Abstract
Background and study aims Endoscopic submucosal dissection (ESD) allows removal of tumors en-bloc. Western adoption of ESD has been hindered by its steep learning curve. Western data regarding ESD learning curve are limited. We analyzed the learning curve of a single endoscopist at a tertiary referral center in the United States.
Patients and methods All consecutive ESDs performed by a single endoscopist at a tertiary referral center in the United States from 2015 through 2022 were identified. Descriptive statistics and CUSUM analysis were used to describe the learning curve for en-bloc, R0 resection, and resection speed.
Results In our study, 503 patients with 515 lesions were included. Severe submucosal fibrosis was found in 17% of the lesions. The rates of en-bloc, R0, and curative resections were 81.9%, 71.1%, and 68.4%, respectively. CUSUM analysis showed that the learning curve plateaued at 268, 347, and 170 cases for en-bloc resection, R0 resection, and achieving a resection speed > 9 cm 2 /hr. Fibrosis significantly affected the R0 resection rate in the regression analysis (95% confidence interval 0.21–0.55). In colonic ESD curve analysis, the learning plateau was reached after 185 cases for both en-bloc and R0 resection.
Conclusions Following ex-vivo training in an animal model, an untutored expert operator achieved competency in ESD between 250 and 350 procedures. Our data can inform development of future training programs in the West.
Keywords: Endoscopy Upper GI Tract; Endoscopic resection (ESD, EMRc, ...); Endoscopy Lower GI Tract; Polyps / adenomas / ...; Colorectal cancer
Introduction
Endoscopic submucosal dissection (ESD) is a minimally invasive, endoscopic resection technique that allows en-bloc resection of premalignant and early malignant lesions throughout the gastrointestinal tract. First described in Japan in 1988 as a method to resect early gastric neoplasia 1 , use of ESD has spread from East to West, with increasing access to the procedure at select U.S. referral centers. Unlike traditional resection methods such as endoscopic mucosal resection (EMR), ESD allows en-bloc resection of lesions. The practice of ESD aligns endoscopists more closely with the principles of surgical oncologic practice, where accurate histologic assessment and clear histologic margins (referred to as R0 resection) are key outcome measures.
Despite the several advantages of ESD over EMR and other resection techniques, adoption has not been as rapid as with other novel endoscopic techniques. Several factors have limited widespread adoption, including long procedure times, lack of dedicated billing codes limiting reimbursement in initial experience, and significant risk of complications compared with EMR 2 . However, perhaps the most significant obstacle to broader adoption has been the steep learning curve and lack of a standardized approach to training in the United States. As a first step toward learning ESD, mastery of EMR and management of related adverse events (AEs) have been recommended by the European Society of Gastrointestinal Endoscopy (ESGE) prior to attempting ESD 3 . Attending ex vivo and in vivo animal labs and participation in live endoscopy training courses have also been suggested as early steps for training in ESD. Observation of expert Japanese endoscopists performing ESD has also been shown to improve procedural competence 4 .
The learning curve for ESD in the United States has not been well-defined. The only reported U.S. learning curve comes from Zhang et al. 5 , who reported their single-center experience with 540 lesions in which R0 resection improved from 45% to > 80% by 250 procedures and approached ~95% by 400 cases. ESD learning curves have been reported elsewhere 6 7 8 . Additional data on learning curves from the United States are needed to validate these previously reported numbers, with such data used to develop training programs and competency evaluations and to provide credentialing bodies and payors with information to accelerate ESD adoption and reimbursement.
In this study, we describe the learning curve for ESD performed by a single operator at a large tertiary referral center in the United States.
Patients and methods
Patients and inclusion and exclusion criteria
All patients older than 18 years undergoing ESD between July 2015 and August of 2022 were identified through a prospectively maintained database and were eligible for inclusion. All ESD procedures were performed by a single operator (M.O) at Baylor St. Luke’s Medical Center in Houston, Texas, United States. Lesions were eligible for inclusion regardless of location (esophagus, stomach, small bowel and colorectal) and both ESD and hybrid ESD procedures were included.
In June 2017, the endoscopist started performing ESD for right-sided colonic lesions and duodenal lesions based on the following inclusion criteria: 1) lesions with prior failed resection of any size, granular lateral spreading tumors ≥ 30 mm, non-granular lateral spreading tumors ≥ 20 mm, and/or any lesion with suspected superficial submucosal invasion based on optical examination; and 2) duodenal neuroendocrine lesions < 2 cm and granular lateral spreading polyps > 3 cm but not involving more than two-thirds of the duodenal circumference.
Exclusion criteria included lesions resected using submucosal tunneling endoscopic resection and endoscopic full-thickness resection for any subepithelial lesions (other than neuroendocrine tumors which often are epithelial lesions with significant subepithelial extension). All patients provided written informed consent for the procedure and institutional review board (IRB) approval from the Baylor College of Medicine IRB (H- 51292) was obtained prior to reviewing patient charts for the purposes of this study.
