Abstract
The microorganisms inhabiting our gastrointestinal tract are critical for human health. Chronic heavy alcohol use can modulate the composition and function of the gut microbiota, thereby exacerbating end-organ damage via the gut-brain axis and the gut-liver axis. In this review, we summarize the bacterial, fungal, and viral gut microbial compositional changes associated with alcohol use and alcohol-associated liver disease and discuss the mechanisms of action by which gut dysbiosis reinforces alcohol use behavior and liver inflammation and injury. We also highlight important pre-clinical and clinical trials that target gut microbial-specific mechanisms for the treatment of alcohol use disorder and alcohol-associated liver disease.
Keywords: Mycobiome, virome, bacterial translocation, intestinal permeability, microbial metabolites
Introduction
The gut microbiome plays a vital role in maintaining human health and alterations to the microbiome (dysbiosis) has been implicated in the pathogenesis of intestinal and extraintestinal diseases[1]. The gut microbiome is comprised of a diverse network of organisms across different domains of life, including bacteria, archaea, fungi, viruses, eukaryotes, and in some cases helminths[2]. Together through cross-domain interactions, including symbiotic or antagonistic competition, they collectively influence their host. The composition of the gut microbiome not only varies across the different sections of the gastrointestinal tract, with several unique communities within the mouth, stomach, small intestine, and colon, but even differs cross-sectionally within the lumen depending on distance from the mucosal epithelium[3]. Most of our understanding of the gut microbiome comes from studying colonic or fecal bacteria, which has the highest density of bacterial organisms. From large-scale collaborative efforts, including the Human Microbiome Project and MetaHit, and technologic advances such as metagenomics, we now have a better understanding of additional domains other than bacteria, including viral, fungal and other multicellular organisms that comprise our human gut microbiome[4].
Bacteria are the most abundant domain found within the human gut microbiome and mainly include members of the phyla Firmicutes (Clostridium, Lactobacillus, and Enterococcus) and Bacteroidetes (Bacteroides and Prevotella), but also include Proteobacteria (Escherichia and Acinetobacter), Actinobacteria (Bifidobacterium), and Verrucomicrobia (Akkermansia) to a lesser extent[2]. The primary archaea within the microbiome include methanogenic species, mainly Methanobrevibacter smithii[1]. The mycobiome or fungi associated with the gut microbiome, mainly include members of the phyla Ascomycota (Candida, Saccharomyces, Aspergillus, and Malassezia)[2]. The virome is mainly composed of bacteriophages and largely consists of the class Caudovirales (Siphoviridae, Myoviridae, and Podoviridae). Helminths, which are nearly absent in modernized countries, still make up a large percentage of the world’s population microbiome and include trematodes (flatworms), nematodes (roundworms), and cestodes (tapeworms). Together, these various domains closely interact with their host to form a dynamic and symbiotic relationship.
Although there is not one single “healthy” gut microbiome, it is hypothesized that there are core functions of the microbiome that contribute to health, which may involve different species within the same phyla (phylotypes) with similar functions[1]. Variation in host age and environment, including diet and lifestyle, influence interindividual variation of the gut microbiome. In healthy conditions, this rich network of trillions of microbes that colonize our gastrointestinal tract plays several key functions, including strengthening the gut wall’s integrity, protecting against pathogens, training and regulating our immune system, breaking down indigestible food products, and producing important nutrients[5]. The short chain fatty acids (SCFAs) butyrate, propionate, and acetate are key nutrients produced from the gut microbiome found to have anti-inflammatory properties in addition to positively impacting colonic epithelial cell health, and various immune and metabolic functions[6]. Additionally, exposure of commensal microbiota and their antigens help educate regulatory T-cells[7]. Better characterization of the gut microbiomes and metabolites in healthy and sick individuals have led to a better understanding of the possible mechanisms and contributions of dysbiosis to the pathogenesis or progression of various diseases, including alcohol use disorder (AUD) and alcohol-associated liver disease (ALD).
Gut bacteria and alcohol use
In 2016, the World Health Organization reported that nearly 3 million deaths, or approximately 5% of all global deaths, were attributed to alcohol consumption[8]. Moreover, alcohol-associated liver disease was estimated to cause 21.5 million years of life lost to disability[9]. Alcohol consumption led to nearly half of the deaths attributed to chronic liver disease and was one of the top two leading indications for liver transplant[10]. Despite the significant social, economic, and medical burdens that alcohol use has on individuals and countries, alcohol consumption has increased across the world over the past few decades, especially recently during the COVID-19 pandemic[10]. In many instances, alcohol use disorder, defined as a loss of control over alcohol intake despite negative psychological, biological, behavioral, and social consequences, goes unrecognized – with one in six adults reporting being asked about their drinking behaviors by their health care providers[11].
