%), and the diagnoses of first wheeze and LRTI correspondingly lower (first wheeze, 7.1% in Scotland vs 10.9% in England and 10.3% in Ireland; LRTI, 5.0% vs 9.9% and 8.8%).
Highest Level of Care
The majority of attendance episodes (11 074 of 17 899; 61.9%) were assessed in the ED (sometimes more than once) and discharged home (Table 1). A total of 6825 (38.1%) attendance episodes from the cohort ultimately resulted in an admission to hospital; 2 children died in the ED. Again, the most common diagnosis assigned to those admitted was bronchiolitis (5300 of 6825; 77.7%). The most common admission location was a hospital ward (5027 of 6825; 73.7%) or an observation unit (1186 of 6825; 17.4%). Of the entire BronchStart cohort, 458 of 17 899 (2.6%) required high-dependency unit (HDU) level care, and a further 154 (0.9%) were admitted to a pediatric intensive care unit (PICU) (Table 1). Testing for RSV was performed commonly for those admitted to a pediatric ward (90.9% tested), to HDU (95.6% tested), or to PICU (96.1% tested), but rarely in the ED (87% untested) or for those admitted to observation units (47% untested). Overall, of the 6825 attendance episodes that resulted in an admission, 5788 (84.8%) were tested for RSV.
Table 1.
Highest Level of Care Provided to Patients in the BronchStart Cohort, by Clinician Diagnosis and Virus Detected by Diagnostic Test
| ED (n = 11 074) |
Observation (n = 1186) |
Ward (n = 5027) |
HDU (n = 458) |
PICU (n = 154) |
Total (n = 17 899) |
|
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| Bronchiolitis | 9180 (83) | 847 (71) | 3918 (78) | 396 (86) | 139 (90) | 14 480 |
| First wheeze | 985 (8.9) | 219 (18) | 661 (13) | 31 (6.8) | 2 (1.3) | 1898 |
| LRTI | 909 (8.2) | 120 (10) | 448 (8.9) | 31 (6.8) | 13 (8.4) | 1521 |
| Virus | ||||||
| RSV | 431 (3.9) | 189 (16) | 1951 (39) | 195 (43) | 85 (55) | 2851 |
| Other | 284 (2.6) | 135 (11) | 728 (14) | 90 (20) | 41 (27) | 1278 |
| Negativea | 768 (6.9) | 310 (26) | 1889 (38) | 153 (33) | 22 (14) | 3142 |
| Untested | 9591 (87) | 552 (47) | 459 (9.1) | 20 (4.4) | 6 (3.9) | 10 628 |
Data are No. (%).
Abbreviations: ED, emergency department; HDU, high-dependency unit; LRTI, lower respiratory tract infection; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
aThis captures patients who were tested specifically for RSV and were negative for the virus.
Of 11 575 initial attendances who were discharged from the ED without admission (Supplementary Table 1 and Supplementary Figure 2), 9928 were discharged and did not reattend at 7 days, 970 reattended but were discharged home again, and 677 reattended and were admitted, meaning that after initial discharge there was a 14.2% (1647 of 11 575) chance of reattendance after initial discharge from an ED, and a 5.9% (677 of 11 575) chance of admission following initial discharge from an ED.
Viral Testing
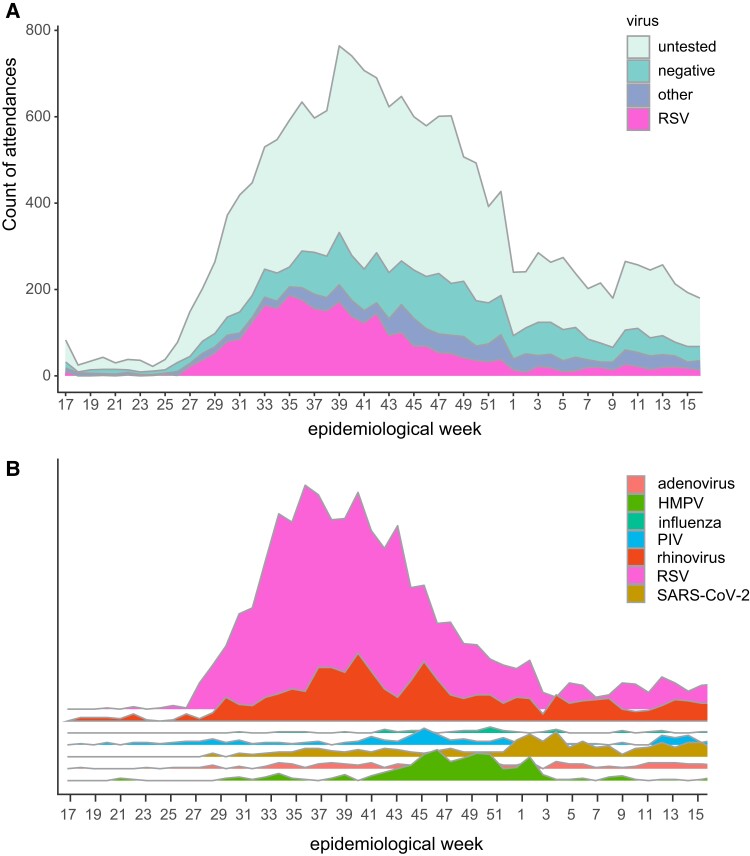
As described above, viral testing was most commonly performed for those admitted to hospital. For the whole cohort, RSV was the most common single pathogen identified (Figure 1A). Bearing in mind that testing for other viral pathogens was less frequent than RSV specific testing, other viral pathogens were also identified in children tested, most commonly rhinovirus (929 of 17 899; 5.2%; Figure 1B), where peaks in infection were similar in timing to RSV; peaks in RSV infection were followed temporally by spikes in human metapneumovirus (257 of 17 899; 1.4%) and then SARS-CoV-2 (287 of 17 899; 1.6%) infection.
