Abstract
Background
Randomized trials conducted in low- and middle-income settings demonstrated efficacy of influenza vaccination during pregnancy against influenza infection among infants <6 months of age. However, vaccine effectiveness (VE) estimates from settings with different population characteristics and influenza seasonality remain limited.
Methods
We conducted a test-negative study in Ontario, Canada. All influenza virus tests among infants <6 months from 2010 to 2019 were identified and linked with health databases to ascertain information on maternal-infant dyads. VE was estimated from the odds ratio for influenza vaccination during pregnancy among cases versus controls, computed using logistic regression with adjustment for potential confounders.
Results
Among 23 806 infants tested for influenza, 1783 (7.5%) were positive and 1708 (7.2%) were born to mothers vaccinated against influenza during pregnancy. VE against laboratory-confirmed infant influenza infection was 64% (95% confidence interval [CI], 50%–74%). VE was similar by trimester of vaccination (first/second, 66% [95% CI, 40%–80%]; third, 63% [95% CI, 46%–74%]), infant age at testing (0 to <2 months, 63% [95% CI, 46%–75%]; 2 to <6 months, 64% [95% CI, 36%–79%]), and gestational age at birth (≥37 weeks, 64% [95% CI, 50%–75%]; < 37 weeks, 61% [95% CI, 4%–86%]). VE against influenza hospitalization was 67% (95% CI, 50%–78%).
Conclusions
Influenza vaccination during pregnancy offers effective protection to infants <6 months, for whom vaccines are not currently available.
Keywords: influenza, pregnancy, vaccination, effectiveness
Influenza vaccination during pregnancy was protective against influenza infection and influenza hospitalization among infants <6 months of age. Because influenza vaccines are not available for this age group, vaccination during pregnancy can offer passive protection to young infants.
Routine influenza immunization of all pregnant individuals has been recommended in Canada since 2007 [1]; similar recommendations have been adopted by many countries since the 2009 H1N1 influenza pandemic [2]. Influenza immunization during pregnancy not only directly protects pregnant people, who are considered at high-risk for severe influenza, but also passively protects newborns through transplacental transfer of maternal anti-influenza antibodies to the fetus [3, 4]. As infants <6 months of age are also at high risk for severe influenza and associated complications [5–7], but are ineligible for influenza vaccination [8], immunization during pregnancy is increasingly viewed as an important strategy for protecting young infants [4].
Vaccine efficacy of influenza immunization during pregnancy against laboratory-confirmed influenza infection among infants <6 months of age pooled across 3 randomized controlled trials (RCTs) conducted in South Africa [9], Mali [10], and Nepal [11] was 35% (95% confidence interval [CI], 19%–47%) [12]. Although several observational studies of influenza vaccine effectiveness (VE) of maternal vaccination against laboratory-confirmed influenza infection among infants <6 months are available from high-resource settings [13–22], estimates vary considerably in magnitude (ranging from 31% to 71%), likely due to differences in influenza seasons and study designs. Similar heterogeneity exists for maternal influenza VE against laboratory-confirmed influenza hospitalization among infants (ranging from 37% to 92%) [13–18, 21, 23–27].
Despite the high-quality RCT evidence showing efficacy of influenza vaccination during pregnancy against influenza infection among infants [12], the trials were limited to 1–2 influenza seasons and conducted in low- and middle-income countries with important differences in influenza seasonality, underlying maternal-infant population characteristics, and health care systems compared with higher-resource temperate settings. In this study, we assessed the VE of maternal influenza vaccination during pregnancy against laboratory-confirmed influenza outcomes among infants <6 months of age in Ontario, Canada.
METHODS
Study Design, Setting, and Population
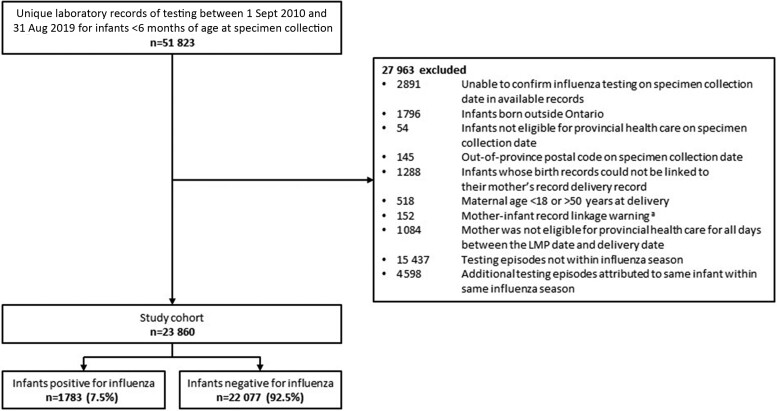
We conducted a test-negative design study among infants <6 months in Ontario—Canada's most populous province with a single-payer publicly funded health care system and approximately 140 000 births each year [28]. This study design is frequently used to evaluate influenza VE because of its ability to reduce biases associated with medical care-seeking behavior compared with other observational designs [29]. We identified all respiratory specimens from infants <6 months collected in any clinical setting (hospitals, emergency departments, physician offices), which were tested for influenza across 9 seasons (2010–2011 to 2018–2019). Only specimens collected during periods of active influenza circulation were included, defined as weeks above a threshold of 5% test positivity for Ontario (dates provided in Supplementary Table 1). For infants with more than 1 influenza test conducted in the same season, we included the earliest positive test (cases) or the earliest test if all results were negative (controls). We excluded infants born outside of Ontario, those not eligible for provincial health insurance on the specimen collection date, and those whose records could not be linked to their mother or whose mother was not continuously eligible for provincial health insurance throughout the entire pregnancy, because this was necessary for ascertainment of influenza vaccination status. Additional exclusions are shown in Figure 1.
Figure 1.
Study flow diagram. aUncertain probability of match between the delivery records of the mother and infant. Abbreviation: LMP, last menstrual period.
Data Sources
All data sources were linked using unique encoded identifiers and analyzed at ICES, an independent, nonprofit research institute whose legal status under Ontario's health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement (https://www.ices.on.ca).
Infant laboratory test results were obtained from a network of public health and hospital-based laboratories across Ontario and included single and multiplex reverse-transcriptase polymerase chain reaction (RT-PCR), viral culture, direct fluorescence assays, and rapid antigen tests. For the 2010–2011 to 2015–2016 seasons, we used previously linked data from the Flu and Other Respiratory Viruses Research (FOREVER) cohort [30], and for 2016–2017 to 2018–2019, we used the Ontario Laboratories Information System and the Public Health Ontario Labware data. We linked these infant test results with the MOMBABY database, which contains integrated mother-infant hospitalization records derived from the birth admission, to identify mother-infant dyads and obtain information about pregnancy and birth characteristics. The Canadian Institute for Health Information's Discharge Abstract Database (hospitalizations), National Ambulatory Care Reporting System (emergency department visits), Same Day Surgery database (same-day surgeries), Ontario Health Insurance Plan Database (physician office visits), and Registered Persons Database (demographics) were used to determine the clinical setting where the infant's respiratory test specimen was collected and to obtain other clinical and sociodemographic information. Several ICES-derived registries were used to identify mothers with preexisting medical complications such as asthma, hypertension, and diabetes. Finally, we used Statistics Canada's Postal Code Conversion File to determine the mother's dissemination area of residence, based on the postal code, to obtain data on rural/urban residence and area-based income quintiles. Details on all data sources are provided in Supplementary Table 2.