Procedure technique
All procedures were completed using Pentax (Montvale, New Jersey, United States) video endoscopes. Lesions were carefully examined under white light and enhanced imaging (Pentax iScan) to identify features suspicious for deep invasion. Once the decision was made to perform ESD, the lesion periphery was marked with the tip of the chosen resection knife using soft coagulation current. Then, mucosal injection using a solution of 0.004% methylene blue mixed with Hespan (6% Hetastarch in 0.9% Sodium Chloride injection) was administered to provide submucosal lift and allow mucosal incision. In esophageal, gastric, and small bowel lesions, 1 mL of 1:10,000 epinephrine was added to each syringe of 10 mL of injection fluid to decrease risk of bleeding. For mucosal incision and dissection, the operator used several knives throughout the timeline of the study, including the Dual Knife, IT Nano (Olympus, Center Valley, Pennsylvania, United States), SB Knife Jr. (Sumitomo Bakelite, Tokyo, Japan), Hybrid T-type Knife (ERBE, Tubingen, Germany), Orise Pro Knife (Boston Scientific, Marlborough, Massachusetts, United States), and the Speedboat RS2 Knife (Creo Medical, Chepstow, United Kingdom). Knife selection was based on availability at the endoscopist facility at different periods of time. To treat visible vessels in certain lesions, the Coagrasper Hemostatic Forceps (Olympus, Center Valley, Pennsylvania, United States) was used. Several electrosurgical generator units were used throughout the study period. The VIO300D and VIO200D (ERBE, Tubingen, Germany) and the Beamer CE200 (CONMED, Utica, New York, United States) were used with varying settings. Electrocautery settings for ERBE and ConMed generators were as described in 9 . In certain cases, a rigidizing overtube (Pathfinder, Neptune Medical, Burlingame, California, United States) or the Dilumen double balloon overtube (Lumendi, Westport, Connecticut, United States) 10 were used in specific cases to provide stability during resection and allow ease of scope removal and reinsertion, if required. Traction was utilized in select cases and included several methods such as clip and line, snare, rubber band, double clip, or implementation of the Dilumen traction device. Utilization of the traction device depended on endoscopist preference and device availability in the hospital for each case. Determination of a hybrid versus conventional ESD approach was per endoscopist preference.
Operator experience
All ESDs were performed by a single operator (M.O.) who completed advanced endoscopy fellowship training in 2011. The operator’s initial experience in ESD occurred from 2012 through 2014, where he performed ESD in an untutored fashion in an ex vivo animal model. During this time period, he attended seven ex vivo animal labs and three in vivo animal labs in the United States. The endoscopist thereafter transitioned to perform his first human cases. To further refine his skills, the operator attended a 1-week observership in Japan in 2015, followed by another observership week in China in 2016 and a third week in Korea in 2018.
Outcomes and definitions
Lesion size was measured following resection using a graded ruler by careful lesion pinning and fixation in formalin. Procedure time was defined as time from scope insertion to scope withdrawal including elective closure time. Hybrid ESD technique was defined as making a circumferential incision around the lesion margins using the ESD knife, then using a snare to remove the remaining lesion after submucosal dissection in one or multiple pieces. On specific occasions, the endoscopist had to use this technique to expedite the procedure in dissecting large lesions or facilitate the resection when ESD was impossible. En-bloc resection was defined as resection of the entire lesion in one piece. R0 resection was defined as lack of tumor extension to both the lateral and vertical margins of the resection specimen. Curative resection was defined as R0 resection absent any lymphovascular or perineural invasion. Resection speed was defined as the lesion surface area in cm 2 divided by total procedure time in hours for en-bloc cases. Resection speed was excluded for 10 subjects who underwent multiple ESDs during the same procedure. AEs were recorded. AEs were defined per the American Society for Gastrointestinal Endoscopy (ASGE) lexicon and AGREE classification 11 12 .
Learning curve endpoints
The main objective of the learning curve analysis was to attain a satisfactory rate of en-bloc resection rate (> 80% of cases), considering it as the primary endpoint. In addition, achieving a R0 resection rate and optimizing resection speed were considered secondary endpoints. Occurrence of AEs and rates of local residual-recurrence were analyzed as short-term and long-term outcomes of the procedures, respectively.
Statistical analysis
Patient and ESD procedure characteristics were summarized by median with minimum and maximum values, or frequency with percentage. Distribution of both resection speed and ESD time was examined by location and sequential blocks using box plots. A CUSUM analysis was used to determine the number of procedures required to reliably achieve en-bloc resection, R0 resection, curative resection, and a resection speed > 9 cm 2 /hr. Among single ESD procedures, non-linear regression with inverse curve fitting was used to examine learning curves for ESD time and resection speed. Among single procedures, the association between procedure characteristics and ESD time was tested using independent mixed-effects linear regression. To account for some patients having multiple procedures, the model included a random intercept for the patient. Significant procedure characteristics were included in a multiple mixed-effects linear regression. P < 0.05 was considered statistically significant. A sensitivity analysis was done for colonic cases. For the sensitivity analysis, there were only three patients who had multiple colonic ESD procedures, and more complex mixed-effects models, or generalized estimated equations, would not consistently be estimable. Therefore, the three repeat colonic ESDs were not included in the regressions so that linear and logistic regression could be used instead. P < 0.05 was considered statistically significant.