Figure 1.
A, Virology testing showed RSV was the main pathogen during the study period (1 May 2021 to 30 April 2022). B, Graphical representation of other viruses identified in attendances in addition to RSV; area is proportional to number of positive tests. Abbreviations: HMPV, human metapneumovirus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Respiratory Support and Pharmacological Therapy in the Admitted BronchStart Cohort
For the admitted cohort, low-flow oxygen therapy was frequently administered for those with a diagnosis of bronchiolitis (2310 of 5301; 43.6%), first episode of wheeze (301 of 913; 33%), or LRTI (273 of 612; 44.6%) (Table 2). High-flow oxygen was administered most commonly for those with a diagnosis of bronchiolitis (922 of 5301; 17.4%), followed by those with LRTI (73 of 612; 11.9%), and less commonly for those with a diagnosis of first episode of wheeze (47 of 913; 5.1%). Antibiotics were administered to over two-thirds of those with a diagnosis of LRTI (434 of 612; 70.9%) and to over a fifth of those with a diagnosis of bronchiolitis (1201 of 5301; 22.7%).
Table 2.
Respiratory Support and Pharmacological Therapies Administered to Admitted BronchStart Patients, by Clinician Diagnosis
| Treatment | Bronchiolitis (n = 5301) | First Wheeze (n = 913) | LRTI (n = 612) |
|---|---|---|---|
| NG fluids | 1975 (37) | 30 (3.3) | 62 (10) |
| IV fluids | 686 (13) | 39 (4.3) | 105 (17) |
| Oxygen, LF | 2310 (43.6) | 301 (33) | 273 (44.6) |
| Oxygen, HF | 922 (17.4) | 47 (5.1) | 73 (11.9) |
| CPAP/BiPAP | 219 (4.1) | 1 (0.1) | 12 (2.0) |
| IMV | 92 (1.7) | 1 (0.1) | 6 (1.0) |
| Antibiotics | 1201 (22.7) | 157 (17.2) | 434 (70.9) |
| MgSO4 | 27 (0.5) | 37 (4.1) | 9 (1.5) |
| Prednisolone | 77 (1.5) | 155 (17) | 34 (5.6) |
| Salbutamol inhaler | 781 (15) | 784 (86) | 249 (41) |
| Salbutamol IV | 14 (0.3) | 8 (0.9) | 1 (0.2) |
Data are No. (%).
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; HF, high flow; IMV, invasive mechanical ventilation; IV, intravenous; LF, low flow; LRTI, lower respiratory tract infection; NG, nasogastric.
