Abstract
The fact that the human epidermal growth factor receptor 2 (HER2)-low group, historically classified as HER2 negative in breast cancer histology, benefited from HER2-targeted treatments similarly to the HER2-positive group indicates that this group has a distinct histology from the HER2-0 group. The effectiveness of cyclin-dependent kinase 4/6 inhibitors, which are the standard first-line treatment for hormone receptor-positive, HER2-negative advanced breast cancer, in this newly defined histological subgroup remains a topic of debate. In our study, we examined the impact of HER2 status on the efficacy of CDK4/6 inhibitors. Our study is a retrospective, multicenter, real-world data analysis. One hundred sixty patients were included in the study. The relationship between HER2 status and other clinical-pathological features, as well as progression-free survival, was examined. Median follow-up was 20.33 ± 0.98 months. The mPFS could not be reached. All patients exhibited positive estrogen receptor expression. Among the patients, 111 (69.4%) were categorized as HER2-0, and 49 (30.6%) as HER2-low. The 24-month progression-free survival rates were similar between HER2-0 and HER2-low patients (60.6% vs 65.3%, hormone receptor: 1.18, CI: 0.67–2.20, P = .554). We established that the mPFS achieved with cyclin-dependent kinase 4/6 inhibitors as a first-line therapy for patients with advanced breast cancer is unaffected by HER2 status.
Keywords: advanced stage breast cancer, HER2 low status, palbociclib, ribociclib
1. Introduction
Breast cancer is the most common cancer in female patients.[1] It is a complex condition comprising various molecular subtypes. Estrogen receptor (ER), progesterone receptor (PR) expression, and human epidermal growth factor receptor 2 (HER2) status are critical factors in both prognosis and treatment selection. Although there has been a notable improvement in survival rates for breast cancer in recent years, advanced-stage breast cancer remains an incurable disease. However, with appropriate medication and supportive care, patients can live for extended periods.
The development of cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors has significantly improved the treatment of hormone receptor (HR)-positive, HER2-negative breast cancer. The combination of CDK 4/6 inhibitors with aromatase inhibitors or fulvestrant has become the established initial therapy for women with advanced-stage HR-positive breast cancer. These combinations have improved median progression-free survival (mPFS) in all patients diagnosed with advanced stage breast cancer, including male patients.[2–7]
HER2-low expression is a subtype that has been the subject of research in recent years and is defined as an HER2 immunohistochemistry (IHC) score of 1+ or 2+ without gene amplification.[8] Novel antibody-drug conjugates targeting HER2 have demonstrated remarkable therapeutic advantages in patients with HER2-low expression.[9,10] Additionally, findings from the DESTINY-Breast04 trial have underscored the efficacy of trastuzumab deruxtecan in HER2-low advanced-stage breast cancer.[11] These promising trial outcomes are reshaping the treatment landscape for breast cancer, sparking increased interest in establishing a HER2-low category in breast cancer evaluation.[12]
In our study, we aimed to determine the effect of HER2-low status on the effectiveness of CDK 4/6 inhibitors.
2. Materials and methods
Our study was a multicenter, retrospective analysis including female patients diagnosed with advanced stage HR-positive, HER2-negative breast cancer. Patients receiving a combination therapy containing palbociclib or ribociclib were included in the study.
Patients who had previously received CDK 4 to 6 inhibitors or who received in the 2nd or subsequent lines of treatment were not included. The baseline clinical features such as age, date of diagnosis, menopause status, CDK 4 to 6 onset date, metastasis sites at the start of CDK 4 to 6 inhibitor therapy, date of disease progression and date of death were recorded. Additionally, pathological features were recorded.
Version 26 of the SPSS was used in the statistical analysis of the study. The χ2 test was used to analyze categorical variables. The Kaplan–Meier method was employed to evaluate the cumulative survival rates of patients with breast cancer. P values <.05 were considered statistically significant.
3. Results
Our study included a total of 160 patients from 5 different tertiary hospitals. The median age of patients was 58.90 ± 12.15 years. All of the patients’ ER expression were positive and PR expression was negative in 9 (5.6%) patients. Among the patients, 111 (69.4%) were categorized as HER2 0, and 49 (30.6%) as HER2 low. Of the patients, 42 (26.2%) were premenopausal, and 118 (73.8%) were postmenopausal. In terms of age, 109 (68.1%) patients were 65 years of age or younger, while 51 (31.9%) patients were over 65. Eighty-three (52.9%) patients had recurrent disease, while 77 (48.1%) patients were metastatic at diagnosis. Visceral metastases were absent in 95 (59.4%) patients but present in 65 (40.6%). Among the patients, 53 (33.1%) had lung metastases, 14 (8.8%) had liver metastases, and 5 (3.1%) had brain metastases. Furthermore, 124 (77.5%) patients had bone metastases, while 36 (22.5%) did not. Treatment consisted of palbociclib for 63 (39.4%) patients and ribociclib for 97 (60.6%) patients. While 121 (75.6%) of the patients used aromatase inhibitors along with CDK 4 to 6 inhibitors, 39 (24.4%) used fulvestrant. Baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics of patients.
