Abstract
The blood-brain barrier (BBB) plays a critical role in maintaining ion and fluid homeostasis, essential for brain metabolism and neuronal function. Regulation of nutrient, water, and ion transport across the BBB is tightly controlled by specialized ion transporters and channels located within its unique cellular components. These dynamic transport processes not only influence the BBB’s structure but also impact vital signaling mechanisms, essential for its optimal function. Disruption in ion, pH, and fluid balance at the BBB is associated with brain pathology and has been implicated in various neurological conditions, including stroke, epilepsy, trauma, and neurodegenerative diseases such as Alzheimer’s disease (AD). However, knowledge gaps exist regarding the impact of ion transport dysregulation on BBB function in neurodegenerative dementias. Several factors contribute to this gap: the complex nature of these conditions, historical research focus on neuronal mechanisms and technical challenges in studying the ion transport mechanisms in in vivo models and the lack of efficient in vitro BBB dementia models. This review provides an overview of current research on the roles of ion transporters and channels at the BBB and poses specific research questions: 1) How are the expression and activity of key ion transporters altered in AD and vascular dementia (VaD); 2) Do these changes contribute to BBB dysfunction and disease progression; and 3) Can restoring ion transport function mitigate BBB dysfunction and improve clinical outcomes. Addressing these gaps will provide a greater insight into the vascular pathology of neurodegenerative disorders.
Keywords: Alzheimer’s disease, Blood-brain barrier, Ion channels, Ion transporters, Vascular dementia
1. Introduction
The blood brain barrier (BBB) is a highly specialized physical and biochemical barrier that regulates the exchange of substances between the brain and the peripheral circulation [1-3]. Its restrictive and selective permeability to substances prevents rapid ionic or metabolic changes within the brain [4]. Unlike a single physical structure, the BBB is a result of functional association between endothelial cells (ECs), basal lamina, pericytes, and astrocytic perivascular endfeet that serves to maintain the structural integrity and ion homeostasis within the brain [1, 4].
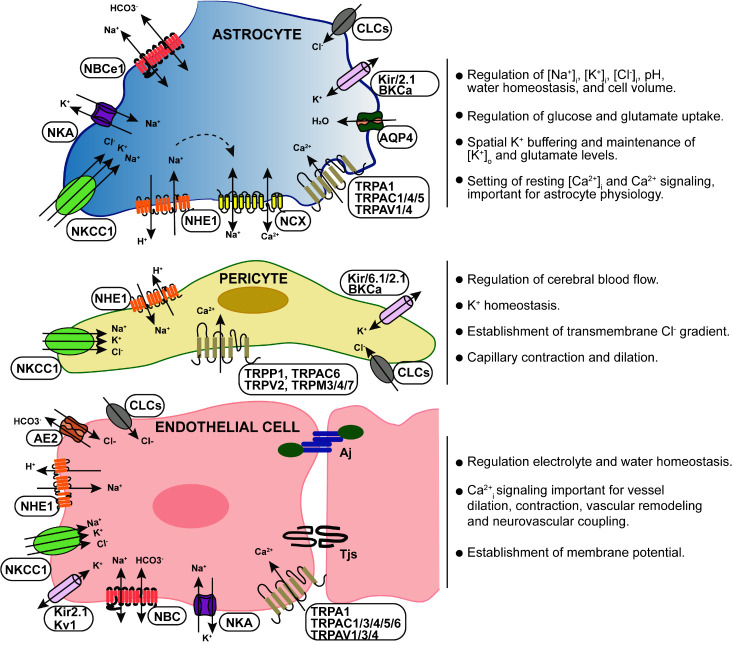
The BBB plays a crucial role in the sustained regulation of ionic composition in the brain’s interstitial fluid [4, 5]. For example, Na+, K+, Ca2+, Cl-, and HCO3- are the major ions in the central nervous system (CNS), which should be kept at an optimal level for neural and synaptic signaling functions [6, 7]. These ions exhibit an asymmetric distribution between luminal and abluminal membranes, with plasma concentrations of Na+ (148-155); K+ (3.9-4.6); Cl- (113-114); Ca2+ (2.5), and cerebrospinal fluid (CSF) Na+ (152-156); K+ (2.99-3); Cl- (126-129); Ca2+ (1.2-2.0) at mmol/ liter of H2O [4]. The transport of these ions across the BBB is tightly regulated by the function of ion channels and transporters within the BBB [6, 7] (Table 1). Importantly, ECs, pericytes, smooth muscle cells and astrocytes express different transporters, receptors, active efflux pumps, ion channels and regulatory molecules. This multifaceted expression allows for the selective transport of ions across the BBB, thereby maintaining ionic balance in the brain [8-10] (Fig. 1). Disruption of ionic gradients at the cellular level can have significant effects on brain function, potentially leading to damage or dysfunction [6, 7, 11].
Table 1.
Key ion channels and transporters within BBB and their function.
| Ion channel/Transporter | Transported ion | Localization | Main function at BBB | ||
|---|---|---|---|---|---|
| ECs | Astrocytes | Pericyte | |||
| Na+-K+-ATPase (NKA) | Na+, K+ | + | + | + | K+ transport, maintenance of [Na+] gradient at the BBB, H2O homeostasis; Regulation of glycolysis and glutamate uptake |
| K+ channels | K+ | + | + | + | Regulation of membrane potential, K+ buffering, CBF and neurovascular coupling |
| Chloride channels | Cl- | + | + | ? | Cl- transport; cell volume regulation |
| TRP Channels | Ca2+ | + | + | + | Regulation of Ca2+ influx, vascular tone, and CBF |
| NHE1 | Na+, H+ | + | + | ? | Removal of intracellular H+; pH regulation |
| NBC | Na+, HCO3- | + | + | ? | pH and HCO3- regulation |
| NKCC1 | Na+, K+, Cl- | + | + | ? | Regulation of Cl-, and K+, homeostasis; H2O transport |
| NCX | Na+, Ca2+ | + | + | ? | Mediates Ca2+ efflux and maintains cellular Ca2+ homeostasis |
| AQP4 | H2O | - | + | - | Regulation of H2O homeostasis |
| AE2 | Cl-, HCO3- | + | ? | ? | Acid loaders; pH regulation |
Figure 1.
Key blood-brain barrier ion transport systems. Ion transporters and channels expressed within the BBB mediates the movement of Na+, K+, Cl-, HCO3-, H+ and Ca2+ into and out of the BBB specific cells. The BBB ion transport is important for regulation of key ionic concentrations in the brain (H+, K+, Ca2+), the uptake and extrusion of trace metals, fluid secretion, intracellular volume, pH and Ca2+ homeostasis.
Many cerebrovascular pathologies are closely associated with BBB dysfunction, where changes at the BBB can lead to or enhance disease development [12, 13]. Emerging new studies indicate that dysfunction in ion transport at the BBB is not only associated with acute brain injuries but also plays a role in various neurological disorders, including stroke, epilepsy, multiple sclerosis, vascular dementia (VaD), and Alzheimer’s disease (AD) [14-16]. Therefore, understanding the mechanisms behind ion transport dysregulation at the BBB is crucial for the development of new therapeutic strategies aimed at addressing various brain diseases. In this review, we present the current knowledge concerning ion transporters and channels expressed by the BBB specific perivascular cells, including their molecular composition, and function in the context of neurological disorders. We also shed light on unanswered questions related to ion transport dysregulation as potential therapeutic targets for neurodegenerative diseases.
2. Overview of the BBB structure and functions
The BBB is formed by a monolayer of tightly sealed microvascular ECs that constitute the walls of the vessels. The abluminal side of the endothelium is covered by a 30-40 nm thick basal lamina, mural cells (such as vascular smooth muscle cells and pericytes), and astrocytic endfeet [1, 2]. Additionally, perivascular macrophages reside between the vascular cells and astrocytic endfeet, providing immune surveillance [2]. ECs are the central elements of the microvasculature that form the BBB. They are characterized by a flattened structure, expression of inter endothelial tight junctions (TJs), and the presence of very few caveolae at the luminal surface [1]. These features contribute to the maintenance of integrity, barrier function, and cellular communication at the BBB. The paracellular transport of substances from blood across the endothelium is regulated by the junctional complexes including TJs, adherens junctions (AJs), and gap junctions that holds the ECs together [2, 17]. The junctional complexes are highly interdependent in regulating EC permeability. The TJs are composed of transmembrane proteins claudin (claudin-1, -3, -5, and -12), occludin, junctional adhesion molecules (A, B, and C) and membrane-associated guanylate kinase protein family zonula occludens (ZO-1, -2, and -3) [18, 19].
The TJs are important to regulate the permeability of the BBB via sealing the paracellular space, preventing diffusion of molecules between the ECs. The claudins, specifically CLDN-5, are the key structural components of the TJs, that contributes to the reduced paracellular ion movement [20]. Mice deficient in CLDN-5 exhibit a selective disruption of the BBB, and increased permeation of tracers less than 1 kDa [21]. Occludin is also present in the filaments of TJs and helps regulate adhesion properties between cells as well as interacting with the inner cellular scaffolding proteins and the actin cytoskeleton [22]. Studies have indicated that downregulation of CLDN-5 and occludin results in decreased barrier tightness and enhanced monocyte migration across the BBB [23]. Loss of CLDN-5 and occludin in the cerebral cortex is found to be associated with synaptic degeneration in post-mortem brains of AD patients [24]. ZOs interact with claudins, occludin, and JAMs to anchor the membrane proteins, tethering them to the actin cytoskeleton [25]. Severe decreases in retinal vascular ZO-1 correlating with increased BBB permeability and abundant arteriolar Aβ40 deposition were identified in AD patients [26].
AJs are intercellular connections that facilitate cell-cell communication and stabilize EC contacts, regulating permeability for large plasma components [19]. They include transmembrane proteins like cadherins, responsible for the adhesion and catenin’s, which support cadherin association and signaling regulation across the BBB. In brain ECs, the primary transmembrane AJ protein is Ve-cadherin, working with catenin, plays an important role in the maintenance of cell-cell adhesion, contact inhibition, cytoskeleton remodeling, and intercellular signaling [22]. The intricate interactions between these transmembrane proteins create a molecular architecture that maintains BBB integrity under mechanical stress. Loss of the AJs can lead to a loss of BBB integrity and its increased permeability seen in various CNS disorders [27].
ECs express transport proteins that facilitate the transport of selective substances, regulate nutrient delivery, and waste removal from brain parenchyma. These transporters use passive and active transport to regulate processes such as signal transduction, endocytosis, transcytosis, and molecular transport critical for transport of nutrients and waste materials within the brain [28]. ECs are equipped with ion transporters/channels (Fig. 1) such as sodium pumps, calcium channels, acid exchangers, proton/bicarbonate transporters and potassium channels. These are critical in regulating the electrophysiological activity of neuronal cells, and maintaining the sodium concentration gradient at the BBB that drives sodium dependent transport processes [29], which are the focus of this review.
Mural cells cover the abluminal surface of the blood vessels and can be classified into two major categories: vascular smooth muscle cells (VSMC) and pericytes. VSMCs are predominantly located in large diameter vessels including arteries, arterioles, and veins, where they contribute to regulation of blood flow [30]. On the other hand, pericytes are typically associated with small diameter vessels, and capillaries, partially covering the endothelium and extending their contacts across hundreds of micrometers of capillary length [31]. Due to their close proximity to ECs, pericytes play a crucial role in regulation of blood flow by controlling the diameter of capillaries through the release of vasoactive substances [32]. Single cell transcriptomics studies have revealed that capillary pericytes express a diverse array of ion channels and G protein coupled receptors, which are involved in mediating the contraction and dilation response to vasoactive mediators [33].
Lastly, astrocytes play a crucial role in the formation and maintenance of the BBB. Astrocytes are the primary contributors to establishing, upkeeping, and repairing the BBB [34, 35]. An important feature of astrocytic interaction with the BBB are the perivascular endfeet that encompass the abluminal surface of ECs and the surrounding mural cells, creating a continuous interface between glial and vascular components. Astrocytic endfeet are highly enriched with ion channels/ transporters, glucose transporters and G-protein coupled receptors [36]. Through these astrocytes dynamically mediate many regulatory functions, including maintenance of BBB integrity, mechanical and metabolic support, endothelial transport, innate immune response, and cerebrovascular regulation [35-37]. Further, astrocytic endfeet processes also secrete basement membrane proteins such as laminins which contribute to stabilizing the BBB [38]. Astrocytes are the major secretors of sonic hedgehog (Shh) Wnt’s, and norrin proteins that were identified as crucial factors of BBB maintenance [39].
Among the BBB ion transporters and channels (Figure 1), the Na+/K+ ATPase mediates the active transport of Na+ and K+ across the BBB that is crucial for maintaining brain water and electrolyte homeostasis [4]. Multiple secondary active transporters such as Na-K-Cl co-transporter, Na/H exchanger, Na-HCO3 co-transporter and Na/Ca exchangers, alongside a variety of ion channels including inward rectifier Kir channels, ATP-sensitive K, and Na channels, function collectively to regulate intracellular ion concentrations, cellular volume, and pH [4, 16]. These transporters and channels play a pivotal role in the vectorial movement across the BBB, maintaining the precise concentrations of Na+, K+, Cl-, and HCO3- in the brain’s extracellular fluid, critical for regulating the electrophysiological activity of neurons. Further, selective expression of channels such as Kir4.1 and aquaporin 4 (AQP4) at the endfeet polarize astrocytes and facilitate the directional flow of K+ and water molecules to maintain ion homeostasis and EC volume [40]. This orchestrated regulation contributes significantly to maintaining the brain's microenvironment and facilitates vital processes such as nutrient transport while preventing the entry of harmful substances into the brain.