Ethics Approval
ICES is a prescribed entity under Ontario's Personal Health Information Protection Act (PHIPA). Section 45 of PHIPA authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to, or planning for all or part of the health system. Projects that use data collected by ICES under section 45 of PHIPA, and use no other data, are exempt from Research Ethics Board review. The use of the data in this project is authorized under section 45 and approved by ICES’ Privacy and Legal Office.
Measures
Infant Influenza Outcomes
Respiratory specimen collection dates from infant laboratory records were combined with dates for health services received by the infants to determine the clinical setting where the specimen was collected. If a specimen collection date was associated with more than 1 clinical setting, we used a hierarchy (intensive care unit [ICU] > hospital ward > emergency department > same-day surgery > physician's office). Influenza tests were attributed to hospital/ICU admissions if the date of specimen collection fell within 3 days prior to the admission date through to the discharge date.
For the primary outcome, infants with a respiratory specimen collected in any clinical setting that yielded influenza were considered cases. For the secondary outcome—influenza hospitalization—case specimens were limited to those attributed to a hospital or ICU admission. The control group for both outcomes comprised infants who tested negative for influenza in any clinical setting.
Maternal Influenza Vaccination During Pregnancy
We considered infants born to mothers who had received an influenza vaccine between the estimated date of the last menstrual period up to 14 days before delivery to be exposed. Infants born to mothers who had received the previous season's influenza vaccine or who were vaccinated <14 days before delivery were considered unexposed in the primary analysis. The date of vaccination was used in combination with the infant's date of birth and gestational age to determine the trimester of vaccination. We used billing claims in the Ontario Health Insurance Plan and Ontario Drug Benefit Databases to ascertain seasonal influenza vaccination in primary care and pharmacies, respectively (Supplementary Table 3).
Other Variables
We obtained the following sociodemographic and clinical characteristics for mothers and infants: maternal age at delivery, neighborhood income, rural residence, maternal preexisting medical comorbidities, seasonal timing of conception and birth, multiple gestation, parity, mode of delivery, maternal obstetrical complications, adequacy of prenatal care, nonobstetric health service utilization 1–2 years prior to the index pregnancy, aggregated diagnosis groups (ADGs) calculated using the Johns Hopkins ACG system version 10.0 in a 2-year period prior to pregnancy, gestational age at birth, birth weight, small-for-gestational age birth, infant sex, and infant age at specimen collection. Supplementary Table 3 provides additional information on all measures, including definitions and specific codes.
Statistical Analysis
We described baseline characteristics by outcome and exposure using descriptive statistics, including standardized mean differences to compare the distributions (absolute differences >0.10 were considered meaningful) [31]. Multivariable logistic regression was used to compare the odds of maternal influenza vaccination during pregnancy among test-positive infant cases to test-negative infant controls, generating adjusted odds ratios (aOR) and 95% CI. Models were adjusted for infant age at test, seasonal timing of birth, prenatal care adequacy, neighborhood income, and influenza season. VE was calculated as (1 − aOR)×100%. For the primary outcome, we conducted subgroup analyses by trimester of vaccination (first/second trimester combined, third trimester), infant age at test (0 to <2 months, 2 to <6 months), gestational age at birth (term birth ≥37 weeks, preterm birth <37 weeks), and by vaccine matched/mismatched seasons (Supplementary Table 4). We conducted several sensitivity analyses to assess the robustness of our main results. First, we reclassified exposure status to “vaccinated” for mothers who had received the previous season's influenza vaccine during pregnancy (as sometimes occurred if an older infant was tested for influenza at the beginning of an influenza season) and for mothers vaccinated <14 days before delivery. We adjusted for several other potential confounders (gestational age at birth, rural/urban residence, maternal preexisting medical comorbidity) to assess whether there was residual confounding by these variables, and we limited the analysis to infants whose respiratory specimen was collected during an emergency department visit or hospitalization with an International Classification of Diseases-Tenth Revision (ICD-10) code indicating acute respiratory illness. For the secondary outcome, we conducted a sensitivity analysis limiting the control group to infants whose specimen was attributed to a hospital or ICU setting. Analyses were conducted using SAS version 9.4 software (SAS Institute, Inc).
RESULTS
Study Population
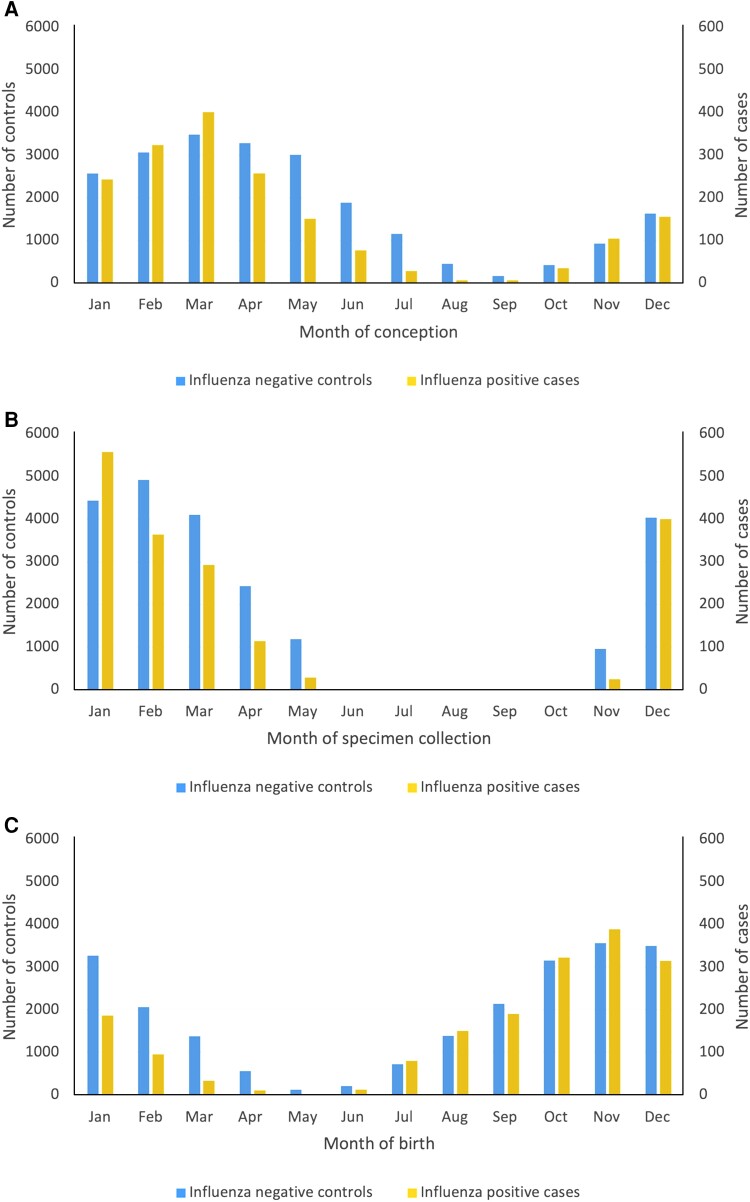
Across 9 influenza seasons, 23 860 infants aged <6 months who were tested for influenza virus during the influenza season met study eligibility criteria (Figure 1). Of these, 1783 infants (7.5%) had a respiratory specimen that yielded influenza (Table 1). Infants with influenza were older than test-negative controls (120 to <180 days at testing, 25.8% vs 20.7%, respectively; median age in days at testing, 74 [IQR, 45–123] vs 63 [IQR, 36–109]), more likely to have been conceived in the winter (50.6% vs 38.8%) and born in the fall (56.6% vs 44.5%), and more likely to have had their specimen collected in an emergency department (40.7% vs 34.0%) (Table 1 and Figure 2). Maternal characteristics were similar between test-positive cases and test-negative controls.
