Abstract
Patients with B-cell lymphomas have altered cellular components of vaccine responses due to malignancy and therapy, and the optimal timing of vaccination relative to therapy remains unknown. Severe acute respiratory syndrome coronavirus 2 vaccines created an opportunity for new insights in vaccine timing because patients were challenged with a novel antigen across multiple phases of treatment. We studied serologic messenger RNA vaccine response in retrospective and prospective cohorts with lymphoma and chronic lymphocytic leukemia, paired with clinical and research immune parameters. Reduced serologic response was observed more frequently during active treatment, but nonresponse was also common within observation and posttreatment groups. Total immunoglobulin A and immunoglobulin M correlated with successful vaccine response. In individuals treated with anti-CD19–directed chimeric antigen receptor–modified T cells, nonresponse was associated with reduced B and T follicular helper cells. Predictors of vaccine response varied by disease and therapeutic group, and therefore further studies of immune health during and after cancer therapies are needed to individualize vaccine timing.
Keywords: lymphoma, SARS-CoV-2 vaccination, antibody response, CAR T cells, vaccine timing
Individuals with lymphoma were at risk for reduced or absent vaccine response, even when untreated. Normal circulating immunoglobulins increased the likelihood of response. CART-19 did not permanently impair response, with the presence of Tfh and B cells predicting receptor-binding domain–specific IgG.
The development of antibody responses after vaccination is considered a hallmark of immunogenicity for most vaccines [1, 2]. In lymphoma and chronic lymphocytic leukemia (CLL) (collectively, lymphomas), vaccine immunogenicity is particularly at risk, because both malignancies and therapies directly impact B cells. In particular, B-cell–directed therapies drastically reduce B-cell numbers and function [3–6]. There is currently no test to determine whether the immune systems of patients with lymphomas are able to coordinate a vaccine response.
Prior to 2020, studies of vaccine responsiveness during lymphoma therapy typically focused on influenza, Streptococcus pneumoniae, and varicella zoster vaccine responses [7–10]. These data primarily studied a memory response in the context of circulating vaccine-specific antibodies and potentially antigen-specific memory B cells. The response to a novel antigen is different and typically requires germinal center formation [11, 12]. Distinguishing new versus memory responses after a boost has been challenging [13], and therefore germinal center–dependent responses to novel antigens had been essentially unstudied in the setting of cancer therapies. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine campaign therefore provided a singular opportunity to ask whether individuals with different lymphoma subtypes and across different stages of therapy would be able to coordinate a de novo antibody response against a novel antigen.
Studies of SARS-CoV-2 vaccine response in lymphomas have demonstrated heterogeneous vaccine immunogenicity, with variations observed depending on therapeutic modality and lymphoma subtype [14–23]. Indeed, individuals with CLL and non-Hodgkin lymphoma (NHL) treated with B-cell–targeting therapies had particularly low serologic vaccine responses within 12 months of vaccination; the UK Multicentre Prospective Observational Study Evaluating COVID-19 Vaccine Immune Responses in Lymphoid Cancer (PROSECO study) further highlighted the disconnect between serologic response and T-cell responses, with T-cell responses increasing over time despite lack of serologic response [18, 24–26]. Still, despite the therapeutic and disease-related barriers to coordinating B-cell:T-cell cross-talk required for serologic response, many individuals with lymphomas successfully developed immunoglobulin G (IgG) specific to SARS-CoV-2.
The heterogeneity of SARS-CoV-2 vaccine response in lymphomas provided an opportunity to ask which immunological features predict whether the immune system is ready to coordinate a response to novel antigen. We assessed predictors of serologic vaccine response across multiple B-cell–altering contexts, including different treatment classes, time from treatment, and clinical measures of immunologic health. We then performed cytometry by time of flight (CyTOF) on whole blood to determine whether higher dimensional measures of immune cell subsets could predict vaccine readiness. Our results indicate that individuals with lymphomas had varying responses to vaccination with reduced or absent serologic responses occurring even in the absence of active treatment and in patients who were treatment naive. Recovery of vaccine responsiveness correlated with time after receipt of B-cell–directed antibody therapies, but clinical and experimental measures of immune health varied by group.
METHODS
Cohort Description
The study included 3 cohorts immunized with Pfizer-BioNTech or Moderna messenger RNA (mRNA) vaccines: (1) a prospective cohort with NHL and CLL (research cohort) with blood collected between 15 February and 9 September 2021; (2) a prospective cohort of healthy adults with blood collected from 21 December 2020 to 29 March 2021 [27, 28] (healthy cohort); and (3) a retrospective observational cohort with NHL and CLL (clinical cohort) with blood collected between 1 January 2020 and 21 November 2021. At enrollment, individuals in the research cohort were planned to receive or were within 1 month of receipt of vaccination. Those with documented prior SARS-CoV-2 infection or antibody positivity before first vaccination were excluded from analyses. Individuals in the clinical cohort were vaccinated before 12 July 2021 and had SARS-CoV-2 antibody levels in the medical record. This research was conducted under an institutional review board–approved protocol in accordance with the Declaration of Helsinki. Individuals in the research and healthy cohorts provided written informed consent. A Health Insurance Portability and Accountability Act (HIPAA) waiver was granted for the clinical cohort.
Research and Healthy Cohort Blood Collection and Serology
Peripheral blood was collected 0–90 days prior to first vaccination, 1–3 weeks after vaccine dose 1, 1–5 months after vaccine dose 2, and, when applicable, 1–2 weeks after third immunization. Enzyme-linked immunosorbent assay (ELISA) was performed on plasma obtained from blood samples, as described previously [29, 30] and in the Supplementary Methods.
Clinical Cohort Blood Collection and Serology
An ELISA was performed in the clinical laboratory using plates coated with 2 μg/mL of the spike protein or receptor-binding domain (RBD), as previously described [29, 30] and as shown in the Supplementary Methods. Results were reported in arbitrary units (AU). The reference ranges were negative (≤0.3), equivocal (0.301–0.699), and positive (≥0.7).