3. BBB dysfunctions in AD and VaD
Disruption/dysfunction of the BBB is considered a hallmark associated with several brain pathologies including neurodegenerative diseases. A large body of evidence indicate that damage to BBB is a characteristic feature of AD, occurring early in the disease process, even before the onset of dementia and neurodegeneration [41, 42]. Brain capillary leakages and perivascular accumulation of blood-derived fibrinogen, thrombin, albumin, and immunoglobulin G (IgG), pericyte and EC degeneration, loss of BBB TJs, and red blood cell extravasation, are some of the features of BBB damage implicated in AD [43].
Activation of inflammatory and oxidative stress signaling pathways are the primary events that cause BBB disruption. Cytokines, amyloid β (Aβ) and tau-proteins are the stimulus of inflammation and increase in proinflammatory mediators and reactive oxygen species (ROS)/reactive nitrogen species (RNS) in ECs, astrocytes and pericytes are the major drivers of BBB damage [44]. At cellular level, degeneration of ECs associated with reduction in EC thickness, length, and density, loss/ damage to TJ proteins, has been detected in AD brains [45]. In addition, pericyte degeneration due to the toxic effects of Aβ also contributes to EC degeneration in AD [46, 47] and facilitates extravasation of toxic plasma proteins into brain parenchyma.
BBB breakdown is also most pronounced in individuals carrying APOE*e4 allele which is shown to be associated with microvascular injury and pericyte degeneration, but the molecular mechanisms are not clear [48, 49]. Increased expression of proinflammatory mediators such as cyclophilin A and matrix metalloproteinase 9 (MMP-9) that are known to degrade BBB TJ proteins are reported to be overexpressed in AD [47]. Changes in the morphology of astrocytes in AD brain have also been shown to cause BBB breakdown [50]. The depolarization of astrocyte endfeet weakens the integrity of BBB, which was reported in the Tg-ArcSwe mouse model of AD [51]. Decreased vascular coverage and loss of AQP4 expression by the endfeet in AD patients and animal models has been shown to be associated with Aβ-accumulation and BBB damage [52, 53]. The perturbed AQP4 expression in the astrocytic end-feet has also been observed to cause inflammation and increase in neurofibrillary tangles in the human AD brain [54].
VaD refers to the loss of cognitive function because of reduced cerebral blood flow (CBF) to the brain, largely caused by damage to the cerebral blood vessels. AD and VaD together account for >70% dementia cases and share similar vascular pathologies and cognitive decline [55]. BBB disruption/dysfunction is considered as a core mechanism in VaD contributing to disease pathology and progression [56]. Loss of EC integrity, degeneration of pericytes, astrocyte endfeet swelling and retraction from the vessel wall, loss of TJs, and brain capillary leakages are the known factors disrupting the BBB integrity subsequently leading brain hypoperfusion [57, 58]. Studies have indicated that oxidative stress, hypoxia, and inflammation are the major drivers of BBB dysfunction and cerebral hypoperfusion. Hypoxia upregulates oxidative stress to produce NO, ROS and free radicals that damage the BBB ECs leading to reduced CBF [59, 60]. Vascular hypoxia causes increased secretion of inflammatory molecules including soluble LRP1, cyclophilin A, MMPs (MMP-2 and MMP-9), IL-1, IL-6, TNF-a and TLR4 that cause damage to the ECs, astrocytes, and surrounding neurons to enhance BBB permeability [61].
In a rat model of VaD (bilateral common carotid artery stenosis (BCAS)), reactive astrocyte-derived lipocalin-2 (Lcn2) in the hippocampus has been shown to mediate BBB damage leading to neuroinflammation and cognitive impairment [62]. Lcn2-deficient mice showed less BBB permeability, cognitive decline, neuronal loss, glial activation, and cytokine production [62]. BBB disruption due to degeneration of pericytes causes disruption in white matter circulation, deposition of fibrinogen, and reduction of CBF that induces further damage to the myelin, axons and oligodendrocytes [63]. Recent studies have shown that, animals subjected to chronic cerebral hypoperfusion upregulates intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1) in the vascular ECs that was associated with cognitive impairment [64].
Together, insights from the literature underscore the significance of BBB dysfunction as a critical factor in neurodegenerative disorders such as AD and VaD. Restoration of BBB function has emerged as a promising therapeutic target to effectively impede the progression of neurodegenerative mechanisms and alleviate the associated clinical deficits.
4. Key ion transporters and channels at the BBB
4.1. Na+/K+-ATPase (NKA)
The NKA is the main transporter that couples metabolic energy to ion transport at the BBB and is highly expressed by the ECs and astrocytes ensheathing the BBB at the abluminal side [4, 29]. NKA is crucial for maintaining optimal ion levels, particularly a high Na+ concentration and low K+ level in the brain interstitial fluid. This balance is crucial for establishing the Na+ concentration gradient at the BBB, facilitating essential Na+ dependent transport processes important for brain water and electrolyte homeostasis [4, 65]. Three different α subunits and two β subunits of NKA have been reported to be expressed at the BBB and within ECs, it is primarily located in the abluminal membranes [4, 5]. However low NKA activity has also been reported in luminal membranes suggesting the presence of different isoforms on these two surfaces [4]. Dysfunction in distinct NKA isoforms plays a crucial role in neurodegenerative diseases; deficiency in α1/α3 subunits accelerates neuronal loss and diminishes pump function. Conversely, the overactivation of the α2 subunit contributes to neuronal loss in AD [66].
In astrocytes, NKA constitutes the major pathway of Na+ efflux, however it is also known to play critical role in the regulation of extracellular K+ concentration ([K+]o) [67]. In a healthy brain, astrocytic NKA activity is required for increased glucose uptake, breakdown of glycogen, stimulation of glycolysis as well as the production of lactate [68]. However, impaired NKA function in astrocytes negatively impacts glutamate clearance, leading to abnormally high neuronal activity affecting memory functions in patients [69, 70]. Reduced NKA activity can also cause increase in intracellular calcium concentration ([Ca2+]i), due to the reverse mode activation of Na+/Ca2+ exchanger (NCX), leading to excitotoxicity [67]. Altered NKA activity in the perivascular astrocytes may disrupt glutamate transport pathways [71], potentially disrupting ionic homeostasis crucial for BBB function, and potentially contributing to the development of neurodegenerative disorders.
Impaired NKA expression and activity have been associated with acute brain injuries and chronic neurodegenerative disorders. In ischemic stroke, reduced NKA activity in neurons and astrocytes leads to accumulation of intracellular sodium (Na+i) that further triggers Na-K-Cl cotransport and Na+/H+ exchanger 1 (NHE1) activation, increasing Na+ influx. With compromised NKA function unable to balance these transport processes, Na+i accumulation worsens, causing EC swelling [16], damaging BBB integrity and leading to brain edema in stroke brains [72]. Similarly, persistent decrease in NKA activity is observed in various traumatic brain injury (TBI) models, contributing to BBB damage [73, 74]. Dysfunctional NKA activity and intracellular Na+ accumulation has been associated with neuroaxonal loss in multiple sclerosis patients [75], sustained membrane depolarization and NMDA receptor overactivation and excitotoxicity in Huntington’s disease (HD) [76]. Additionally, significantly decreased NKA activity has been observed in motor neurons of the (SOD1) G93A mouse model of Amyotrophic lateral sclerosis [77]. However, further studies are needed to precisely determine the impact of dysfunctional NKA activity on BBB function.
4.2. Ca2+ channels/transporters
Ca2+ channels and their downstream signaling mechanisms play critical roles in mediating the integrity of the BBB and contribute significantly to CBF regulation [78, 79]. ECs express many different types of Ca2+ channels, including Ca2+ transporters like the NCX, Ca2+ ATPase, P2X receptors, L-Type Ca2+ channels important for the regulation of BBB integrity and CBF [29, 80]. Transient receptor potential (TRP) channels are another important class of Ca2+ influx channels expressed abundantly by ECs and astrocytes [81, 82]. Among the 7 different subfamilies, the TRPC, TRPV, TRPM, and TRPA1 are known to modulate BBB function. TRPC channels are the best characterized and have been proposed to mediate EC [Ca2+]i which is a crucial second messenger that leads to various vascular responses, including changes in vascular tone, alteration in BBB permeability, vascular remodeling and oxidative damage [83]. TRPC6 is mainly activated by diacylglycerol, mechanical stretch, and exposure to ROS to cause Ca2+ influx that facilitates increases in EC permeability [84]. TRPC3 overexpression/hyperactivation in ECs has been associated with increased BBB permeability and vasogenic edema observed in response to status epilepticus in mice [85]. Activation of TRPA1 within capillary ECs, contribute to Ca2+ mediated dilation of arterioles and increased blood flow in the somatosensory cortex of the mouse brain, indicating its important role in mediating neurovascular coupling [86].
Astrocytes express TRPA1, TRPC1/4/5, and TRPV4 channels (reviewed in [82]). The TRPA1 channels in hippocampal astrocytes mediate spotty Ca2+ transients that contribute to resting [Ca2+]i [87]. The TRPC1/4and 5 channels provide a pathway for store operated Ca2+ entry (SOCE) and contribute to modulating cytosolic Ca2+ signals and generating a substantial Na+ entry [82]. TRPV1 in astrocytes are preferentially localized in the endfeet and its activation trigger expression of early gene, c-Fos involved in cell proliferation and differentiation [88]. TRPV4, also expressed in astrocytic endfeet are activated in response to hypo-osmotic stress and cell swelling, stimulate Ca2+ induced Ca2+ release and amplify neurovascular coupling response [89]. Under pathological states, astrocytic TRP channels can be activated by ROS, RNS, proinflammatory factors, and pathological markers of neurodegenerative diseases, such as Aβ, which disrupts the Ca2+ homeostasis [82]. Astrocytic Ca2+ overload can cause excessive activation of astrocytes that can cause BBB disruption by releasing pro-inflammatory factors. Capillary pericytes express 11 different isoforms of TRP channels (TRPC1/3/4/6, TRPM3/4/7, TRPML1, TRPP1, TRPP3 and TRPV2). Among these isoforms, members of TRPC subfamily and TRPM7 play essential roles in mediating SOCE intracellular Ca2+ signaling in pericytes. Pericytes exhibit sensitivity to a range of mechanical perturbations, and TRPP1, TRPV2, TRPC1/6 and TRPM4 channels are implicated in mechanosensation- evoked Ca2+ entry influencing the vascular tone (reviewed extensively in [33]).
Abnormal Ca2+ signaling due to dysfunctional Ca2+ channels and transporters has been studied extensively in ischemic stroke models. Studies using nifedipine, (Ca2+ channel blocker), Ca2+ chelators, or inhibition of protein kinase C (PKC) have shown decreased Ca2+i levels, reducing EC permeability and BBB damage in cultured ECs and ex vivo brain slices during ischemic conditions [90-92]. Dysfunctional TRP channels, particularly TRPM2 and TRPM4, have been implicated in ischemic stroke. Inhibition of TRPM2 preserved EC integrity, reduced brain edema and preserved TJs [93], while pharmacological inhibition of TRPM4 mitigated oncotic cell death [94] and decreased BBB permeability and improved cerebral perfusion [95]. The upregulation of sulfonylurea receptor 1 (SUR1)-TRPM4 channel has been seen in microvascular ECs, astrocytes and pericytes in both humans with stroke and rat models of ischemic stroke [96-98]. Activation of SUR1-TRPM4 channels is shown to mediate PAR-1 and Ca2+ dependent secretion of MMP9 by the brain ECs, causing BBB damage [99]. Pharmacological blockade of SUR1 channel decreased MMP9 secretion, mitigated BBB damage, and reduced hemorrhagic transformation in stroke models [99-102].
The involvement of Ca2+ channels in neurodegenerative disorders has been extensively documented [103]. In mouse models, the application of neurotoxins mimicking Parkinson’s disease (PD) leads to the downregulation of TRPC1 and store operated Ca2+ influx resulting in the death of dopaminergic neurons. Conversely, the activation or overexpression of TRPC1 protects against neurotoxin mediated cytotoxicity [104]. TRPC1 acting as a potential store operated Ca2+ channel is upregulated in response to mutant huntingtin protein expression in mouse model of HD, inducing neuronal damage. Inhibition of store operated Ca2+ entry or knockdown of TRPC1 downstream regulator STIM2 provides neuroprotection and rescues dendritic spine deficiency [105].
4.3. K+ channels
K+ channels, such as inwardly rectifying potassium channels (Kir), two-pore domain K+ channels (K2P), KCa channels and voltage-gated K+ channels (Kv) are known to play important roles in the regulation of BBB permeability and control of CBF [106]. The inward rectifier Kir2.1 channel is the primary K+ channel type in brain capillary ECs. Along with voltage-gated potassium channel Kv1, it contributes to the establishment of membrane potential and mediates K+ efflux into the brain interstitial fluid [107]. Recent findings highlight that EC Kir2.1 are amplifiers of retrograde electrical signaling in the cerebral vasculature, which is critical for K+ evoked increases in CBF [107, 108]. Action potential induced extracellular K+ released from perivascular neurons and astrocytes activates Kir2.1 in ECs, resulting in a rapidly propagating endothelial hyperpolarization, vessel dilation and a subsequent increase in local CBF [107, 109].