Table 1.
Characteristics of Study Population According to Infant Influenza Test Results and Maternal Influenza Vaccination During Pregnancy
| Characteristic | Infants <6 mo of Age Tested for Influenza | Maternal Influenza Vaccination During Pregnancy | ||||
|---|---|---|---|---|---|---|
| Influenza-Positive Cases (n = 1783) | Influenza-Negative Controls (n = 22 077) |
Standardized Mean Difference | Vaccinated (n = 1708) |
Not Vaccinateda (n = 22 152) |
Standardized Mean Difference | |
| Maternal influenza vaccination during pregnancy | ||||||
| Not vaccinateda | 1741 (97.6) | 20 411 (92.5) | 0.24 | … | 22 152 (100) | … |
| Vaccinated | 42 (2.4) | 1666 (7.5) | 0.24 | 1708 (100) | … | … |
| Trimester of pregnancy at maternal influenza vaccination | ||||||
| Not vaccinateda | 1741 (97.6) | 20 411 (92.5) | 0.24 | … | 22 152 (100) | … |
| 1st trimester, <14 wk | 0 (0.0) | 48 (0.2) | 0.07 | 48 (2.8) | … | … |
| 2nd trimester, 14–27 wk | 13 (0.7) | 674 (3.1) | 0.17 | 687 (40.2) | … | … |
| 3rd trimester, ≥28 wk | 29 (1.6) | 944 (4.3) | 0.16 | 973 (57.0) | … | … |
| Influenza season of test | ||||||
| 2010–2011 | 197 (11.0) | 2309 (10.5) | 0.02 | 111 (6.5) | 2395 (10.8) | 0.15 |
| 2011–2012 | 97 (5.4) | 1565 (7.1) | 0.07 | 114 (6.7) | 1548 (7.0) | 0.01 |
| 2012–2013 | 289 (16.2) | 3133 (14.2) | 0.06 | 169 (9.9) | 3253 (14.7) | 0.15 |
| 2013–2014 | 233 (13.1) | 3029 (13.7) | 0.02 | 264 (15.5) | 2998 (13.5) | 0.05 |
| 2014–2015 | 202 (11.3) | 3119 (14.1) | 0.08 | 210 (12.3) | 3111 (14.0) | 0.05 |
| 2015–2016 | 245 (13.7) | 2340 (10.6) | 0.10 | 243 (14.2) | 2342 (10.6) | 0.11 |
| 2016–2017 | 165 (9.3) | 2241 (10.2) | 0.03 | 143 (8.4) | 2263 (10.2) | 0.06 |
| 2017–2018 | 161 (9.0) | 2105 (9.5) | 0.02 | 178 (10.4) | 2088 (9.4) | 0.03 |
| 2018–2019 | 194 (10.9) | 2236 (10.1) | 0.02 | 276 (16.2) | 2154 (9.7) | 0.19 |
| Infant age at test | ||||||
| Median age, d (IQR) | 74 (45–123) | 63 (36–109) | 0.23 | 40 (22–61) | 67 (38–114) | 0.74 |
| 0 to <60 d (<2 m) | 698 (39.1) | 10 348 (46.9) | 0.16 | 1256 (73.5) | 9790 (44.2) | 0.62 |
| 60 to <120 d (2 to <4 m) | 625 (35.1) | 7158 (32.4) | 0.06 | 402 (23.5) | 7381 (33.3) | 0.22 |
| 120 to <180 d (4 to <6 m) | 460 (25.8) | 4571 (20.7) | 0.12 | 50 (2.9) | 4981 (22.5) | 0.61 |
| Seasonal timing of conception | ||||||
| Spring, 21 Mar to 20 Jun | 618 (34.7) | 8854 (40.1) | 0.11 | 1115 (65.3) | 8357 (37.7) | 0.57 |
| Summer, 21 Jun to 20 Sep | 48 (2.7) | 2285 (10.4) | 0.31 | 331 (19.4) | 2002 (9.0) | 0.30 |
| Fall, 21 Sep to 20 Dec | 214 (12.0) | 2364 (10.7) | 0.04 | 8 (0.5) | 2570 (11.6) | 0.48 |
| Winter, 21 Dec to 20 Mar | 903 (50.6) | 8574 (38.8) | 0.24 | 254 (14.9) | 9223 (41.6) | 0.62 |
| Seasonal timing of birth | ||||||
| Spring, 21 Mar to 20 Jun | 23 (1.3) | 1161 (5.3) | 0.22 | 181 (10.6) | 1003 (4.5) | 0.23 |
| Summer, 21 Jun to 20 Sep | 350 (19.6) | 3588 (16.3) | 0.09 | 0 (0.0) | 3938 (17.8) | 0.66 |
| Fall, 21 Sep to 20 Dec | 1010 (56.6) | 9826 (44.5) | 0.24 | 497 (29.1) | 10 339 (46.7) | 0.37 |
| Winter, 21 Dec to 20 Mar | 400 (22.4) | 7502 (34.0) | 0.26 | 1030 (60.3) | 6872 (31.0) | 0.62 |
| Infant sex | ||||||
| Female | 778 (43.6) | 9285 (42.1) | 0.03 | 722 (42.3) | 9341 (42.2) | 0 |
| Male | 1005 (56.4) | 12 792 (57.9) | 0.03 | 986 (57.7) | 12 811 (57.8) | 0 |
| Multiple gestation | 91 (5.1) | 1213 (5.5) | 0.02 | 100 (5.9) | 1204 (5.4) | 0.02 |
| Gestational age at delivery, wk, median (IQR) | 39 (38–40) | 39 (37–40) | 0.10 | 39 (38–40) | 39 (37–40) | 0.03 |
| Preterm birth, <37 wk | 246 (13.8) | 3490 (15.8) | 0.06 | 250 (14.6) | 3486 (15.7) | 0.03 |
| Birthweight, grams, median (IQR) | 3300 (2920–3666) | 3295 (2875–3670) | 0.04 | 3335 (2930–3697) | 3290 (2875–3665) | 0.07 |
| Small for gestational age at birth, <10th percentile | 207 (11.6) | 2373 (10.7) | 0.03 | 152 (8.9) | 2428 (11.0) | 0.07 |
| ≥1 previous delivery, parous | 474 (26.6) | 6115 (27.7) | 0.03 | 488 (28.6) | 6101 (27.5) | 0.02 |
| Cesarean delivery | 584 (32.8) | 7428 (33.6) | 0.02 | 581 (34.0) | 7431 (33.5) | 0.01 |
| Rural residence | 30 (1.7) | 554 (2.5) | 0.06 | 18 (1.1) | 566 (2.6) | 0.11 |
| Neighborhood income quintile | ||||||
| 1, lowest | 412 (23.1) | 5381 (24.4) | 0.03 | 301 (17.6) | 5492 (24.8) | 0.18 |
| 2 | 365 (20.5) | 4469 (20.2) | 0.01 | 328 (19.2) | 4506 (20.3) | 0.03 |
| 3 | 379 (21.3) | 4370 (19.8) | 0.04 | 341 (20.0) | 4408 (19.9) | 0 |
| 4 | 348 (19.5) | 4350 (19.7) | 0 | 383 (22.4) | 4315 (19.5) | 0.07 |
| 5, highest | 268 (15.0) | 3400 (15.4) | 0.01 | 349 (20.4) | 3319 (15.0) | 0.14 |