Comorbidities in the BronchStart Cohort
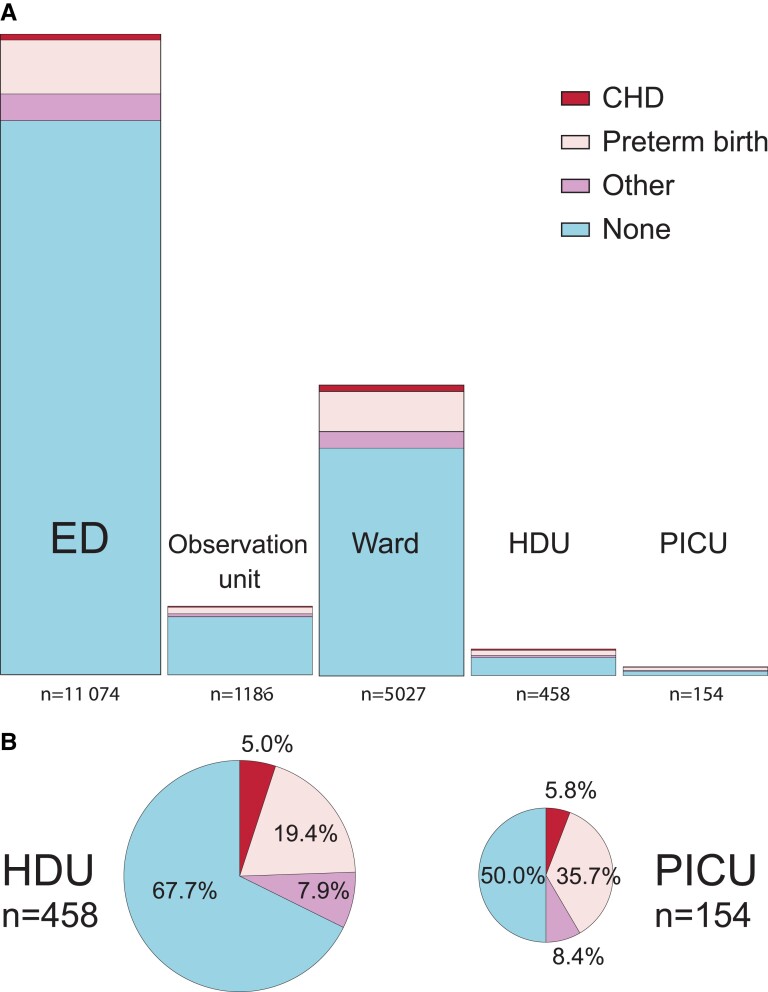
Of the BronchStart cohort attendances, 3011 (16.8%) were classified as having 1 or more comorbidities, including preterm birth. Of the cohort, 1970 (11%) were born preterm, 183 (1%) had chronic lung disease of prematurity, 264 (1.5%) had congenital heart disease, and 22 (0.1%) had a neuromuscular disorder. The proportion of comorbidities increased with level of care received. Of those seen and discharged from an ED, 86.4% had no documented comorbidity; this fell to 50% of patients admitted to a PICU (Figure 2 and Table 3). Prematurity was the most common comorbidity at all levels of care, including those admitted to HDU (99 of 458, 19.4%) and PICU (55 of 154, 35.7%). The most common comorbidity documented apart from prematurity or congenital heart disease in the HDU/PICU cohort was Down syndrome (11 of 612 patients, 1.8%).
Figure 2.
A, Highest level of care for BronchStart attendances including proportion of children with comorbidities. B, Children with comorbidities in HDU and PICU admissions. Data from Table 3, area of plots scaled to number of cases. Patients were ranked hierarchically so that each patient could only be assigned 1 category of comorbidity; congenital heart disease subsumed preterm birth or other comorbidities, and preterm birth other comorbidities. Abbreviations: CHD, congenital heart disease; ED, emergency department; HDU, high-dependency unit; PICU, pediatric intensive care unit.
Table 3.
Comorbidities and Highest Level of Care Received in the BronchStart Cohort
| Highest Level of Care | Preterm | CLD | CHD | NMD | Other | None | Total |
|---|---|---|---|---|---|---|---|
| ED | 963 (8.7) | 66 (0.6) | 104 (0.9) | 8 (0.1) | 571 (5.2) | 9573 (86.4) | 11 074 |
| Observation unit | 119 (10) | 12 (1) | 19 (1.6) | 0 (0) | 64 (5.4) | 1002 (84.5) | 1186 |
| Ward | 731 (14.5) | 77 (1.5) | 109 (2.2) | 9 (0.2) | 434 (8.6) | 3926 (78.1) | 5027 |
| HDU | 99 (21.6) | 20 (4.4) | 23 (5) | 3 (0.7) | 59 (12.9) | 310 (67.7) | 458 |
| PICU | 58 (37.7) | 8 (5.2) | 9 (5.8) | 2 (1.3) | 36 (23.4) | 77 (50) | 154 |
Data are No. (%). Patients could have more than 1 comorbidity and thus row totals do not add to 100%.
Abbreviations: CHD, congenital heart disease; CLD, chronic lung disease; ED, emergency department; HDU, high-dependency unit; NMD, neuromuscular disorder; PICU, pediatric intensive care unit.
Burden of Respiratory Support for Children With Comorbidities
Those born preterm had a 5.12-fold higher risk of invasive mechanical ventilation than those without comorbidities, and were more likely to be admitted, receive oxygen, receive high-flow oxygen therapy, be admitted to HDU/PICU, or receive invasive mechanical ventilation (Table 4). We also examined the relationship between the degree of prematurity and the risk of admission. We found that the highest risk of admission was for those born at <28 weeks, who had an odds ratio of admission of 3.28 (95% CI, 2.48–4.36) compared to those born at term and aged 12–23 months (Supplementary Table 2).