Ethanol is absorbed by the gastrointestinal tract via simple diffusion. Small amounts of ethanol are absorbed and undergo first pass metabolism in the stomach, but chronic heavy alcohol consumption, even in the absence of liver disease, affects gut bacterial composition, increases intestinal permeability, and leads to activation of systemic inflammatory cascade pathways[12]. Alcohol use is associated with decreased amounts of key commensal bacteria, including Roseburia, Faecalibacterium, Blautia, Bacteriodes, and Lachnospiraceae, Akkermansia, Lactobacilli, Bifidobacteria, and Enterococci[13]. Conversely, there is a higher abundance of Proteobacteria, a phylum which encompasses many known human pathogens such as Klebsiella, Enterobacter, Citrobacter, Salmonella, Escherichia coli, Shigella, Proteus, and Serratia in the Enterobacteriaceae family. This alcohol-induced gut dysbiosis can be at least partially reversed with abstinence[12, 13]. However, those with alcohol use disorder and alcohol dependence demonstrate further changes to their gut microbiome, including decreased abundance of bacteria with anti-inflammatory properties such as Bifidobacterium species, and bacteria from the Clostridiales, Lachnospiraceae, and Ruminococcaceae families known to produce metabolites important to gut health such as SCFAs, like butyrate[12, 13].
Studies suggest that the gut dysbiosis caused by both chronic heavy alcohol consumption and binge drinking may in fact perpetuate alcohol use disorder by increasing craving and psychological parameters such as anxiety and depression. A study of young adults without AUD found that binge drinking was associated with alterations in microbiome composition, which in turn correlated with alcohol craving[14]. In particular, modulation of the levels of SCFAs, such as decreased acetate degradation, increased acetate synthesis, and decreased butyrate production were linked to higher levels of alcohol craving both at baseline and at 3-month follow-up[14]. Another study of patients with chronic AUD undergoing a short-term detoxification program found that only a subset of patients with AUD developed increased gut permeability and that increased permeability was associated with higher scores of depression, anxiety, and alcohol craving in patients after 3 weeks of abstinence[12]. Mice colonized with gut microbiota from patients with AUD demonstrated increased voluntary alcohol preference, reduced sociability, and increased depression-like behavior and stress levels in behavioral tests, as well as increased neuroinflammation and decreased myelin-related gene expression[15, 16].
While the mechanism of action is still not well understood, different mechanisms involving the tryptophan-kynurenine pathway, γ-Aminobutyric acid (GABA)-specific neural circuits, short chain fatty acids, and histone modifications have been implicated (Figure 1). A study of patients with AUD before and after abstinence found that serum levels of kynurenine, a tryptophan metabolite, were elevated after abstinence, and higher levels of kynurenine were associated with lower levels of alcohol craving during abstinence[17]. Gut microbiota can directly metabolize tryptophan or stimulate indoleamine 2,3-dioxygenase 1 to indirectly upregulate tryptophan metabolism to kynurenine, which is thought to modulate glutamate neurotransmission to reduce alcohol-seeking and relapse behavior. Modulation of GABA, the main inhibitory neurotransmitter in the mammalian cortex, by gut microbial metabolism has also been implicated. Indeed, numerous gut microbes have the ability to produce neurotransmitters such as GABA, serotonin, and dopamine, which may be able to cross the blood-brain barrier during states of increased permeability to act directly on the brain, or indirectly deliver signals via the vagus nerve[18]. In mice colonized with gut microbiota from patients with alcohol use disorder, decreased gene expression of the alpha 1 subunit of GABA type A receptor, the most common GABA receptor in the brain, was observed in the prefrontal cortices[16]. Another way gut microbiota may modulate alcohol use is via histone modifications, epigenetic modifications, such as via incorporation of the alcohol metabolite acetate into the brain in histone acetylation or through production of butyrate, a short chain fatty acid that also inhibits histone deacetylases[19]. Changes in the gut microbiota in response to alcohol use may also indirectly modulate histones, such as by decreasing hepatic β-hydroxybutyrate synthesis[15]. β-hydroxybutyrate exerts neuroprotective effects by inhibiting histone deacetylases to increase expression of brain-derived neurotrophic factor (BDNF) and Forkhead box class O 3a (FOXO3A) and prevent endoplasmic reticulum stress.
Figure 1.