| Missing | 11 (0.6) | 107 (0.5) | 0.02 | 6 (0.4) | 112 (0.5) | 0.02 |
| Maternal age at delivery, y | ||||||
| <20 | 33 (1.9) | 585 (2.6) | 0.05 | 34 (2.0) | 584 (2.6) | 0.04 |
| 20–24 | 182 (10.2) | 3070 (13.9) | 0.11 | 145 (8.5) | 3107 (14.0) | 0.18 |
| 25–29 | 457 (25.6) | 5960 (27.0) | 0.03 | 406 (23.8) | 6011 (27.1) | 0.08 |
| 30–34 | 669 (37.5) | 7624 (34.5) | 0.06 | 647 (37.9) | 7646 (34.5) | 0.07 |
| ≥35 | 442 (24.8) | 4838 (21.9) | 0.07 | 476 (27.9) | 4804 (21.7) | 0.14 |
| Any maternal preexisting comorbiditiesb | 414 (23.2) | 5377 (24.4) | 0.03 | 442 (25.9) | 5349 (24.1) | 0.04 |
| Asthma | 318 (17.8) | 4317 (19.6) | 0.04 | 364 (21.3) | 4271 (19.3) | 0.05 |
| Hypertension | 53 (3.0) | 658 (3.0) | 0.0 | 58 (3.4) | 653 (2.9) | 0.03 |
| Diabetes | 68 (3.8) | 631 (2.9) | 0.05 | 53 (3.1) | 646 (2.9) | 0.01 |
| Heart disease | 14 (0.8) | 146 (0.7) | 0.01 | 7 (0.4) | 153 (0.7) | 0.04 |
| Any maternal obstetrical complications | 465 (26.1) | 6048 (27.4) | 0.03 | 481 (28.2) | 6032 (27.2) | 0.02 |
| Preeclampsia/eclampsia | 37 (2.1) | 595 (2.7) | 0.04 | 57 (3.3) | 575 (2.6) | 0.04 |
| Premature rupture of membranes | 208 (11.7) | 2857 (12.9) | 0.04 | 236 (13.8) | 2829 (12.8) | 0.03 |
| Gestational diabetes | 139 (7.8) | 1548 (7.0) | 0.03 | 113 (6.6) | 1574 (7.1) | 0.02 |
| Gestational hypertension | 74 (4.2) | 1023 (4.6) | 0.02 | 80 (4.7) | 1017 (4.6) | 0 |
| Hypertension complicating pregnancy | 14 (0.8) | 152 (0.7) | 0.01 | 16 (0.9) | 150 (0.7) | 0.03 |
| Placenta abruptio | 25 (1.4) | 448 (2.0) | 0.05 | 28 (1.6) | 445 (2.0) | 0.03 |
| Placenta previa | 19 (1.1) | 242 (1.1) | 0 | 14 (0.8) | 247 (1.1) | 0.03 |
| Prenatal care adequacy | ||||||
| Intensive | 128 (7.2) | 1655 (7.5) | 0.01 | 162 (9.5) | 1621 (7.3) | 0.08 |
| Adequate | 774 (43.4) | 9363 (42.4) | 0.02 | 862 (50.5) | 9275 (41.9) | 0.17 |
| Intermediate | 620 (34.8) | 7283 (33.0) | 0.04 | 502 (29.4) | 7401 (33.4) | 0.09 |
| Inadequate | 180 (10.1) | 2659 (12.0) | 0.06 | 125 (7.3) | 2714 (12.3) | 0.17 |
| No care | 81 (4.5) | 1117 (5.1) | 0.02 | 57 (3.3) | 1141 (5.2) | 0.09 |
| ≥1 Nonobstetric hospitalization in the 2 y prior to LMP | 109 (6.1) | 1609 (7.3) | 0.05 | 90 (5.3) | 1628 (7.3) | 0.09 |
| No. of outpatient GP/FP visits in 1 y prior to pregnancy, median (IQR) | 4 (1–7) | 3 (1–6) | 0.08 | 4 (2–7) | 3 (1–6) | 0.14 |
| Percent of visits in 1 y prior to LMP to the same GP/FP, median (IQR) | 75 (50–100) | 80 (50–100) | 0.06 | 83 (54–100) | 80 (50–100) | 0.02 |
| Sum of aggregated diagnosis groups, median (IQR)c | 5 (3–8) | 5 (3–8) | 0.01 | 5 (3–8) | 5 (3–8) | 0.04 |
| No. of aggregated diagnosis groupsc | ||||||
| 0 | 69 (3.9) | 850 (3.9) | 0 | 49 (2.9) | 870 (3.9) | 0.06 |
| 1–2 | 277 (15.5) | 3017 (13.7) | 0.05 | 229 (13.4) | 3065 (13.8) | 0.01 |
| 3–4 | 372 (20.9) | 4934 (22.3) | 0.04 | 384 (22.5) | 4922 (22.2) | 0.01 |
| ≥5 | 1065 (59.7) | 13 276 (60.1) | 0.01 | 1046 (61.2) | 13 295 (60.0) | 0.03 |
| Clinical setting where infant test specimen was collected | ||||||
| Emergency department | 726 (40.7) | 7510 (34.0) | 0.14 | 531 (31.1) | 7705 (34.8) | 0.08 |
| Hospital ward | 879 (49.3) | 10 771 (48.8) | 0.01 | 884 (51.8) | 10 766 (48.6) | 0.06 |
| Intensive care unit | 54 (3.0) | 2106 (9.5) | 0.27 | 191 (11.2) | 1969 (8.9) | 0.08 |
| Physician office | 91 (5.1) | 1263 (5.7) | 0.03 | 80 (4.7) | 1274 (5.8) | 0.05 |
| Missingd | 33 (1.9) | 427 (1.9) | 0.01 | 22 (1.3) | 438 (2.0) | 0.05 |
Data are No. (%) except where indicated.
Abbreviations: FP, family physician; GP, general physician; HIV, human immunodeficiency virus; IQR, interquartile range; LMP, last menstrual period.
aA total of 460 mothers who were vaccinated within 2 weeks of delivery (n = 215; 201 test-negative controls and 14 test-positive cases) or with previous season's vaccine (n = 245; 231 test-negative controls and 14 test-positive cases) were considered as unvaccinated in the main analysis.
bComposite of asthma, chronic hypertension, preexisting diabetes, heart disease, and immune disorder (including HIV). Sum of individual conditions does not equal the total number of individuals with any individual condition, as categories were not mutually exclusive.
cAggregated Diagnosis Groups coded using the Johns Hopkins ACG System version 10.0.
dInfants whose test specimens could not be attributed to a clinical setting where collection occurred were included in the main analysis of the primary outcome, but excluded from any analyses that required a specific clinical setting for specimen collection.