Table 4.
Treatments Administered and Highest Level of Care for Those Born Preterm Compared to Those With no Comorbidities in the BronchStart Cohort
| Outcome | No Comorbidities (n = 14 888) |
Preterm (n = 1970)a |
RRb | (95% CI)b |
|---|---|---|---|---|
| Admitted | 5316 (36) | 1007 (51) | 1.43 | (1.36–1.5) |
| Oxygen, LF | 2262 (15) | 528 (27) | 1.76 | (1.62–1.91) |
| Oxygen, HF | 754 (5.1) | 221 (11) | 2.22 | (1.92–2.55) |
| CPAP/BiPAP | 151 (1.0) | 75 (3.8) | 3.75 | (2.84–4.91) |
| IMV | 59 (0.4) | 40 (2.0) | 5.12 | (3.42–7.6) |
| Admit HDU | 327 (2.2) | 117 (5.9) | 2.70 | (2.19–3.31) |
| Admit PICU | 77 (0.5) | 58 (2.9) | 5.69 | (4.05–7.96) |
Data are No. (%).
Abbreviations: BiPAP, bilevel positive airway pressure; CI, confidence interval; CPAP, continuous positive airway pressure; HDU, high-dependency unit; HF, high flow; IMV, invasive mechanical ventilation; LF, low flow; PICU, pediatric intensive care unit; RR, relative risk.
aPreterm group includes those who had associated comorbidities, aged 0–23 months.
bRelative risk and CIs calculated using log binomial regression models.
Participants with congenital heart disease were more likely to receive higher levels of care than those with no comorbidities (Supplementary Table 3), except for invasive mechanical ventilation, where although there was a high relative risk this did not reach statistical significance.
Inferred National Treatment Burden
On average, there were 58 590 annual admissions for children in England and Scotland aged 0–11 months with a diagnosis of bronchiolitis, LRTI, or wheeze in the period 2016–2019 (Supplementary Table 4). Comparing demographic variables for the BronchStart cohort to those for the HES and SMR01 admissions, age and socioeconomic were similar (Supplementary Table 5), but the BronchStart cohort had a higher proportion of infants in the 0–3-month age group (41.3% vs 32.3% in HES and 33.4% in SMR01). Of the admitted BronchStart cohort tested for RSV, 2423 of 5792 (41.8%) were positive for RSV (1907 of 3912, 48.7% in those aged 0–11 months; 516 of 1880, 27.4% of those aged 12–23 months). Assuming an RSV positivity of 48.7% for the 0–11-month cohort gives an estimate of 28 561 (95% CI, 27 637–29 486) RSV-positive admissions with these diagnoses per year for this age group. Using these estimates, we inferred that in an average year, RSV infections in England and Scotland were associated with 12 167 infants receiving low-flow oxygen, 4998 high-flow oxygen, and 6198 a course of antibiotics during their admission (Table 5).
Table 5.
Inferred Treatment Burden for England and Scotland for Bronchiolitis, Lower Respiratory Tract Infection, and First Episode of Wheeze in Infants 0–11 Months
| Treatment | BronchStart Treatments | % Treated In BronchStart Cohort | % Attributable to RSV | Estimated RSV-Specific Treatments Annually (95% CI) |
|---|---|---|---|---|
| NG fluids | 1884 | 41.6 | 20.3 | 11 881 (11 497–12 266) |
| IV fluids | 606 | 13.4 | 6.5 | 3827 (3703–3951) |
| Oxygen, LF | 1925 | 42.6 | 20.8 | 12 167 (11 773–12 561) |
| Oxygen, HF | 791 | 17.5 | 8.5 | 4998 (4836–5160) |
| CPAP/BiPAP | 207 | 4.6 | 2.2 | 1314 (1271–1356) |
| Antibiotics | 983 | 21.7 | 10.6 | 6198 (5997–6399) |
| Total | 4524 | 100 | 48.7 | 28 561 (27 637–29 486) |
An annual admissions total was calculated for 2016–2019 using the Hospital Episode Statistics (England) and Scottish Morbidity Record 01 (Scotland) datasets.
Abbreviations: BiPAP, bilevel positive airway pressure; CI, confidence interval; CPAP, continuous positive airway pressure; HF, high flow; IV, intravenous; LF, low flow; NG, nasogastric; RSV, respiratory syncytial virus.