Whole Blood CyTOF
Whole blood (300 µL) sampled between 14 days and 4 months after the second dose of vaccination was added to the Maxpar Direct Immune Profiling Assay kit (Standard BioTools) and acquired on the Fluidigm Helios instrument (CyTOF). Data were analyzed using the OMIQ cytometry analysis platform (https://app.omiq.ai/) (Supplementary Methods).
RESULTS
Individuals With Lymphoma Have Impaired Vaccine Responses
To compare immune responses in patients with lymphomas to those of healthy adults, we enrolled 71 patients with NHL/CLL who planned to receive or were within 1 month of receipt of SARS-CoV-2 immunization (Table 1, “Research Cohort”). Control subjects were healthy adults who had blood collected, processed, and analyzed in parallel [27]. Patients with NHL/CLL underwent sample collection at time points aligned with clinical visits (Figure 1A); 24 and 65 patients had specimens available after the first and second vaccine dose, respectively. Forty patients (56%) received Pfizer-BioNTech and 31 (44%) received Moderna (Table 1). At a median of 2.1 weeks after dose 1, patients with NHL/CLL demonstrated a reduced frequency of seroconversion (6/24 [25%] vs 32/33 [97%]) compared to healthy controls (Figure 1B, left panel). Six weeks (range, 0.5–23 weeks) after dose 2, the proportion of patients with lymphoma who developed detectable RBD-specific IgG increased but remained significantly lower than healthy controls (33/65 [51%] vs 32/32 [100%]) (Figure 1B, right panel). Even when serologic response was detectable in lymphoma, 28 of 33 responders (85%) developed RBD-specific IgG that fell below the 25th quartile of healthy individuals (Figure 1B, right panel). As a group, no B-cell NHL subtype was associated with a normal serologic vaccine response, although some individuals with NHL/CLL demonstrated normal or near-normal anti-RBD concentrations (Figure 1C).
Table 1.
Patient Characteristics
| Characteristic | All Patients (n = 273) |
Clinical Cohort (n = 202) |
Research Cohort (n = 71) |
|---|---|---|---|
| Age, y, median (range) | 67 (24–91) | 68 (24–91) | 67 (40–82) |
| Sex, female, n (%) | 96 (35) | 71 (35) | 25 (35) |
| non-Hodgkin lymphoma subtypes | |||
| iNHL | 82 (30) | 57 (28) | 25 (35) |
| FL | 48 (18) | 33 (16) | 15 (21) |
| MZL | 28 (10) | 19 (9) | 9 (13) |
| LPL | 6 (2) | 5 (3) | 1 (1) |
| CLL/SLL | 74 (27) | 52 (26) | 22 (31) |
| CLL | 69 (25) | 47 (23) | 22 (31) |
| SLL | 2 (1) | 2 (1) | … |
| PLL | 2 (1) | 2 (1) | … |
| Richter's | 1 (<1) | 1 (0.5) | … |
| DLBCL | 49 (18) | 27 (13) | 22 (31) |
| DLBCL | 40 (15) | 20 (10) | 20 (26) |
| PMBL | 2 (1) | 2 (1) | … |
| t-iNHL | 6 (2) | 4 (2) | 2 (3) |
| THRLBCL | 1 (<1) | 1 (<1) | … |
| MCL | 18 (7) | 16 (8) | 2 (3) |
| PTLD | 1 (<1) | 1 (1) | … |
| TCL | 24 (9) | 24 (12) | … |
| CTCL | 14 (5) | 14 (7) | … |
| ALCL | 4 (1.5) | 4 (2) | … |
| PTCL NOS | 2 (1) | 2 (1) | … |
| AITL | 1 (<1) | 1 (<1) | … |
| ATLL | 2 (1) | 2 (1) | … |
| ENTKL | 1 (<1) | 1 (<1) | … |
| Hodgkin lymphoma | 25 (9) | 25 (12) | … |
| No. of prior therapies, median (range) | 2 (0–15) | 2 (0–15) | 1 (0–9) |
| Disease status | |||
| Active disease | 146 (53) | 110 (54) | 36 (51) |
| Complete remission | 127 (47) | 92 (46) | 35 (49) |
| Active treatment | 128 (47) | 93 (46) | 35 (49) |
| Most recent therapy | |||
| Untreated | 59 (22) | 29 (14) | 30 (42) |
| Anti-CD20 | 39 (14) | 39 (19) | … |
| R-chemo | 36 (13) | 32 (16) | 4 (6) |
| CAR T cells | 35 (13) | 7 (3) | 28 (39) |
| BTKi | 34 (12) | 25 (12) | 9 (13) |
| Chemo | 22 (8) | 22 (11) | … |
| Anti-PD-1 | 13 (5) | 13 (6) | … |
| R-lenalidomide | 2 (1) | 2 (1) | … |
| Anti-PD-1/chemo | 5 (2) | 5 (2) | … |
| Venetoclax | 4 (2) | 4 (2) | … |
| Venetoclax + anti-CD20 | 2 (1) | 2 (1) | … |
| BTKi + anti-CD20 | 4 (2) | 4 (2) | … |
| Lenalidomide | 4 (2) | 4 (2) | … |
| Mogamulizumab | 4 (2) | 4 (2) | … |
| Othera | 10 (4) | 10 (5) | … |
| Vaccine type | |||
| Pfizer | 147 (54) | 107 (53) | 40 (56) |
| Moderna | 126 (46) | 95 (47) | 31 (44) |
| Time from diagnosis to vaccine 1, mo (range) | 78 (−4 to 373) | 83 (−4 to 373) | 41 (0–256) |
| ALC (1000/μL), median (range) | 1.0 (0–216) | 1.0 (0–216) | 2 (0–80) |
| CD19 (cells/μL), median (range) | 20 (0–1000) | 176 (9–544) | 9 (0–1000) |
| IgM (mg/dL), median (range) | 37 (3–501) | 40 (8–344) | 28 (3–501) |
| IgG (mg/dL), median (range) | 681 (110–1800) | 713 (110–1800) | 590 (159–942) |
| IgA (mg/dL), median (range) | 76 (1–508) | 72 (1–394) | 79 (1–508) |
| CD4 (cells/μL), median (range) | 416 (28–1442) | 447 (28–1442) | 364 (53–949) |
| CD8 (cells/μL), median (range) | 290 (21–1276) | 264 (21–1276) | 365 (21–676) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: anti-CD20, rituximab or obinutuzumab; ALC, absolute lymphocyte count; anti-PD-1, anti-PD-1 antibody; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; BTKi, Bruton tyrosine kinase inhibitor; CAR T cells, chimeric antigen receptor–modified T-cells targeting CD1; CLL, chronic lymphocytic leukemia; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; ENTKL, extranodal natural killer/T-cell lymphoma; FL, follicular lymphoma; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; iNHL, indolent non-Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; mo, months; MZL, marginal zone lymphoma; No., number; PLL, prolymphocytic leukemia; PMBL, primary mediastinal large B-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma, not otherwise specified; PTLD, post-transplant lymphoproliferative disorder; R-chemo, rituximab chemotherapy; R-lenalidomide, rituximab-lenalidomide; SLL, small lymphocytic lymphoma; TCL, T-cell lymphoma; THRLBCL, T/histiocyte-rich large B-cell lymphoma; t-iNHL, transformed indolent non-Hodgkin lymphoma.