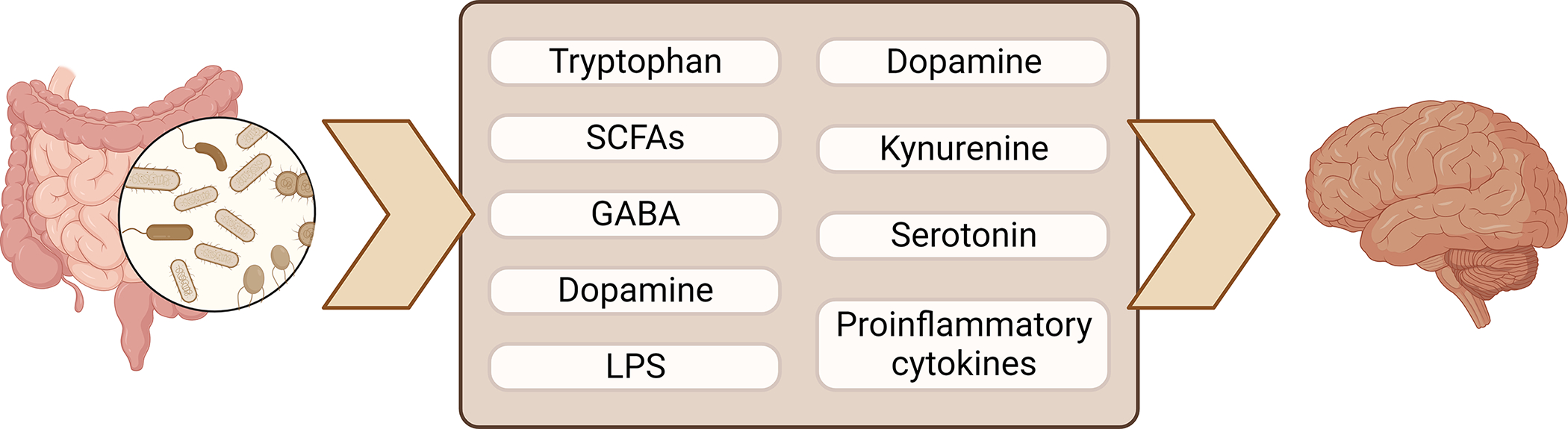
Gut microbiota produce important metabolites that modulate the central nervous system. GABA, γ-Aminobutyric acid; LPS, lipopolysaccharide; SCFAs, short chain fatty acids. Figure created using Biorender.
Several rodent studies have treated chronic alcohol use by targeting the gut-brain axis and using antibiotics to modulate the gut microbiome. In rats, various antibiotics, including ceftriaxone, ampicillin, cefazolin, and amoxicillin, have demonstrated efficacy in reducing relapse-like drinking, which is described as alcohol intake when given one or two weeks after chronic alcohol consumption. Antibiotics have also been shown to decrease voluntary drinking, alcohol reward, and alcohol withdrawal symptoms[20]. In addition to significantly changing microbiome composition in patients with chronic alcohol consumption, ceftriaxone and other beta-lactams have neuromodulatory effects via increase of glutamate transporter 1 in the CNS, thereby increasing the reuptake of the excitatory neurotransmitter glutamate and reducing glutamate toxicity. Despite this, a randomized control trial using minocycline to treat humans with heavy alcohol use did not show reductions in alcohol-induced craving after alcohol administration[21]. Nevertheless, treatment of patients with alcohol use disorder and cirrhosis with fecal microbiota transplantation did demonstrate short-term reduction in alcohol craving and consumption, in association with favorable changes to the microbiome, as compared to placebo treatment[22], suggesting that the gut microbiome is an attractive target for further therapeutic exploration in alcohol use disorder.
Gut bacteria and alcohol-associated liver disease
Although there is a dose-response relationship between alcohol consumption and the various stages of ALD, ranging from asymptomatic steatosis to cirrhosis, there is significant interindividual variation in who develops progressive liver disease, with about 20–30% patients developing liver injury and fibrosis[23]. In the natural history of ALD, patients develop cirrhosis when liver fibrosis progresses to the point of distorting the liver architecture and forming nodules. Patients can also develop alcoholic hepatitis, a more acute syndrome characterized by jaundice and associated with very high mortality in severe forms. Gut microbial dysbiosis is an important risk factor in both the development and progression of ALD.
Similar to the compositional changes in gut microbiota seen in patients with AUD, those with alcohol-associated liver disease had a reduction in abundance of healthy commensal organisms such as Bacteroidetes, Firmicutes, and Ruminococcaceae species, and an increase in Enterobacteriaceae, Veillonellaceae, and Streptococcaceae species as compared with healthy controls[24–26]. Patients with alcohol-associated cirrhosis had similarly depleted commensal bacteria, however they notably had increased amounts of oral microbiota as well as Lactobacillus and Bifidobacterium species, which differs from those without cirrhosis[13]. In healthy individuals, an intact intestinal barrier allows for absorption of essential nutrients while containing gut microbes and harmful microbial products within the gut lumen. This intestinal barrier is comprised of a mucus layer, which contains antimicrobial molecules secreted by intestinal epithelial cells and Paneth cells and immunoglobulin A produced by plasma cells, and epithelial cells bound together by adherens and tight junctions[27]. Chronic alcohol use not only is associated with enrichment of more potentially pathologic gut microbiota, but also facilitates the breakdown of the intestinal barrier, leading to increased gut microbial translocation into the liver via the portal vein, which supplies the majority of the blood flow to the liver (Figure 2).