Capillary pericytes express extremely high levels of Kir6.1 and, to a lesser extent, Kir2.2 and Kir2.1 channels [33]. The Kir6.1 and SUR 2 subunits constitute the vascular form of ATP sensitive potassium channels (KATP) channels, which play a major role in coupling membrane hyperpolarization to CBF control [33, 110]. KATP channels in capillary pericytes can be modulated by glucose concentration, adenosine, and endothelin-1 (ET-1). A decrease in cellular glucose activates KATP channels due to lower ATP:ADP ratio, which causes pericyte hyperpolarization and transmits the signal to capillary ECs, increasing blood flow via a Kir2.1 channel-dependent mechanism [111]. Endogenous adenosine from neurons and glial cells can also stimulate KATP channels through the A2AR-Gαs-AC-PKA signaling cascade to conduct electrical response, which enhances CBF [112]. However, ET-1 would inhibit KATP channels to prevent hyperpolarization and dilation of pericytes, even diminish gap junctions between pericytes [31]. The role of Kir2.2 in pericytes remains poor explored, but it was predicted to propagate hyperpolarizing signals from capillary pericytes to upstream vessels [33]. Recently, RNA sequencing transcriptomic analysis of brain vascular pericytes indicated that Kir4.1 functionally expressed in pericytes are engaged in maintenance of K+ homeostasis [113]. Further studies are needed to explore the role of K+ channels in pericytes.
In astrocytes, four types of K+ channels including Kir4.1, K2P channels, large-conductance KCa (BK) and Kv channels have been identified, of which Kir4.1 and BK are abundantly expressed in endfeet [106]. Kir4.1 plays a pivotal role in “K+ buffering”, translocating excess extracellular K+ into the capillaries, and maintains water/K+ homeostasis together with AQP4 in astrocyte endfeet [114]. In addition, BK channels were found co-localized with AQP4 in perivascular astrocytic endfeet of rat hippocampus and cerebellum, suggesting the involvement of BK channels in K+ redistribution and regulation of CBF [106]. Astrocytic Ca2+ waves triggered by neuronal activity can activate BK channels directly at the endfeet, resulting in extracellular K+ accumulation and rapidly induce both vasodilation and vasoconstriction [80, 115].
Disfunction of Kv channel function has been associated with neuronal hyperexcitability and implicated in neurodevelopmental and neurodegenerative disorders [116]. In animal models of PD, inactive Kv channels are linked to reduced dopamine release. Mice over expressing mutant α-synuclein showed increased action potential firing of dopaminergic neurons and disrupted dopamine release which was attributed to the impaired Kv channels [117]. KCa3.1 channels are the major K+ channels in the ECs and astrocytic endfeet and are shown to be involved in ischemia induced K+ fluxes leading to EC swelling and subsequent BBB disruption [118]. A recent study has demonstrated that treatment with KCa3.1 channel inhibitor TRAM-34 significantly reduced brain edema induced by ischemic stroke in rats [118]. The involvement of BBB specific K+ channels in neurodegenerative diseases will be discussed in section 5.1.3.
4.4. Cl- channels
Cl- channels are expressed abundantly in astrocytes, pericytes and ECs wherein they play important roles in control of membrane potential, cell volume homeostasis and regulation of cellular processes such as proliferation and apoptosis [33, 119, 120]. Cl- transport across the BBB is important to facilitate movement of major cations such as Na+, K+ and Ca2+ and thus plays a critical role in ion regulation [121]. Among the 9 different voltage gated Cl- channels (ClCs) identified in mammalian tissue, ClC-2, -3, -6 and -7 are the major members expressed in the brain [121]. Astrocytes express ClC-2, Ca2+ activated Cl- channel bestrophin1 (Best1), maxi Cl- channels (MAC), and volume regulated anion channel (VRAC) (reviewed in [119]). The role of astrocytic Cl- channels in BBB function is not clear. However, ClC-2 is expressed in the astrocytic endfeet surrounding the blood vessels, where it is predicted to regulate Cl- and blood flow. Research indicates that genetic knockout of ClC-2 in mice results in extensive reactive astrogliosis, brain inflammation and increased BBB permeability during the aging process [122]. Capillary pericytes express the Ca2+ activated Cl- channel, TMEM16A or anoctamin and several members of voltage dependent ClC family: ClC-2, -3, -4, -6 and -7 [30]. These channels play an important role in regulation of membrane potential by coupling to several Ca2+ sources, including IP3 receptor and TRP channels that modulate the vascular function. For example, Ca2+ mediated activation of TMEM16A causes membrane depolarization, amplifies capillary pericyte contraction and reduced CBF after ischemia in mice [123]. Pharmacological inhibition of TMEM16A reduced the ischemia-evoked contraction and death of pericytes, improved postischemic CBF, and reduced brain hypoxia and infarct size after ischemia [123]. This study highlighted the potential therapeutic value of TMEM16A inhibition to improve CBF in conditions of impaired microvascular blood flow. TMEM16A is also expressed in ECs and forms Ca2+ activated Cl- channels to modulate resting membrane potential and [Ca2+]i. These changes regulate cell proliferation, migration, control of membrane potential, regulation of cell volume, and smooth muscle contraction. Importantly, its activity also contributes to the regulation of BBB trans-endothelial permeability [124].
The CNS Cl- channels not only regulate neuronal excitability but also indirectly impact neuronal functions by modulating gliotransmitter release from astrocytes, influencing neurological disorders (reviewed extensively by Wang et al., 2023 [125]). Disruption of ClC-2 expression for example is associated with astrocyte activation and progressive neurodegeneration in aging mice [126]. Dysregulated Cl- channels cause excessive neurotransmitter release, leading to neuronal impairment. This dysregulation, particularly during astrocytic swelling, contributes significantly to neurological disorder pathogenesis, impacting tissue damage and secondary neurotoxicity. Cl- channels, like VRAC and Best1, modulate the release of excitatory amino acids, exacerbating neuronal function impairments during acute brain injuries [127]. Furthermore, Cl- channels participate in cell apoptosis via endoplasmic reticulum (ER) stress, a mechanism associated with neurological disorders like AD, PD, and amyotrophic lateral sclerosis (ALS). Blocking chloride channels shows promise in preventing apoptosis through the ER-stress pathway, suggesting therapeutic potential against neurodegenerative diseases [128].
4.5. Na+/H+ exchangers (NHEs)
NHEs are a family of membrane transporter proteins, encoded by the SLC9A gene subfamily and NHE1 is the best characterized member of this family expressed abundantly in the brain [129]. It mediates the exchange of extracellular Na+ for intracellular H+ (H+i)and plays an important role in regulating intracellular pH (pHi), as well as cellular volume, important for various physiological processes [130]. It is essentially quiescent at normal pHi and can be activated by intracellular acidification, osmotic cell shrinkage, growth factors and hormones, and pathological conditions such as hypoxia and ischemia [131]. In response to these triggers, NHE1 together with the bicarbonate transporters functions to extrude excessive protons from the cell. However, increased NHE1 activity also causes an overload of [Na+]i that forces the NCX into reverse mode and increases [Ca2+]i to mediate a cascade of signaling events that can have deleterious effects on cell survival [132]. In the BBB, NHEs are mainly expressed in ECs, pericytes, and astrocytes. In ECs, ~80% of NHE1 protein is located in the luminal membranes of ECs and contribute to pHi recovery after intracellular acidification, which can be inhibited by NHE1 specific inhibitor HOE642 [133]. Ischemia stimulates EC NHE1 activity that leads to increased [Na+]i and EC swelling contributing to ischemia-induced BBB damage. Recent studies have found that functional NHE1 expression in ECs can be activated in response to high extracellular K+, which contributes to breaches in BBB integrity and therefore increased drug uptake [134].
There are some studies that demonstrate the presence of the NHE1 in BBB ECs [134, 135]. However, research on the expression and function of NHEs in pericytes remains limited. In cultured human brain microvascular pericytes, pHi is predominantly regulated by NHE1 [136]. Extracellular acidosis induces NHE1 activity and generates Ca2+i oscillation via release of Ca2+ from the ER stores suggesting NHE1 operation in reverse mode (H+ influx in exchange of Na+ extrusion). It has been proposed that cytosolic Ca2+ increases mediate the phosphorylation of CaMKII and cAMP responsive element-binding protein (CREB), which initiates gene transcription to regulate a variety of cellular responses in human CNS pericytes [137].
In the astrocytes, NHE1 is the most important ion transporter responsible not only for pHi regulation but also for the maintenance of [Na+]i [132]. The steady-state pHi in mouse cortical astrocytes is reported at 7.0-7.2 maintained by NBC and NHE1 activities [138]. Acidic astrocytic pHi stimulates a rapid increase in NHE1 activity disrupts Na+ and Ca2+ homeostasis, reduces Na+ dependent glutamate uptake. These ionic perturbations induce astrocyte swelling and promotes release of glutamate, proinflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and matrix metallopeptidase (MMP)-9 which has a detrimental effect on BBB integrity [139, 140]. Targeted deletion of Nhe1 gene in GFAP+ reactive astrocytes resulted in reduced endothelial transcytosis, swelling of astrocyte end feet and ECs, structural disruptions of TJs and overall BBB impairment following ischemic stroke in mice. This specific loss of Nhe1 in astrocytes led to reduced astrogliosis, diminished infarct volume, and improved cerebral perfusion post ischemic stroke [139]. Moreover, in a model of chronic cerebral hypoperfusion, which replicates the vascular contributions to cognitive impairment and dementia (VCID), the inhibition of astrocytic NHE1 resulted in the reduction of reactive astrocyte gliosis, preservation of white matter and hippocampal integrity, and enhancement of cognitive function by decreasing ROS production and inflammation [141].
Abnormal NHE1 activation has been linked to various neurological disorders such as epilepsy, dementia, and ischemic stroke [7]. Preclinical studies have shown deleterious effects of sustained NHE1 activation during cerebral ischemia and reperfusion. Inhibition of NHE1 either pharmacologically or via genetic knock down, has shown neuroprotection and aiding in neurological recovery [139, 142]. NHE1 is also known to modulate the electrical properties of neurons potentially triggering seizure activity. Studies in mice with nonfunctional NHE1 gene have shown the development of epileptic seizures, ataxia, and decreased growth [143]. Moreover, increased NHE1 activity in astrocytes triggered by HIV-linked gp-120 and cytokines, potentially leads to elevated pHi and enhanced release of glutamate through reversal of Na+-dependent glutamate transporters, contributing to the development of AIDS-related dementia [144].
4.6. Na+-dependent HCO3- transporter 1 (NBCe1)
The NBCe1 protein is expressed in astrocytes in the brain and is the major regulator of intracellular and extracellular pH [145]. NBCe1 mediates the electrogenic transport of Na+ and HCO3- across plasma membranes and exists in five splice variants, NBCe1 (-A through -E), which are abundantly expressed in the brain [146]. These variants share common traits, including voltage dependence, independent of Na+ and Cl-, and susceptibility to inhibition by stilbene compounds. In astrocytes, NBCe1 acts with a stoichiometry of 1Na+:2HCO3- and can operate in an outward mode, extruding Na+ and HCO3- and causing intracellular acidification [138]. However, reverse mode activation of NBCe1 can occur in astrocytes in response to membrane depolarization, elevated extracellular HCO3-, and Na+ concentrations, leading to HCO3- and Na+ influx, and astrocyte swelling [147]. A recent study has indicated that NBCe1 in astrocytes is required for its normal morphological complexity and BBB function [148]. Targeted Nbce1 gene deletion in Aldh1l1+ astrocytes has shown to reduce astrocyte processes, and astrogenesis coupled with increased frequency of Ca2+ waves in the astrocyte soma [148]. Astrocytic Nbce1 knockout brains also showed altered vasculature with increased blood vessel diameter and volume. Moreover, the ECs exhibited a 2-fold increase in EC marker expression and a notable upregulation of AQP4 protein expression. Additionally, the blood vessels displayed a deficiency in the coverage of TJ proteins ZO-1 and claudin 5, hinting at a plausible role in in modulating BBB integrity [148]. These findings collectively point towards abnormal vasculature largely due to the deficiency of NBCe1 in astrocytes. However, the specific role of NBCe1 in neurological disorders remains elusive.
4.7. Na-K-2Cl cotransporter-1 (NKCC1)
The NKCC1 belongs to the SLC12 family of cation-chloride cotransporters (CCCs), expressed abundantly in the brain. It couples Na+, K+ and Cl- transport into the cell, with a stoichiometry ratio of 1Na+:1K+:2Cl- [149] and play important roles in the regulation of intracellular chloride concentration [Cl-]i. NKCC1 is highly expressed by multiple cell types, including immature neurons, astrocytes, oligodendrocytes, ECs and epithelial cells of the choroid plexus [149]. In all these cells, the activity of NKCC1 regulates [Cl-]i to maintain their cellular volume amidst fluctuations in extracellular osmolarity and intracellular solute content. This mechanism is crucial to prevent excessive swelling or shrinkage that could compromise cellular structural integrity. Disruption in NKCC1 expression and activity can alter [Cl-]i homeostasis, consequently disrupting cell volume and altering the regulated transport of ions across the blood-brain and epithelial barriers.