aOther includes allogeneic hematopoietic stem cell transplantation, intron A, retinoid, radiation, PI3K inhibitors.
Figure 1.
Serologic response to vaccination in patients with lymphoma. A, Schema of research and healthy cohort blood draw timepoints. B, Anti–receptor-binding domain (RBD) immunoglobulin G (IgG) after vaccine dose 1 and dose 2 in the research and healthy cohorts. Dotted line reflects the limit of anti-RBD IgG detection. Proportion positive indicated at bottom. P values derived from Fisher exact tests. C, Anti-RBD IgG by disease subtype after second vaccination. P value derived from Kruskal–Wallis test of difference across all groups. The iNHL group was comprised of 17 patients not receiving active treatment and 7 active treatment patients, the chronic lymphocytic leukemia group comprised 8 patients receiving active treatment and 11 untreated patients, and the diffuse large B-cell lymphoma group predominantly comprised patients status post anti-CD19–directed chimeric antigen receptor–modified T cells (n = 16) whereas the other 4 patients were not receiving active treatment. D, Description of clinical cohort blood draw timepoints (left). Anti-RBD IgG by disease subtype in the clinical cohort. Proportion of positive patients indicated at bottom (right). P value derived from Kruskal–Wallis test of difference across all groups. Abbreviations: AU, arbitrary units; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; IgG, immunoglobulin G; iNHL, indolent non-Hodgkin lymphoma; MCL, mantle cell lymphoma; RBD, receptor-binding domain; PTLD, posttransplant lymphoproliferative disease; TCL, T-cell lymphoma.
Although both anti-spike and anti-RBD antibodies correlate with neutralizing antibody levels, most neutralizing antibodies target a portion of RBD [31], and the ratio of anti-RBD to total anti-spike IgG can roughly estimate the quality of the antibody response. In individuals with lymphoma who generated an antibody response, the anti-RBD to anti-spike ratio was significantly lower than in healthy controls (Supplementary Figure 1A). However, it remained unclear whether functional differences exist among patients with RBD-binding IgG. Thus, we assessed neutralizing antibodies in a subset of patients with detectable RBD-specific IgG (Supplementary Table 1). For the Alpha variant (D614G), when RBD-specific IgG was detectable, serological responses correlated strongly with neutralizing antibody titers (focus reduction neutralization titer 50% [FRNT50]), and correlations were similar across healthy and lymphoma cohorts (Supplementary Figure 1B). When tested against the Delta variant (B.1.617.2), the correlations were reduced in general, with some individuals having a discordant RBD IgG and FRNT50 status. The correlations were less similar between cohorts but were not statistically different (Supplementary Figure 1C). Together, these data suggested that the rate of seroconversion and the concentration of RBD-specific IgG are decreased in lymphoma but, when present, RBD-specific IgG correlated with neutralizing antibodies.
To understand differences between lymphoma subtypes more broadly, we examined a larger clinical cohort for whom anti-RBD IgG was measured using a clinical assay. Two hundred two patients were enrolled (Table 1, “Clinical Cohort”). All received an mRNA-based vaccine. Blood samples were obtained at a median of 9 weeks (range, 0.5–34 weeks) after the second vaccine dose; 117 of 202 patients (58%) seroconverted (Supplementary Figure 1D). In patients who developed an antibody response following vaccination, responses appeared durable based on cross-sectional analysis over the first 30 weeks (Supplementary Figure 1D). As with the research cohort, anti-RBD IgG responses varied by lymphoma subtype (Figure 1D).
Therapeutic Class and Time From Therapy Completion Differentially Impact Serologic Responses
Decreased vaccine responses in individuals with lymphoma could be due to the presence of malignancy and/or due to the effect of cancer therapy. We therefore assessed the effect of active treatment on humoral response to immunization (Figure 2A). As shown in Table 1, 93 of 202 patients (46%) in the clinical cohort were receiving active treatment (within 5 half-lives of therapeutic agent, 3 months of last chemotherapy dose, 6 months of last anti-CD20 antibody) prior to vaccination, or were status post anti-CD19–directed chimeric antigen receptor–modified T cells (CART-19) without progressive lymphoma. Active treatment was associated with a reduced seroconversion rate (34% vs 78%) (Figure 2B). Antibody responses were lower in patients receiving active treatment in CLL, indolent NHL (iNHL), mantle cell lymphoma (MCL), and T-cell lymphoma (TCL), whereas there was no difference for Hodgkin lymphoma (HL) or diffuse large B-cell lymphoma (DLBCL) (Figure 2C). For patients not receiving active treatment, individuals with CLL had significantly lower antibody levels after 2 vaccine doses compared to those with DLBCL, iNHL, HL, and TCL.