Results
Patient and lesion characteristics
Patient, lesion, and procedure characteristics are summarized in Table 1 . A total of 503 patients and 515 lesions were included, with 265 males (52.7 %) and a median age of 66 years (range, 27–90 years). Mean lesions size was 39.8 ± 18.7 mm. A total of 119 patients (23.6%) were on anticoagulant or antiplatelet therapy, which was discontinued before the procedure with or without bridging, based on individual patient risk for thrombosis or cardiac events, and resumed within 24 hours post-procedure ( Table 1 ).
Table 1 Patient, lesion, and procedure characteristics.
| Characteristic | Overall, 503 patients (lesions = 515) |
| SD, standard deviation; ESD, endoscopic submucosal dissection. | |
| Gender | |
|
265 (52.7%) |
| Age (y) | |
|
66 (27–90) |
| Anticoagulant/antiplatelet use | 119 (23.6%) |
| Endoscopic size of lesions (mean ± SD) | 39 ± 18.7 mm |
| Lesion location | N = 515 |
|
221 (42.9%) |
|
111 (21.5%) |
|
61 (11.8%) |
|
45 (8.7%) |
|
42 (8.1%) |
|
17 (3.3%) |
|
16 (3.1%) |
|
2 (0.3%) |
| Subepithelial tumor | 13 (2.5%) |
| Lesion characteristics | |
|
42 (8.1%) |
|
40 (7.7%) |
|
88 (17.0%) |
| Paris classification | N = 515 lesions |
|
228 (44.2%) |
|
130 (25.2%) |
|
28 (5.4%) |
|
109 (21.1%) |
|
20 (3.8%) |
| Procedure characteristics | |
|
119 lesions |
|
98 (82.3%) |
|
110 (21.2%) |
|
60 (11.6%) |
|
57 (11.06%) |
| ESD techniques | N = 515 |
|
405 (78.8%) |
|
110 (21.2%) |
| Closure methods | N = 515 |
|
101 (19.6%) |
|
322 (62.5%) |
|
92 (17.8%) |
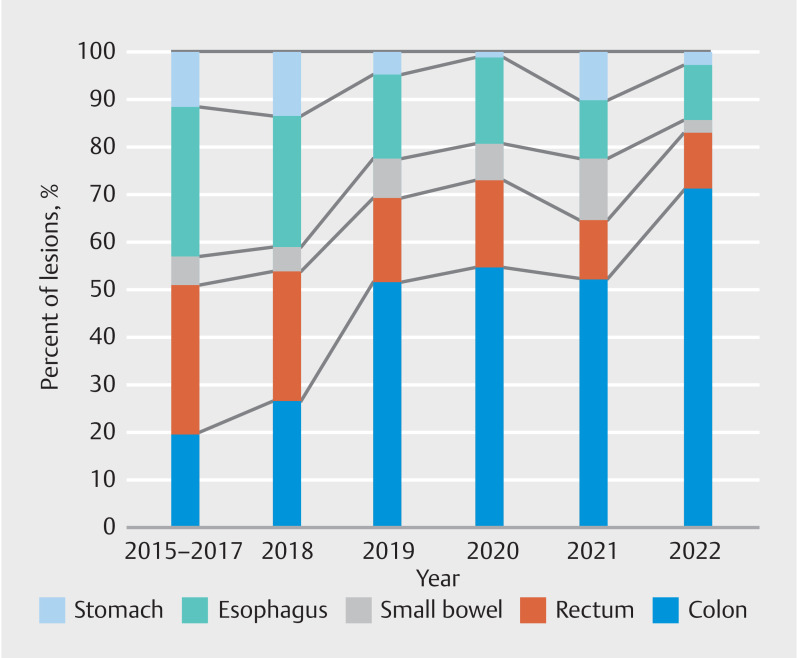
Ten patients (2%) underwent multiple ESDs during their initial index procedure. Lesions were most commonly located in the colon (n = 221, 42.9%), followed by the esophagus (n = 111, 21.5%), rectum (n = 61, 11.8%), duodenum (n = 45, 8.7%), stomach (n = 42, 8.1%), ileocecal valve (n = 17, 3.3%), appendix (n = 16, 3.1%), and jejunum (n = 2, 0.3%) ( Fig. 1 ). A total of 13 lesions were subepithelial tumors. Previous manipulation (incomplete resection, previous attempt at EMR) was noted in 42 lesions (8.1%), previous tattoos that extended under the lesion or to the lesion edge were noted in 40 lesions (7.9 %). Severe submucosal fibrosis as determined by the endoscopist was noted in 88 lesions (17 %). Paris II a+ c (depressed) component was found in 109 lesions (21.1%).
Fig. 1.
Distribution of lesions per year by location.