NKCC1 is expressed abundantly in BBB ECs where it is predominantly located in the luminal membrane of the ECs and plays an important role in the formation of ionic edema by loading Na+ into ECs [16]. Its presence in the ECs of leptomeningeal vasculature at the capillary level is shown to play role in CSF production throughout the CNS [150]. Highest levels of NKCC1 expression have been detected in the ECs of embryonic mouse cortex indicating its role in BBB development [151]. Hypoxia, aglycemia, hyperglycemia and moderate to severe ischemia induces increased expression of NKCC1 in ECs which along with NKA increases secretion of Na+ from blood into the brain parenchyma leading to edema [152, 153]. In addition to acting as an ion transporter, NKCC1 also participates in interactions with the actin cytoskeleton [154]. F-actin stress fibers present in injured ECs leads to endothelial contraction and disruption of TJ protein ZO-1, which in turn leads to BBB damage [155]. Studies have shown that NKCC1 knockdown or its inhibition leads to decreased expression of F-actin in ECs highlighting its role in regulating cytoskeleton dynamics [156].
In capillary pericytes, NKCC1 protein expression has been detected in CD13+ cells in the perinatal mouse brain indicating its role in the development of BBB [157]. Additionally, NKCC1 mRNA expression has been confirmed in the capillary pericytes of the adult brain cortex. In both pericytes and the smooth muscle cells within the vasculature, there exists a high [Cl-]i concentration, maintained by the actions of plasma membrane NKCC1 and the Cl-/HCO3- exchanger AE2 [158]. Recent studies highlight NKCC1’s significant role in the regulation of pericytes contractility [123]. Inhibition of NKCC1 activity in mouse cortical slices with its potent inhibitor bumetanide have demonstrated the attenuation of endothelin-1 mediated capillary contraction indicating the dependency of capillary pericyte contractility on the transmembrane Cl- gradient established by NKCC1 activity [123].
NKCC1 protein is abundantly expressed in astrocyte cell soma as well as processes and plays a crucial role in maintaining [Na+]i, [K+]i and [Cl-]i [157]. Unlike neurons, astrocytes preserve high activity of NKCC1 throughout development and therefore, have higher intracellular [Cl-]i [159]. The NKCC1 function in astrocytes is critical for regulating cell volume and counteracting the astrocytic shrinkage due to osmotic stress [7]. Astrocytic NKCC1 activation is also associated with elevated extracellular K+ concentrations, resulting in cellular swelling due to co-transport of water [160]. In adult brain, astrocytic NKCC1 has been implicated in withdrawal of fine astrocytic processes from synapses following long-term potentiation induction, highlighting its role in synaptic modifications associated with a memory trace [161]. Increased NKCC1 expression in astrocytes has been seen in various pathological conditions and overstimulation of NKCC1 leads to astrocyte swelling, which subsequently contributes to BBB damage, cerebral edema, and neurological dysfunctions [7].
Upregulation of NKCC1 is a common feature associated in various neurological conditions such as stroke, hemorrhage, TBI, spinal cord injury and brain tumors. Inhibition of NKCC1 using bumetanide (BMT) has shown protective effects by decreasing inflammatory response, neuronal damage, and edema formation in TBI and spinal cord injury models [162, 163]. Additionally, NKCC1 upregulation in choroid plexus was detected in a rat model of posthemorrhagic hydrocephalus, and intraventricular BMT injection reduced CSF hypersecretion [164]. High expression of NKCC1 is detected in several human gliomas associated with poor prognosis. Inhibition of NKCC1 by BMT reduced glioma cell migration and invasion of peritumor tissue in vivo and promoted apoptosis [165, 166]. In rodent models of HD, upregulation of NKCC1 was observed, and BMT administration rescued altered GABAergic transmission, cognition, and motor deficits [167]. Moreover, BMT attenuated motor deficits in PD mouse models [168]. NKCC1 upregulation has also been reported in HD patients, and BMT ameliorated motor symptoms in PD patients.
4.8. Na+-Ca2+ exchanger (NCX)
NCX is a plasma membrane antiporter, which mainly maintains cytosolic Ca2+ homeostasis in cells. All three isoforms of NCX (NCX1—NCX3/SLC8A1—A3) are expressed in adult CNS, which can be detected in heterogeneous cell populations, including neurons, astrocytes and epithelial cell [169]. All NCXs were often identified around blood vessels where perivascular astrocytic endfeet and ECs are located. In astrocytes, NCXs are enriched at distal processes surrounding synapses, with NCX1 transcript being the most predominant [7, 170]. As an electrogenic transporter, NCX regulates Na+- and Ca2+ dependent signaling, as well as membrane potentials in the brain. It has been identified that NCX operates in forward mode at low [Na+]i, which mediates influx of 3 Na+ and 1 Ca2+ extrusion across the cell membrane. However, when Na+i is elevated, NCX can operate in the reverse mode which couples Na+ efflux to the Ca2+ influx [7, 171]. In astrocytes and ECs, NCX is involved in the regulation of Na+i and Ca2+i dynamics, important for BBB integrity and function. Ca2+ induced endothelial cytoskeleton contractions were proposed to prolong BBB rupture following osmotic stress [172]. Pharmacological studies show NCX blockers have a synergistic impact on BBB opening during hypertonic infusions and alter the generation of brain vasogenic edema following radio frequency injuries [170]. [Ca2+]i transients in astrocyte endfeet can cause cerebrovascular vasoconstriction, which suggests that NCX activity in astrocytic endfeet may play an important role in the glial control of brain microcirculation [170]. In addition, NCX participates in the activation of resident cells in the brain, and leads to the destruction of the BBB. NCX in astrocytes is proven to be involved in Ca2+ induced ROS generation, DNA ladder formation, and nuclear condensation [173]. Ca2+ overload in brain microvascular ECs increases brain ROS levels by NADPH oxidase 5 (NOX5), which contributes to BBB breakdown [174].
Changes in the activity of NCX isoforms have been implicated in various CNS pathologies, including stroke, multiple sclerosis, hypoxia ischemic encephalopathy, and AD, suggesting their potential as a pharmacological target [175]. In mice, NCX1 overexpression/activation has demonstrated to reduce ischemic volume and improve neurological deficits following ischemic stroke [176]. Knockdown of NCX3 in mice has been shown to impair oligodendrocyte proliferation, leading to the early onset of experimental autoimmune encephalomyelitis accompanied by worsened clinical symptoms [177]. Exposure of neuronal cultures to Aβ1-42 induces calpain mediated cleavage of NCX3 isoform that generates hyper functional form of the antiporter that, by increasing Ca2+ content into ER, delays caspase 12 activation and neuronal death [178]. The direct research implicating the role of NCX in BBB regulation is currently limited.
5. Role of BBB ion channels and transporters in neurodegenerative disorders
It has been well established that both astrocytes and ECs play vital roles in maintaining the functionality and integrity of the BBB. Astrocytic endfeet contribute significantly to BBB regulation by maintaining ion and water balance. Additionally, soluble factors derived from astrocytes have been found to induce essential aspects of BBB function [179]. ECs on the other hand, facilitate the bidirectional transport across the brain through ion transporters, protein and peptide carriers and active efflux transport. Moreover, TJ proteins between brain ECs also greatly uphold the structure of BBB.
Compelling evidence indicates that following acute brain injuries like ischemic stroke and brain trauma, rapid activation of ion channels and transporters within the ECs and perivascular astrocytes disrupts ion homeostasis and contributes to inflammation, increased BBB permeability and brain damage as previously reviewed [7, 11]. However, the impact of dysregulated ion channels and transporters within BBB-specific cells in neurodegenerative diseases including AD and vascular dementia remains understudied.
5.1. Ion channels and ion transporters in BBB dysfunction in AD and VaD
In AD, there is emerging evidence suggesting aberrant activities of ion transporters/channels play a role in glial cell activation causing neuroinflammation and neuronal damage. Moreover, they contribute to impaired perivascular clearance of Aβ and disrupts CBF leading to neurodegeneration [180]. In addition, Aβ fibrils and aggregates deposit in the extracellular spaces of the brain tissue and around the vasculature of the brain, causing cerebral amyloid angiopathy (CAA) which damages the vessel wall leading to BBB permeability, vessel occlusion or rupture [181]. In the context of AD and the BBB, our understanding of ion channel and ion transporter dysfunction primarily stems from the interactions between the BBB cell types and accumulated Aβ plaques [181]. For VaD, dysfunction of ion channels and transporter primarily arises from chronic cerebral hypoperfusion, which impairs the BBB due to alterations in the microenvironment and neuronal toxicity impacting BBB specific cells that express essential ion channels and transporters [182]. The vascular pathology associated with VaD results in chronic cerebral hypoperfusion, reducing the availability of ions from the bloodstream for perivascular cells to meet the demands of the brain parenchyma [182]. Nonetheless, it is important to note that there remains limited literature providing explicit evidence for alterations in ion channel/transporter expression and activities within the perivascular cells of the BBB in AD and VaD brains. Evidence suggests that the disruption of Na+, K+, Ca2+ and Cl- homeostasis particularly in astrocytes and ECs contributes to a proinflammatory cascade that precedes the onset of AD development [180]. The underlying mechanisms include compromised perivascular drainage and impaired BBB transport and vascular dysfunction [183]. A post-mortem study demonstrated significant BBB permeability and evidence of cerebral hypoperfusion in patients with AD, VaD, and mixed AD, this clinical evidence supports the hypothesis that vascular pathology may influence the ionic regulation of BBB specific cells as a compensatory response to reduced CBF [184]. Studies focusing on astrocytes and ECs, at the BBB have discovered homeostatic deficits in ion channels/transporters contributing to AD/VaD pathology. Table 2 presents an overview of key BBB ion channels/transporters and their implication in AD and VaD.
Table 2.
Ion channels and transporters within BBB and their dysregulation in AD and VaD.
| Ion channel/ transporter | Transported ion | BBB cells | Changes in AD/VaD | Refs |
|---|---|---|---|---|
| Na+-K+-ATPase (NKA) | Na+, K+ | Endothelial cells (ECs), Astrocytes (As), and Pericytes | Reduced expression and activity in As and ECs in mouse AD models. | [186] |
| Reduced activity in VaD brain. | [190] | |||
| Aβ binding to NKA causing vessel constriction in vessels isolated from AD patient brains and EC damage in EC cultures. | [189] | |||
| Kv1 | K+ | ECs, Smooth muscle cells | No reports. | [107] |
| Kv3.4 | K+ | As | Upregulation in as exposed to Aβ oligomers and in As of Tg2576 AD mice. | [221] |
| KCa3.1 and BK channels | K+ | ECs, As | Increased activity in As, Reactive gliosis, pro inflammatory cytokine production in SAMP8 mouse model of AD. Decreased EC BK activity, capillary constriction and reduced CBF in APP23 mouse model of AD. | [218], [220]. |
| Kir2.1 | K+ | ECs | Kir2.1 overexpression, reduced vasodilation, and neurovascular coupling in APP; 3xTg-AD and 5xFAD mice. | [224], [225]; [226]. |
| Kir4.1 | K+ | As | Reduced expression in As in VCID mouse model, Postmortem human AD brains, and in APPSw (Tg2576) and APPSwDI AD mice. | [216],[214] |
| Kir6.2 | K+ | As | Increased expression in reactive as in human postmortem AD samples and in 3xTg-AD mice. | [213] |
| CaV1.2 | Ca2+ | As | Increased expression in reactive As in Aβ PP751 mice. | [195] |
| TRPV1 | Ca2+ | As | Aβ induced increased expression in As, Increase in iNOS, COX2 and TNF-α production. | [227] |
| TRPV4 | Ca2+ | As, ECs | Aβ induced increased activation in As, oxidative stress and astrocyte damage in mouse brain slices; proinflammatory cytokine production. | [205] |
| TRPC1 | Ca2+ | As | Increased activation in As from Tg5469 AD mice, Ca2+i overload, inflammation. | [228] |
| TRPM2 | Ca2+ | As, ECs | Increased activation in ECs, Ca2+i overload, and EC dysfunction. | [208] |
| TRPA1 | Ca2+ | ECs | Mediates increase in Ca2+i in EC line bEnd3 exposed to Aβ42 oligomers. Increased expression within ECs in 5xFAD mice associated with BBB disruption. | [209] |
| NHE1 | Na+, H+ | As, ECs | Increased expression in reactive As in BCAS model. | [141] |
| NKCC1 | Na+, K+, Cl- | As, ECs | No reports | |
| NCX | Na+, Ca2+ | As, ECs | No reports | |
| NBCe1 | Na+, HCO3- | As | No reports |
5.1.1. BBB specific NKA dysregulation in AD/VaD
Significant research has firmly established a connection between AD and reduced levels of NKA, resulting in Na+ and K+ imbalance and loss of Ca2+ homeostasis. This ionic dysfunction significantly contributes to vascular dysfunction, inflammation, and neurodegeneration [185-187]. However, despite this understanding, the precise cellular identities involved in these mechanisms remain unclear. Ionic imbalances have been observed in cultured mouse astrocytes, when exposed to Aβ25-35 and Aβ1-40 leading to a 2-3 fold increased [Na+]i and 1.5 fold increase in [K+]i [186]. These alterations closely resemble the levels detected in the brain tissues of individuals with AD indicating dysfunctional NKA [186]. The Aβ treated astrocytes also exhibited decreased expression of NKA indicating its depressed activity contributing to ion imbalance [186]. Conversely, a recent study has revealed increased levels of α2 subunit of NKA (α2 -NKA) within astrocytes in postmortem AD human brain tissue [188]. In a mouse model of tauopathy, pharmacological inhibition of α2-NKA suppressed the neuroinflammation and brain atrophy and α2-NKA knockdown prevented the accumulation of tau pathology [188]. Recently, in an in vitro blood cell cultures and ex vivo blood vessels, it has been demonstrated that patient derived Aβ assemblies bind to the α3 subunit of NKA in caveolae on ECs and induces ROS production and activates PKC. The increased PKC stimulates inactive form of endothelial nitric oxide (eNOS) that causes vessel constriction and EC damage [189]. In a recent study, Philbert et al., [190] utilized a plasma mass spectrometry (ICP-MS) to measure the concentrations of Na+ and K+ in the human post-mortem tissues from VaD cases, revealing increased Na+ and reduced K+ levels in hippocampal tissue, potentially due to dysfunction of NKA contributing to hippocampal atrophy and cognitive impairment in VaD [190]. These findings raise the possibility that reduced activity of the NKA may underlie the observed alterations in Na+ and K+ levels. Moreover, in a separate investigation led by Adav et al., [191], an in-depth analysis of the proteomic composition of post-mortem brain samples from VaD patients indicated a substantial increase in NKA protein expression, accompanied by the deamidation of both catalytic and regulatory subunits of the NKA protein. These findings emphasize the central role of NKA in preserving ion homeostasis and raise the possibility that the specific functions of various NKA isoforms in distinct cell types may bear critical implications for the pathophysiology of both AD and VaD.