DISCUSSION
The BronchStart study captured data on 17 899 attendance episodes for children aged 0–23 months of age attending EDs with serious respiratory disease, gathering information on comorbidities, respiratory support, and pharmacological therapies administered. The most common diagnosis assigned was bronchiolitis, and RSV was the most common pathogen identified; however, only 48.7% of those admitted aged 0–11 months who were tested were positive for RSV. Risk factors for admission and escalation of therapy in the BronchStart cohort included prematurity and congenital heart disease. However, in our cohort for all levels of care, excluding PICU, most attendance episodes did not have any documented comorbidities. For PICU admissions, 50% of attendances were documented as having comorbidities, of which preterm birth was the most common. Using the BronchStart dataset to infer the impact of disease, we confirmed that bronchiolitis and other respiratory diagnosis in infants under 1 year of age constitute a significant burden on secondary health care services.
With BronchStart we have demonstrated the utility of a rapidly rolled out research study to capture disrupted childhood disease presentations. The same research model is applicable to other conditions and other locations and could be rapidly implemented to understand changes in RSV and other respiratory diseases, following the introduction of any anti-RSV intervention.
Another strength of our study is the large number of cases recruited prospectively—we believe that this is the largest prospective study of serious respiratory infections in children in the 0–23-month age group. We collected detailed information on viral testing, treatments administered, and outcomes across EDs and hospital wards, at a point just before the likely introduction of RSV immunization, and following the disruption caused by the COVID-19 pandemic. In addition, we used prospectively collected data to provide estimates of the treatment burden of RSV-specific respiratory disease in the 0–11-month age group, at a national level in England and Scotland.
Recruitment to the study was limited to PERUKI study centers, and therefore we could not guarantee that the cases recruited were representative of England, Scotland, and Ireland as a whole. Using annual incidence based on calendar years rather than seasons is another limitation. Comparison of BronchStart admissions to the HES and SMR-01 datasets for admissions with the same diagnoses showed that BronchStart admitted cases were more likely to be younger than admissions in England and Scotland in a prepandemic period. Discrepancies in the ages represented may be due to the fact that in the study we captured patients who were admitted with serious respiratory disease following ED presentation, and may therefore have missed data on those presenting via other pathways, or due to sampling biases within EDs.
Comparison of diagnoses from England, Scotland, and Ireland shows some differences in the proportions of the presenting conditions (bronchiolitis/first episode of wheeze/LRTI). While Scotland showed a generally higher prevalence of bronchiolitis presentations this is possibly explained by different socioeconomic, health care structures, and clinician interpretation of national guidance on diagnosis and management. Variance in admission rates between countries of the European Union has been previously reported, with Scotland demonstrating a higher rate of admissions compared with England, Spain, The Netherlands, and Finland [25].
Heterogeneity in testing approaches limits estimates of respiratory disease caused by other pathogens in this cohort. Our study does not include primary care prescription data, so our estimate relates solely to the antibiotic burden for RSV infection in admitted children. Another limitation is that we did not include children with suspected bacterial infection (sepsis, empyema) that may have developed secondary to RSV infection [26, 27], and thus we may have underestimated the burden of infection in this cohort. As the BronchStart study collected patient data following the lifting of COVID-19 restrictions, the percentage of cases positive for RSV, and the treatment burden, may not be representative of a typical year once RSV seasonality starts to resume normal prepandemic patterns.
We found that a lower proportion of admissions were positive for RSV than has been noted in previous studies [28, 29], which, however, focused on infants and children with bronchiolitis rather than all serious respiratory viral infections. However, despite this lower proportion, we estimate that in a typical year a large number of infants with RSV infection in England and Scotland will receive feeding support, low-flow oxygen, high-flow oxygen, and a course of antibiotics. As well as the direct costs of treatment, these have significant implications in terms of environmental impact [30] and the costs of antimicrobial resistance [31]. The impact of the COVID-19 pandemic on RSV epidemiology produced an interesting country to country variance in both timing and frequency of RSV infection rates. The Netherlands reported year-round transmission [32], whilst France reported clustering of transmission delayed from the anticipated peak season at a lower rate than prepandemic years [33]. Our data, similar to that reported by France, shows clustering of transmission delayed from the anticipated peak season.
We found, as reported previously, that risk factors for admission and escalation of care in the BronchStart cohort included prematurity, congenital heart disease, and other comorbidites [34, 35]. Although part of this is likely to relate to national guidance [36] to have lower admission thresholds for those with comorbidities, our dataset confirms the greater risk for those infants of requiring additional support, including critical care support. Our study is similar in scale to one recently published with data from Denmark [37], which captured information on 1932 RSV-positive admissions in children aged 0–11 months in the 2021–2022 season (compared to an estimated 2203 RSV-positive admissions in the BronchStart cohort for the same age group). However, through the prospective collection of data, we were able to collect more granular information on treatments administered for our cohort.
