Abstract
Background
We assessed associations between binding antibody (bAb) concentration <5 days from symptom onset and testing positive for COVID-19 among patients in a test-negative study.
Methods
From October 2021 to June 2022, study sites in 7 states enrolled patients aged ≥6 months presenting with acute respiratory illness. Respiratory specimens were tested for SARS-CoV-2. In blood specimens, we measured concentrations of anti-SARS-CoV-2 antibodies against the spike protein receptor binding domain (RBD) and nucleocapsid antigens from the ancestral strain in standardized bAb units (BAU). Percentage change in odds of COVID-19 by increasing anti-RBD bAb was estimated via logistic regression as (1 – adjusted odds ratio of COVID-19) × 100, adjusting for COVID-19 mRNA vaccine doses, age, site, and high-risk exposure.
Results
Out of 2018 symptomatic patients, 662 (33%) tested positive for acute SARS-CoV-2 infection. Geometric mean RBD bAb levels were lower among COVID-19 cases than SARS-CoV-2 test-negative controls during the Delta-predominant period (112 vs 498 BAU/mL) and Omicron-predominant period (823 vs 1189 BAU/mL). Acute-phase ancestral spike RBD bAb levels associated with 50% lower odds of COVID-19 were 1968 BAU/mL against Delta and 3375 BAU/mL against Omicron; thresholds may differ in other laboratories.
Conclusions
During acute illness, antibody concentrations against ancestral spike RBD were associated with protection against COVID-19.
Keywords: antibodies, COVID-19, correlates of protection, SARS-CoV-2 infection
From October 2021 to June 2022, we assessed the association between antibody concentration and COVID-19 illness among patients enrolled in a test-negative study in 7 US states. We found that higher anti–receptor-binding domain antibodies in patients were associated with protection against symptomatic COVID-19.
COVID-19 vaccine trials and immunologic studies have evaluated neutralizing antibodies as potential immune correlates of protection from COVID-19 illness [1, 2]. Concentrations of immunoglobulin G (IgG) binding antibody (bAb) against ancestral SARS-CoV-2 spike protein and receptor-binding domain (RBD) have also been shown to correlate with protection [3]. Immune correlates of protection following vaccination are important for immunogenicity studies and potential evaluation of new COVID-19 vaccines and formulations [1, 2, 4]. Assessing protective antibody levels in the population may help not only vaccine evaluation but also prediction of susceptibility to and protection against emerging variants [5]. Immune correlates are continually reevaluated as levels of protection mediated by antibodies vary with time and emergence of new SARS-CoV-2 variants.
Observational studies of licensed vaccines can contribute to understanding immune biomarkers associated with protection against COVID-19 illness. Observational test-negative design studies are widely used to evaluate influenza and COVID-19 vaccine effectiveness (VE) [6, 7] and may be used to estimate antibody levels proximal to illness onset, which may correlate with protection [8]. Test-negative design COVID-19 VE studies systematically enroll and test symptomatic patients who seek medical care for an acute respiratory illness [7, 9]. Reduction in the odds of laboratory-confirmed illness provides an estimate of VE against disease end points. Blood specimens collected at enrollment can be used in serologic assays to measure antibody titers early in infection. COVID-19 mRNA vaccines elicit antibodies against RBD but not against SARS-CoV-2 nucleocapsid (N) protein [10, 11]; thus, the presence of anti-N antibodies indicates past SARS-CoV-2 infection among vaccinated and unvaccinated individuals while anti-RBD antibodies may result from either prior SARS-Cov-2 infection or vaccination. In this report, we assessed associations between anti–SARS-CoV-2 RBD and N protein antibody concentrations during acute respiratory illness and odds of COVID-19 among patients enrolled in a COVID-19 VE study.
MATERIALS AND METHODS
Study Population and Sample Collection
Ambulatory patients aged ≥1 year presenting within 10 days of respiratory illness onset were enrolled from participating health care facilities across 7 study sites in the US Flu Vaccine Effectiveness Network, as previously described [12, 13]. Epidemiologic data collected from enrolled patients included age, date of illness onset, reported symptoms, documented COVID-19 vaccination history including dates of COVID-19 vaccination, and dates of prior positive COVID-19 test results recorded in electronic medical records. Respiratory specimens (nasal/nasopharyngeal and throat swabs) were tested for SARS-CoV-2 by real-time reverse-transcription polymerase chain reaction. A subset of these specimens was sequenced for SARS-CoV-2 lineage at the US Centers for Disease Control and Prevention (CDC). Patients were classified by test results as COVID-19 cases or SARS-CoV-2 test-negative controls. SARS-CoV-2 variant infection was determined by genomic sequencing or categorized by predominant variant during 2 periods as previously described [12–14]: Delta (1 October–24 December 2021) or Omicron BA.1–5 (25 December 2021–29 June 2022).
At enrollment, research staff at each study site collected blood specimens from participants by finger stick and absorbed drops on Whatman 903 filter paper cards. Filter paper blood spots were dried at room temperature, packed with desiccant, and sent to the CDC. An acute blood specimen had to be collected from a patient within 5 days of symptom onset for inclusion in the analysis (Supplementary Figures 1 and 2) [15]. This activity was reviewed and approved by the CDC and each US Flu Vaccine Effectiveness Network site's institutional review board.