5.1.2. BBB Ca2+ channels/transporter dysregulation in AD/VaD
Most studies investigating ionic dysregulation in AD brains highlights the involvement of astrocytic Ca2+ dysfunction [192, 193]. Dysregulated astrocytic Ca2+ transporters/channels are the major drivers of neuroinflammation observed in AD. Though specific endfeet Ca2+ dynamics and dysfunctional channel or transporters at the BBB in AD brains has not been reported in the literature so far, it is noteworthy that the astrocytic Ca2+ signaling constitutes an intricate machinery, well organized in cell soma and fine processes contacting the vasculature orchestrating the BBB pathophysiology. The microdomain Ca2+ changes have been observed in the endfeet that are mediated through voltage gated Ca2+ channels, TRP channels, SOCE entry or reversed NCX [194]. The Ca2+ changes mediated by aberrant activities of these channels/transporters induce gene expression changes, astrocyte metabolism and reactive astrogliosis that can impact the BBB in AD brains [193]. Increased activity of L type voltage gated Ca2+ channels has been implicated in pathogenesis of dementia and AD. In a recent study, Daschil et al., has reported that CaV1.2 α1-subunits are highly expressed in reactive astrocytes of 12-month-old transgenic AD mice associated with increased amyloid-β-load [195]. Abnormally large Ca2+ signals have been detected in astrocytes isolated from triple transgenic AD mice indicating defects in Ca2+ handling [196]. Hyperactivity of [Ca2+]i has also been observed in vivo within the plaque associated reactive astrocytes in the brains of animal models of AD [197, 198]. The activation of astroglial purinergic receptors is also implicated in these aberrant Ca2+ changes and an increased frequency of astroglial Ca2+ oscillations in the pre plaque stage is associated with instability of BBB ECs affecting the vascular tone and local blood flow [199, 200]. Vascular dysfunction is also noted upon exposure of astrocytes to Aβ that increased the frequency of spontaneous Ca2+ responses associated with arteriolar lumen diameter instability [199-201]. In these studies, the Ca2+ changes were characterized by oscillating cycles of vessel relaxation and constriction [200]. Importantly, the vascular responses corresponded with increased frequency and amplitude of evoked intercellular astrocytic Ca2+ waves [197] and was found prominent in the reactive astrocytes close to amyloid plaques [199].
Another path to [Ca2+]i overload in astrocytes is shown to be through Aβ-activated TRP channels that include TRPA1, TRPC6, TRPV1 and TRPV4 [114]. This specific path of Ca2+ overload leads the astrocytes to express pro-inflammatory factors, activating NF-kB, NFAT and serine/threonine-protein phosphatase 2B (PP2B), inducing an inflammatory phenotype akin to reactivity, which can prove detrimental to the integrity of the BBB [202]. Aβ also mediates activation of astrocytic TRPC1 channels which interacts with Orai1 and stromal interacting molecule, ST1MI that drives the SOCE causing sustained Ca2+ signals that can cause inflammation [203]. Additionally, TRPC6 is known to exhibit protective effects on astrocytes including inhibiting astrocyte Ca2+ hyperactivity and suppressing the astrocytic inflammatory response [204]. Elevated levels of astrocytic TRPV4 channels in AD brains are implicated in Ca2+ mediated astrogliosis, ROS production, and increases in IL-1β, TNF-α, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX) 2 that induces inflammatory BBB damaging response [205]. Ca2+ induced EC dysfunction in AD and VaD is shown to be mediated mainly through the TRP channels [206, 207]. It has also been demonstrated that extracellular Aβ accumulation in AD brains induces oxidative stress in brain ECs, which activates the DNA repair enzyme poly-ADPR polymerase [208]. The increased production of poly-ADPR activates TRPM2 channel, thereby inducing an EC Ca2+ overload, EC dysfunction and BBB impairment [208]. In a recent study, Yang et al., [209] reported that exposure of EC line, bEnd3 to Aβ42 oligomer induces Ca2+ dysregulation mediated by TRPA1 channels. In addition, their findings indicated elevated TRPA1 expression within ECs in 5xFAD mice, was associated with Aβ accumulation and BBB dysfunction [209].
These studies strongly indicate that Ca2+ signaling in astrocytes and ECs is dysregulated within AD brains. Aberrant Ca2+ signaling leads to disruption in cellular structures, alter protein expression, increase secretion of pro inflammatory mediators that can impact BBB permeability. Besides TRP channels, dysregulated Ca2+ due to abnormal BBB transporter activities discussed in section 4.2 remain unexplored in AD and VaD. Given the important roles that these channels and transporters play in BBB disruption in acute brain injuries, further research is required to understand their potential impact on chronic neurodegenerative diseases like AD.
In the context of VaD, there is a growing interest in Ca2+ channels as potential therapeutic targets, although they are not conventionally studied within the specific cell types of the BBB. The hypoperfusion observed in VaD could potentially benefit from Ca2+ channel blockers, as they have the capacity to relax smooth muscle cells in the cerebral vasculature. Using BBB permeable Ca2+ channel blockers may prove valuable in mitigating excitotoxicity, a phenomenon that leads to neuronal death seen in both VaD [210]. Additionally, recent study by Taylor et al., [211], investigating the CBF component of VaD in a hypertension induced VaD model has shed light on the uncoupling of Ca2+ sparked BK Channel mediated vasodilation mechanisms. These mechanisms, which normally promote hyperpolarization and vasodilation, become uncoupled, and leads to the increase in vessel constriction in hypertension models [211]. Given VaD’s reduced blood flow and vascular disease components, it is plausible that downstream effects could influence Ca2+ ion concentrations and alter the activities of Ca2+ channels and transporters. Therefore, it would be worthwhile to explore the activities of Ca2+ channels and transporter in perivascular cells at the BBB in the context of VaD.
5.1.3. BBB K+ channels in AD
Abnormal expression of astrocytic KATP and Kir channels is known to contribute to the pathogenesis of AD [212, 213]. Kir6.2 subunits were significantly increased in reactive astrocytes in both patients with AD and in 3xTg-AD mice hippocampus [213]. However, their contribution to BBB pathophysiology is not clear. A postmortem study indicated that reduced Kir4.1 expression in patients with AD exhibited moderate to severe CAA [214]. In mice with varying degrees of CAA, a decrease in Kir4.1 expression was seen in astrocytes at the neurovascular unit indicating its role in loss of BBB integrity and vessel occlusion/rupture [214]. The loss of astrocyte Kir4.1 in AD pathology is similar to the loss of AQP4 at the BBB; both anchor to DP71 dystrophin protein in astrocyte terminal protrusions and play important roles in maintaining BBB integrity [215].
In vascular cognitive impairment and dementia mouse models, Sudduth et al. [216] demonstrated the destruction of astrocyte terminal protrusions and a decrease in DP71, AQP4, and Kir4.1 localization along with neuroinflammation and cognitive dysfunction. AQP4, Kir4.1 and dystrophin 1 were also reduced in autopsied brain tissue from individuals with AD that also display moderate and severe CAA [214]. While reductions in Kir4.1 may occur secondary to changes in the BBB due to increase in Aβ deposition, such reductions in expression may play a role in overall disease progression as well as the development of co-morbidities such as seizures [217] in AD patients. Thus, Kir4.1 may represent a potential therapeutic target in the progression of AD. In addition, increased activity of intermediate-conductance KCa, KCa3.1 in astrocytes is linked to Ca2+ overload, astrocyte activation and gliosis [218]. Inhibiting KCa3.1 channel activity with pharmacological agents reduces reactive astrogliosis and production of pro-inflammatory factors, IL-1β and TNF-α [218]. Proinflammatory cytokines can damage the BBB and increase its permeability through activation and destruction of TJs of microvascular ECs [219] and this needs to be further evaluated in the context of AD/VaD conditions. Recently reduced activity of endothelial BK channels was seen in APP23 AD mice that exhibited enhanced cerebral vessel constriction and reduced blood flow to the brain [220] indicated compromised vessel function. Recently, Kv3.4 subunit, mediating fast inactivating K+ currents, was shown to be upregulated in reactive astrocytes in both in vitro and in vivo AD models. Selective knockdown of Kv3.4 expression significantly downregulated both reactive astrogliosis and Aβ trimers in the brain of AD transgenic mice [221]. Thus, it is reasonable to predict that changes in K+ channel function may alter K+ buffering and signaling at the vasculature leading to further brain impairment.
5.1.4. BBB NHE1 in AD and VaD
NHE1 is specifically found in glial cells of cortical and hippocampal regions of the brain, areas often implicated in AD development and pathology [144]. So far there is no direct evidence to indicate NHE1 mediated BBB impairment in AD or VaD models. However, in an experimental vascular dementia model (BCAS), NHE1 protein is shown to be upregulated in reactive astrocytes and pharmacological inhibition of NHE1 protein with its potent inhibitor HOE642 significantly attenuated astrogliosis and improved hippocampal integrity and cognitive function [141]. The underlying mechanism is that blocking NHE1-mediated H+ efflux acidifies astrocytic cytosolic environment, which dampened NOX activity [222]. This led to suppression of NOX-mediated ROS production and pro-inflammatory transcriptome in reactive astrocytes. Considering the well-established role of inflammatory reactive astrocytes in BBB dysfunction [139, 223], it is speculated that increased NHE1 activity in reactive astrocytes in AD and VaD brains could potentially lead to vascular inflammation, contributing to BBB dysfunction associated with cognitive impairment. However further research is necessary to establish a direct correlation between increased NHE1 activity in perivascular astrocytes/ECs and BBB damage in AD or VaD.
6. Conclusions and Perspectives
The ion channels and transporters expressed by BBB ECs, astrocytes, and pericytes play key roles in controlling the precise concentrations of Na+, K+, Ca2+, and Cl- across the BBB, providing an optimal medium for neuronal function and synaptic signaling. Dysfunction in these ion channels and transporters leads to osmotic cell swelling, activation of proinflammatory mediators, disruption of TJ proteins, a common phenomenon in acute brain injuries. However, their role in neurodegenerative diseases like AD and VaD, and their contribution to long-term impairments, requires further investigation. Our understanding of how these channels influence disease mechanisms is limited, especially in chronic neurodegenerative conditions. Since ion dysregulation precedes amyloid pathology, irreversible neuronal damage or cognitive decline, detection of dysregulated BBB specific ion channel and transporters could facilitate early identification of neurodegenerative changes. Further research should focus on characterizing these channels in healthy and diseased states, understanding their contribution to BBB integrity or dysfunction. Structure-function studies and exploring drug targets for restoring ion balance in neurological disorders are warranted. Future research directions should explore pharmacological interventions specifically tailored to these BBB channels to restore ionic equilibrium.
Acknowledgements
This work was supported by a NIH grant: NIH R01 NS110755-01A1 (G.B); and NIH R21 NS131754-01A1 (G.B).
Funding Statement
This work was supported by a NIH grant: NIH R01 NS110755-01A1 (G.B); and NIH R21 NS131754-01A1 (G.B).