| All patients | HER2 0 group (N:111) | HER2 low group (N:49) |
||
|---|---|---|---|---|
| Characteristics | N (%) | N (%) | N (%) | P value |
| Age ≤65 >65 |
109 (68.1) 51 (31.9) |
77 (69.4) 34 (30.6) |
32 (65.3) 17 (34.7) |
.713 |
| Estrogen receptor expression Positive Negative |
160 (100) ‐(0) |
111 (100) |
49 (100) |
1 |
| Progesterone receptor expression Positive Negative |
151 (94.4) 9 (5.6) |
106 (95.5) 5 (4.5) |
45 (91.8) 4 (8.2) |
.355 |
| Menopause status Premenopausal Postmenopausal |
42 (26.2) 118 (73.8) |
29 (26.1) 82 (73.9) |
13 (26.5) 36 (73.5) |
.957 |
| Disease status De novo metastatic Recurrent disease |
77 (48.1) 83 (52.9) |
52 (46.8) 59 (53.2) |
25 (51) 24 (49) |
.732 |
| Visceral metastases Presence Absence |
65 (40.6) 95 (59.4) |
45 (40.5) 66 (59.5) |
20 (40.8) 29 (59.2) |
1 |
| Bone metastases Presence Absence |
124 (77.5) 36 (22.5) |
86 (77.5) 25 (22.5) |
38 (77.6) 11 (22.4) |
1 |
| Treatment agent Palbociclib Ribociclib |
63 (39.4) 97 (60.6) |
38 (34.2) 73 (65.8) |
25 (51) 24 (49) |
.054 |
| Endocrine therapy Aromatase inhibitors Fulvestrant |
121 (75.6) 39 (24.4) |
81 (73.0) 30 (27.0) |
40 (81.6) 9 (18.4) |
.318 |
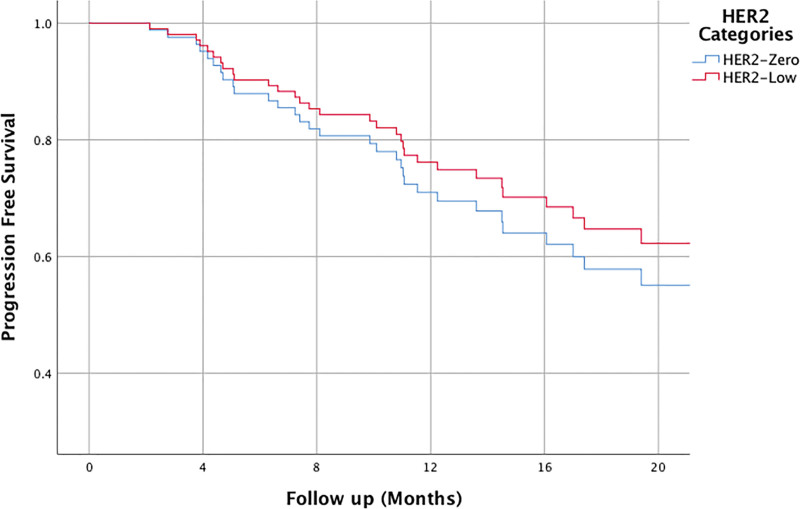
The median follow-up period was approximately 20.33 ± 0.98 months, during which disease progression was observed in 55 patients, resulting in the death of 12 patients. The progression-free survival (PFS) time could not be determined during the follow-up period, but the PFS rate at 24 months was 62.3%. There were no differences in PFS rates between patients ≤65 and >65 years old (PFS rate at 24 months: 60.7% vs 66.3%, hazard ratio [HR]: 1.34, confidence interval [CI]: 0.73–2.46, P = .341). Additionally, no significant differences were observed in PFS rates between HER2-negative patients and HER2 low patients (PFS rate at 24 months: 60.6% vs 65.3%, HR: 1.09, CI: 0.82–1.44, P = .554). (The relationship between PFS and HER2 status is presented in Fig. 1.) Premenopausal and postmenopausal patients have similar 24 months PFS rate (PFS rate at 24 months: 69.3% vs 60.0%, HR: 0.93, CI: 0.68–1.27, P = .668). PFS rate was statistically significantly worse in de novo metastatic patients than in recurrent metastatic patients (PFS rate at 24 months: 70.9% vs 54.2%, HR: 1.90, CI: 1.09–3.30, P = .022). For patients with visceral organ metastases, the PFS rate was numerically lower, but this difference was statistically insignificant compared to patients without visceral metastases (PFS rate at 24 months: 56.6% vs 66.9%, HR: 1.43, CI: 0.84–2.43, P = .184). There was no difference in PFS between patients with and without bone metastases (PFS rate at 24 months: 67.7% vs 61.2%, HR: 0.87, CI: 0.62–1.24, P = .462). Additionally, there were no differences in terms of PFS in the presence of lung, liver metastases.