Serologic Assays
Dried blood spots (DBSs) have been shown to provide similar results to venipuncture for SARS-CoV-2 antibody testing [16–18]. SARS-CoV-2 antibody concentrations were measured with the FlexImmArray SARS-CoV-2 Human IgG Antibody Test (Tetracore): a microsphere-based assay including (1) antigens from nucleocapsid and spike RBD proteins of the SARS-CoV-2 ancestral WA1 strain Hu-1 and (2) human IgG calibrator serum for each antigen [19]. Diluted samples (1:300) were incubated with conjugated microspheres, and fluorescent anti-human IgG-phycoerythrin was used as the reporter. Readings in sample median fluorescence intensity (MFI) ratios as compared with calibrator sera were obtained with Luminex MAGPIX (Luminex Corporation). According to manufacturer specifications, MFI ratios ≥1.2 were defined as seropositive. MFI ratios were standardized to bAb units (BAU) calibrated against the World Health Organization’s anti–SARS-CoV-2 immunoglobulin international standard (20/150) by linear regression [20]. Antibody concentration (BAU per milliliter) was multiplied by a dilution factor of 300 for analyses (anti-RBD seropositivity cutoff, 15.9 BAU/mL; anti-N seropositivity cutoff, 6.9 BAU/mL).
Statistical Analysis
Analyses were restricted to patients with a known date of specimen collection and SARS-CoV-2 result based on real-time reverse-transcription polymerase chain reaction. Patients were excluded if they received 1 dose or >4 doses of COVID-19 mRNA vaccine, any non-mRNA vaccine dose, or a COVID-19 vaccine dose of unknown type. Demographic characteristics, COVID-19 vaccination status, and prior SARS-CoV-2 infection history were compared between patients testing SARS-CoV-2 positive at enrollment and patients who tested negative. Geometric mean concentration (GMC) for anti-RBD and anti-N antibodies was compared across patients by case status, COVID-19 mRNA vaccine doses received (unvaccinated, 2, 3, or 4 doses), and evidence of prior SARS-CoV-2 infection, defined as electronic medical record documentation of ≥1 prior positive SARS-CoV-2 test result or anti-N seropositivity (≥6.9 BAU/mL) in acute sera. Prior infection was documented from 17 March 2020 to 12 June 2022. Distributions of anti-RBD and anti-N bAb levels were plotted by COVID-19 case and test-negative control status and number of COVID-19 vaccines received.
Odds of acute COVID-19–positive cases vs test-negative controls were estimated by anti-RBD concentration (BAU per milliliter) in a logistic regression model adjusted for COVID-19 mRNA vaccine doses (2, 3, or 4 doses vs unvaccinated), age (with cubic terms), study site, calendar time (illness onset week), and high-risk SARS-CoV-2 exposure (health care worker or contact of laboratory-confirmed COVID-19 case). Model covariates were defined a priori according to previous analyses [12, 13] and maintained in multivariate models if inclusion changed the main effect estimate by >5% or significantly improved the model fit by the log-likelihood ratio test (Supplementary Table 1). Percentage change in relative odds of symptomatic COVID-19 was calculated as follows: (1 – adjusted odds ratio) × 100. Anti-RBD bAb concentration thresholds associated with 50% lower relative odds of COVID-19 were estimated from model parameters. Adjusted estimates were stratified by COVID-19 variant period, with stratified models adjusting for the covariates described previously.
Next, we evaluated how the percentage of COVID-19 cases and test-negative controls with anti-RBD bAb levels differed by anti-N bAb levels (<10 BAU/mL, low; 10–99 BAU/mL, medium; ≥100 BAU/mL, high), where higher anti-N antibody concentration indicated more recent prior SARS-CoV-2 infection. Percentages of participants with anti-RBD bAb concentrations above the threshold for a 50% reduction in odds of COVID-19 were estimated by anti-N bAb level, COVID-19 mRNA vaccine doses, and evidence of prior SARS-CoV-2 infection.
For a subset of COVID-19 cases, geometric mean anti-RBD and anti-N bAb concentrations and percentages of specimens of specimens above the 50% threshold concentrations were quantified in paired acute and convalescent specimens collected 21 to 56 days apart. All statistical analyses were performed with R version 4.0.3 (R Foundation for Statistical Computing).
RESULTS
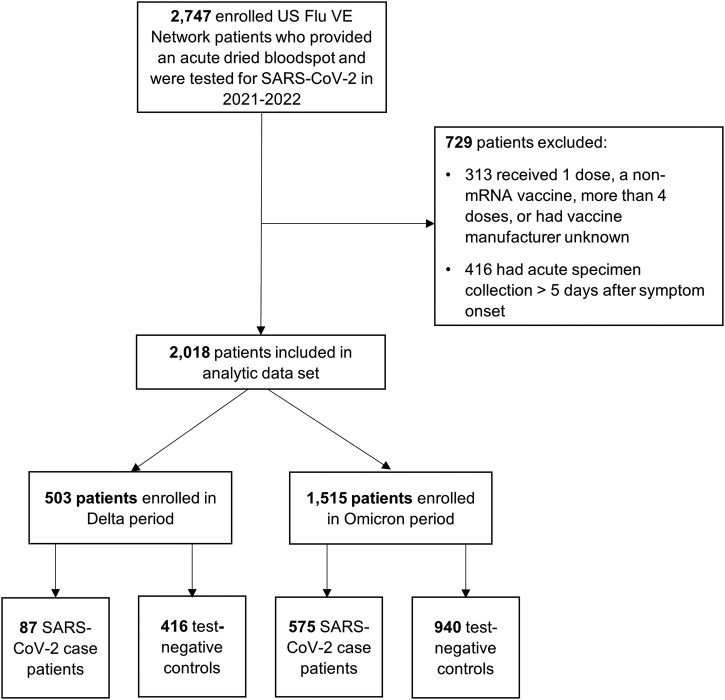
A total of 2018 enrollees in the US Flu Vaccine Effectiveness Network had blood specimens collected within 5 days of symptom onset and were included in analyses (Figure 1): 662 (33%) COVID-19 cases and 1356 (67%) test-negative controls. SARS-CoV-2 positivity varied by variant period. Of 503 patients enrolled during the Delta variant–predominant period, 87 (17%) tested positive for SARS-CoV-2, as compared with 575 (38%) who tested positive during the Omicron period among 1515 patients (Table 1). Among COVID-19 cases enrolled between 1 October and 24 December 2021 (Delta variant period), 20% had evidence of SARS-CoV-2 infection vs 42% enrolled between 25 December 2021 and 29 June 2022 (Omicron BA.1–5 period). A total of 48 SARS-CoV-2–positive specimens collected during the Omicron-predominant period were genetically characterized: Omicron BA.2 lineage accounted for 38 cases, followed by BA.1 (n = 5), BA.2.12.1 (n = 3), BA.4 (n = 1), and BA.5 (n = 1).