Figure 2.
Active treatment is associated with decreased immunogenicity of severe acute respiratory syndrome coronavirus 2 vaccines. A, Description of treated versus untreated patients within the clinical cohort. B, Anti–receptor-binding domain (RBD) immunoglobulin G (IgG) after vaccine dose 2 in patients undergoing active treatment versus those not undergoing active treatment. P value derived from Fisher exact test. Dashed lines represent thresholds between positive samples, equivocal samples, and negative samples, as indicated. C and D, Anti-RBD IgG in active treatment versus no active treatment across disease subtypes (C) and across therapies (D). P values indicated at the top, derived from Wilcoxon rank-sum tests with corrections for multiple comparisons. Abbreviations: AU, arbitrary units; BTKi, Bruton tyrosine kinase inhibitor; CART, chimeric antigen receptor–modified T cells; CD20, anti-CD20 monoclonal antibody; CLL, chronic lymphoid leukemia; DLBCL, diffuse large cell B-cell lymphoma; HL, Hodgkin lymphoma; IgG, immunoglobulin G; iNHL, indolent non-Hodgkin lymphoma, len, lenalidomide; MCL, mantle cell lymphoma; none, no therapy received; ns, not significant; PD-1, anti-PD-1 monoclonal antibody; PTLD, posttransplant lymphoproliferative disease; RBD, receptor-binding domain; R-chemo, anti-CD20 monoclonal antibody with chemotherapy; TCL, T-cell lymphoma; ven, venetoclax.
We next investigated the effect of specific therapies on seroconversion. Table 1 describes the most recently administered lymphoma therapies. Lack of response was observed in patients receiving anti-CD20 monoclonal antibodies, anti-CD20 based chemoimmunotherapy, and chemotherapy (Figure 2D). Notably, among patients receiving active treatment, we observed that patients receiving anti-PD-1 antibody therapy had consistently high rates of response.
Monoclonal antibody therapy targeting CD20 and CART-19 are associated with a long half-life and prolonged B-cell depletion. We therefore assessed vaccine response as a function of time from the last anti-CD20 antibody dose and from CART-19 infusion. Time from anti-CD20 therapy positively correlated with vaccine-induced anti-RBD IgG in both patients receiving anti-CD20 antibody monotherapy (Supplementary Figure 1E) and concomitant anti-CD20 chemoimmunotherapy (Supplementary Figure 1F). Conversely, time from chemotherapy alone was not correlated with response, suggesting that immune reconstitution was rapid and active treatment alone is the primary driver of nonresponse (Supplementary Figure 1G).
Patients in remission after CART-19 therapy generally had poorer antibody responses for a significantly longer duration after completion of therapy. For the first 1–2 years after CART-19 infusion, anti-RBD IgG was negative. In some patients, nonresponse occurred many years after CART-19 infusion, although other patients demonstrated seroconversion 1–2 years post-CART-19 (Supplementary Figure 1H). This observation is consistent with known timing of B-cell recovery after CART-19 therapy [32].
Clinical Measures of Immune Health and Response to mRNA Vaccination
We next asked whether immune features around the time of immunization could predict vaccine responsiveness. Indeed, patients with normal concentrations of total immunoglobulins before vaccination were more likely to mount a detectable antibody response; however, this association was not evident for T-cell or T-cell subset counts (Figure 3A–D, and Supplementary Figure 2A–C, top rows). Similarly, in analyses of correlation, absolute CD19 and immunoglobulin levels positively correlated with RBD-specific IgG (Figure 3A–D and Supplementary Figure 2A–C, bottom rows). No measured immunologic parameter fully discriminated between response and nonresponse.
Figure 3.
Baseline immune cell frequencies in the blood were associated with vaccine immunogenicity. A–D (top row), Research cohort anti–receptor-binding domain (RBD) immunoglobulin G (IgG) by normal versus below normal clinical measures of immune status (top row, proportions of positive tests for normal and low clinical values noted at bottom). P values are derived from Fisher exact tests. A–D (bottom row), Correlation between anti-RBD IgG versus values of clinically measured immunologic parameters. P values are derived from Spearman correlation tests. Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; ns, not significant; RBD, receptor-binding domain.
mRNA Vaccine Response in DLBCL Patients After CAR T-Cell Therapy Is Associated With B-Cell Reconstitution
To gain a deeper understanding of immune features predictive of vaccine response, we performed a high-parameter immunologic assessment (per Supplementary Table 2) in individuals with DLBCL who were status post CART-19 infusion (DLBCL–CART-19). We assessed 14 individuals with DLBCL–CART-19 (5 responders and 9 nonresponders, Supplementary Table 3), and 23 healthy donor peripheral blood samples using CyTOF. Data were analyzed using t-distributed stochastic neighbor embedding and FlowSOM approaches to define and quantify cell populations that differed between responders and nonresponders (Figure 4A, Supplementary Figure 2D). These approaches highlighted a loss of participating cells from cluster 3 in DLBCL–CART-19 nonresponders when compared to healthy controls and DLBCL–CART-19 responders (Figure 4A–D). Cluster 3 contained 10%–15% of B cells in responders and expressed varying degrees of CD38, IgD, CCR6, CD45RA, HLA-DR, and CD27, reflecting activated B cells (Figure 4C and 4D). Together these data are consistent with B cells serving as a driving predictor of vaccine response in DLBCL–CART-19 patients.