Figure 1.
The relationship between HER2 status and progression free survival.
The comparison between palbociclib treatment and ribociclib treatment also did not reveal significant differences in terms of PFS (PFS rate at 24 months: 60.3% vs 63.9%, HR: 1.06, CI: 0.81–1.39, P = .652). PFS was found to be 31.83 ± 3.17 months in patients using aromatase inhibitors together with CDK 4-6 inhibitors, while it was 18.96 ± 1.50 months in patients using fulvestrant together (HR: 0.49, CI: 0.27–0.88, P = .017). The relationship between PFS and clinical and pathological factors is presented in Table 2.
Table 2.
The relationship between PFS and clinical-pathological features.
| Clinical factors | HR | 95% CI | P value |
|---|---|---|---|
| Age ≤65 vs >65 |
1.34 | 0.73–2.46 | .331 |
| Progesterone receptor expression Positive vs negative |
1.03 | 0.36–2.94 | .955 |
| Human epidermal growth factor receptor 2 HER2 0 vs HER2 low |
1.09 | 0.82–1.44 | .554 |
| Menopause status Premenopausal vs postmenopausal |
0.93 | 0.68–1.27 | .668 |
| Disease status De novo metastatic vs recurrent disease |
1.90 | 1.09–3.30 | .022 |
| Visceral metastases Presence vs absence |
1.43 | 0.84–2.43 | .184 |
| Bone metastases Presence vs absence |
0.87 | 0.62–1.24 | .462 |
| Treatment agent Palbociclib vs ribociclib |
1.06 | 0.81–1.39 | .652 |
| Endocrine therapy Aromatase inhibitors vs fulvestrant |
0.49 | 0.27–0.88 | .017 |
The bold values indicate statistically significant data.
The distribution of patients in the HER2-negative group and HER2 low group is outlined in Table 1. No significant differences were observed in terms of age, PR status, menopausal status, de novo or recurrent disease status, site of metastases, the type of CDK 4-6 agent given or hormonotherapy agent used with CDK 4 to 6 inhibitor (P-values: .713, .355, .957, .626, 0.974, .978, .861, .992, .054, .318, respectively).
4. Discussions
The incorporation of CDK 4 to 6 inhibitors alongside aromatase inhibitors or fulvestrant represents the contemporary standard in managing HR-positive, HER2-negative advanced breast cancer. Nonetheless, it is noteworthy that in phase 3 trials assessing the efficacy of CDK 4 to 6 inhibitors, patients categorized as HER2 negative included individuals with HER2 low expression. Regrettably, a distinct analysis specific to the subset of patients with HER2 low expression was not conducted.[2,13–15] Following the findings of the Destiny 04 study, which demonstrated the efficacy of trastuzumab deruxtecan in the cohort characterized as HER2 low, there is ongoing discussion regarding whether this group represents a distinct subtype compared to HER2-negative patients. This debate extends to its potential impact on the efficacy of CDK 4 to 6 inhibitors.[11,16–19] Our study findings indicate that there is no discernible correlation between the efficacy of CDK 4 to 6 inhibitors and the HER2 low status.
While a mPFS rate of approximately 24 months was observed with CDK 4 to 6 inhibitors, the occurrence of disease progression in some patients within a notably shorter timeframe prompted further analyses among progressing patients. Findings from the PALOMA 2 and 3 trials, utilizing tissue samples analyzed with The EdgeSeq Oncology Biomarker Panel, indicated superior efficacy among Luminal A and Luminal B subtypes.[20] When utilizing NanoString technology to assess the intrinsic subtypes of tissue samples from patients enrolled in the MONALEESA studies, it was observed that ribociclib conferred benefit to all patients, with the exception of those exhibiting basal-like characteristics.[21] These studies have underscored the heterogeneity among patients categorized as HR-positive and HER2-negative, revealing differences in intrinsic subtypes and varied efficacies of CDK 4 to 6 inhibitors across these subtypes. The recent recognition of the heterogeneity within the HER2-negative group, encompassing both HER2 0 and HER2 low patients, has prompted investigations into the association between HER2 expression levels and the activity of CDK 4 to 6 inhibitors.