Procedure characteristics
An endoscope stabilization device was used in 119 procedures (98 [82.3%] used the Lumendi platform and 21 [21.2%] used the Pathfinder rigidizing overtube). Traction was used in 60 procedures (11.6%). A hybrid ESD/EMR resection was used in 110 lesions (21.2%). The coagulation grasper hemostatic device was needed for hemostasis in 57 lesions (11%). Median resection time was 81 minutes (interquartile range [IQR] 59–115). Closure was achieved in 414, 80.3% of lesions ( Table 1 )
Resection and pathological outcomes
Table 2 summarizes primary resection outcomes. En-bloc resection was achieved in 422 lesions (81.9%). Median entire procedure resection speed was 6.9 cm 2 /hr (IQR 4.0–11.8) with a median resection area of 8.75 cm 2 (IQR 5–15.3). Overall, R0 resection was achieved in 71.1% of lesions (n = 367), with curative resection achieved in 68.4% (n = 353). On pathological assessment, a total of 125 lesions were found to harbor malignancy (24.2%), 360 were premalignant (69.9%) and 30 lesions (5.8%) had neuroendocrine pathology.
Table 2 Resection and pathological outcomes.
| Characteristic | 503 patients (515 lesions) |
| Primary outcome | |
|
422 (81.9%) |
|
367 (71.1%) |
|
353 (68.4%) |
| Procedure time in minutes (median) | 82 ± 49.8 minutes |
| Resection speed (overall) | 6.9 ± 7.2 cm 2 /hr |
| Pathological assessment | |
|
125 (24.2%) |
|
360 (69.9%) |
|
30 (5.8%) |
| Submucosal invasion | 28 (5.4%) |
| Lymphovascular invasion | 27 (5.2%) |
| Specimen size (cm) | |
|
3.3 cm |
|
2.5 cm |
| Adverse events – intraprocedure | |
|
2 (0.4%) |
| Adverse events – post-procedure | |
|
6 (1%) |
|
7 (1.3%) |
|
3 (0.5%) |
| AGREE classification | N = 18 |
|
1 (5.5%) |
|
15 (83.5%) |
|
1 (5.5%) |
|
1 (5.5%) |
| Additional surgery after non-curative resection | 26 (5%) |
Learning curve analysis for all cases
Cases were sequentially divided into blocks consisting of 50 lesions for learning curve analysis, similar to previously published data 5 . CUSUM analysis demonstrated that a rapid learning curve followed by a plateau for en-bloc resection was achieved at approximately 265 cases. En-bloc resection consistently above 80% was achieved after five blocks (250 cases) ( Fig. 2 ). As for R0 resection rates, an R0 resection rate consistently above 70% was achieved by block 8 (400 cases) and CUSUM analysis ( Fig. 2 ) showed a learning plateau was achieved at approximately 347 cases. Finally, the curative resection learning curve very closely mirrored R0 resection, with CUSUM analysis showing a curative resection learning plateau at 348 cases ( Fig. 2 ).
Fig. 2.
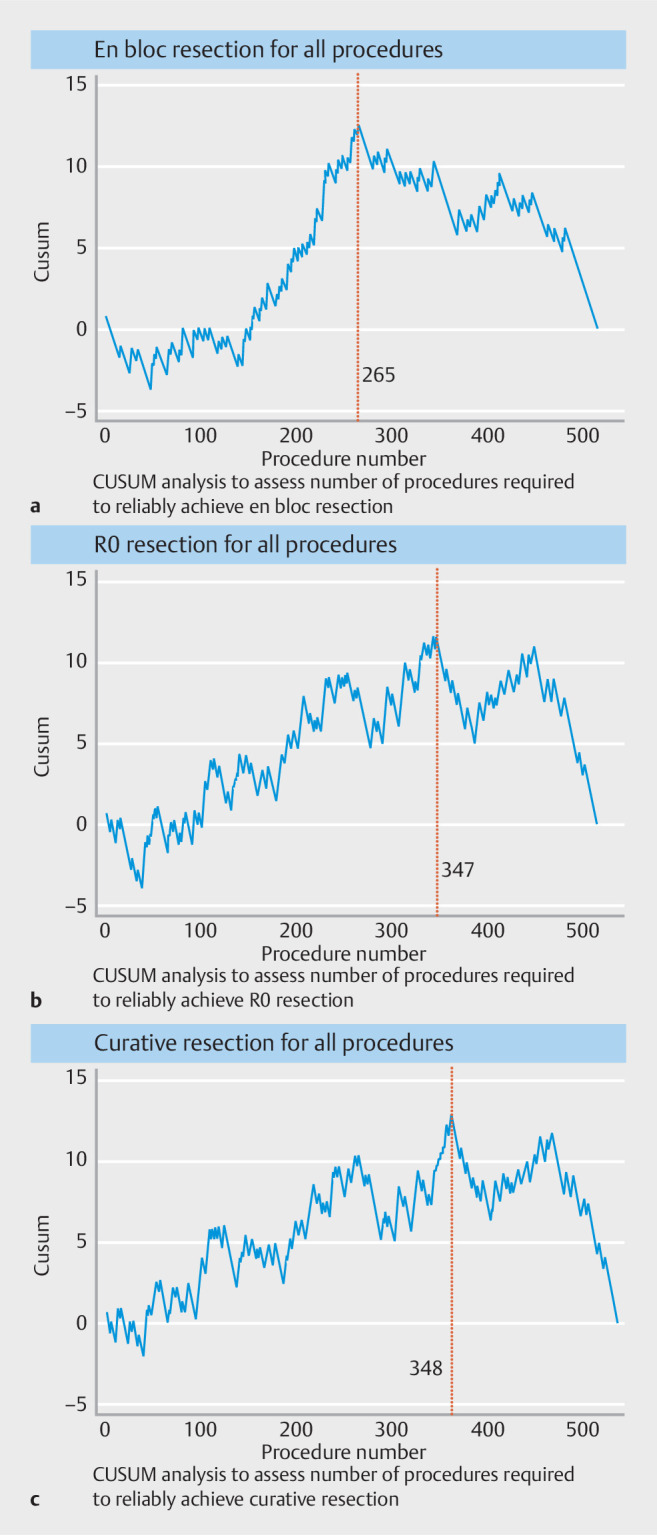
CUSUM analysis assessing the number of procedures required to study outcomes.