Figure 1.
US Flu Vaccine Effectiveness Network enrollment for 2021–2022 season. The number of patients enrolled in the network and included in the final analytic data set are shown, detailing each exclusion criterion applied. The Delta-predominant period was from 1 October to 24 December 2021 and the Omicron-predominant period from 25 December 2021 to 29 June 2022.
Table 1.
Characteristics of COVID-19 Cases and SARS-CoV-2 Test-Negative Controls With Acute Respiratory Illness by SARS-CoV-2 Molecular Test Result
| Delta Perioda | Omicron Periodb | |||||
|---|---|---|---|---|---|---|
| Characteristic | Test-Negative Controls (n = 416) | Cases (n = 87) | P Valuec | Test-Negative Controls (n = 940) | Cases (n = 575) | P Valued |
| Age, y | 34 (1–83) | 38 (7–72) | .699 | 38 (3–82) | 40 (5–82) | .006 |
| Sexe | .779 | .245 | ||||
| Female | 250 (60) | 51 (59) | 623 (66) | 364 (63) | ||
| Male | 165 (40) | 36 (41) | 316 (34) | 210 (37) | ||
| Race/ethnicityf | .240 | .009 | ||||
| White, non-Hispanic | 250 (60) | 54 (64) | 597 (64) | 321 (57) | ||
| Black, non-Hispanic | 18 (4.3) | 6 (7.1) | 38 (4.1) | 23 (4.1) | ||
| Asian, non-Hispanic | 38 (9.2) | 7 (8.2) | 69 (7.4) | 71 (13) | ||
| Other, non-Hispanic | 16 (3.9) | 6 (7.1) | 37 (4.0) | 26 (4.6) | ||
| Hispanic | 92 (22) | 12 (14) | 191 (20) | 123 (22) | ||
| Days from symptom onset | 3 (0–5) | 2 (0–5) | .239 | 2 (0–5) | 2 (0–5) | <.001 |
| COVID-19 vaccination status | .029 | .660 | ||||
| Unvaccinated | 82 (20) | 28 (32) | 160 (17) | 85 (15) | ||
| 2 doses | 234 (56) | 45 (52) | 233 (25) | 150 (26) | ||
| 3 doses | 100 (24) | 14 (16) | 521 (55) | 326 (57) | ||
| 4 doses | 0 (0) | 0 (0) | 26 (2.8) | 14 (2.4) | ||
| Evidence of prior SARS-CoV-2 infectiong | .002 | <.001 | ||||
| No | 262 (63) | 70 (80) | 345 (37) | 333 (58) | ||
| Yes | 154 (37) | 17 (20) | 595 (63) | 242 (42) | ||
| Self-reported presence of ≥1 medical conditionh | 92 (23) | 23 (28) | .335 | 257 (28) | 142 (25) | .243 |
| Health care worker or close contact with confirmed COVID-19 case | 115 (28) | 41 (47) | <.001 | 406 (43) | 314 (55) | <.001 |
| Study site | .046 | <.001 | ||||
| California | 194 (47) | 37 (43) | 243 (26) | 207 (36) | ||
| Pennsylvania | 93 (22) | 29 (33) | 132 (14) | 57 (9.9) | ||
| Tennessee | 52 (12) | 8 (9.2) | 83 (8.8) | 46 (8.0) | ||
| Texas | 50 (12) | 4 (4.6) | 201 (21) | 83 (14) | ||
| Wisconsin | 27 (6.5) | 9 (10) | 72 (7.7) | 27 (4.7) | ||
| Michigan | 0 (0) | 0 (0) | 76 (8.1) | 51 (8.9) | ||
| Washington | 0 (0) | 0 (0) | 133 (14) | 104 (18) | ||
Data are presented as median (range) or No. (%).
aThe Delta-predominant period was defined as 1 October to 24 December 2021.
bThe Omicron-predominant period was defined as 25 December 2021 to 29 June 2022.
cWilcoxon rank sum test, Pearson χ2 test, Fisher exact test.
dWilcoxon rank sum test, Pearson χ2 test.
eMissing sex, n = 3.
fMissing race/ethnicity, n = 23.
gEvidence of prior SARS-CoV-2 infection was defined as electronic medical record documentation of prior positive SARS-CoV-2 test results or anti-nucleocapsid binding antibody levels in acute sera indicative of prior infection (≥6.9 binding antibody units/mL). Prior infection was documented from 17 March 2020 to 12 June 2022.
hMissing self-reported presence of ≥1 medical condition, n = 63.
During the Delta- and Omicron-predominant periods, COVID-19 cases had lower anti-RBD and anti-N bAb concentrations as compared with test-negative controls (Table 2). Anti-RBD GMCs increased by number of COVID-19 mRNA vaccine doses and decreased by time since COVID-19 vaccination among cases and test-negative controls (Supplementary Figure 3). Higher anti-RBD bAb concentrations were also associated with evidence of prior SARS-CoV-2 infection.
Table 2.