In the existing literature, only 4 studies have investigated the impact of HER2 low status on the efficacy of CDK 4 to 6 inhibitors. Among these studies, limitations were observed, including a relatively small sample size of 54 patients receiving CDK 4 to 6 inhibitors in the initial phase and a predominance of approximately 85% of patients receiving palbociclib in the study conducted by Bao et al. Importantly, these studies collectively found that mPFS was statistically significantly shorter in the HER2 low group.[17] In this particular study, the incidence of HER2 low status was observed to be 77%, whereas in our study and other similar investigations, it hovered around 30%. However, the exclusion of patients solely treated in the first-line setting, coupled with the low number of patients receiving first-line treatment, presents challenges in assessing the impact of HER2 low status. In our study, comprising 160 patients treated in the first line, the utilization of palbociclib and ribociclib was relatively more balanced. Notably, in our study, mPFS appeared comparable between the HER2 0 and HER2 low groups.
In the study with a comparable patient cohort size to ours, comprising 165 patients treated with palbociclib, a comparison was made between those classified as HER2 0 or HER2 low. mPFS was reported as 23 months for the HER2 0 group and 19 months for the HER2 low group, revealing no significant difference between the 2 groups.[18] In the Italian study, it was observed that both mPFS and mOS were notably shorter in the HER2 low group when compared to the HER2 0 group.[19] In the study conducted by Çalişkan et al, the efficacy of CDK 4 to 6 inhibitors was assessed separately for first-line and subsequent-line treatments. Interestingly, regardless of the treatment line, no significant association was found between HER2 status and the activity of CDK 4 to 6 inhibitors.[16] Indeed, emerging evidence indicates that HER2 low status may possess distinct characteristics compared to HER2 0 patients. Retrospective data suggest that HER2 0 patients exhibit a higher rate of pathological complete response with neoadjuvant therapy, whereas HER2 low patients tend to present with more aggressive histological features, particularly among early-stage breast cancer patients.[22–25] However, these distinctions have not been conclusively demonstrated in the context of advanced breast cancer.[26–28]
With CDK 4 to 6 inhibitors becoming the standard first-line treatment in routine clinical practice, attention turned to the potential role of the interaction between the HER2–HER3 axis and ER in drug resistance. Consequently, a hypothesis emerged suggesting that HER2 low status might exhibit reduced responsiveness to CDK 4 to 6 inhibitors. However, conflicting results have been reported in the limited number of studies conducted, primarily due to the absence of a standardized method for determining HER2 low status and the lack of objective evaluation, which includes challenges such as variations in assessments by different pathologists.[16–19,29]
Our limitations are that our study was retrospective and multicenter, and that HER2 status was evaluated by different pathologists. However, the similar distribution of patients receiving palbociclib and ribociclib, the fact that all of them were used in first-line treatment, and the similar clinical characteristics of HER2 0 and HER2 low patients make the results of our study reliable. Whether the differences caused by HER2 low status in HER2-targeted treatments are also related to the effectiveness of CDK 4 to 6 inhibitors is an issue that will be discussed in the future and should be investigated in prospective, randomized controlled studies.
Author contributions
Conceptualization: Hasan Cagri Yildirim, Deniz Can Guven, Ozan Yazici, Yüksel Urun, Ahmet Ozet, Erhan Gokmen, Berna Oksuzoglu, Sercan Aksoy.
Data curation: Hasan Cagri Yildirim, Mustafa Buyukkor, Gözde Kavgaci, Buket Şahin Celik, Kadriye Bir Yucel, Bengü Dursun, Elvin Chalabiyev, Funda Yilmaz, Saadet Sim Yildirim, Fatih Kus, Fatih Tay, Asli Gecgel, Bariş Koksal.
Formal analysis: Hasan Cagri Yildirim.
Funding acquisition: Hasan Cagri Yildirim.
Investigation: Hasan Cagri Yildirim.
Methodology: Hasan Cagri Yildirim.
Project administration: Hasan Cagri Yildirim.
Resources: Hasan Cagri Yildirim.
Software: Hasan Cagri Yildirim.
Supervision: Hasan Cagri Yildirim, Yüksel Urun, Ahmet Ozet, Erhan Gokmen, Sercan Aksoy.
Validation: Hasan Cagri Yildirim.
Visualization: Hasan Cagri Yildirim, Ozan Yazici.