Although we found a significant admission and treatment burden for RSV, our estimates vary considerably from other recently published data. Using national surveillance data, Bardsley et al [38] estimate a mean of 49 674 winter (weeks 40–10) and 23 508 summer (weeks 11–39) admissions (mean annual total = 73 182) for children under 1 year of age attributable to RSV disease in England in the period 2015–2020, which is over double what we estimate in our study (28 561 admissions) for both England (population 56.5 million) and Scotland (population 5.4 million) [39], and more than triple what was estimated in a previous study [40], albeit from a slightly earlier time period. The definitions we used to capture RSV-associated disease are likely to be less sensitive than those used by Bardsley et al, as they included infants admitted with a primary diagnosis of bronchiolitis (International Classification of Diseases-Tenth Revision [ICD10] code J21), pneumonia (J12–18), unspecified LRTI (J22), bronchitis (J20), or upper respiratory tract infection (J00–06), which may explain some of the difference in the 2 estimates. Another possible difference may relate to the fact that Bardsley et al used “RSV-associated disease presentations” [41] rather than direct hospital-based testing data, as used in our study, where results from both point-of-care testing and reverse transcription polymerase chain reaction (RT-PCR) were used to infer an RSV-specific burden.
We found that following discharge from an ED after presentation with symptoms of bronchiolitis, LRTI or wheeze, 14.2% of 0–23 month olds represented to the same ED in the following 7 days, and 5.9% were admitted, which should help clinicians in counselling parents in this situation.
Our data suggest that even highly effective immunization against RSV is likely to result in a reduction, rather than elimination, of seasonal admissions for respiratory viral infections in infants. This is because in our study only a proportion of serious respiratory viral presentations in infants were associated with RSV infection. Our study supports previous findings that comorbidities, including those born preterm and congenital heart disease, increase the probability of being admitted, of receiving treatment whilst an inpatient, and of escalation of care to HDU or PICU [42]. However, an immunization approach that only targets high-risk infants is unlikely to impact significantly on overall hospital burden, as we found that 77.9% of admissions at all levels of care had no comorbidities.
CONCLUSION
Our study provides an up-to-date estimate of the impact of acute respiratory disease due to viral infections in England and Scotland in those 0–23 months of age during a period of postpandemic return to RSV circulation, to guide immunization decisions. Although RSV was the major pathogen in this cohort, over 50% of admissions for serious respiratory disease due to viral infection in those aged <1 year of age were not associated with the virus. Whilst prematurity and congenital heart disease were risk factors for admission to hospital, HDU and PICU, the majority of these admissions, for all levels of care, were in previously healthy term-born infants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Thomas C Williams, Child Life and Health, University of Edinburgh, Edinburgh, United Kingdom; Department of Paediatric Respiratory and Sleep Medicine, Royal Hospital for Children and Young People, Edinburgh, United Kingdom.
Robin Marlow, Emergency Department, Bristol Royal Hospital for Children, Bristol, United Kingdom.
Pia Hardelid, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom.
Mark D Lyttle, Emergency Department, Bristol Royal Hospital for Children, Bristol, United Kingdom; Research in Emergency Care Avon Collaborative Hub, University of the West of England, Bristol, United Kingdom.
Kate M Lewis, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom.
Chengetai D Mpamhanga, Child Life and Health, University of Edinburgh, Edinburgh, United Kingdom.
Steve Cunningham, Department of Paediatric Respiratory and Sleep Medicine, Royal Hospital for Children and Young People, Edinburgh, United Kingdom; Centre for Inflammation Research, University of Edinburgh, Edinburgh, United Kingdom.
Damian Roland, Paediatric Emergency Medicine Leicester Academic Group, Leicester Royal Infirmary, Leicester, United Kingdom; Sapphire Group, Health Sciences, University of Leicester, Leicester, United Kingdom.