Figure 4.
Severe acute respiratory syndrome coronavirus 2 mRNA vaccine response in diffuse large cell B-cell lymphoma (DLBCL) after chimeric antigen receptor–modified T-cell therapy is associated with B-cell reconstitution. A, FlowSOM clusters of mononuclear cells (lymphocytes and monocytes) concatenated from each DLBCL cohort (nonresponder, n = 9 and responder, n = 5) and healthy cohort (n = 23). B, Projection of indicated protein onto t-SNE map. C, Boxplot of mononuclear cell frequencies from each cohort in each FlowSOM cluster in (B). D, Median fluorescence intensity of each marker in each FlowSOM cluster (row scaled z-score). Significance was determined by unpaired Wilcoxon test: *P < .05, **P < .01, ***P < .001, ****P < .0001. Abbreviations: CM, central memory; DLBCL, diffuse large cell B-cell lymphoma; EM, effector memory; EMRA, effector memory cells expressing CD45RA; IgG, immunoglobulin G; NK, natural killer; RBD, receptor-binding domain; t-SNE, t-distributed stochastic neighbor embedding.
To characterize B-cell activation states in DLBCL–CART-19 vaccine responders, we analyzed B cells with 14-marker FlowSOM clustering (per Supplementary Table 2). We observed distinct differences in B-cell subset distribution between DLBCL–CAR-T-19 responders and nonresponders (Supplementary Figure 3A and 3B). Responders showed enrichment in clusters 2 (naive B cells), 4 (circulating pre-class-switched memory B cells), and 6 (non-class-switched memory B cells) (Supplementary Figure 3A–E). In contrast, nonresponders were enriched for cluster 3, early plasmablast precursors (Supplementary Figures 3A–E). We observed a positive correlation between time from CART-19 infusion and 3 B-cell subsets (naive, pre-class-switched and non-class switched memory B cells), when all patients (composite; responders and nonresponders) were analyzed (Supplementary Figure 4A, 4C, and 4D, respectively). In contrast, early plasmablast precursors (cluster 3) negatively correlate with time from CART-19 infusion when all patients (composite) were analyzed (Supplementary Figure 4B). Together, these data suggest that pre- and non-class-switched memory B subsets are associated with the ability to coordinate a vaccine response after CART-19, and that pre-class-switched memory B cells may represent the most important predictive feature of vaccine response.
CD4 T-Cell Populations and Vaccine Response
Next, we analyzed nonnaive CD4+ T cells (per Supplementary Table 2) given the importance of T follicular helper (Tfh) cells in generating high-affinity antibodies. Indeed, nonresponders demonstrated reduced participation in a cluster marked by CXCR5, CD38, CCR7, and CD27, consistent with Tfh (Figure 5A–D, Supplementary Figure 2E). FlowSOM clustering revealed 2 additional clusters with differential participation of cells in DLBCL–CART-19 responders compared to the nonresponders (clusters 3 and 5, Figure 5A and 5C). In comparison to healthy donors and DLBCL–CART-19 responders, the frequency of cells in cluster 3 was increased in nonresponders, expressing markers (CCR4+CCR6+CD161+) resembling cells of Th17-like phenotype [33] (Figure 5A–D). In contrast, cluster 5 was increased in responders, expressing markers consistent with activated circulatory Tfh with a central memory phenotype (Figure 5A–D). We also assessed the correlation between the frequency of Tfh and time from CART-19 therapy, as well as B-cell subsets enriched in responders or nonresponders (Supplementary Figure 4E–I). We observed statistically significant positive and negative correlation between non-class-switched memory B cells (cluster 6) and early plasmablast precursors (cluster 3) with Tfh frequency (Supplementary Figure 4G and 4I), highlighting the contribution of Tfh and early plasmablast precursors to vaccine response and nonresponse, respectively.
Figure 5.
Increased T follicular helper (Tfh) frequency is associated with vaccine response in diffuse large cell B-cell lymphoma after chimeric antigen receptor–modified T-cell therapy. A, FlowSOM cluster of non-naive CD4+ T cells from each indicated cohort. B, Projection of each indicated protein onto the t-SNE map in (A). C, Frequencies of nonnaive CD4+ T cells from each cohort in each FlowSOM cluster in (A). Responder (n = 5), nonresponder (n = 9), and healthy (n = 23). D, Median fluorescence intensity of each marker in each FlowSOM cluster (row scaled z-score). Markers expression by cluster 3 (CD161+CXCR3–CCR6+CCR6+, Th17-like cells) and cluster 5 (CCR7+CD38+CXCR3+CXCR5+CD27+CD45RO+, activated circulatory Tfh with a central memory phenotype). Significance was calculated by unpaired Wilcoxon test: *P < .05, **P < .01, ***P < .001, ****P < .0001. Abbreviations: DLBCL, diffuse large cell B-cell lymphoma; IgG, immunoglobulin G; RBD, receptor-binding domain; t-SNE, t-distributed stochastic neighbor embedding.