Writing – original draft: Hasan Cagri Yildirim, Deniz Can Guven, Yüksel Urun, Ahmet Ozet, Erhan Gokmen, Berna Oksuzoglu, Sercan Aksoy.
Writing – review & editing: Hasan Cagri Yildirim, Berna Oksuzoglu, Sercan Aksoy.
Abbreviations:
- CDK 4/6
- cyclin-dependent kinase 4/6
- ER
- estrogen receptor
- HER2
- human epidermal growth factor receptor 2
- HR
- hormone receptor
- PFS
- progression-free survival
- PR
- progesterone receptor
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of Hacettepe University.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Yildirim HC, Buyukkor M, Kavgaci G, Celik BŞ, Yucel KB, Dursun B, Chalabiyev E, Yilmaz F, Yildirim SS, Kus F, Tay F, Gecgel A, Koksal B, Guven DC, Yazici O, Urun Y, Ozet A, Gokmen E, Oksuzoglu B, Aksoy S. The impact of human epidermal growth factor receptor-2 (low) status on the efficacy of first line cyclin-dependent kinase 4/6 inhibitors in advanced breast cancer. Medicine 2024;103:30(e38828).
References
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- [2].Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36. [DOI] [PubMed] [Google Scholar]
- [3].Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer. 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yildirim HC, Mutlu E, Chalabiyev E, et al. Clinical outcomes of cyclin-dependent kinase 4–6 (CDK 4–6) inhibitors in patients with male breast cancer: a multicenter study. The Breast. 2022;66:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yildirim HC, Kapar C, Koksal B, et al. Efficacy of first-line CDK 4-6 inhibitors in premenopausal patients with metastatic breast cancer and the effect of dose reduction due to treatment-related neutropenia on efficacy: a Turkish Oncology Group (TOG) study. J Chemother. 2024:1–7. [DOI] [PubMed] [Google Scholar]
- [7].Yildirim HC, Kutlu Y, Mutlu E, et al. The efficacy of palbociclib and ribociclib in the first-line treatment of metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer in male patients: a Turkish oncology group (TOG) study. Int J Clin Oncol. 2024;29:258–65. [DOI] [PubMed] [Google Scholar]
- [8].Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Laboratory Med. 2018;142:1364–82. [DOI] [PubMed] [Google Scholar]
- [9].Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38:1887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banerji U, van Herpen CM, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–35. [DOI] [PubMed] [Google Scholar]
- [11].Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang H, Karakas C, Tyburski H, et al. HER2-low breast cancers: Current insights and future directions. Semin Diagn Pathol. 2022;39:305–12. Elsevier; [DOI] [PubMed] [Google Scholar]
- [13].Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–50. [DOI] [PubMed] [Google Scholar]
- [14].Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46. [DOI] [PubMed] [Google Scholar]
- [15].Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–72. [DOI] [PubMed] [Google Scholar]
- [16].Yildirim EC, Atag E, Coban E, et al. The effect of low HER2 expression on treatment outcomes in metastatic hormone receptor positive breast cancer patients treated with a combination of a CDK4/6 inhibitor and endocrine therapy: A multicentric retrospective study. The Breast. 2023;70:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bao KK, Sutanto L, Shirley S, Cheung KM, Chan JC. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor–positive, ERBB2-negative metastatic breast cancer. JAMA Network Open. 2021;4:e2133132–e2133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carlino F, Diana A, Ventriglia A, et al. HER2-low status does not affect survival outcomes of patients with Metastatic Breast Cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers. 2022;14:4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zattarin E, Sposetti C, Leporati R, et al. Abstract HER2-02: HER2-02 HER2-low status is associated with worse clinical outcomes in hormone receptor-positive, HER2-negative advanced breast cancer patients treated with first-line cyclin-dependent kinase 4/6 inhibitors plus endocrine therapy. Cancer Res. 2023;83:HER2–02. [Google Scholar]
- [20].Finn RS, Cristofanilli M, Ettl J, et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res Treat. 2020;184:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. npj Breast Cancer. 2021;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–61. [DOI] [PubMed] [Google Scholar]
- [24].Rossi V, Sarotto I, Maggiorotto F, et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist. 2012;17:1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eggemann H, Ignatov T, Burger E, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. 2015;22:725–33. [DOI] [PubMed] [Google Scholar]
- [26].Agostinetto E, Rediti M, Fimereli D, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers. 2021;13:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hein A, Hartkopf AD, Emons J, et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer (Oxford, England : 1990). 2021;155:1–12. [DOI] [PubMed] [Google Scholar]
- [29].Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer (Oxford, England: 1990). 2022;163:35–43. [DOI] [PubMed] [Google Scholar]