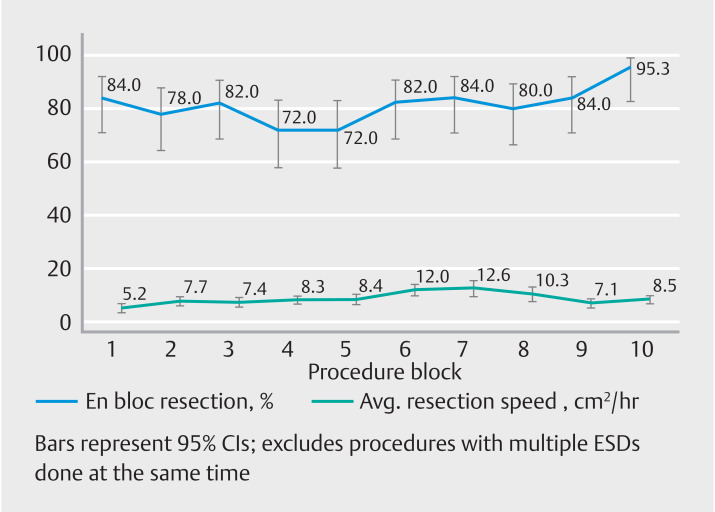
A resection speed > 9 cm 2 /hr was reliably achieved after 31 en-bloc single procedures located in the esophagus, and 71 en-bloc single procedures located in the colon. Resection speed increased from a mean of 5.2 cm 2 /hr to 9 cm 2 /hr by block 5 (250 cases) and peaked at 12.6 cm 2 /hr by block 7 (350 cases). Fig. 3 demonstrates the resection speed and en-bloc rates by block. AEs including all lesions per block were illustrated in Fig. 4 .
Fig. 3.
Block-level analysis of resection speed and en-bloc rates.
Fig. 4.
Adverse events for all lesions per block.
In analysis of independent regression results for ESD time and its associated factors (lesion size, multiple knife used, using of overtubes, tattooing, fibrosis, depressed morphology [IIa +c] and using coagulation grasper), procedures with lesion length > 4 cm had an average ESD time that was 31.1 minutes longer (95% confidence interval [CI] 22.5–39.7) compared with procedures with length < 4 cm. In addition, procedures utilizing multiple knives had an average ESD time that was extended by 28.7 minutes (95% CI 18.8–38.5) compared with procedures using one knife. Moreover, presence of fibrosis (adjusted coefficient 19.2, 95% CI 8.9–29.5) and Paris IIa+IIc classification (adjusted coefficient 12.5, 95% CI 2.8–22.3) were also associated with a longer mean ESD time compared with procedures without fibrosis and those with different Paris grades, respectively.
Table 3 presents independent regression analyses exploring associations with resection speed > 9 cm 2 /hr. Cases with a lesion length > 4 cm had 9.16 times higher odds of achieving a resection speed > 9 cm 2 /hr (95% CI 5.53, 15.17) compared with cases with a lesion length ≤ 4 cm. Furthermore, when compared with esophageal lesions, cases located in the jejunum or duodenum (adjusted odds ratio [aOR] 0.14, 95% CI 0.04–0.56), colon (aOR 0.53, 95% CI 0.29–0.98), or appendix or inhibitory concentration value (aOR 0.08, 95% CI 0.02–0.40) exhibited decreased odds of achieving a resection speed > 9 cm 2 /hr.