Geometric Mean Concentrations for Anti-SARS-CoV-2 RBD and N Antigens by COVID-19 Case Status, COVID-19 Vaccination Status, and Laboratory-Confirmed Prior SARS-CoV-2 Infection
| Spike Protein RBD | N Protein | |||||||
|---|---|---|---|---|---|---|---|---|
| Test-Negative Control | SARS-CoV-2 Case | Test-Negative Control | SARS-CoV-2 Case | |||||
| Variable | Antibody Response a / Tested, No. (%) | Geometric Mean Concentration (95% CI) | Antibody Response a / Tested, No. (%) | Geometric Mean Concentration (95% CI) | Antibody Response b / Tested, No. (%) | Geometric Mean Concentration (95% CI) | Antibody Response b / Tested, No. (%) | Geometric Mean Concentration (95% CI) |
| Delta period | 381/416 (92) | 497.8 (398.6–621.6) | 63/87 (72) | 112.1 (57.9–217.2) | 148/416 (36) | 4.0 (3.3–4.8) | 17/87 (20) | 2.1 (1.5–3.0) |
| COVID-19 vaccination status | ||||||||
| Unvaccinated | 58/82 (71) | 67.7 (35.3–130.1) | 5/28 (18) | 3.1 (1.3–7.4) | 33/82 (41) | 5.3 (3.0–9.4) | 4/28 (14) | 1.2 (.7–2.1) |
| 2 doses | 226/234 (97) | 494.1 (395.5–616.7) | 45/45 (100) | 468.3 (319.2–687.1) | 63/234 (27) | 2.8 (2.2–3.5) | 7/45 (16) | 2.1 (1.2–3.6) |
| 3 doses | 97/100 (97) | 2600.4 (1960.9–3448.6) | 13/14 (93) | 1522.0 (411.0–5635.9) | 52/100 (52) | 7.1 (5.8–8.9) | 6/14 (43) | 7.0 (3.3–15.0) |
| 4 doses | … | … | … | … | … | … | … | … |
| Time since last COVID-19 vaccination, d | ||||||||
| <90 | 103/105 (98) | 2800.3 (2244.1–3494.4) | 15/16 (94) | 1463.9 (474.1–4519.8) | 56/105 (53) | 7.3 (5.9–9.1) | 7/16 (44) | 7.5 (3.3–17.1) |
| ≥90 | 220/229 (96) | 460.6 (365.9–579.9) | 43/43 (100) | 449.8 (302.4–669.0) | 59/229 (26) | 2.7 (2.2–3.4) | 6/43 (14) | 1.9 (1.1–3.3) |
| Evidence of prior SARS-CoV-2 infection | ||||||||
| Yes | 154/154 (100) | 1903.7 (1560.3–2322.6) | 16/17 (94) | 1093.8 (359.2–3330.4) | … | … | … | … |
| No | 227/262 (87) | 226.3 (168.6–303.7) | 47/70 (67) | 64.5 (31.1–133.5) | … | … | … | … |
| Omicron period | 886/940 (94) | 1189.0 (1049.7–1346.8) | 530/575 (92) | 822.7 (689.9–981.1) | 580/940 (62) | 15.5 (13.6–17.8) | 228/575 (40) | 5.7 (5.0–6.5) |
| COVID-19 vaccination status | ||||||||
| Unvaccinated | 115/160 (72) | 115.8 (74.0–181.2) | 50/85 (59) | 41.8 (21.8–80.3) | 106/160 (66) | 29.5 (19.0–45.9) | 38/85 (45) | 6.2 (3.8–10.2) |
| 2 doses | 231/233 (99) | 1323.6 (1115.3–1570.8) | 143/150 (95) | 522.5 (394.0–693.0) | 141/233 (61) | 17.4 (12.9–23.4) | 39/150 (26) | 3.2 (2.4–4.2) |
| 3 doses | 514/521 (99) | 2192.0 (1977.2–2427.8) | 323/326 (99) | 2078.0 (1860.3–2321.1) | 316/521 (61) | 12.3 (10.6–14.3) | 142/326 (44) | 7.0 (6.0–8.1) |
| 4 doses | 26/26 (100) | 3658.7 (3112.4–4301.0) | 14/14 (100) | 3250.3 (2389.2–4421.9) | 17/26 (65) | 11.3 (7.8–16.4) | 9/14 (64) | 14.7 (5.9–36.8) |
| Time since last COVID-19 vaccination, d | ||||||||
| <90 | 198/202 (98) | 2760.8 (2345.4–3249.8) | 89/91 (98) | 2660.4 (2020.3–3503.2) | 149/202 (74) | 17.0 (13.6–21.1) | 56/91 (62) | 10.6 (8.0–14.2) |
| ≥90 | 573/578 (99) | 1687.8 (1524.9–1868.1) | 391/399 (98) | 1187.4 (1033.1–1364.7)) | 325/578 (56) | 12.6 (10.7–14.8) | 134/399 (34) | 4.9 (4.2–5.7) |
| Evidence of prior SARS-CoV-2 infectionc | ||||||||
| Yes | 577/595 (97) | 1976.5 (1756.0–2224.8) | 233/242 (96) | 1689.0 (1363.2–2092.6) | … | … | … | … |
| No | 309/345 (90) | 494.9 (387.0–632.9) | 297/333 (89) | 487.8 (381.0–624.6) | … | … | … | … |
Geometric mean concentrations are presented as BAU/mL.
Abbreviations: bAb, binding antibody; BAU, binding antibody units; N, nucleocapsid; RBD, receptor-binding domain.
aTetracore cutoff (anti-RBD BAU/mL) = 15.9.
bTetracore cutoff (anti-N BAU/mL) = 6.9.
cEvidence of prior SARS-CoV-2 infection was defined as electronic medical record documentation of prior positive SARS-CoV-2 test results or anti-N bAb levels in acute sera indicative of prior infection (≥6.9 BAU/mL). Prior infection was documented from 17 March 2020 to 12 June 2022. A total of 253 individuals had electronic medical record documentation of prior positive SARS-CoV-2 test results. Anti-N bAb–level measurements are not shown for this variable because anti-N bAb levels were included in the calculation of prior infection.