Figure 2.
Bar graphs displaying the estimated date of conception (A), specimen collection date (B), and date of birth (C) of influenza positive cases and influenza negative controls, by month.
Overall, 1708 (7.2%) infants were born to mothers who were vaccinated against influenza during pregnancy with the current season's vaccine at <14 days before delivery. Infants born to vaccinated mothers were more likely to be conceived in the spring or summer compared to those born to unvaccinated mothers (84.7% vs 46.7%, respectively), and to be younger at the time of specimen collection (<60 days at testing, 73.5% vs 44.2%; median age in days at testing, 40 [IQR, 22–61] vs 67 [IQR, 38–114]). They were also more likely to be born to older mothers (≥35 years, 27.9% vs 21.7%), mothers living in higher-income neighborhoods (fifth quintile, 20.4% vs 15.0%), and mothers who had more frequent prenatal care visits (intensive/adequate prenatal care, 60.0% vs 49.2%) (Table 1).
Vaccine Effectiveness
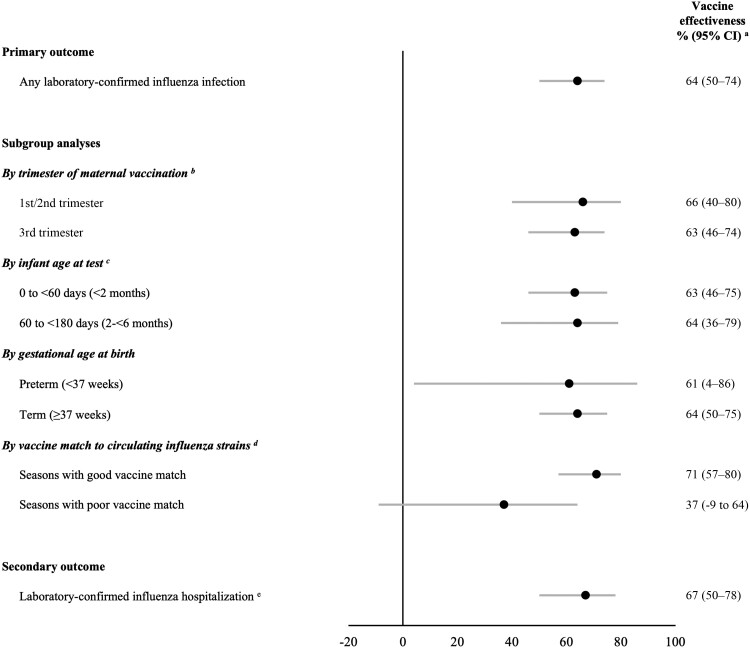
Across seasons, estimated effectiveness of influenza vaccination during pregnancy was 64% (95% CI, 50%–74%) against laboratory-confirmed influenza infection among infants (Figure 3 and Supplementary Table 5). VE was similar among infants <2 months of age (63%; 95% CI, 46%–75%) and those 2 to <6 months (64%; 95% CI, 36%–79%); we were unable to separately estimate VE for infants 4 to <6 months due to small numbers. VE was also similar among infants born at term (64%; 95% CI, 50%–75%) and preterm (61%; 95% CI, 4%–86%) gestation, as well as according to vaccination during the first/second trimester (66%; 95% CI, 40%–80%) or third trimester (63%; 95% CI, 46%–74%) of pregnancy. During 7 seasons in which influenza vaccines were well matched to dominant circulating strains, VE was 71% (95% CI, 57%–80%); conversely, in 2 poorly matched seasons (2014–2015 and 2017–2018), it was 37% (95% CI, −9% to 64%). Twenty-four test-positive cases were hospitalized; VE against laboratory-confirmed influenza hospitalization was 67% (95% CI, 50%–78%).
Figure 3.
Estimated effectiveness of maternal influenza vaccination during pregnancy against laboratory-confirmed influenza among infants <6 months of age (additional data provided in Supplementary Table 5). aVaccine effectiveness was estimated as 1 minus the adjusted odd ratio, comparing the odds of maternal influenza vaccination during pregnancy among test-positive infant cases relative to test-negative infant controls. Odds ratios were adjusted for infant age at test, season of birth, prenatal care adequacy, neighborhood income, and influenza season (with the exception of the subgroup analysis by infant age at test, for which odds ratios were adjusted for season of birth, prenatal care adequacy, neighborhood income, and influenza season). bResults for first and second trimester vaccinations were combined due to small cell counts. cResults for 60 to <120 days and 120 to <180 days were combined due to small cell counts. dInfluenza vaccines in 2014–2015 and 2017–2018 were not considered well matched against circulating influenza strains (see Supplementary Table 4). eCase specimens were limited to those collected during an admission to a hospital ward or intensive care unit. Controls were infants who tested negative for influenza in any clinical setting. Abbreviation: CI, confidence interval.
Results from sensitivity analyses are provided in Table 2. Estimated VE was somewhat lower than the primary analysis when mothers who received the previous season's vaccine (n = 245) or were vaccinated <14 days before delivery (n = 251) were reclassified as vaccinated. Additional adjustments for infant gestational age at birth, rural/urban residence, and maternal preexisting medical comorbidity did not change the VE estimates. When the study population was limited to records of infants whose specimen was attributed to a hospitalization or emergency department visit coded as an acute respiratory illness, estimated VE was 71% (95% CI, 58%–80%). For the secondary outcome, when the test-negative control group was limited to infants whose specimen was attributed to a hospital or ICU setting, estimated VE did not change.
Table 2.
Sensitivity Analyses for Estimated Effectiveness of Maternal Influenza Vaccination During Pregnancy Against Laboratory-Confirmed Influenza Among Infants <6 Months of Age
| Sensitivity Analysis | Influenza-Positive Cases | Influenza-Negative Controls | Adjusted OR (95% CI)a | Vaccine Effectiveness, % (95% CI)a,b |
|---|---|---|---|---|
| Primary outcome | ||||
| Reclassify those who received previous season's vaccine as vaccinated | 56/1783 (3.1) | 1897/22 077 (8.6) | 0.41 (.31–.54) | 59 (46–69) |
| Reclassify those vaccinated within 14 d of birth as vaccinated | 56/1783 (3.1) | 1867/22 077 (8.5) | 0.41 (.31–.54) | 59 (46–69) |
| Reclassify those who received previous season's vaccine and those vaccinated within 14 d of birth as vaccinated | 70/1783 (3.9) | 2098/22 077 (9.5) | 0.44 (.35–.57) | 56 (43–65) |
| Add gestational age at birth, wk, to fully adjusted model | 42/1783 (2.4) | 1666/22 077 (7.5) | 0.36 (.26–.49) | 64 (51–74) |
| Add rural/urban status to fully adjusted model | 42/1783 (2.4) | 1666/22 077 (7.5) | 0.35 (.26–.48) | 65 (52–74) |
| Add any maternal preexisting medical comorbidity to fully adjusted model | 42/1783 (2.4) | 1666/22 077 (7.5) | 0.35 (.26–.49) | 65 (51–74) |
| Limit to health care encounters with an ARI code, hospitalizations and ED visitsc | 30/1381 (2.2) | 793/9852 (8.0) | 0.29 (.20–.42) | 71 (58–80) |
| Secondary outcome | ||||
| Limit control group to hospitalized infants who tested negative for influenzad | 24/933 (2.6) | 1051/12 877 (8.2) | 0.34 (.22–.51) | 66 (49–78) |
Data are number of infants born to vaccinated mothers/total number of infants (%).