Third Vaccine Dose Induced Seroconversion in a Subset of Patients on Bruton Tyrosine Kinase Inhibition
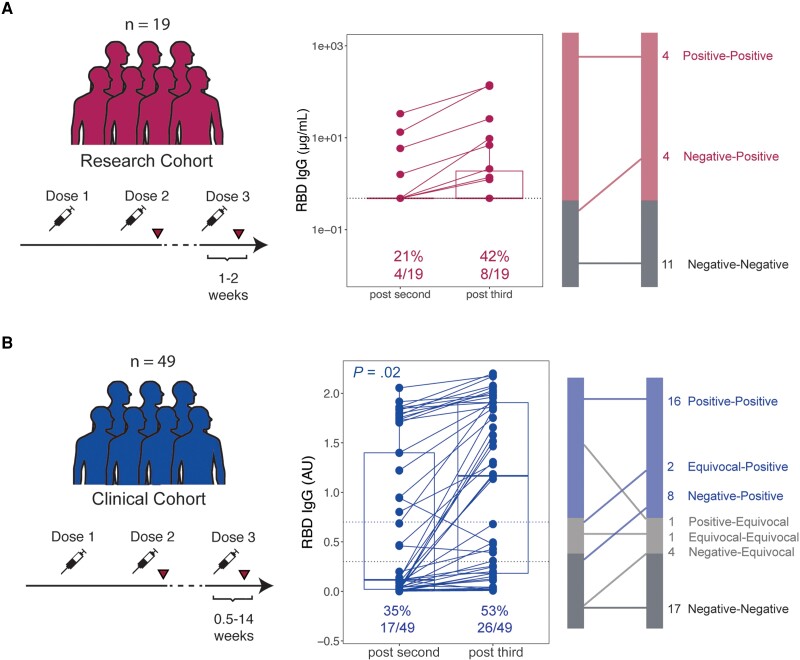
Finally, we assessed serologic anti-RBD IgG responses in a subset of patients with lymphoma who received a third dose of vaccine (Supplementary Table 4). In the research cohort, 19 patients received a third vaccine dose and had RBD-specific IgG assessed at a median of 15 days (range, 5–94 days) after vaccination. Only 4 patients were seropositive before the third dose (Figure 6A). Of the remaining 15 patients, 4 (27%) converted from antibody negative to positive, whereas 11 of 15 (73%) remained negative for RBD-specific IgG. First-time SARS-CoV-2 infection was documented in the medical record for 37 patients in the research cohort. Of these, 1 infection occurred between doses 1 and 2; 3 occurred between doses 2 and 3; and 33 infections occurred after dose 3. The date of further vaccines was not recorded; the median time from vaccine dose 3 to infection was 368 days (range, 13–827 days).
Figure 6.
Serologic response to third severe acute respiratory syndrome coronavirus 2 vaccination in patients with lymphoma. Paired research cohort (A) and clinical cohort (B) anti–receptor-binding domain (RBD) immunoglobulin G (IgG) responses after second and third vaccines doses, with graphical representation of changes at right. P value derived from Fisher exact test.
In the clinical cohort, 49 patients received a third mRNA vaccine dose and underwent subsequent clinical assessment of RBD-specific IgG (Figure 6B). Prior to the third dose, 17 of 49 patients (35%) were seropositive. At a median of 5 weeks after third vaccination (range, 0.5–14 weeks), anti-RBD IgG concentration increased. Ten of 32 (31%) seronegative (including equivocal) patients developed detectable RBD-specific IgG. The remaining 22 of 32 (69%) seronegative patients continued to have undetectable or equivocal RBD-specific IgG even after the third dose.
The disease and treatment status for patients who did and did not respond to a third vaccine dose are listed in Supplementary Tables 5 and 6. Of 68 total patients assessed in the research and clinical cohorts (Supplementary Table 5), 65 (96%) maintained the same treatment status (eg, continued the same therapy or did not receive therapy) between doses 2 and 3. The majority of patients who converted to positive after receiving a third dose were not actively receiving treatment (9/14 [64%]), but 5 (36%) patients were receiving a Bruton tyrosine kinase inhibitor (BTKi) for iNHL, MCL, or CLL. Only 2 of 33 (6%) patients who remained negative or equivocal after the third dose were not on active therapy, and both had active disease. The only patient who demonstrated a decrease in RBD-specific IgG (titers decreased from positive to equivocal) initiated anti-CD20 therapy between doses 2 and 3. In summary, across both cohorts, a third dose of vaccine seroconverted 21% (14/68) of patients who were seronegative or equivocal after dose 2. Patients who derived this benefit were predominantly those who were untreated (4/14 [29%]), had completed therapy (4/14 [29%]), or were receiving a BTKi (5/14 [36%]).
DISCUSSION
The generation of protective immunity requires a responsive immune system. Studies of vaccine responsiveness in lymphomas prior to 2021 were limited to measuring vaccine responsiveness to recall antigens and therefore studied processes that could draw from memory responses. Here, we leveraged SARS-CoV-2 mRNA vaccination to dissect the clinical and immunologic predictors of de novo serologic responsiveness in lymphoma. Serologic response in lymphoma was both less common and reduced in magnitude when compared to healthy individuals. Of note, while serologic response was reduced during active treatment, treatment status alone was insufficient to predict responsiveness. When lymphoma subtypes and treatment approaches were analyzed in composite, total serum levels of immunoglobulin A (IgA) and immunoglobulin M (IgM) were weakly correlated with vaccine response. Cellular predictors of response performed within a uniform population of individuals with DLBCL–CART-19 indicate that, in addition to the frequency of B cells, the frequency of CXCR5+ Tfh-like cells was associated with serologic vaccine responsiveness post-CART-19 therapy.
Although nonresponse was observed for many individuals without active therapy, active therapy remained a strong predictor of impaired response. Our data suggest that the timing of vaccination relative to therapy is an important factor in successful response to vaccination. Consistent with other studies, the effects of anti-CD20 antibodies and chemotherapy were reduced with time, with gradual increases in anti-RBD–specific antibodies as the weeks from anti-CD20 treatment increased [26, 34, 35]. Untreated patients may benefit from vaccination at diagnosis or as far in advance of treatment as clinical care allows. However, the time required to initiate and sustain adequate B-cell selection and plasma cell formation before initiation of B-cell–targeting therapies has not been defined and would likely require several weeks. For patients who received CART-19 therapy, the window of nonresponse was much longer than the 3 months post-CART-19 currently recommended by published consensus guidelines; instead, vaccine responsiveness may take 1–2 years after CART-19 infusion [36]. Conversely, therapies such as PD-1 blockade had no discernible effect on RBD-specific IgG. The variability in vaccine responsiveness during therapy, observation, and across therapeutic classes suggests that other estimates of vaccine readiness are needed.