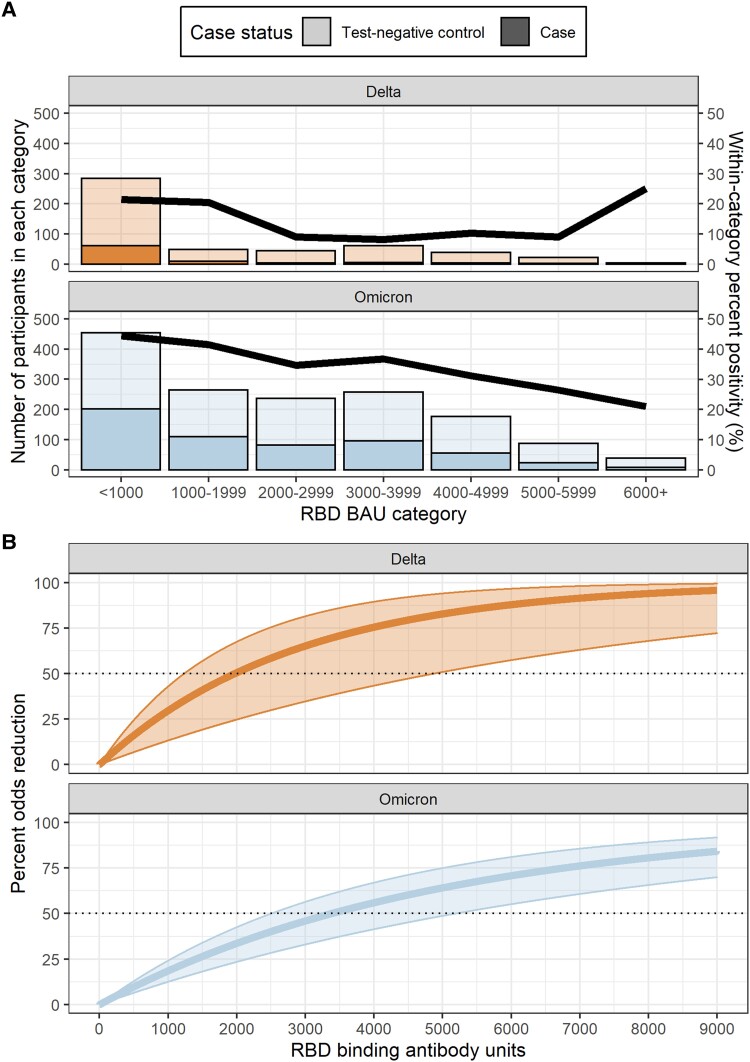
During both variant periods, relative odds of COVID-19 cases were lower among patients with higher acute-phase anti-RBD bAb concentrations (Figure 2A). However, anti-RBD bAb concentration thresholds for 50% lower odds of COVID-19 with Delta variant infection (1968 BAU/mL) were below those for Omicron variant–associated illness (3375 BAU/mL; Figure 2B). Larger percentages of COVID-19 cases and test-negative controls with moderate vs high anti-N bAb concentrations had anti-RBD bAb levels above the 50% threshold associated with reduced odds of illness, and percentages of cases and controls above the 50% threshold rose with the number of COVID-19 mRNA vaccine doses received and evidence of prior SARS-CoV-2 infection (Table 3).
Figure 2.
Association between SARS-CoV-2 antispike receptor-binding domain (RBD) immunoglobulin G antibodies and likelihood of symptomatic COVID-19. A, Bars indicate the number of COVID-19 cases (darker shading) and test-negative controls (lighter shading) within each anti-RBD binding antibody unit (BAU) category. The line represents SARS-CoV-2 real-time reverse-transcription polymerase chain reaction test positivity within each anti-RBD binding antibody category. Results are stratified by variant period: Delta (orange) and Omicron (blue). The Delta-predominant period was from 1 October to 24 December 2021 and the Omicron period from 25 December 2021 to 29 June 2022. B, The percentage odds reduction in COVID-19 illness by anti-RBD binding antibody level is stratified by variant period: Delta (orange) and Omicron (blue). Percentage odds reduction was estimated as (1 – adjusted odds ratio) × 100 per the adjusted odds ratio produced by a logistic regression model adjusted for COVID-19 vaccination status, age, study site, illness onset week, and high-risk SARS-CoV-2 exposure.
Table 3.
Likelihood of Symptomatic COVID-19 by Anti-N Binding Antibody Levels, COVID-19 Vaccination Status, and Prior SARS-CoV-2 Infection
| Delta Perioda | Omicron Periodb | |||||
|---|---|---|---|---|---|---|
| Variable | Cases/Total | Anti-RBD bAb ≥1968 BAU/mLc/ Controls | Anti-RBD bAb ≥1968 BAU/mLc/ Cases | Cases/Total | Anti-RBD bAb ≥3375 BAU/mLd/ Controls | Anti-RBD bAb ≥3375 BAU/mLd/ Cases (%) |
| Anti-N bAb levels | ||||||
| Low, <10 BAU/mL | 76/389 (20) | 83/313 (27) | 10/76 (13) | 402/847 (47) | 84/445 (19) | 71/402 (18) |
| Medium, 10–99 BAU/mL | 7/79 (9) | 54/72 (75) | 4/7 (57) | 133/430 (31) | 156/297 (53) | 64/133 (48) |
| High, ≥100 BAU/mL | 4/35 (11) | 19/31 (61) | 2/4 (50) | 40/238 (17) | 84/198 (42) | 16/40 (40) |
| COVID-19 vaccination status | ||||||
| Unvaccinated | 28/110 (25) | 13/82 (16) | 1/28 (4) | 85/245 (35) | 14/160 (9) | 6/85 (7) |
| 2 doses | 45/279 (16) | 56/234 (24) | 6/45 (13) | 150/383 (39) | 59/233 (25) | 14/150 (9) |
| 3 doses | 14/114 (12) | 87/100 (87) | 9/14 (64) | 326/847 (38) | 233/521 (45) | 122/326 (37) |
| 4 doses | … | … | … | 14/40 (35) | 18/26 (69) | 9/14 (64) |
| Evidence of prior SARS-CoV-2 infectione | ||||||
| No | 70/332 (21) | 50/262 (19) | 6/70 (9) | 333/678 (49) | 28/345 (8) | 41/333 (12) |
| Yes | 17/171 (10) | 106/154 (69) | 10/17 (59) | 242/837 (29) | 296/595 (50) | 110/242 (45) |
Data are presented as No. (%).