Abbreviations: ARI, acute respiratory infection; CI, confidence interval; ED, emergency department; ICD-10, CM International Classification of Diseases-Tenth Revision; OR, odds ratio.
aAdjusted for infant age at test, season of birth, prenatal care adequacy, neighborhood income, and influenza season.
bVaccine effectiveness was estimated as 1 minus the adjusted OR, comparing the odds of maternal influenza vaccination during pregnancy among test-positive infant cases relative to test-negative infant controls.
cARI codes: ICD-10 codes B34, B97, J00-J06, J09-J18, J20-J22, J39.8, J39.9, J40, J45, J80, J81, J96, J98, R05, R06.0-R6.2, R06.4, R06.7, R06.8, R07.0, R07.1, R09.2, R09.3, R50, R53.
dCase and control specimens were limited to those collected during an admission to a hospital ward or intensive care unit.
DISCUSSION
In this test-negative study of 23 860 infants across 9 influenza seasons in Ontario, Canada, we found that influenza vaccination during pregnancy was effective at preventing laboratory-confirmed influenza infection and influenza hospitalization among infants <6 months of age. Taken together with RCT evidence that influenza vaccination during pregnancy reduces the incidence of all-cause severe pneumonia [32] and hospitalization for all-cause lower respiratory tract infection in infants [33], our study supports influenza immunization during pregnancy as an effective strategy to protect young infants from influenza-related morbidity during their first 6 months of life.
Our findings are generally consistent with other observational studies from high-resource settings [13–22]. Nevertheless, estimates across the other studies vary considerably in magnitude—ranging from 31% [20] to 71% [15]—likely due to differences in influenza seasons (ie, match between circulating strains and vaccine strains), testing patterns, study designs (ie, prospective cohort study [13, 19, 20, 22], retrospective cohort study [14, 18, 21], test-negative case-control study [17], and screening study [15, 16]), or possibly due to underlying differences in study setting (United States [13, 14], United Kingdom [15, 16], Greece [19, 20], Denmark [17], Australia [18, 21], and Japan [22]). The most similar study to ours, with respect to study design and time period, was a population-based test-negative study conducted in Denmark from 2010 to 2017, in which the estimated effectiveness of influenza vaccination during pregnancy was 57% (95% CI, 25%–75%) against laboratory-confirmed influenza infection among infants <6 months [17]. Variability in vaccine efficacy against infant influenza infection was also observed across the RCTs conducted in South Africa (49%; 95% CI, 12%–70%) [9], Mali (33%; 95% CI, 4%–54%) [10], and Nepal (30%; 95% CI, 5%–48%) [11], as well as an earlier RCT from Bangladesh (63%; 95% CI, 5%–85%) [34].
To our knowledge, effectiveness of vaccination during pregnancy against influenza infection among preterm infants has not been previously reported. We found similar VE among infants born at preterm and term gestation (61% and 64%, respectively). Although the confidence interval for estimated VE among preterm infants was wide, it is compelling that influenza vaccination in pregnancy has the potential to protect preterm infants from influenza, as has been demonstrated for earlier Tdap vaccination during pregnancy against pertussis infection among preterm infants [35]. We did not observe a qualitative difference in VE according to trimester of maternal influenza vaccination. Transplacental transfer of maternal anti-influenza antibodies is known to start in the early second trimester and increase with advancing gestation [36, 37]. Despite that later gestational timing of influenza vaccination increases the transfer of maternal antibodies to the fetus [37], few studies have evaluated the clinical impact of gestational timing of vaccination on influenza VE among young infants and, those studies that have, were limited by small cell sizes precluding conclusive interpretation of their findings [21, 23, 25]. Unlike the pooled RCT results that demonstrated higher efficacy of vaccination during pregnancy at younger infant ages (0 to <2 months, 56% [95% CI, 28%–73%]; 2 to <4 months, 39% [95% CI, 11%–58%]; 4 to <6 months, 19% [95% CI, −9% to 40%] [12]), we did not observe a difference in estimated VE among infants 0 to <2 months of age and those 2 to <6 months (63% and 64%, respectively), which was unexpected because the concentration of maternal anti-influenza antibodies in infant sera decays over time after birth [38, 39]. This finding may be due to the fact that very few infants 4 to <6 months of age were born to vaccinated mothers, therefore, the estimate for the 2 to <6 month age group primarily reflects VE for infants 2 to <4 months of age, which is a time period during which infants would still be expected to be well protected by maternal immunization [40].
Extant evidence on maternal influenza VE against laboratory-confirmed influenza hospitalization of infants <6 months of age is derived from observational studies [13–18, 21, 23–27], as the incidence was too low to be assessed in the RCTs [24]. Similar to VE against infection, estimates against laboratory-confirmed influenza hospitalization also range considerably in magnitude, from 29% [24] to 92% [13]. Three of the previously published studies used a test-negative design and were conducted in similar years to those in our study, originating from Denmark [17], Australia [23], and South Africa [24]. While the study from Denmark used similar population-based sources of routinely collected laboratory and maternal-newborn data to the sources we used in Ontario and obtained a similar estimate (61%; 95% CI, 26%–80% [17]), the other 2 were prospectively conducted and used clinical criteria for enrollment. The Australian study, conducted across 12 large pediatric hospitals, included infants <6 months admitted with signs of acute respiratory illness and tested for influenza. Estimated effectiveness of influenza vaccination during pregnancy against influenza hospitalization among infants <6 months was 37% (95% CI, 2%–60%) across the 4 influenza seasons, but higher among infants 0 to <2 months (49%; 95% CI, 11%–71%) and in 2018 (98%; 95% CI, 57%–100%), when the vaccine formulation for the Southern hemisphere was well matched to circulating strains [23]. The South African study was conducted in 4 hospital sites from 2015 to 2018. Infants admitted to hospital with an acute respiratory illness were prospectively identified and tested for influenza. Overall VE was 29% (95% CI, −33% to 32%); however, when limited to only vaccine-matched strains, it was 65% (95% CI, 12%–86%) [24]. While we expected higher VE against influenza hospitalization than against any influenza infection in our study, the estimates were similar in magnitude, and had overlapping confidence intervals. However, it is important to note that 95% of testing was performed in the emergency department or after hospital admission and more than 50% occurred in infants who were ultimately admitted, thus, our results reflect VE against relatively more severe infections, and actual VE against any influenza infection may be lower.