Our studies of immunologic predictors of vaccine responsiveness across all groups yielded both cellular and serologic correlates. First, the ability of B cells to class switch and generate IgA and IgM-secreting plasma cells and therefore normal levels of total serum IgA and IgM performed slightly better than absolute T and B cells counts as a correlate of vaccine response. Still, these measures may perform differently depending upon therapeutic context, and the study of clinical immunologic predictors was limited as these tests were not uniformly ordered. The research cohort more uniformly captured exploratory measures of immune health using CyTOF. When the largest cohort of uniform diagnosis and treatment was studied (DLBCL–CART-19), low or absent B-cell frequency was the strongest predictor of nonresponse, a finding that would likely be available in clinical B-cell counts as well. This observation is consistent with recently published data [37, 38]. Beyond B cells, the loss of Tfh in blood was also associated with nonresponse. Tfh cells in blood are recent emigres from lymphoid tissues and may also reflect the end product of productive T- and B-cell interactions [39, 40]; the presence of circulating Tfh cells may therefore serve as an additional measure of healthy T- and B-cell interactions. Of note, while antibody responses can be important in disease prevention, vaccines also elicit T-cell responses that are critical in infection control [14, 18, 41]. Our study does not capture SARS-CoV-2–specific T-cell responses. Other studies suggest that the absence of a serologic response in a B-cell–deficient individual can be dissociated from effective T-cell immunization [38, 42]. In addition, comparison of responses between patients after infection and immunization versus immunization alone would further test whether defects in antibody response also apply to replicating virus.
In summary, our study suggests that optimal timing of vaccination in patients with lymphoma may be impacted by lymphoma subtype, treatment received, and time from last therapy. While several immunological measures were correlated with vaccine response, there remains substantial need to develop clinical predictors and immunologic features of immune health and vaccine readiness in all patients. Further study with uniform cohorts and deeper analyses of cell types and states is needed. These in-depth studies may inform alternative strategies to induce more robust vaccine responses in patients with lymphoma and achieve actionable measures of immunologic function in settings of immunocompromise.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Elise A Chong, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Kingsley Gideon Kumashie, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia.
Emeline R Chong, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
Joseph Fabrizio, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
Aditi Gupta, Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Jakub Svoboda, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Stefan K Barta, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Kristy M Walsh, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
Ellen B Napier, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
Rachel K Lundberg, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
Sunita D Nasta, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
James N Gerson, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Daniel J Landsburg, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Joyce Gonzalez, Immune Health.
Andrew Gaano, Immune Health.
Madison E Weirick, Department of Microbiology.
Christopher M McAllister, Department of Microbiology.
Moses Awofolaju, Department of Microbiology.
Gavin N John, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia.
Shane C Kammerman, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia.
Josef Novacek, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia.
Raymone Pajarillo, Center for Cellular Immunotherapies.
Kendall A Lundgreen, Department of Microbiology.
Nicole Tanenbaum, Department of Microbiology.
Sigrid Gouma, Department of Microbiology.
Elizabeth M Drapeau, Department of Microbiology.
Sharon Adamski, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Kurt D’Andrea, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Ajinkya Pattekar, Center for Cellular Immunotherapies; Department of Pathology and Laboratory Medicine.
Amanda Hicks, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Scott Korte, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Harsh Sharma, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Sarah Herring, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Justine C Williams, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Jacob T Hamilton, Institute for Immunology; Department of Pathology and Laboratory Medicine.
Paul Bates, Department of Microbiology.
Scott E Hensley, Department of Microbiology.
Eline T Luning Prak, Immune Health; Department of Pathology and Laboratory Medicine.
Allison R Greenplate, Institute for Immunology; Department of Pathology and Laboratory Medicine.
E John Wherry, Institute for Immunology; Department of Pathology and Laboratory Medicine; Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
Stephen J Schuster, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania.
Marco Ruella, The Richard Berman Family Innovations Center in CLL and Lymphomas, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania; Division of Hematology/Oncology, Hospital of the University of Pennsylvania; Center for Cellular Immunotherapies; Institute for Immunology.
Laura A Vella, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia; Department of Pathology and Laboratory Medicine.
Notes
Acknowledgments. The authors thank Peter and Martha (Muff) Morse whose philanthropy supported this research. The authors also thank all patients and healthy participants and their families and caregivers, as well as the clinical research team and medical personnel who managed patient care.
Author contributions. E. A. C., K. M. W., E. J. W., L. A. V., M. R., and S. J. S. designed the research. E. A. C., A. R. G., E. J. W., S. J. S., L. A. V., M. R., and S. J. S. oversaw the research. E. A. C., E. R. C., J. F., and A. G. collected the data. M. E. W., C. M. M., M. A., N. T., S. G., and E. M. D. performed the research serology experiments. E. L. P. oversaw the optimization and validation of the clinical laboratory RBD assays. J. G. and A. G. performed the IgG RBD clinical laboratory assays. S. E. H. oversaw the optimization and validation of the research RBD assays. K. A. L. performed the neutralization experiments. P. B. oversaw the optimization and validation of the neutralization assays. E. A. C., K. G. K., G. N. G., S. C. K., J. N., A. L. G., and L. A. V. analyzed the data. E. A. C., J. S., S. K. B., E. B. N., R. K. L., S. D. N., J. N. G., D. J. L., and S. J. S. managed patients. A. P., A. H., S. K., H. S., S. H., J. T. H., and R. P. processed peripheral blood samples. S. A., K. D., J. C. W., A. P., A. H., S. K., H. S., S. H., and J. T. H. managed the research sample database. E. A. C., K. G. K., M. R., and L. A. V. wrote the manuscript. L. A. V., K. G. K., G. N. G., S. C. K., and J. N. created the figures. All authors reviewed and edited the manuscript. All authors had access to the original data and approved this manuscript for publication.