Abbreviations: bAb, binding antibody; N, nucleocapsid; RBD, receptor-binding domain.
aThe Delta-predominant period was defined as 1 October to 24 December 2021.
bThe Omicron-predominant period was defined as 25 December 2021 to 29 June 2022.
c50% reduction in odds of symptomatic COVID-19 cutoff for anti-RBD binding antibody levels during the Delta period: 1968 BAU/mL.
d50% reduction in odds of symptomatic COVID-19 cutoff for anti-RBD binding antibody levels during the Omicron period: 3375 BAU/mL.
eEvidence of prior SARS-CoV-2 infection was defined as electronic medical record documentation of prior positive SARS-CoV-2 test results or anti-N bAb levels in acute sera indicative of prior infection (≥6.9 BAU/mL). Prior infection was documented from 17 March 2020 to 12 June 2022. Anti-N bAb-level measurements are not shown for this variable because anti-N bAb levels were included in the calculation of prior infection.
A total of 104 COVID-19 cases enrolled during the Omicron variant–predominant period had blood specimens collected during acute and convalescent phases of illness. At enrollment, 27 (26%) patients had anti-RBD bAb concentrations ≥3375 BAU/mL, associated with 50% reduction in odds of COVID-19 (GMC, 1257.8; 95% CI, 923.9–1712.3). In convalescent specimens collected 21 to 56 days later, 73 (27%) patients had anti-RBD bAb concentrations ≥3375 BAU/mL (GMC, 3188.5; 95% CI, 2638.7–3853.0). GMCs of anti-N bAbs in acute- and convalescent-phase specimens increased from 5.5 BAU/mL (95% CI, 4.3–7.1) to 259.4 BAU/mL (95% CI, 200.6–335.4).
DISCUSSION
In this observational study, symptomatic patients with higher levels of IgG antibodies against SARS-CoV-2 spike and nucleocapsid proteins were less likely to have laboratory-confirmed COVID-19 than those with lower antibody levels against either protein. Patients with anti-RBD bAb concentrations ≥1968 BAU/mL in the acute phase of illness had 50% lower odds of testing positive for COVID-19 associated with the Delta variant as compared with those with antibody levels below this threshold. Against Omicron variant–associated SARS-CoV-2 infection, bAb levels against ancestral spike RBD ≥3375 BAU/mL correlated to 50% lower odds of laboratory-confirmed COVID-19.
Using SARS-CoV-2 test-negative control data collected from the test-negative design, we found that a higher concentration of anti-RBD IgG antibodies was correlated with lower odds of COVID-19 illness [21]. Studies from COVID-19 vaccine trials have correlated anti–SARS-CoV-2 bAb levels against ancestral spike and RBD antigens with virus-neutralizing antibody levels, which likely play a key role in protection [3]. The test-negative design provides efficient enrollment of patients with laboratory-confirmed illness (depending on the proportion of COVID-19 illness among patients seeking care) and an uninfected comparison group of patients seeking care for similar illness [8, 22]. The design can be limited in that differences in antibody levels between vaccinated and unvaccinated individuals could be due to confounders other than vaccination, and it assumes that acute-phase antibodies estimate preexisting antibody levels and minimal anamnestic response. We accounted for these limitations by controlling for confounding covariates in models and limiting analysis to specimens collected within 5 days of symptom onset. While distributions of anti-RBD IgG antibody concentrations in the current study largely overlapped between COVID-19 cases and test-negative controls, higher antibody levels were associated with a lower likelihood of COVID-19 illness. In addition, among cases with specimens collected during convalescence, >70% had anti-RBD bAb concentrations above the 50% threshold for lower odds of infection. These results suggest that test-negative studies may provide a means of estimating correlates of protection as new SARS-CoV-2 variants emerge.
The role that anti-N bAbs play in protection against COVID-19 is less clear. COVID-19 cases and test-negative controls with high anti-RBD bAb levels did not always have high anti-N levels; this could be due to differences in timing of prior infection relative to vaccination [10, 11], as anti-N antibody responses to infection have been observed to differ among vaccinated vs unvaccinated persons [15]. We were unable to assess time from most recent SARS-CoV-2 infection for all patients; however, anti-N antibody levels may also reflect a shorter time interval between prior and current SARS-CoV-2 infection. Future studies are needed to assess the role that anti-N antibodies play in protection against COVID-19.
The current analysis was aided by the collection of DBS specimens from symptomatic patients at the time of clinical presentation. In a previous analysis, the presence of anti-N antibody in acute-phase blood spot specimens classified 5 times as many patients with prior SARS-CoV-2 infection as those that were self-reported or documented in electronic medical records [12, 13]. DBS specimens were recognized early in the COVID-19 pandemic as alternatives to venous blood collection for anti–SARS-CoV-2 binding assays [17, 23, 24]. Self-collected DBSs that could be shipped by mail facilitated SARS-CoV-2 seroprevalence studies [25–32] and longitudinal household cohort studies [27, 33]. In our study, the bAb concentrations against ancestral spike RBD and N antigens quantified from acute-phase DBSs that correlated with 50% lower odds of COVID-19 were consistent with those reported from COVID-19 vaccine trials based on standardized Meso Scale Diagnostics quantitative binding assays [1, 3, 5, 22]. Studies evaluating bAb from DBS and serum specimen types may provide additional tools for evaluating correlates of protection against future SARS-CoV-2 variants [2, 5, 34].