References
- [1].Daneman R, Prat A (2015). The blood-brain barrier. Cold Spring Harb Perspect Biol, 7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kadry H, Noorani B, Cucullo L (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS, 17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Langen UH, Ayloo S, Gu C (2019). Development and Cell Biology of the Blood-Brain Barrier. Annu Rev Cell Dev Biol, 35:591-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hladky SB, Barrand MA (2016). Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS, 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hladky SB, Barrand MA (2014). Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS, 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Putten M, Fahlke C, Kafitz KW, Hofmeijer J, Rose CR (2021). Dysregulation of Astrocyte Ion Homeostasis and Its Relevance for Stroke-Induced Brain Damage. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Song S, Luo L, Sun B, Sun D (2020). Roles of glial ion transporters in brain diseases. Glia, 68:472-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, et al. (2016). Analysis of the brain mural cell transcriptome. Sci Rep, 6:35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mokgokong R, Wang S, Taylor CJ, Barrand MA, Hladky SB (2014). Ion transporters in brain endothelial cells that contribute to formation of brain interstitial fluid. Pflugers Arch, 466:887-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Verkhratsky A, Rose CR (2020). Na(+)-dependent transporters: The backbone of astroglial homeostatic function. Cell Calcium, 85:102136. [DOI] [PubMed] [Google Scholar]
- [11].Boscia F, Begum G, Pignataro G, Sirabella R, Cuomo O, Casamassa A, et al. (2016). Glial Na(+) -dependent ion transporters in pathophysiological conditions. Glia, 64:1677-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu YC, Sonninen TM, Peltonen S, Koistinaho J, Lehtonen S (2021). Blood-Brain Barrier and Neurodegenerative Diseases-Modeling with iPSC-Derived Brain Cells. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Knox EG, Aburto MR, Clarke G, Cryan JF, O'Driscoll CM (2022). The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry, 27:2659-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jia Y, Wang X, Chen Y, Qiu W, Ge W, Ma C (2021). Proteomic and Transcriptomic Analyses Reveal Pathological Changes in the Entorhinal Cortex Region that Correlate Well with Dysregulation of Ion Transport in Patients with Alzheimer's Disease. Mol Neurobiol, 58:4007-4027. [DOI] [PubMed] [Google Scholar]
- [15].Shah K, Abbruscato T (2014). The role of blood-brain barrier transporters in pathophysiology and pharmacotherapy of stroke. Curr Pharm Des, 20:1510-1522. [DOI] [PubMed] [Google Scholar]
- [16].O'Donnell ME (2014). Blood-brain barrier Na transporters in ischemic stroke. Adv Pharmacol, 71:113-146. [DOI] [PubMed] [Google Scholar]
- [17].Zlokovic BV (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 57:178-201. [DOI] [PubMed] [Google Scholar]
- [18].Zihni C, Mills C, Matter K, Balda MS (2016). Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol, 17:564-580. [DOI] [PubMed] [Google Scholar]
- [19].Dejana E, Orsenigo F (2013). Endothelial adherens junctions at a glance. J Cell Sci, 126:2545-2549. [DOI] [PubMed] [Google Scholar]
- [20].Gunzel D, Yu AS (2013). Claudins and the modulation of tight junction permeability. Physiol Rev, 93:525-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. (2003). Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol, 161:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV (2016). Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers, 4:e1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, et al. (2006). Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood, 107:4770-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yamazaki Y, Shinohara M, Shinohara M, Yamazaki A, Murray ME, Liesinger AM, et al. (2019). Selective loss of cortical endothelial tight junction proteins during Alzheimer's disease progression. Brain, 142:1077-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gonzalez-Mariscal L, Tapia R, Chamorro D (2008). Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta, 1778:729-756. [DOI] [PubMed] [Google Scholar]
- [26].Shi H, Koronyo Y, Fuchs DT, Sheyn J, Jallow O, Mandalia K, et al. (2023). Retinal arterial Abeta(40) deposition is linked with tight junction loss and cerebral amyloid angiopathy in MCI and AD patients. Alzheimers Dement, 19:5185-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harris ES, Nelson WJ (2010). VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol, 22:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ayloo S, Gu C (2019). Transcytosis at the blood-brain barrier. Curr Opin Neurobiol, 57:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev, 99:21-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature, 554:475-480. [DOI] [PubMed] [Google Scholar]
- [31].Hartmann DA, Coelho-Santos V, Shih AY (2022). Pericyte Control of Blood Flow Across Microvascular Zones in the Central Nervous System. Annu Rev Physiol, 84:331-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hamilton NB, Attwell D, Hall CN (2010). Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hariharan A, Weir N, Robertson C, He L, Betsholtz C, Longden TA (2020). The Ion Channel and GPCR Toolkit of Brain Capillary Pericytes. Front Cell Neurosci, 14:601324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Verkhratsky A, Pivoriunas A (2023). Astroglia support, regulate and reinforce brain barriers. Neurobiol Dis, 179:106054. [DOI] [PubMed] [Google Scholar]
- [35].Verkhratsky A, Parpura V, Li B, Scuderi C (2021). Astrocytes: The Housekeepers and Guardians of the CNS. Adv Neurobiol, 26:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Diaz-Castro B, Robel S, Mishra A (2023). Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu Rev Neurosci, 46:101-121. [DOI] [PubMed] [Google Scholar]
- [37].Michinaga S, Koyama Y (2019). Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yao Y, Chen ZL, Norris EH, Strickland S (2014). Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun, 5:3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, et al. (2014). Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest, 124:3825-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Salman MM, Kitchen P, Halsey A, Wang MX, Tornroth-Horsefield S, Conner AC, et al. (2022). Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain, 145:64-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Montagne A, Zhao Z, Zlokovic BV (2017). Alzheimer's disease: A matter of blood-brain barrier dysfunction? J Exp Med, 214:3151-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat Neurosci, 21:1318-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV (2016). Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochim Biophys Acta, 1862:887-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang X, Hussain B, Chang J (2021). Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther, 27:36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zlokovic BV (2011). Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci, 12:723-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013). Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol, 23:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, et al. (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab, 36:216-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. (2013). Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun, 4:2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [49].Hultman K, Strickland S, Norris EH (2013). The APOE varepsilon4/varepsilon4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J Cereb Blood Flow Metab, 33:1251-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cai Z, Wan CQ, Liu Z (2017). Astrocyte and Alzheimer's disease. J Neurol, 264:2068-2074. [DOI] [PubMed] [Google Scholar]
- [51].Yang J, Lunde LK, Nuntagij P, Oguchi T, Camassa LM, Nilsson LN, et al. (2011). Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer's disease. J Alzheimers Dis, 27:711-722. [DOI] [PubMed] [Google Scholar]
- [52].Lan YL, Zou S, Chen JJ, Zhao J, Li S (2016). The Neuroprotective Effect of the Association of Aquaporin-4/Glutamate Transporter-1 against Alzheimer's Disease. Neural Plast, 2016:4626593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rasmussen MK, Mestre H, Nedergaard M (2018). The glymphatic pathway in neurological disorders. Lancet Neurol, 17:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zeppenfeld DM, Simon M, Haswell JD, D'Abreo D, Murchison C, Quinn JF, et al. (2017). Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol, 74:91-99. [DOI] [PubMed] [Google Scholar]
- [55].Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. (2008). Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol, 7:812-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ueno M, Chiba Y, Matsumoto K, Murakami R, Fujihara R, Kawauchi M, et al. (2016). Blood-brain barrier damage in vascular dementia. Neuropathology, 36:115-124. [DOI] [PubMed] [Google Scholar]
- [57].Ueno M, Chiba Y, Murakami R, Matsumoto K, Fujihara R, Uemura N, et al. (2019). Disturbance of Intracerebral Fluid Clearance and Blood-Brain Barrier in Vascular Cognitive Impairment. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ (2020). Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front Aging Neurosci, 12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu H, Zhang J (2012). Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci, 122:494-499. [DOI] [PubMed] [Google Scholar]
- [60].Zhang X, Wu B, Nie K, Jia Y, Yu J (2014). Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem Int, 65:23-29. [DOI] [PubMed] [Google Scholar]
- [61].Hussain B, Fang C, Chang J (2021). Blood-Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Front Neurosci, 15:688090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kim JH, Ko PW, Lee HW, Jeong JY, Lee MG, Kim JH, et al. (2017). Astrocyte-derived lipocalin-2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia, 65:1471-1490. [DOI] [PubMed] [Google Scholar]
- [63].Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. (2018). Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med, 24:326-337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [64].Khan MB, Hoda MN, Vaibhav K, Giri S, Wang P, Waller JL, et al. (2015). Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res, 6:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hertz L, Song D, Xu J, Peng L, Gibbs ME (2015). Role of the Astrocytic Na(+), K(+)-ATPase in K(+) Homeostasis in Brain: K(+) Uptake, Signaling Pathways and Substrate Utilization. Neurochem Res, 40:2505-2516. [DOI] [PubMed] [Google Scholar]
- [66].Zhang X, Lee W, Bian JS (2022). Recent Advances in the Study of Na(+)/K(+)-ATPase in Neurodegenerative Diseases. Cells, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Felix L, Delekate A, Petzold GC, Rose CR (2020). Sodium Fluctuations in Astroglia and Their Potential Impact on Astrocyte Function. Front Physiol, 11:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brown AM, Ransom BR (2007). Astrocyte glycogen and brain energy metabolism. Glia, 55:1263-1271. [DOI] [PubMed] [Google Scholar]
- [69].Capuani C, Melone M, Tottene A, Bragina L, Crivellaro G, Santello M, et al. (2016). Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med, 8:967-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Iure A, Mazzocchetti P, Bastioli G, Picconi B, Costa C, Marchionni I, et al. (2019). Differential effect of FHM2 mutation on synaptic plasticity in distinct hippocampal regions. Cephalalgia, 39:1333-1338. [DOI] [PubMed] [Google Scholar]
- [71].Illarionova NB, Brismar H, Aperia A, Gunnarson E (2014). Role of Na,K-ATPase alpha1 and alpha2 isoforms in the support of astrocyte glutamate uptake. PLoS One, 9:e98469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D (2009). Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda), 24:257-265. [DOI] [PubMed] [Google Scholar]
- [73].Lima FD, Oliveira MS, Furian AF, Souza MA, Rambo LM, Ribeiro LR, et al. (2009). Adaptation to oxidative challenge induced by chronic physical exercise prevents Na+,K+-ATPase activity inhibition after traumatic brain injury. Brain Res, 1279:147-155. [DOI] [PubMed] [Google Scholar]
- [74].Della-Pace ID, Souza TL, Grauncke ACB, Rambo LM, Ribeiro LR, Cipolatto RP, et al. (2019). Modulation of Na(+)/K(+)- ATPase activity by triterpene 3beta, 6beta, 16beta-trihidroxilup-20 (29)-ene (TTHL) limits the long-term secondary degeneration after traumatic brain injury in mice. Eur J Pharmacol, 854:387-397. [DOI] [PubMed] [Google Scholar]
- [75].Biller A, Pflugmann I, Badde S, Diem R, Wildemann B, Nagel AM, et al. (2016). Sodium MRI in Multiple Sclerosis is Compatible with Intracellular Sodium Accumulation and Inflammation-Induced Hyper-Cellularity of Acute Brain Lesions. Sci Rep, 6:31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fao L, Coelho P, Rodrigues RJ, Rego AC (2022). Restored Fyn Levels in Huntington's Disease Contributes to Enhanced Synaptic GluN2B-Composed NMDA Receptors and CREB Activity. Cells, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ellis DZ, Rabe J, Sweadner KJ (2003). Global loss of Na,K-ATPase and its nitric oxide-mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci, 23:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ma X, Liu W (2019). Calcium signaling in brain microvascular endothelial cells and its roles in the function of the blood-brain barrier. Neuroreport, 30:1271-1277. [DOI] [PubMed] [Google Scholar]
- [79].Dalal PJ, Muller WA, Sullivan DP (2020). Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am J Pathol, 190:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Longden TA, Hill-Eubanks DC, Nelson MT (2016). Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab, 36:492-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Earley S, Brayden JE (2015). Transient receptor potential channels in the vasculature. Physiol Rev, 95:645-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Verkhratsky A, Reyes RC, Parpura V (2014). TRP channels coordinate ion signalling in astroglia. Rev Physiol Biochem Pharmacol, 166:1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kwan HY, Huang Y, Yao X (2007). TRP channels in endothelial function and dysfunction. Biochim Biophys Acta, 1772:907-914. [DOI] [PubMed] [Google Scholar]
- [84].Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, et al. (2010). Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem, 285:23466-23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ryu HJ, Kim JE, Kim YJ, Kim JY, Kim WI, Choi SY, et al. (2013). Endothelial transient receptor potential conical channel (TRPC)-3 activation induces vasogenic edema formation in the rat piriform cortex following status epilepticus. Cell Mol Neurobiol, 33:575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, et al. (2021). Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci, 15:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mannari T, Morita S, Furube E, Tominaga M, Miyata S (2013). Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia, 61:957-971. [DOI] [PubMed] [Google Scholar]