PERUKI:
Karena Fraser, James Baker, Helen Bailie, Meriel Tolhurst-Cleaver, Rob Stellman, Stuart Hartshorn, Jessica Watson, Roisin Begley, Sakura Hingley, Manali Dutta, Gemma Ramsden, Eleanor Ryan, Sheena Durnin, Stanley Koe, Gergely Halasz, Steve Brearey, Darren Ranasinghe, Mudiyur Gopi, Claudia Spalding, Sylvester Gomes, Gracita Woods, Patrick Aldridge, Vicky Owens, Hemantha Balehithlu, Simon Richardson, David Hartin, Rachael Mitchell, Alice Downes, Damian Roland, Sabrina Sequeira, Jo Tillett, Simon Dowson, Jo Tomlinson, Adebayo Da Costa, Alfred Sime, Claire Kirby, Adam Lawton, Ruth Wear, Christopher Gough, Sharryn Gardner, Zena Haslam, Craig Rimmer, Jiske Steensma, Sahana Rao, Heather Deall, Sharon Hall, Catriona Middleton, Emily Walton, Friyana Dastur Mackenzie, Manish Thakker, Gisela Robinson, Graham Johnson, Shye Wong, Cynthia Diaba, Steve Foster, Jen Browning, Lynsey Rooney, Kirsty Challen, Michael Rosser, Pratiksha Patel, Amy Spicer, Lorna Bagshaw, Seb Gray, Sally Gibbs, Niall Mullen, Louise Fairley, Jane Bayreuther, David James, Heather Jarman, Clare O'Leary, Linda Clerihew, Raine Astin-Chamberlain, Sarah Trippick, Lawrence Armstrong, Joanne Mulligan, Sophie Keers, Benjamin Cahill, Misbah Mohammad, Richard Burridge, Sarah Wilson, Amutha Anpananthar, Erum Jamall, and David Lacy
Notes
Acknowledgments . We thank colleagues across the PERUKI Network for data collection for this study. We thank Khalil Abudahab, Anthony Underwood, and David Aanenson at Microreact for support in creating the dashboard, and Linda Wijlaars for assistance in data extraction. We thank Mai Baquedano for technical support in the launch of the REDCap survey tool and ongoing data management, and Darren Goble for information management and technology support, including maintenance of the server and development of a data flow pipeline for the BronchStart outputs. We thank Elizabeth Whittaker for input at the project planning stage. We thank the RESCEU investigators for their support, and Simon Drysdale, Mathieu Bangert, and John Paget for their review of an initial manuscript.
The use of Hospital Episode Statistics dataset was approved by the Health and Social Care Information Centre for the purpose of this study (DARS-NIC-393510-D6H1D-v1.11). Reused with the permission of the Health and Social Care Information Centre. Research at University College London, Great Ormond Street Institute of Child Health is supported by the National Institute for Health Research (NIHR) Great Ormond Street Hospital Biomedical Research Centre. This research benefits from and contributes to the NIHR Children and Families Policy Research Unit, but was not commissioned by the NIHR Policy Research Programme. This work uses data provided by patients and collected by the English NHS as part of their care and support. We thank Monica McGibbon at Public Health Scotland for providing Scottish Morbidity Record (SMR01) data on admissions in Scotland.
Disclaimer . The results reported herein reflect only the authors’ view and not of the European Commission (EC). As such, the EC is not responsible for any use that may be made of the information contained in this publication.
Financial support . This work was supported by the Respiratory Syncytial Virus Consortium in Europe (RESCEU). RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 116019. This Joint Undertaking receives support from the European Union Horizon 2020 Research and Innovation Programme and European Federation of Pharmaceutical Industries and Associations.
Data availability . The BronchStart dataset will be held for a predetermined period of time and is available to be considered to be shared on request to the authors. Source data from Hospital Episode Statistics dataset are not openly available. Researchers wishing to access the data need to apply to the Health and Social Care Information Centre for England (now NHS England).
References
- 1. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7:e1031–45. [DOI] [PubMed] [Google Scholar]
- 3. Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016; 374:62–72. [DOI] [PubMed] [Google Scholar]
- 4. Lewis KM, De Stavola B, Hardelid P. Geospatial and seasonal variation of bronchiolitis in England: a cohort study using hospital episode statistics. Thorax 2020; 75:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Brien KL, Baggett HC, Brooks WA, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019; 394:757–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams TC, MacRae C, Swann OV, et al. Indirect effects of the COVID-19 pandemic on paediatric healthcare use and severe disease: a retrospective national cohort study. Arch Dis Child 2021; 106:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadambari S, Goldacre R, Morris E, Goldacre MJ, Pollard AJ. Indirect effects of the COVID-19 pandemic on childhood infection in England: population based observational study. BMJ 2022; 376:e067519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia R, Lu L, Su L, et al. Resurgence of respiratory syncytial virus infection during COVID-19 pandemic among children in Shanghai, China. Front Microbiol 2022; 13:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams T, Sinha I, Barr I, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Euro Surveill 2021; 26:2100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foley DA, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019–related public health measures. Clin Infect Dis 2021; 73:e2829–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. [DOI] [PubMed] [Google Scholar]
- 12. Williams TC, Kim S, Spiro DJ, Campbell H. Preparing for the future implementation of respiratory syncytial virus vaccines. Lancet Respir Med 2020; 8:233–5. [DOI] [PubMed] [Google Scholar]
- 13. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386:837–46. [DOI] [PubMed] [Google Scholar]
- 14. Medicines and Healthcare Products Regulatory Agency . Marketing authorisations granted 1–14 November 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1119040/Marketing_authorisations_granted_1_-_14_November_2022.pdf. Accessed 1 March 2023.