These findings are subject to several limitations. First, results are limited to mild to moderate ambulatory illness as well as the population that has access to care and would seek care when ill. Immune markers associated with protection against severe disease should be investigated. Second, few individuals received 4 COVID-19 mRNA vaccine doses, and small sample sizes limited our ability to compare 50% thresholds for a reduction in odds of COVID-19 stratified by vaccine doses. In addition, exposures to SARS-CoV-2 and preventive measures may differ among COVID-19 cases and patients uninfected by SARS-CoV-2, and residual confounding may remain after controlling for high-risk exposure. Except for the subset of 104 paired acute and convalescent samples, DBSs used in this study were collected at 1 time point during acute illness. Acute-phase antibody titers may reflect early antibody rise in some individuals, resulting in an overestimation of the antibody response at the time of infection for cases; however, analyses were restricted to acute-phase specimens collected within 5 days of symptom onset to limit the influence of early antibody rise. Use of DBSs in this multiplex microsphere assay was previously validated against qualitative serologic assays [19] but not against standardized assays widely used to quantify bAb levels [35]. Antibody levels were assessed against ancestral RBD and N antigens rather than against antigens representative of SARS-CoV-2 variants circulating at the time of infection. Threshold antibody concentrations to achieve 50% protection will vary depending on IgG affinity for SARS-CoV-2 variant virus antigens. This study was designed to assess applicability of the test-negative study design to interrogate antibody levels associated with SARS-CoV-2–associated illness. Validation of specimen types used in standardized serologic assays is needed.
Overall, these results suggest a role for observational studies designed to assess VE in evaluating immune correlates of protection. Standardization of serologic assays and ongoing immunogenicity studies with well-characterized sera will be needed to update correlates of protection against circulating SARS-CoV-2 variants and facilitate approval of new vaccines [4]. With multiple licensed and recommended COVID-19 vaccines, observational studies incorporating immune markers can complement immunogenicity studies in evaluation of relative VE.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Kelsey M Sumner, US Centers for Disease Control and Prevention, Atlanta, Georgia; Epidemic Intelligence Service, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Ruchi Yadav, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Emma K Noble, US Centers for Disease Control and Prevention, Atlanta, Georgia; Oak Ridge Institute for Science and Education, Oak Ridge, Tennessee.
Ryan Sandford, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Devyani Joshi, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Sara Y Tartof, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
Karen J Wernli, Kaiser Permanente Washington Health Research Institute, Seattle, Washington.
Emily T Martin, School of Public Health, University of Michigan, Ann Arbor, Michigan.
Manjusha Gaglani, Baylor Scott & White Health, Temple, Texas; Baylor College of Medicine–Temple, Temple, Texas; College of Medicine, Texas A&M University, Temple, Texas.
Richard K Zimmerman, University of Pittsburgh, Pittsburgh, Pennsylvania.
H Keipp Talbot, Vanderbilt University Medical Center, Nashville, Tennessee.
Carlos G Grijalva, Vanderbilt University Medical Center, Nashville, Tennessee.
Edward A Belongia, Marshfield Clinic Research Institute, Marshfield, Wisconsin.
Jessie R Chung, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Eric Rogier, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Melissa M Coughlin, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Brendan Flannery, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Notes
Acknowledgments. We acknowledge Hannah Berger, Gina Burbey, Brianna Freund, Jennifer King, Tamara Kronenwetter-Koepel, Karen McGreevey, Vicki Moon, Carla Rottscheit, and Kelly Scheffen from the Marshfield Clinic; Bruno Lewin, Ana Florea, Jennifer Ku, Vennis Hong, Harp Takhar, Sally Shaw, Jeniffer Kim, Britta Amundsen, Ashley McDaniel, Raul Calderon, Gabriela Jimenez, Alicia Torres, Alexandria Reyes, Korina Chen, and Susie Flores from Kaiser Permanente Department of Health Research Science; C. Hallie Phillips, Erika Kiniry, Stacie Wellwood, Kathryn Moser, Brianna Wickersham, Matt Nguyen, Rachael Doud, and Suzie Park from Kaiser Permanente Washington Health Research Institute; Dayna Wyatt, Stephanie Longmire, Meredith Denny, Zhouwen Liu, and Yuwei Zhu of Vanderbilt University Medical Center; Michael Smith, Chandni Raiyani, Kayan Dunnigan, Kempapura Murthy, Mufaddal Mamawala, Amanda McKillop, Eric Hoffman, Martha Zayed, Ashley Graves, Kimberley Walker, Marcus Volz, Arundhati Rao, Manohar Mutnal, Michael Reis, Tresa McNeal, Keith Stone, Jason Ramm, Madhava Beeram, Sharla Russell, Jeremy Ray, Deborah Price, Jason Ettlinger, Courtney Shaver, Monica Bennett, Elisa Priest, Natalie Settele, Jennifer Thomas, and Alejandro Arroliga from Baylor Scott & White Health; and Sara S. Kim and Manish Patel of the US CDC.
Data availability. All data produced in the present study are available upon reasonable request to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US CDC. Some authors are federal employees of the US government, and this work was prepared as part of their official duties. Title 17 USC 105 provides that “copyright protection under this title is not available for any work of the United States Government.” All authors have reviewed and approved of this version of the manuscript.