Strengths of this study include the province-wide linked data sources, enabling us to define a large maternal-infant study population with ascertainment of laboratory-confirmed influenza infections among infants and influenza vaccination during pregnancy. Our study provides estimates of the effectiveness of influenza vaccination during pregnancy against influenza infection and hospitalization of infants <6 months from a Canadian setting; previously, no such estimates had been available for this population and our results are likely generalizable within Canada and possibly to other high-income settings with similar influenza seasonality. Our study also has limitations. First, secondary use of routinely collected laboratory data (as opposed to prospectively collected data with clinical criteria for testing) for test-negative studies can introduce bias if the clinical decision to test is associated with vaccination status and influenza positivity [29]. However, if maternal influenza vaccination status during pregnancy influenced a care provider's decision to test an infant for influenza, a simulation study demonstrated that the probability of being tested would have to be at least twice as high in vaccinated compared with unvaccinated subjects to meaningfully bias VE estimates [41]. There was, however, a higher proportion of test-positive case infants who were hospitalized and/or had a diagnosis code for acute respiratory illness, compared with the test-negative controls, which could suggest variability in testing protocols (eg, some test-negative infants may have been tested for other purposes, such as hospitalization for an unrelated illness or surgery). Next, we used billing claims from primary care and pharmacies to ascertain influenza vaccination during pregnancy and obtained an overall vaccination coverage rate of 7.2%. An Ontario validation study from 2007 to 2009 estimated the sensitivity of primary care claims for influenza vaccination to be 42% among pregnant women [42]. Although we do not know the sensitivity of the vaccination variable derived for our study, it is likely to be somewhat improved due to the addition of claims from vaccinations received in pharmacies. There are limited published coverage data in the Ontario pregnant population against which to compare the vaccination rates from our study―in a survey conducted by the Public Health Agency of Canada, influenza vaccine coverage during pregnancy in Ontario in the 2018–2019 influenza season was estimated to be 43.3% in a sample of 554 pregnant individuals [43]. However, based on other work we have conducted on Tdap vaccination during pregnancy in Ontario, it is possible that the survey methodology and small respondent sample overestimated coverage (ie, 40% Tdap coverage in 2018–2019 estimated by the survey [43] vs 24.9% Tdap coverage estimated in our previous work using Ontario's province-wide health administrative databases [44]). Nevertheless, it is possible that nondifferential misclassification of maternal influenza vaccination status biased our estimates toward the null. Moreover, the low proportion of vaccinated individuals limited the study's power to evaluate VE stratified by smaller gestational age categories or by influenza season. While we had information on many potential confounding variables, we cannot rule out residual confounding by other factors such as maternal smoking or breastfeeding. Finally, we were unable to generate estimates by influenza type/subtype, as this information was not consistently available in the data sources used in this study.
CONCLUSIONS
In this population-based study across 9 recent influenza seasons, maternal influenza vaccination during pregnancy protected infants <6 months from both laboratory-confirmed influenza infection and influenza hospitalization. Given that infants in this age group have a high risk for severe influenza illness and complications, but cannot be vaccinated against influenza themselves, maternal influenza immunization during pregnancy is an important strategy for protecting young infants during their first 6 months of life.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Deshayne B Fell, School of Epidemiology and Public Health, University of Ottawa, Ottawa, Ontario, Canada; Research Institute, Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada.
Margaret Russell, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Stephen G Fung, Research Institute, Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada.
Sarah Swayze, Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada.
Hannah Chung, Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada.
Sarah A Buchan, Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada; Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Centre for Vaccine Preventable Diseases, University of Toronto, Toronto, Ontario, Canada.
Weston Roda, Mathematical and Statistical Sciences, University of Alberta, Edmonton, Alberta, Canada.
Christa Smolarchuk, Analytics and Performance Reporting Branch, Health Standards, Quality and Performance Division, Alberta Health, Edmonton, Alberta, Canada.
Kumanan Wilson, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada; Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; Bruyère Research Institute, Ottawa, Ontario, Canada.
Natasha S Crowcroft, Department of Immunization, Vaccines, and Biologicals, World Health Organization, Geneva, Switzerland.
Kevin L Schwartz, Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada; Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Department of Medicine, Unity Health Toronto, Toronto, Ontario, Canada.
Jonathan B Gubbay, Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada; Department of Pediatrics, University of Toronto, Toronto, Ontario, Canada; Department of Pediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada.
Allison J McGeer, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada; Department of Microbiology, Sinai Health System, Toronto, Ontario, Canada.
Marek Smieja, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada.
David C Richardson, Department of Pathology and Laboratory Medicine, William Osler Health System, Brampton, Ontario, Canada; Department of Medicine, William Osler Health System, Brampton, Ontario, Canada.
Kevin Katz, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada; Division of Infection Prevention and Control, North York General Hospital, Toronto, Ontario, Canada; Shared Hospital Laboratory, North York General Hospital, Toronto, Ontario, Canada.
George Zahariadis, Newfoundland and Labrador Public Health Laboratory, St John's, Newfoundland and Labrador, Canada.
Aaron Campigotto, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada; Division of Microbiology, Department of Paediatric Laboratory Medicine, Hospital for Sick Children, Toronto, Ontario, Canada.
Samira Mubareka, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada; Department of Laboratory Medicine & Pathobiology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
J Dayre McNally, Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Department of Pediatrics, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Timothy Karnauchow, Children's Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Department of Pathology and Laboratory Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Nathan Zelyas, Alberta Public Health Laboratory, Alberta Precision Laboratories, Edmonton, Alberta, Canada; Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, Alberta, Canada.
Lawrence W Svenson, Analytics and Performance Reporting Branch, Health Standards, Quality and Performance Division, Alberta Health, Edmonton, Alberta, Canada; Division of Preventive Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada; School of Public Health, University of Alberta, Edmonton, Alberta, Canada; Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Jeffrey C Kwong, Populations and Public Health, ICES, Toronto and Ottawa, Ontario, Canada; Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada; University Health Network, Toronto, Ontario, Canada.
Notes
Acknowledgment. This article used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from the Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health (MOH) Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information provided by the Ontario MOH and the Canadian Institute for Health Information. We thank IQVIA Solutions Canada, Inc for use of their Drug Information File.
Author contributions. D. B. F., M. L. R., and J. C. K. conceptualized the study, obtained the grant funding, oversaw the data analysis, interpreted the findings, and drafted the manuscript. H. C. and S. S. conducted the data analysis, and contributed to the study methodology, interpretation, and final manuscript. S. F. assisted with drafting the manuscript. All authors contributed to the interpretation of findings and critical review and revision of the final manuscript, approve the final manuscript as submitted, and agree to be accountable for all aspects of the work.
Data availability. The datasets from this study are held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (eg, health care organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS (email, das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors on request, understanding that the computer programs may rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Financial support. This work was supported by the Canadian Immunization Research Network through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944); and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. J. C. K. is supported by a Clinician Scientist Award from the University of Toronto Department of Family and Community Medicine. Funding to pay the Open Access publication charges for this article was provided by the Canadian Immunization Research Network.