- 15. European Medicines Agency . Beyfortus summary of product characteristics, 2022. https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_en.pdf. Accessed 21 June 2023. [Google Scholar]
- 16. Pfizer . Pfizer announces positive top-line data of phase 3 global maternal immunization trial for its bivalent respiratory syncytial virus (RSV) vaccine candidate. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-global. Accessed 20 November 2022.
- 17. Roland D, Williams T, Lyttle MD, et al. Features of the transposed seasonality of the 2021 RSV epidemic in the UK and Ireland: analysis of the first 10 000 patients. Arch Dis Child 2022; 107:1062–3. [DOI] [PubMed] [Google Scholar]
- 18. Lyttle MD, O'Sullivan R, Hartshorn S, Bevan C, Cleugh F, Maconochie I. Pediatric emergency research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child 2014; 99:602–3. [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 20. Williams TC, Lyttle MD, Cunningham S, et al. Study pre-protocol for “BronchStart—the impact of the COVID-19 pandemic on the timing, age and severity of respiratory syncytial virus (RSV) emergency presentations; a multi-centre prospective observational cohort study”. Wellcome Open Res 2021; 6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2023. [Google Scholar]
- 22. Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. R J 2021; 13:570–80. [Google Scholar]
- 23. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003; 157:940–3. [DOI] [PubMed] [Google Scholar]
- 24. Lewis K, De Stavola B, Hardelid P. Is socioeconomic position associated with bronchiolitis seasonality? A cohort study. J Epidemiol Commun Health 2021; 75:76–83. [DOI] [PubMed] [Google Scholar]
- 25. Wildenbeest JG, Billard MN, Zuurbier RP, et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med 2023; 11:341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guy R, Henderson KL, Coelho J, et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill 2023; 28:2200942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holdstock V, Twynam-Perkins J, Bradnock T, et al. National case series of group A streptococcus pleural empyema in children: clinical and microbiological features. Lancet Infect Dis 2023; 23:154–6. [DOI] [PubMed] [Google Scholar]
- 28. Hasegawa K, Goto T, Hirayama A, et al. Respiratory virus epidemiology among U.S. infants with severe bronchiolitis: analysis of two multi-center, multi-year cohort studies. Pediatr Infect Dis J 2019; 38:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J 2013; 32:950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One 2016; 11:e0157014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mestrovic T, Robles Aguilar G, Swetschinski LR, et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Heal 2022; 7:e897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Löwensteyn YN, Zheng Z, Rave N, et al. Year-round RSV transmission in The Netherlands following the COVID-19 pandemic: a prospective nationwide observational and modeling study. J Infect Dis 2023; 228:1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delestrain C, Danis K, Hau I, et al. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr Pulmonol 2021; 56:3669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homaira N, Mallitt KA, Oei JL, et al. Risk factors associated with RSV hospitalisation in the first 2 years of life, among different subgroups of children in NSW: a whole-of-population-based cohort study. BMJ Open 2016; 6:e011398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kristensen K, Hjuler T, Ravn H, Simoẽs EAF, Stensballe LG. Chronic diseases, chromosomal abnormalities, and congenital malformations as risk factors for respiratory syncytial virus hospitalization: a population-based cohort study. Clin Infect Dis 2012; 54:810–7. [DOI] [PubMed] [Google Scholar]
- 36. National Institute for Health and Care Excellence . Bronchiolitis in children: diagnosis and management. https://www.nice.org.uk/guidance/ng9/chapter/1-Recommendations#assessment-and-diagnosis. Accessed 2 April 2021. [PubMed]
- 37. Nygaard U, Hartling UB, Nielsen J, et al. Hospital admissions and need for mechanical ventilation in children with respiratory syncytial virus before and during the COVID-19 pandemic: a Danish nationwide cohort study. Lancet Child Adolesc Heal 2023; 7:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bardsley M, Morbey RA, Hughes HE, et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis 2022; 23:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Office for National Statistics . Population estimates for the UK, England, Wales, Scotland and Northern Ireland: mid-2021. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2021. Accessed 15 February 2023.
- 40. Reeves RM, Hardelid P, Panagiotopoulos N, Minaji M, Warburton F, Pebody R. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: a data linkage modelling study. J Infect 2019; 78:468–75. [DOI] [PubMed] [Google Scholar]
- 41. Bardsley M, Morbey RA, Hughes HE, et al. Estimating hospital admissions due to respiratory syncytial virus in children—authors’ reply. Lancet Infect Dis 2023; 23:33–4. [DOI] [PubMed] [Google Scholar]
- 42. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol 2017; 52:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.