Table 3 Multiple generalized estimating equation regression for resection speed > 9 cm2/hr .
| Adjusted odds ratio | 95% confidence interval | P value | ||
| Lesion length > 4 cm | 9.16 | 5.53 | 15.17 | 0.000 |
| Location | 0.003 | |||
|
Reference | . | . | . |
|
0.70 | 0.28 | 1.76 | 0.447 |
|
0.14 | 0.04 | 0.56 | 0.005 |
|
0.53 | 0.29 | 0.98 | 0.044 |
|
0.08 | 0.02 | 0.40 | 0.002 |
|
0.97 | 0.43 | 2.20 | 0.942 |
| Fibrosis | 0.42 | 0.16 | 1.09 | 0.074 |
| Previous resection | 0.53 | 0.16 | 1.70 | 0.282 |
| Ink tattooing | 0.34 | 0.07 | 1.62 | 0.176 |
Analyzing the learning curve in colonic cases (excluding rectum, appendix and IC valve)
When colonic cases were analyzed independently, en-bloc resection rate was at a plateau between 70% and 78% for the first four blocks (first 200 colonic cases); however, rates consistently above 90% were achieved in block 5 (250 cases). CUSUM analysis demonstrated similar findings with en-bloc learning curve plateau achieved by 185 colonic cases. As for R0 resection, rates ranged between 62% and 76% throughout the first four blocks, with peak R0 resection rate of 87.7% achieved in block 5 (250 cases). CUSUM analysis demonstrated that an R0 learning curve plateau was achieved in approximately 185 cases. Finally, the curative resection learning curve again very closely mirrored the R0 resection learning curve, with a plateau between 62% and 76% until block 5 (250 cases), where the rates increased to 92%.
Resection speed for colonic cases increased from a mean of 5.9 cm 2 /hr in block 1 to 11 cm 2 /hr by block 3 (150 colonic cases).
Examining independent regression results for time of (ESD) and its associations. On average, procedures with a lesion length > 4 cm demonstrated an ESD time that was 26.5 minutes longer (95% CI 15.9–37.1) compared with procedures without such lesion length, while considering the influence of using multiple knives or a hemostatic device. Furthermore, procedures utilizing multiple knives had an average ESD time that was extended by 26.39 minutes (95% CI 13.7–39.05) compared with procedures with use of one knife. However, after accounting for lesion length and number of knives used, the association between use of a hemostatic device and ESD time was no longer statistically significant ( P = 0.072).
Independent regression analyses of associations with R0 resection
When considering number of knives used, colonic cases involving fibrosis were found to be significantly less likely to achieve R0 resection (aOR 0.42, 95% CI 0.20–0.90) compared with cases without fibrosis. Moreover, in colonic ESDs, those utilizing multiple knives during the same procedure were also less likely to achieve R0 resection compared with cases using less than one knife (aOR 0.42, 95% CI 0.20–0.90).
Adverse events
A total of 18 AEs were identified, two intraprocedure and 16 post-procedure. During the first half of the study, there were nine AEs reported in 250 cases with a 3.6% rate of AEs. In the second half, seven AEs occurred among 253 patients, resulting in an AE rate of 2.7%. The majority of AEs (83%) were classified as Grade III a ( Table 2 ).
Two intraprocedure perforations occurred during the study period, both of which were primarily repaired with clips and endoscopic suturing, with both patients recovering uneventfully.
Six patients had delayed perforations detected after the procedure: one developed an esophageal leak after esophageal ESD for adenocarcinoma, which was repaired with an over-the-scope clip (OTSC) and esophageal stenting; however, the patient ultimately required esophagectomy considering a non-curative resection. There was one duodenal perforation closed with an OTSC, a second duodenal perforation in which previous clips were removed and OTSCs were used to close the defect. In another patient, a contained duodenal perforation was treated by using OTSCs and antibiotics. Another patient presented with sepsis 3 days after colonic ESD, was found to have a colonic perforation that required right hemicolectomy, and had had uneventful recovery. A 72-year-old man with severe cardiac and renal disease who underwent a colonic ESD for a 4-cm tubular adenoma with high-grade dysplasia was found to have post-coagulation electrocautery syndrome 3 days after the procedure, underwent urgent laparotomy, and suffered an intraoperative cardiac arrest and died. However, three patients had esophageal stenosis that required endoscopic dilation. Finally, seven patients (1.3%) had delayed bleeding requiring endoscopic management.
Discussion
In this study of 515 lesions resected by ESD, an analysis of the untutored learning curve of a single advanced endoscopist at a tertiary academic center in the United States showed that the learning plateau for en-bloc and R0 resection was achieved at between approximately 250 to 350 cases.
In the current study, we observed that utilizing different devices, traction methods, and ESD techniques resulted in a technical success rate, as indicated by the en-bloc resection rate, of 81.8%. This rate was achieved after performing 264 procedures, and it progressively increased to 95.3% after completion of the ninth block of cases (450 cases). However, the R0 resection rate, which indicates absence of positive vertical or lateral margins, was achieved after 347 procedures. Initially, presence of cautery artifacts from knife incisions contributed to positive lateral margins, resulting in a lower R0 resection rate. Nevertheless, over time and by adopting adequate margin techniques, the R0 resection rate increased in subsequent years, particularly becoming more pronounced in the latest two blocks of cases. In addition, we had a relatively low AE rate. This can be partially attributed to the fact that over 80% of resection beds after ESD were closed using various methods, such as clipping or suturing. While many endoscopists may choose to forego closure of large defects, our decision to pursue closure when feasible stems from our patient population and prohibitive costs of routine post-procedure admission in the United States. Patients referred to our practice often come from rural areas, several hours away by car, and any delayed AE would likely be difficult to address locally. We have previously shown that same-day discharge is feasible in the majority of patients undergoing ESD 13 , and that even with lesions involving more than 50% of the luminal circumference, bleeding rates can be low with appropriate closure 14 .