References
- 1. National Advisory Committee on Immunization . Statement on seasonal influenza vaccine for 2011–2012: An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Can Commun Dis Rep 2011; 37:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ortiz JR, Perut M, Dumolard L, et al. . A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF joint reporting form on immunization. Vaccine 2016; 34:5400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kay AW, Blish CA. Immunogenicity and clinical efficacy of influenza vaccination in pregnancy. Front Immunol 2015; 6:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abu-Raya B, Madhi SA, Omer SB, et al. . Global perspectives on immunization against SARS-CoV-2 during pregnancy and priorities for future research: an international consensus paper from the World Association of Infectious Diseases and Immunological Disorders. Front Immunol 2021; 12:808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nair H, Brooks WA, Katz M, et al. . Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 6. Poehling KA, Edwards KM, Weinberg GA, et al. . The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 7. Bhat N, Wright JG, Broder KR, et al. . Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005; 353:2559–67. [DOI] [PubMed] [Google Scholar]
- 8. Fiore AE, Uyeki TM, Broder K, et al. . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 9. Madhi SA, Cutland CL, Kuwanda L, et al. . Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371:918–31. [DOI] [PubMed] [Google Scholar]
- 10. Tapia MD, Sow SO, Tamboura B, et al. . Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinhoff MC, Katz J, Englund JA, et al. . Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis 2017; 17:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Omer SB, Clark DR, Madhi SA, et al. . Efficacy, duration of protection, birth outcomes, and infant growth associated with influenza vaccination in pregnancy: a pooled analysis of three randomised controlled trials. Lancet Respir Med 2020; 8:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eick AA, Uyeki TM, Klimov A, et al. . Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 2011; 165:104–11. [DOI] [PubMed] [Google Scholar]
- 14. Shakib JH, Korgenski K, Presson AP, et al. . Influenza in infants born to women vaccinated during pregnancy. Pediatrics 2016; 137:e20152360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dabrera G, Zhao H, Andrews N, et al. . Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Euro Surveill 2014; 19:1–4. [DOI] [PubMed] [Google Scholar]
- 16. Walker JL, Zhao H, Dabrera G, et al. . Assessment of effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants in England, 2013–2014 and 2014–2015. J Infect Dis 2020; 221:16–20. [DOI] [PubMed] [Google Scholar]
- 17. Mølgaard-Nielsen D, Fischer TK, Krause TG, Hviid A. Effectiveness of maternal immunization with trivalent inactivated influenza vaccine in pregnant women and their infants. J Intern Med 2019; 286:469–80. [DOI] [PubMed] [Google Scholar]
- 18. Rowe SL, Leder K, Perrett KP, et al. . Maternal vaccination and infant influenza and pertussis. Pediatrics 2021; 148:e2021051076. [DOI] [PubMed] [Google Scholar]
- 19. Maltezou HC, Asimakopoulos G, Stavrou S, et al. . Effectiveness of quadrivalent influenza vaccine in pregnant women and infants, 2018–2019. Vaccine 2020; 38:4625–31. [DOI] [PubMed] [Google Scholar]
- 20. Maltezou HC, Stavros S, Asimakopoulos G, et al. . Effectiveness of maternal vaccination with quadrivalent inactivated influenza vaccine in pregnant women and their infants in 2019–2020. Expert Rev Vaccines 2022; 21:983–92. [DOI] [PubMed] [Google Scholar]
- 21. Foo D, Sarna M, Pereira G, Moore HC, Regan AK. Longitudinal, population-based cohort study of prenatal influenza vaccination and influenza infection in childhood. Vaccine 2022; 40:656–65. [DOI] [PubMed] [Google Scholar]
- 22. Ohfuji S, Deguchi M, Tachibana D, et al. . Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217:878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McRae J, Blyth CC, Cheng AC, Quinn HE, Wood N, Macartney KK. Preventing severe influenza in Australian infants: maternal influenza vaccine effectiveness in the PAEDS-FluCAN networks using the test-negative design. Vaccine 2022; 40:2761–71. [DOI] [PubMed] [Google Scholar]
- 24. Nunes MC, Walaza S, Meiring S, et al. . Effectiveness of influenza vaccination of pregnant women for prevention of maternal and early infant influenza-associated hospitalizations in South Africa: a prospective test-negative study. Open forum Infect Dis 2022; 9:ofac552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 2010; 51:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poehling K, Szilagyi PG, Staat M, et al. . Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204:S141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazagatos C, Godoy P, Muñoz Almagro C, Pozo F, Larrauri A, IVE in Pregnant Women Working Group . Effectiveness of influenza vaccination during pregnancy to prevent severe infection in children under 6 months of age, Spain, 2017–2019. Vaccine 2020; 38:8405–10. [DOI] [PubMed] [Google Scholar]
- 28. Statistics Canada . Table: 13-10-0428-01; Live births and fetal deaths (stillbirths), by type of birth (single or multiple), 2022. 10.25318/1310042801-eng. Accessed 14 December 2023. [DOI]
- 29. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 30. Kwong JC, Buchan SA, Chung H, et al. . Can routinely collected laboratory and health administrative data be used to assess influenza vaccine effectiveness? Assessing the validity of the Flu and Other Respiratory Viruses Research (FOREVER) cohort. Vaccine 2019; 37:4392–400. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009; 38:1228–34. [Google Scholar]
- 32. Omer SB, Clark DR, Aqil AR, et al. . Maternal influenza immunization and prevention of severe clinical pneumonia in young infants: analysis of randomized controlled trials conducted in Nepal, Mali and South Africa. Pediatr Infect Dis J 2018; 37:436–40. [DOI] [PubMed] [Google Scholar]
- 33. Nunes MC, Cutland CL, Jones S, et al. . Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis 2017; 65:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaman K, Roy E, Arifeen SE, et al. . Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555–64. [DOI] [PubMed] [Google Scholar]
- 35. Tessier E, Campbell H, Ribeiro S, et al. . Impact of extending the timing of maternal pertussis vaccination on hospitalized infant pertussis in England, 2014–2018. Clin Infect Dis 2021; 73:e2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marchant A, Sadarangani M, Garand M, et al. . Maternal immunisation: collaborating with mother nature. Lancet Infect Dis 2017; 17:e197–208. [DOI] [PubMed] [Google Scholar]
- 37. Cuningham W, Geard N, Fielding JE, et al. . Optimal timing of influenza vaccine during pregnancy: a systematic review and meta-analysis. Influenza Other Respir Viruses 2019; 13:438–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nunes MC, Cutland CL, Jones S, et al. . Duration of infant protection against influenza illness conferred by maternal immunization. JAMA Pediatr 2016; 170:840–7. [DOI] [PubMed] [Google Scholar]
- 39. Amin AB, Nunes MC, Tapia MD, et al. . Immunogenicity of influenza vaccines administered to pregnant women in randomized clinical trials in Mali and South Africa. Vaccine 2020; 38:6478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nunes MC, Madhi SA. Prevention of influenza-related illness in young infants by maternal vaccination during pregnancy. F1000Res 2018; 7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jackson ML, Phillips CH, Benoit J, et al. . The impact of selection bias on vaccine effectiveness estimates from test-negative studies. Vaccine 2018; 36:751–7. [DOI] [PubMed] [Google Scholar]
- 42. Schwartz KL, Jembere N, Campitelli MA, Buchan SA, Chung H, Kwong JC. Using physician billing claims from the Ontario Health Insurance Plan to determine individual influenza vaccination status: an updated validation study. CMAJ Open 2016; 4:E463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Public Health Agency of Canada . Vaccination during pregnancy in Canada: results from the 2019 survey of vaccination during pregnancy. Ottawa, Canada, 2023. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/survey-vaccination-during-pregnancy/full-report-2019.html. Accessed 14 December 2023.
- 44. Fakhraei R, Fung SG, Petrcich W, et al. . Trends and characteristics of Tdap vaccination during pregnancy in Ontario, Canada: a retrospective cohort study. CMAJ Open 2022; 10:E1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.