- [89].Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT (2013). TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A, 110:6157-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rakkar K, Bayraktutan U (2016). Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochim Biophys Acta, 1862:56-71. [DOI] [PubMed] [Google Scholar]
- [91].Fleegal MA, Hom S, Borg LK, Davis TP (2005). Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol, 289:H2012-2019. [DOI] [PubMed] [Google Scholar]
- [92].Selvatici R, Falzarano S, Franceschetti L, Cavallini S, Marino S, Siniscalchi A (2006). Differential activation of protein kinase C isoforms following chemical ischemia in rat cerebral cortex slices. Neurochem Int, 49:729-736. [DOI] [PubMed] [Google Scholar]
- [93].Zhang Y, Zhang X, Park TS, Gidday JM (2005). Cerebral endothelial cell apoptosis after ischemia-reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab, 25:868-877. [DOI] [PubMed] [Google Scholar]
- [94].Wei S, Low SW, Poore CP, Chen B, Gao Y, Nilius B, et al. (2020). Comparison of Anti-oncotic Effect of TRPM4 Blocking Antibody in Neuron, Astrocyte and Vascular Endothelial Cell Under Hypoxia. Front Cell Dev Biol, 8:562584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chen B, Wei S, Low SW, Poore CP, Lee AT, Nilius B, et al. (2023). TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. (2006). Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med, 12:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mehta RI, Tosun C, Ivanova S, Tsymbalyuk N, Famakin BM, Kwon MS, et al. (2015). Sur1-Trpm4 Cation Channel Expression in Human Cerebral Infarcts. J Neuropathol Exp Neurol, 74:835-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, et al. (2012). Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab, 32:525-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gerzanich V, Kwon MS, Woo SK, Ivanov A, Simard JM (2018). SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One, 13:e0195526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al. (2012). Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol, 72:799-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, et al. (2012). Does inhibiting Sur1 complement rt-PA in cerebral ischemia? Ann N Y Acad Sci, 1268:95-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, et al. (2014). Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care, 20:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rather MA, Khan A, Wang L, Jahan S, Rehman MU, Makeen HA, et al. (2023). TRP channels: Role in neurodegenerative diseases and therapeutic targets. Heliyon, 9:e16910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Arshad A, Chen X, Cong Z, Qing H, Deng Y (2014). TRPC1 protects dopaminergic SH-SY5Y cells from MPP+, salsolinol, and N-methyl-(R)-salsolinol-induced cytotoxicity. Acta Biochim Biophys Sin (Shanghai), 46:22-30. [DOI] [PubMed] [Google Scholar]
- [105].Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, et al. (2016). Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington's Disease Mouse Model. J Neurosci, 36:125-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Seifert G, Henneberger C, Steinhauser C (2018). Diversity of astrocyte potassium channels: An update. Brain Res Bull, 136:26-36. [DOI] [PubMed] [Google Scholar]
- [107].Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, et al. (2017). Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci, 20:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Moshkforoush A, Ashenagar B, Harraz OF, Dabertrand F, Longden TA, Nelson MT, et al. (2020). The capillary Kir channel as sensor and amplifier of neuronal signals: Modeling insights on K(+)-mediated neurovascular communication. Proc Natl Acad Sci U S A, 117:16626-16637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Negri S, Faris P, Soda T, Moccia F (2021). Endothelial signaling at the core of neurovascular coupling: The emerging role of endothelial inward-rectifier K(+) (K(ir)2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow. Int J Biochem Cell Biol, 135:105983. [DOI] [PubMed] [Google Scholar]
- [110].Hosford PS, Christie IN, Niranjan A, Aziz Q, Anderson N, Ang R, et al. (2019). A critical role for the ATP-sensitive potassium channel subunit K(IR)6.1 in the control of cerebral blood flow. J Cereb Blood Flow Metab, 39:2089-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hariharan A, Robertson CD, Garcia DCG, Longden TA (2022). Brain capillary pericytes are metabolic sentinels that control blood flow through a K(ATP) channel-dependent energy switch. Cell Rep, 41:111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sancho M, Klug NR, Mughal A, Koide M, Huerta de la Cruz S, Heppner TJ, et al. (2022). Adenosine signaling activates ATP-sensitive K(+) channels in endothelial cells and pericytes in CNS capillaries. Sci Signal, 15:eabl5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang H, Zhang X, Hong X, Tong X (2021). Homogeneity or heterogeneity, the paradox of neurovascular pericytes in the brain. Glia, 69:2474-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wang S, Wang B, Shang D, Zhang K, Yan X, Zhang X (2022). Ion Channel Dysfunction in Astrocytes in Neurodegenerative Diseases. Front Physiol, 13:814285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT (2010). Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A, 107:3811-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Noh W, Pak S, Choi G, Yang S, Yang S (2019). Transient Potassium Channels: Therapeutic Targets for Brain Disorders. Front Cell Neurosci, 13:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, et al. (2014). Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci, 34:13586-13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chen YJ, Wallace BK, Yuen N, Jenkins DP, Wulff H, O'Donnell ME (2015). Blood-brain barrier KCa3.1 channels: evidence for a role in brain Na uptake and edema in ischemic stroke. Stroke, 46:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Elorza-Vidal X, Gaitan-Penas H, Estevez R (2019). Chloride Channels in Astrocytes: Structure, Roles in Brain Homeostasis and Implications in Disease. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gascoigne DA, Drobyshevsky A, Aksenov DP (2021). The Contribution of Dysfunctional Chloride Channels to Neurovascular Deficiency and Neurodegeneration. Front Pharmacol, 12:754743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002). Molecular structure and physiological function of chloride channels. Physiol Rev, 82:503-568. [DOI] [PubMed] [Google Scholar]
- [122].Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hubner CA, et al. (2007). Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci, 27:6581-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Korte N, Ilkan Z, Pearson CL, Pfeiffer T, Singhal P, Rock JR, et al. (2022). The Ca2+-gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J Clin Invest, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Suzuki T, Yasumoto M, Suzuki Y, Asai K, Imaizumi Y, Yamamura H (2020). TMEM16A Ca(2+)-Activated Cl(-) Channel Regulates the Proliferation and Migration of Brain Capillary Endothelial Cells. Mol Pharmacol, 98:61-71. [DOI] [PubMed] [Google Scholar]
- [125].Wang Z, Choi K (2023). Pharmacological modulation of chloride channels as a therapeutic strategy for neurological disorders. Front Physiol, 14:1122444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cortez MA, Li C, Whitehead SN, Dhani SU, D'Antonio C, Huan LJ, et al. (2010). Disruption of ClC-2 expression is associated with progressive neurodegeneration in aging mice. Neuroscience, 167:154-162. [DOI] [PubMed] [Google Scholar]
- [127].Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA (2014). LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol, 592:4855-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shen M, Wang L, Wang B, Wang T, Yang G, Shen L, et al. (2014). Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt. Cell Death Dis, 5:e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Xinhan L, Matsushita M, Numaza M, Taguchi A, Mitsui K, Kanazawa H (2011). Na+/H+ exchanger isoform 6 (NHE6/SLC9A6) is involved in clathrin-dependent endocytosis of transferrin. Am J Physiol Cell Physiol, 301:C1431-1444. [DOI] [PubMed] [Google Scholar]
- [130].Xu H, Ghishan FK, Kiela PR (2018). SLC9 Gene Family: Function, Expression, and Regulation. Compr Physiol, 8:555-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Luo J, Sun D (2007). Physiology and pathophysiology of Na(+)/H(+) exchange isoform 1 in the central nervous system. Curr Neurovasc Res, 4:205-215. [DOI] [PubMed] [Google Scholar]
- [132].Leng T, Shi Y, Xiong ZG, Sun D (2014). Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog Neurobiol, 115:189-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sipos H, Torocsik B, Tretter L, Adam-Vizi V (2005). Impaired regulation of pH homeostasis by oxidative stress in rat brain capillary endothelial cells. Cell Mol Neurobiol, 25:141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liktor-Busa E, Blawn KT, Kellohen KL, Wiese BM, Verkhovsky V, Wahl J, et al. (2020). Functional NHE1 expression is critical to blood brain barrier integrity and sumatriptan blood to brain uptake. PLoS One, 15:e0227463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lam TI, Wise PM, O'Donnell ME (2009). Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol Cell Physiol, 297:C278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nakamura K, Kamouchi M, Kitazono T, Kuroda J, Matsuo R, Hagiwara N, et al. (2008). Role of NHE1 in calcium signaling and cell proliferation in human CNS pericytes. Am J Physiol Heart Circ Physiol, 294:H1700-1707. [DOI] [PubMed] [Google Scholar]
- [137].Nakamura K, Kamouchi M, Arimura K, Nishimura A, Kuroda J, Ishitsuka K, et al. (2012). Extracellular acidification activates cAMP responsive element binding protein via Na+/H+ exchanger isoform 1-mediated Ca(2)(+) oscillation in central nervous system pericytes. Arterioscler Thromb Vasc Biol, 32:2670-2677. [DOI] [PubMed] [Google Scholar]
- [138].Theparambil SM, Deitmer JW (2015). High effective cytosolic H+ buffering in mouse cortical astrocytes attributable to fast bicarbonate transport. Glia, 63:1581-1594. [DOI] [PubMed] [Google Scholar]
- [139].Begum G, Song S, Wang S, Zhao H, Bhuiyan MIH, Li E, et al. (2018). Selective knockout of astrocytic Na(+) /H(+) exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia, 66:126-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Cengiz P, Kintner DB, Chanana V, Yuan H, Akture E, Kendigelen P, et al. (2014). Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One, 9:e84294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Liu Q, Bhuiyan MIH, Liu R, Song S, Begum G, Young CB, et al. (2021). Attenuating vascular stenosis-induced astrogliosis preserves white matter integrity and cognitive function. J Neuroinflammation, 18:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Metwally SAH, Paruchuri SS, Yu L, Capuk O, Pennock N, Sun D, et al. (2023). Pharmacological Inhibition of NHE1 Protein Increases White Matter Resilience and Neurofunctional Recovery after Ischemic Stroke. Int J Mol Sci, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, et al. (1999). Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol, 276:C788-795. [DOI] [PubMed] [Google Scholar]
- [144].Verma V, Bali A, Singh N, Jaggi AS (2015). Implications of sodium hydrogen exchangers in various brain diseases. J Basic Clin Physiol Pharmacol, 26:417-426. [DOI] [PubMed] [Google Scholar]
- [145].Theparambil SM, Ruminot I, Schneider HP, Shull GE, Deitmer JW (2014). The electrogenic sodium bicarbonate cotransporter NBCe1 is a high-affinity bicarbonate carrier in cortical astrocytes. J Neurosci, 34:1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, et al. (2008). Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience, 155:818-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Larsen BR, MacAulay N (2017). Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia, 65:1668-1681. [DOI] [PubMed] [Google Scholar]
- [148].Ye Q, Jo J, Wang CY, Oh H, Choy TJ, Kim K, et al. (2023). Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a NO-CCL2-CCR2 pathway. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Pressey JC, de Saint-Rome M, Raveendran VA, Woodin MA (2023). Chloride transporters controlling neuronal excitability. Physiol Rev, 103:1095-1135. [DOI] [PubMed] [Google Scholar]
- [150].Li Q, Aalling NN, Forstera B, Erturk A, Nedergaard M, Mollgard K, et al. (2020). Aquaporin 1 and the Na(+)/K(+)/2Cl(-) cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system. Fluids Barriers CNS, 17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Loo L, Simon JM, Xing L, McCoy ES, Niehaus JK, Guo J, et al. (2019). Single-cell transcriptomic analysis of mouse neocortical development. Nat Commun, 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Simard JM, Kahle KT, Gerzanich V (2010). Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg, 113:622-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Klug NR, Chechneva OV, Hung BY, O'Donnell ME (2021). High glucose-induced effects on Na(+)-K(+)-2Cl(-) cotransport and Na(+)/H(+) exchange of blood-brain barrier endothelial cells: involvement of SGK1, PKCbetaII, and SPAK/OSR1. Am J Physiol Cell Physiol, 320:C619-C634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Schiapparelli P, Guerrero-Cazares H, Magana-Maldonado R, Hamilla SM, Ganaha S, Goulin Lippi Fernandes E, et al. (2017). NKCC1 Regulates Migration Ability of Glioblastoma Cells by Modulation of Actin Dynamics and Interacting with Cofilin. EBioMedicine, 21:94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, et al. (2016). Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun, 7:10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Luo WD, Min JW, Huang WX, Wang X, Peng YY, Han S, et al. (2018). Vitexin reduces epilepsy after hypoxic ischemia in the neonatal brain via inhibition of NKCC1. J Neuroinflammation, 15:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kurki SN, Uvarov P, Pospelov AS, Trontti K, Hubner AK, Srinivasan R, et al. (2023). Expression patterns of NKCC1 in neurons and non-neuronal cells during cortico-hippocampal development. Cereb Cortex, 33:5906-5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bulley S, Jaggar JH (2014). Cl(-) channels in smooth muscle cells. Pflugers Arch, 466:861-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Untiet V, Kovermann P, Gerkau NJ, Gensch T, Rose CR, Fahlke C (2017). Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation. Glia, 65:388-400. [DOI] [PubMed] [Google Scholar]