Many colonic lesions undergoing ESD in the United States are located in the right side of the colon, often in challenging positions for endoscopists. Due to the higher prevalence of obesity in the United States and a high incidence of previously manipulated polyps, we utilized different endoscope stabilization devices throughout the study. Use of stabilization devices in colonic ESD often facilitates en-bloc resection and can be thought of as an important aid to the procedure rather than a “crutch.”
Data on the ESD learning curve in the United States are quite limited, owing to the small number of centers performing ESD and lack of a standardized training pathway. The largest experience to date comes from Zhang et al. 5 where, in an analysis similar to ours, the untutored learning curve for a single, expert endoscopist was described. Approximately 250 cases were needed to achieve proficiency benchmarks including en-bloc resection > 90% and R0 resection > 80%, with continued improvements in R0 resection to > 95% at 400 cases. To our knowledge, no other US-based learning curve analyses have been published to date. However, the learning curve for less challenging lesions (stomach and rectum lesions) is different, as described in a small 2018 study in Germany on 50 patients. The learning curve was assessed after a formal training program consisting of observation, followed by animal model training, and then supervised ESD, finally leading to independent ESD. The study found that R0 started at 86.7% for the first 15 lesions and peaked at 100% for the last 15 lesions 15 .
The largest European learning curve experience comes from Sweden 16 , where in 301 colorectal lesions (57% rectal), improvements in R0 resection rate were from 60% in the first 60 cases, to ~80% by 300 cases.
Our findings are similar to the above Western experiences, supporting the validity of our study. However, a few key points unique to our experience are worth highlighting. Approximately 16.9% of lesions in our analysis were previously manipulated with incomplete previous resection or had a tattoo extending to the lesion base, both of which are established risk factors for submucosal fibrosis and subsequent lower en-bloc and R0 resection. This has been shown to be common in the United States 5 and steepens the learning curve compared with other regions such as Japan, where less challenging (non-manipulated, gastric, or rectal) lesions are more prevalent. In addition, countries with more robust public health care systems are more likely able to create clear guidelines for referral of lesions to expert endoscopists as soon as they are discovered, compared with the United States, where lesions are often tackled first by community endoscopists and on some occasions only referred to expert centers as a last resort before surgical referral. Interestingly, referral of complex benign colonic lesions for surgical resection is increasing in the United States 17 .
Finally, lesions in our analysis increased in complexity gradually throughout the study period, likely owing to a growing referral base and increased proficiency allowing the endoscopist to tackle more challenging lesions.
Establishing learning curve metrics for ESD in the United States is critically important for several reasons. It is an important step toward development of formalized training protocols and programs. We believe that ex vivo training for 20 hours with three tutored live animal labs is a sufficient amount of training to attempt ESD, with the first three live human cases preferably proctored. Establishing formal training pathways allows faster adoption of ESD with several downstream benefits, most important of which is organ preservation for patients: A 2020 study by Moon et al. showed that laterally spreading colorectal lesions continue to be referred for surgical resection in the United States, with at least 90% of those ultimately undergoing successful endoscopic resection when appropriately referred back to an expert endoscopist 18 . In addition, wider adoption of ESD will support efforts to establish a dedicated billing code for the procedure as a step toward appropriate reimbursement. Once this is achieved, ESD may evolve into a mainstream procedure offered routinely to benefit patients. At this time, widespread adoption of ESD is limited by the additional time and effort compared with EMR, which are often not reimbursed 19 , disincentivizing endoscopists from learning and implementing ESD in their practice 20 .
Our study has several strengths, including the large number of included lesions and various subanalyses of learning curves, by both location and complexity. A few limitations are inherent in the retrospective nature of the study, including use of total procedure time as a surrogate for more accurate lesion dissection time “defined as the time from first injection to last treatment with the knife” because this was not available for all procedures. Future prospective studies accounting for this may allow more accurate estimates of the number of procedures needed to achieve a resection speed > 9 cm 2 /hr. In addition, the expert endoscopist (M.O.) continued to perform other third space procedures, including esophageal and gastric per-oral endoscopic myotomy and endoscopic full-thickness resection, throughout the study period, which may have influenced the trajectory of the learning curve because many of the involved techniques are shared among all third space endoscopy procedures. Finally, different locations in the gastrointestinal tract have different learning curves, yet our study assessed the learning curve of ESD across various locations, which may be considered a limitation. However, we were able to demonstrate the feasibility of achieving proficiency in different locations with just 250 cases performed by a single endoscopist. Also, owing to the study’s retrospective design, it was not possible to discern whether the endoscopist chose the hybrid ESD technique to expedite the procedure or because ESD was deemed impossible.