Data availability. Requests for data should be directed to the corresponding author. All requests for raw and analyzed preclinical data and materials are promptly reviewed by the University of Pennsylvania to determine if they are subject to intellectual property or confidentiality obligations. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a material transfer agreement.
Financial support. E. A. C. was supported by the Lymphoma Research Foundation under a Postdoctoral Fellowship Grant and Clinical Investigator Career Development Award. E. L. P. was supported by the National Institutes of Health (NIH) (grant number P30-CA016520). E. J. W. was supported by the NIH (grant numbers AI105343, AI112521, AI082630, AI201085, AI123539, and AI117950). E. J. W. was also supported by the Parker Institute for Cancer Immunotherapy, which supports the cancer immunology program at the University of Pennsylvania. M. R. was supported by a Lymphoma Research Foundation Career Development Award; Gilead Research Scholar Award; Gabrielle's Angel Foundation; Emerson Collective Award; Laffey-McHugh Foundation; Parker Institute for Cancer Immunotherapy; and the National Cancer Institute (grant numbers 1K99CA212302 and R00CA212302). L. A. V. was supported by the Doris Duke Foundation (grant number 2021190) and the NIH (grant number K08AI136660).
References
- 1. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plotkin SA. Correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401–9. [DOI] [PubMed] [Google Scholar]
- 3. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer 2018; 17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alu A, Lei H, Han X, Wei Y, Wei X. BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies. J Hematol Oncol 2022; 15:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361–5. [DOI] [PubMed] [Google Scholar]
- 6. Ali A, Goy A, Dunleavy K. CAR T-cell therapy in highly aggressive B-cell lymphoma: emerging biological and clinical insights. Blood 2022; 140:1461–9. [DOI] [PubMed] [Google Scholar]
- 7. Nordøy T, Aaberge IS, Husebekk A, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol 2002; 19:71–8. [DOI] [PubMed] [Google Scholar]
- 8. Centkowski P, Brydak L, Machała M, et al. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J Clin Immunol 2007; 27:339–46. [DOI] [PubMed] [Google Scholar]
- 9. Dagnew AF, Ilhan O, Lee W-S, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019; 19:988–1000. [DOI] [PubMed] [Google Scholar]
- 10. Tsigrelis C, Ljungman P. Vaccinations in patients with hematological malignancies. Blood Rev 2016; 30:139–47. [DOI] [PubMed] [Google Scholar]
- 11. Kaneko N, Kuo H-H, Boucau J, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2020; 183:143–57.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laidlaw BJ, Ellebedy AH. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol 2022; 22:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiepers A, van ‘t Wout MFL, Greaney AJ, et al. Molecular fate-mapping of serum antibody responses to repeat immunization. Nature 2023; 615:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 2021; 5:3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant Cell Ther 2021; 27:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benda M, Mutschlechner B, Ulmer H, et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol 2021; 195:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021; 39:1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021; 137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol 2021; 96:1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia 2021; 35:2703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021; 11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Re D, Barrière J, Chamorey E, et al. Low rate of seroconversion after mRNA anti-SARS-CoV-2 vaccination in patients with hematological malignancies. Leuk Lymphoma 2021; 62:3308–10. [DOI] [PubMed] [Google Scholar]
- 23. Fendler A, Shepherd STC, Au L, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer 2021; 2:1321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiedmeier-Nutor JE, Iqbal M, Rosenthal AC, et al. Response to COVID-19 vaccination post-CAR T therapy in patients with non-Hodgkin lymphoma and multiple myeloma. Clin Lymphoma Myeloma Leuk 2023; 23:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang A, Akhtar A, Linderman SL, et al. Humoral responses against SARS-CoV-2 and variants of concern after mRNA vaccines in patients with non-Hodgkin lymphoma and chronic lymphocytic leukemia. J Clin Oncol 2022; 40:3020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim SH, Stuart B, Joseph-Pietras D, et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer 2022; 3:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol 2021; 6:eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccination induces durable immune memory to SARS-CoV-2 with continued evolution to variants of concern. bioRxiv [Preprint]. Posted online 23 August 2021. doi: 10.1101/2021.08.23.457229 [DOI]
- 29. Anderson EM, Diorio C, Goodwin EC, et al. SARS-CoV-2 antibody responses in children with MIS-C and mild and severe COVID-19. J Pediatric Infect Dis Soc 2020; 10:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flannery DD, Gouma S, Dhudasia MB, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol 2020; 5:eabd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584:115–9. [DOI] [PubMed] [Google Scholar]
- 32. Chong EA, Ruella M, Schuster SJ. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med 2021; 384:673–4. [DOI] [PubMed] [Google Scholar]
- 33. Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends Immunol 2012; 33:505–12. [DOI] [PubMed] [Google Scholar]
- 34. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol 2013; 33:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Society of Hematology. ASH-ASTCT COVID-19 vaccination for HCT and CAR T-cell recipients. 2022. https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients. Accessed 29 August 2023.
- 37. Okamoto A, Fujigaki H, Iriyama C, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv 2022; 6:3230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atanackovic D, Kreitman RJ, Cohen J, et al. T cell responses against SARS-CoV-2 and its Omicron variant in a patient with B cell lymphoma after multiple doses of a COVID-19 mRNA vaccine. J Immunother Cancer 2022; 10:e004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vella LA, Buggert M, Manne S, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest 2019; 129:3185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goenka R, Barnett LG, Silver JS, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol 2011; 187:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021; 54:2133–42.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atanackovic D, Luetkens T, Omili D, et al. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell-depleted lymphoma after CART therapy. Blood 2022; 140:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.