- [160].Macaulay N, Zeuthen T (2012). Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res, 37:2299-2309. [DOI] [PubMed] [Google Scholar]
- [161].Henneberger C, Bard L, Panatier A, Reynolds JP, Kopach O, Medvedev NI, et al. (2020). LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron, 108:919-936 e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lu KT, Huang TC, Tsai YH, Yang YL (2017). Transient receptor potential vanilloid type 4 channels mediate Na-K-Cl-co-transporter-induced brain edema after traumatic brain injury. J Neurochem, 140:718-727. [DOI] [PubMed] [Google Scholar]
- [163].Zhang M, Cui Z, Cui H, Cao Y, Zhong C, Wang Y (2016). Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci, 17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. (2017). Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med, 23:997-1003. [DOI] [PubMed] [Google Scholar]
- [165].Luo L, Guan X, Begum G, Ding D, Gayden J, Hasan MN, et al. (2020). Blockade of Cell Volume Regulatory Protein NKCC1 Increases TMZ-Induced Glioma Apoptosis and Reduces Astrogliosis. Mol Cancer Ther, 19:1550-1561. [DOI] [PubMed] [Google Scholar]
- [166].Luo L, Wang J, Ding D, Hasan MN, Yang SS, Lin SH, et al. (2020). Role of NKCC1 Activity in Glioma K(+) Homeostasis and Cell Growth: New Insights With the Bumetanide-Derivative STS66. Front Physiol, 11:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Dargaei Z, Bang JY, Mahadevan V, Khademullah CS, Bedard S, Parfitt GM, et al. (2018). Restoring GABAergic inhibition rescues memory deficits in a Huntington's disease mouse model. Proc Natl Acad Sci U S A, 115:E1618-E1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Lozovaya N, Eftekhari S, Cloarec R, Gouty-Colomer LA, Dufour A, Riffault B, et al. (2018). GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat Commun, 9:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Annunziato L, Pignataro G, Boscia F, Sirabella R, Formisano L, Saggese M, et al. (2007). ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann N Y Acad Sci, 1099:413-426. [DOI] [PubMed] [Google Scholar]
- [170].Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S (2007). Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium, 41:221-234. [DOI] [PubMed] [Google Scholar]
- [171].Khananshvili D (2013). The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Mol Aspects Med, 34:220-235. [DOI] [PubMed] [Google Scholar]
- [172].Rapoport SI (2000). Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol, 20:217-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Chen S, Shao L, Ma L (2021). Cerebral Edema Formation After Stroke: Emphasis on Blood-Brain Barrier and the Lymphatic Drainage System of the Brain. Front Cell Neurosci, 15:716825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Casas AI, Kleikers PW, Geuss E, Langhauser F, Adler T, Busch DH, et al. (2019). Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J Clin Invest, 129:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Annunziato L, Secondo A, Pignataro G, Scorziello A, Molinaro P (2020). New perspectives for selective NCX activators in neurodegenerative diseases. Cell Calcium, 87:102170. [DOI] [PubMed] [Google Scholar]
- [176].Molinaro P, Sirabella R, Pignataro G, Petrozziello T, Secondo A, Boscia F, et al. (2016). Neuronal NCX1 overexpression induces stroke resistance while knockout induces vulnerability via Akt. J Cereb Blood Flow Metab, 36:1790-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Casamassa A, La Rocca C, Sokolow S, Herchuelz A, Matarese G, Annunziato L, et al. (2016). Ncx3 gene ablation impairs oligodendrocyte precursor response and increases susceptibility to experimental autoimmune encephalomyelitis. Glia, 64:1124-1137. [DOI] [PubMed] [Google Scholar]
- [178].Pannaccione A, Secondo A, Molinaro P, D'Avanzo C, Cantile M, Esposito A, et al. (2012). A new concept: Abeta1-42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J Neurosci, 32:10609-10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010). Structure and function of the blood-brain barrier. Neurobiol Dis, 37:13-25. [DOI] [PubMed] [Google Scholar]
- [180].Harilal S, Jose J, Parambi DGT, Kumar R, Unnikrishnan MK, Uddin MS, et al. (2020). Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res Bull, 160:121-140. [DOI] [PubMed] [Google Scholar]
- [181].Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, Jansen JFA, Backes WH (2018). Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci Biobehav Rev, 90:26-33. [DOI] [PubMed] [Google Scholar]
- [182].Rajeev V, Fann DY, Dinh QN, Kim HA, De Silva TM, Lai MKP, et al. (2022). Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics, 12:1639-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Gireud-Goss M, Mack AF, McCullough LD, Urayama A (2021). Cerebral Amyloid Angiopathy and Blood-Brain Barrier Dysfunction. Neuroscientist, 27:668-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tayler H, Miners JS, Guzel O, MacLachlan R, Love S (2021). Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer's disease, vascular dementia and mixed dementia. Brain Pathol, 31:e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].de Lores Arnaiz GR, Ordieres MG (2014). Brain Na(+), K(+)-ATPase Activity In Aging and Disease. Int J Biomed Sci, 10:85-102. [PMC free article] [PubMed] [Google Scholar]
- [186].Vitvitsky VM, Garg SK, Keep RF, Albin RL, Banerjee R (2012). Na+ and K+ ion imbalances in Alzheimer's disease. Biochim Biophys Acta, 1822:1671-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Ohnishi T, Yanazawa M, Sasahara T, Kitamura Y, Hiroaki H, Fukazawa Y, et al. (2015). Na, K-ATPase alpha3 is a death target of Alzheimer patient amyloid-beta assembly. Proc Natl Acad Sci U S A, 112:E4465-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Mann CN, Devi SS, Kersting CT, Bleem AV, Karch CM, Holtzman DM, et al. (2022). Astrocytic alpha2-Na(+)/K(+) ATPase inhibition suppresses astrocyte reactivity and reduces neurodegeneration in a tauopathy mouse model. Sci Transl Med, 14:eabm4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Sasahara T, Satomura K, Tada M, Kakita A, Hoshi M (2021). Alzheimer's Abeta assembly binds sodium pump and blocks endothelial NOS activity via ROS-PKC pathway in brain vascular endothelial cells. iScience, 24:102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Philbert SA, Xu J, Scholefield M, Church SJ, Unwin RD, Cooper GJS (2022). Contrasting Sodium and Potassium Perturbations in the Hippocampus Indicate Potential Na(+)/K(+)-ATPase Dysfunction in Vascular Dementia. Front Aging Neurosci, 14:822787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Adav SS, Qian J, Ang YL, Kalaria RN, Lai MK, Chen CP, et al. (2014). iTRAQ quantitative clinical proteomics revealed role of Na(+)K(+)-ATPase and its correlation with deamidation in vascular dementia. J Proteome Res, 13:4635-4646. [DOI] [PubMed] [Google Scholar]
- [192].Cascella R, Cecchi C (2021). Calcium Dyshomeostasis in Alzheimer's Disease Pathogenesis. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Verkhratsky A (2019). Astroglial Calcium Signaling in Aging and Alzheimer's Disease. Cold Spring Harb Perspect Biol, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Verkhratsky A, Nedergaard M (2018). Physiology of Astroglia. Physiol Rev, 98:239-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Daschil N, Obermair GJ, Flucher BE, Stefanova N, Hutter-Paier B, Windisch M, et al. (2013). CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-beta plaques in an Alzheimer's disease mouse model. J Alzheimers Dis, 37:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ronco V, Grolla AA, Glasnov TN, Canonico PL, Verkhratsky A, Genazzani AA, et al. (2014). Differential deregulation of astrocytic calcium signalling by amyloid-beta, TNFalpha, IL-1beta and LPS. Cell Calcium, 55:219-229. [DOI] [PubMed] [Google Scholar]
- [197].Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009). Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science, 323:1211-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zott B, Busche MA, Sperling RA, Konnerth A (2018). What Happens with the Circuit in Alzheimer's Disease in Mice and Humans? Annu Rev Neurosci, 41:277-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Delekate A, Fuchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun, 5:5422. [DOI] [PubMed] [Google Scholar]
- [200].Takano T, Han X, Deane R, Zlokovic B, Nedergaard M (2007). Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer's disease. Ann N Y Acad Sci, 1097:40-50. [DOI] [PubMed] [Google Scholar]
- [201].Chow SK, Yu D, Macdonald CL, Buibas M, Silva GA (2010). Amyloid beta-peptide directly induces spontaneous calcium transients, delayed intercellular calcium waves and gliosis in rat cortical astrocytes. ASN Neuro, 2:e00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Lee KI, Lee HT, Lin HC, Tsay HJ, Tsai FC, Shyue SK, et al. (2016). Role of transient receptor potential ankyrin 1 channels in Alzheimer's disease. J Neuroinflammation, 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Hong C, Jeong B, Park HJ, Chung JY, Lee JE, Kim J, et al. (2020). TRP Channels as Emerging Therapeutic Targets for Neurodegenerative Diseases. Front Physiol, 11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Liu L, Chen M, Lin K, Xiang X, Yang J, Zheng Y, et al. (2020). TRPC6 Attenuates Cortical Astrocytic Apoptosis and Inflammation in Cerebral Ischemic/Reperfusion Injury. Front Cell Dev Biol, 8:594283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Bai JZ, Lipski J (2014). Involvement of TRPV4 channels in Abeta(40)-induced hippocampal cell death and astrocytic Ca(2+) signalling. Neurotoxicology, 41:64-72. [DOI] [PubMed] [Google Scholar]
- [206].Balasubramanian P, DelFavero J, Ungvari A, Papp M, Tarantini A, Price N, et al. (2020). Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev, 64:101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Negri S, Sanford M, Shi H, Tarantini S (2023). The role of endothelial TRP channels in age-related vascular cognitive impairment and dementia. Front Aging Neurosci, 15:1149820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, et al. (2014). The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nat Commun, 5:5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Yang E, Kaddoumi A (2021). Role of endothelial TRPA1 expression in blood-brain barrier dysfunction. Alzheimer's & Dementia, 17:e058550. [Google Scholar]
- [210].Nimmrich V, Eckert A (2013). Calcium channel blockers and dementia. Br J Pharmacol, 169:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Taylor JL, Walsh KR, Mosneag IE, Danby TGE, Luka N, Chanda B, et al. (2023). Uncoupling of Ca(2+) sparks from BK channels in cerebral arteries underlies hypoperfusion in hypertension-induced vascular dementia. Proc Natl Acad Sci U S A, 120:e2307513120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lv J, Xiao X, Bi M, Tang T, Kong D, Diao M, et al. (2022). ATP-sensitive potassium channels: A double-edged sword in neurodegenerative diseases. Ageing Res Rev, 80:101676. [DOI] [PubMed] [Google Scholar]
- [213].Griffith CM, Xie MX, Qiu WY, Sharp AA, Ma C, Pan A, et al. (2016). Aberrant expression of the pore-forming K(ATP) channel subunit Kir6.2 in hippocampal reactive astrocytes in the 3xTg-AD mouse model and human Alzheimer's disease. Neuroscience, 336:81-101. [DOI] [PubMed] [Google Scholar]
- [214].Wilcock DM, Vitek MP, Colton CA (2009). Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience, 159:1055-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Eide PK, Hansson HA (2018). Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol, 44:474-490. [DOI] [PubMed] [Google Scholar]
- [216].Sudduth TL, Weekman EM, Price BR, Gooch JL, Woolums A, Norris CM, et al. (2017). Time-course of glial changes in the hyperhomocysteinemia model of vascular cognitive impairment and dementia (VCID). Neuroscience, 341:42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Scarmeas N, Honig LS, Choi H, Cantero J, Brandt J, Blacker D, et al. (2009). Seizures in Alzheimer disease: who, when, and how common? Arch Neurol, 66:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Yi M, Yu P, Lu Q, Geller HM, Yu Z, Chen H (2016). KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer's disease. Mol Cell Neurosci, 76:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Willis CL, Brooks TA, Davis TP (2008). Chronic inflammatory pain and the neurovascular unit: a central role for glia in maintaining BBB integrity? Curr Pharm Des, 14:1625-1643. [DOI] [PubMed] [Google Scholar]
- [220].Taylor JL, Pritchard HAT, Walsh KR, Strangward P, White C, Hill-Eubanks D, et al. (2022). Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer's disease. Proc Natl Acad Sci U S A, 119:e2204581119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Boscia F, Pannaccione A, Ciccone R, Casamassa A, Franco C, Piccialli I, et al. (2017). The expression and activity of K(V)3.4 channel subunits are precociously upregulated in astrocytes exposed to Abeta oligomers and in astrocytes of Alzheimer's disease Tg2576 mice. Neurobiol Aging, 54:187-198. [DOI] [PubMed] [Google Scholar]
- [222].Liu R, Wang J, Chen Y, Collier JM, Capuk O, Jin S, et al. (2022). NOX activation in reactive astrocytes regulates astrocytic LCN2 expression and neurodegeneration. Cell Death Dis, 13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kim H, Leng K, Park J, Sorets AG, Kim S, Shostak A, et al. (2022). Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat Commun, 13:6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Lacalle-Aurioles M, Trigiani LJ, Bourourou M, Lecrux C, Hamel E (2022). Alzheimer's disease and cerebrovascular pathology alter inward rectifier potassium (K(IR) 2.1) channels in endothelium of mouse cerebral arteries. Br J Pharmacol, 179:2259-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Hakim MA, Behringer EJ (2020). Development of Alzheimer's Disease Progressively Alters Sex-Dependent KCa and Sex-Independent KIR Channel Function in Cerebrovascular Endothelium. J Alzheimers Dis, 76:1423-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT (2021). PIP(2) Improves Cerebral Blood Flow in a Mouse Model of Alzheimer's Disease. Function (Oxf), 2:zqab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Benito C, Tolon RM, Castillo AI, Ruiz-Valdepenas L, Martinez-Orgado JA, Fernandez-Sanchez FJ, et al. (2012). beta-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-alpha, PPAR-gamma and TRPV1, but not CB(1) or CB(2) receptors. Br J Pharmacol, 166:1474-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Linde CI, Baryshnikov SG, Mazzocco-Spezzia A, Golovina VA (2011). Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein. Am J Physiol Cell Physiol, 300:C1502-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]