Conclusions
In conclusion, our analysis of 515 lesions resected by ESD found that an untutored expert endoscopist achieved competency for en-bloc, R0, and curative resection at between approximately 250 and 350 procedures. The learning curve for ESD is steep but comparable to other complex endoscopic procedures.
Footnotes
Conflict of Interest Mai Khalaf: has no conflicts to disclose. Fares Ayoub: has no conflicts to disclose. Mohamed O Othman: Consultant for Olympus America, AbbVie, Boston Scientific Corporation, Lumendi, Apollo, Conmed and Medtronic. Research grants from Olympus America, AbbVie, Boston Scientific Corporation, Lucid Diagnostic and US Biotest. Salmaan Jawaid: consultant for ConMed, Creo Medical, and Lumendi. Kristin Staggers: has no conflicts to disclose. Johanna W El-Haj: has no conflicts to disclose.
Supplementary Material
References
- 1.Hirao M, Masuda K, Asanuma T et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 2.Russo P, Barbeiro S, Awadie H et al. Management of colorectal laterally spreading tumors: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E239–E259. doi: 10.1055/a-0732-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libânio D, Pimentel-Nunes P, Bastiaansen B et al. Endoscopic submucosal dissection techniques and technology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2023;55:361–389. doi: 10.1055/a-2031-0874. [DOI] [PubMed] [Google Scholar]
- 4.Draganov PV, Chang M, Coman RM et al. Role of observation of live cases done by Japanese experts in the acquisition of ESD skills by a western endoscopist. World J Gastroenterol. 2014;20:4675–4680. doi: 10.3748/wjg.v20.i16.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Ly EK, Nithyanand S et al. Learning curve for endoscopic submucosal dissection with an untutored, prevalence-based approach in the United States. Clin Gastroenterol Hepatol. 2020;18:580–INF. doi: 10.1016/j.cgh.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Hotta K, Oyama T, Shinohara T et al. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302–306. doi: 10.1111/j.1443-1661.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinbrück I, Faiss S, Dumoulin FL et al. Predictive factors for the outcome of unsupervised endoscopic submucosal dissection during the initial learning curve with prevalence-based indication. Dig Dis Sci. 2023;68:3614–3624. doi: 10.1007/s10620-023-08026-9. [DOI] [PubMed] [Google Scholar]
- 8.Wagner A, Neureiter D, Kiesslich T et al. Single-center implementation of endoscopic submucosal dissection (ESD) in the colorectum: Low recurrence rate after intention-to-treat ESD. Dig Endosc. 2018;30:354–363. doi: 10.1111/den.12995. [DOI] [PubMed] [Google Scholar]
- 9.Jawaid S, Keihanian T, Khalaf M et al. Settings of a novel electrosurgical generator to enable efficient and safe submucosal endoscopic procedures. Endosc Int Open. 2023;11:E743–E751. doi: 10.1055/a-2085-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail MS, Bahdi F, Mercado MO et al. ESD with double-balloon endoluminal intervention platform versus standard ESD for management of colon polyps. Endosc Int Open. 2020;8:E1273–E1279. doi: 10.1055/a-1226-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nass KJ, Zwager LW, van der Vlugt M et al. Novel classification for adverse events in GI endoscopy: the AGREE classification. Gastrointest Endosc. 2022;95:1078–INF. doi: 10.1016/j.gie.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Cotton PB, Eisen GM, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y-I. Ambulatory endoscopic submucosal dissection for gastrointestinal neoplasms: trends and associated factors in the United States. Clin Gastroenterol Hepatol. 2024 doi: 10.1016/j.cgh.2023.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Keihanian T, Zabad N, Khalaf M et al. Safety and efficacy of a novel suturing device for closure of large defects after endoscopic submucosal dissection (with video) Gastrointest Endosc. 2023;98:381–391. doi: 10.1016/j.gie.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Ebigbo A, Probst A, Römmele C et al. Step-up training for colorectal and gastric ESD and the challenge of ESD training in the proximal colon: results from a German Center. Endosc Int Open. 2018;6:E524–E530. doi: 10.1055/a-0584-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rönnow CF, Uedo N, Toth E et al. Endoscopic submucosal dissection of 301 large colorectal neoplasias: outcome and learning curve from a specialized center in Europe. Endosc Int Open. 2018;6:E1340–E1348. doi: 10.1055/a-0733-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peery AF, Cools KS, Strassle PD et al. Increasing rates of surgery for patients with nonmalignant colorectal polyps in the United States. Gastroenterology. 2018;154:1352–INF. doi: 10.1053/j.gastro.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon N, Aryan M, Khan W et al. Effect of referral pattern and histopathology grade on surgery for nonmalignant colorectal polyps. Gastrointest Endosc. 2020;92:702–INF. doi: 10.1016/j.gie.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal S, Ali A, Razzaq A et al. Lack of proper reimbursement is hampering adoption of minimally invasive gastrointestinal endoscopy in North America. World J Gastrointest Endosc. 2020;12:49–52. doi: 10.4253/wjge.v12.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AY. Training in endoscopic submucosal dissection from a Western perspective. Techniq Gastrointest Endosc. 2017;19:159–169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.