Financial support. This work was supported by the US Centers for Disease Control and Prevention (grants 75D30121C11529, 75D30121C12339, 75D30121C12246, 75D30121C11513, 75D30121C12279, 75D30121C11909, 75D30121C11519); National Institutes of Health (grant UL1TR001857); and National Center for Advancing Translational Sciences (Clinical Translational Science Award 5UL1TR002243-03).
References
- 1. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 3. Benkeser D, Montefiori DC, McDermott AB, et al. Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial. Sci Transl Med 2023; 15:eade9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research . Emergency use authorization for vaccines to prevent COVID-19: guidance for industry: appendix 2. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19. Accessed 12 June 2023.
- 5. Khoury DS, Docken SS, Subbarao K, Kent SJ, Davenport MP, Cromer D. Predicting the efficacy of variant-modified COVID-19 vaccine boosters. Nat Med 2023; 29:574–8. [DOI] [PubMed] [Google Scholar]
- 6. Dean NE, Hogan JW, Schnitzer ME. COVID-19 vaccine effectiveness and the test-negative design. N Engl J Med 2021; 385:1431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014; 13:1571–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Follmann DA, Dodd L. Immune correlates analysis using vaccinees from test negative designs. Biostatistics 2022; 23:507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Interim guidelines for COVID-19 antibody testing. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html. Accessed 31 August 2023.
- 11. Fox T, Geppert J, Dinnes J, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2022; 11:CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SS, Chung JR, Talbot HK, et al. Effectiveness of two and three mRNA COVID-19 vaccine doses against Omicron- and Delta-related outpatient illness among adults, October 2021–February 2022. Influenza Other Respir Viruses 2022; 16:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tartof SY, Xie F, Yadav R, et al. Prior SARS-CoV-2 infection and COVID-19 vaccine effectiveness against outpatient illness during widespread circulation of SARS-CoV-2 Omicron variant, US Flu VE Network. Influenza Other Respir Viruses 2023; 17:e13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambrou AS, Shirk P, Steele MK, et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) variants—United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Follmann D, Janes HE, Chu E, et al. Kinetics of the antibody response to symptomatic SARS-CoV-2 infection in vaccinated and unvaccinated individuals in the blinded phase of the mRNA-1273 COVID-19 vaccine efficacy trial. Open Forum Infect Dis 2023; 10:ofad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulchandani R, Brown B, Brooks T, et al. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys high throughput immunoassay. J Clin Virol 2021; 136:104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sims MD, Podolsky RH, Childers KL, et al. Dried blood spots are a valid alternative to venipuncture for COVID-19 antibody testing. J Immunol Methods 2023; 513:113420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zava TT, Zava DT. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis 2021; 13:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell KF, Carlson CM, Nace D, et al. Evaluation of a multiplex bead assay against single-target assays for detection of IgG antibodies to SARS-CoV-2. Microbiol Spectr 2022; 10:e0105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhuo R, Charlton C, Plitt S, et al. Comparison of SARS-CoV-2 spike antibody quantitative titer reporting using the World Health Organization international standard units by four commercial assays. J Clin Virol 2022; 156:105292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benkeser D, Fong Y, Janes HE, et al. Immune correlates analysis of a phase 3 trial of the AZD1222 (ChAdOx1 nCoV-19) vaccine. NPJ Vaccines 2023; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cholette F, Mesa C, Harris A, et al. Dried blood spot specimens for SARS-CoV-2 antibody testing: a multi-site, multi-assay comparison. PLoS One 2021; 16:e0261003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turgeon CT, Sanders KA, Rinaldo P, et al. Validation of a multiplex flow immunoassay for detection of IgG antibodies against SARS-CoV-2 in dried blood spots. PLoS One 2021; 16:e0252621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown L, Byrne RL, Fraser A, et al. Self-sampling of capillary blood for SARS-CoV-2 serology. Sci Rep 2021; 11:7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cowgill KD, Erosheva EA, Elder A, Miljacic L, Buskin S, Duchin JS. Anti-SARS-CoV-2 seroprevalence in King County, WA—cross-sectional survey, August 2020. PLoS One 2022; 17:e0272783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Hoog MLA, Sluiter-Post JGC, Westerhof I, et al. Longitudinal household assessment of respiratory illness in children and parents during the COVID-19 pandemic. JAMA Netw Open 2022; 5:e2237522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karp DG, Danh K, Espinoza NF, Seftel D, Robinson PV, Tsai CT. A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots. Sci Rep 2020; 10:20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matias WR, Fulcher IR, Sauer SM, et al. Disparities in SARS-CoV-2 infection by race, ethnicity, language, and social vulnerability: evidence from a citywide seroprevalence study in Massachusetts, USA. J Racial Ethn Health Disparities 2023; 11:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roxhed N, Bendes A, Dale M, et al. Multianalyte serology in home-sampled blood enables an unbiased assessment of the immune response against SARS-CoV-2. Nat Commun 2021; 12:3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang X, Sharma A, Pasic M, et al. Assessment of SARS-CoV-2 seropositivity during the first and second viral waves in 2020 and 2021 among Canadian adults. JAMA Netw Open 2022; 5:e2146798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valentine-Graves M, Hall E, Guest JL, et al. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process and patient confidence in specimens. PLoS One 2020; 15:e0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oelsner EC, Krishnaswamy A, Balte PP, et al. Collaborative cohort of cohorts for COVID-19 research (C4R) study: study design. Am J Epidemiol 2022; 191:1153–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A COVID-19 milestone attained—a correlate of protection for vaccines. N Engl J Med 2022; 387:2203–6. [DOI] [PubMed] [Google Scholar]
- 35. Khoury DS, Schlub TE, Cromer D, et al. Correlates of protection, thresholds of protection, and immunobridging among persons with SARS-CoV-2 infection. Emerg Infect Dis 2023; 29:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.