Figure 2.
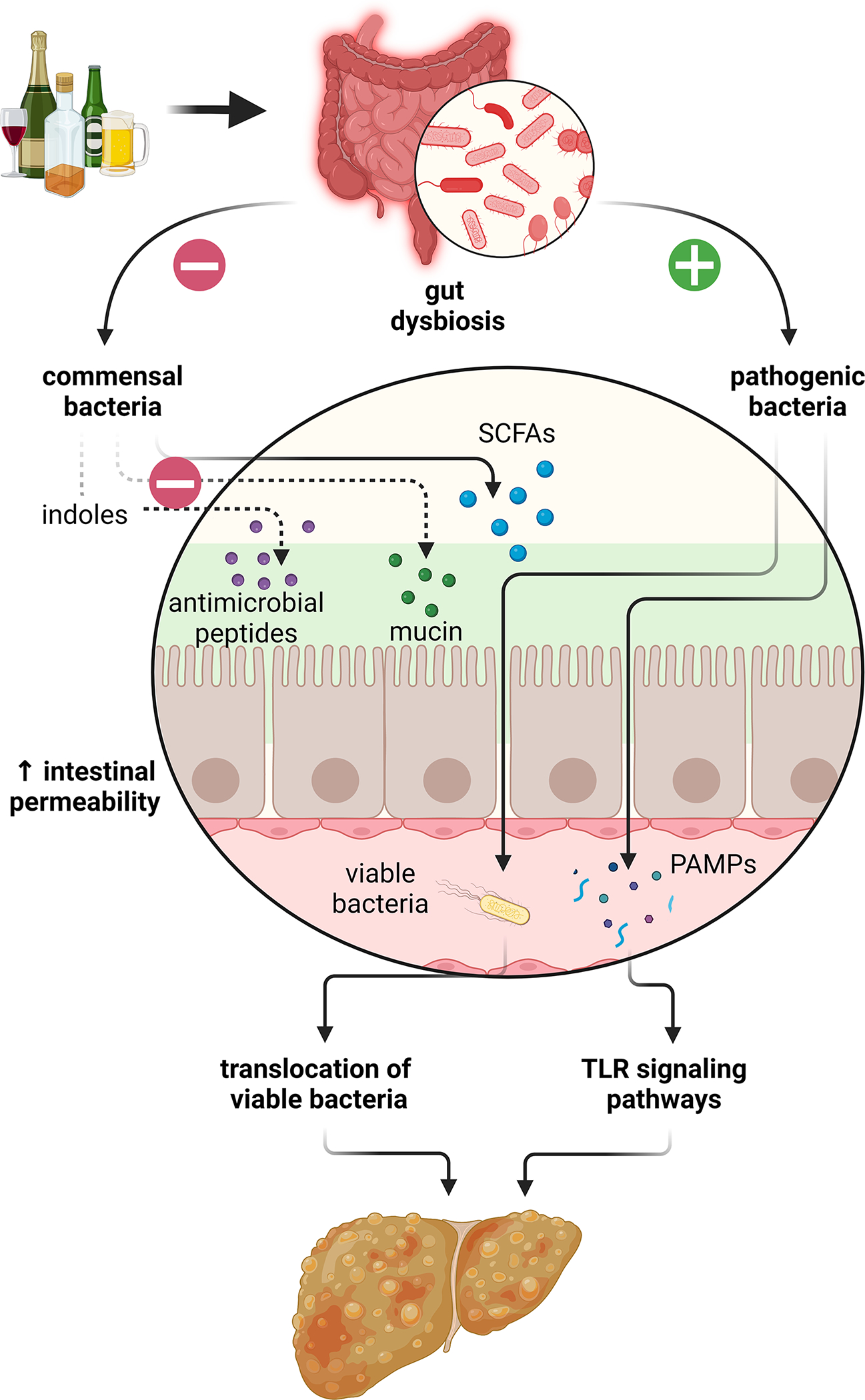
Chronic alcohol use leads to gut dysbiosis, which results in increased intestinal permeability, translocation of gut bacteria and pathogen-associated molecular patterns (PAMPs), and increased liver inflammation and fibrosis via toll-like receptor (TLR) signaling pathways. SCFAs, short chain fatty acids. Figure created using Biorender.
Translocation of bacterial lipopolysaccharide (LPS) and other bacterial endotoxins, such as pathogen-associated molecular patterns (PAMPs), results in activation of pro-inflammatory cascades such as the toll-like receptor (TLR) signaling pathways. Different TLRs recognize specific bacterial components; for example, TLR2 recognizes Gram-positive bacterial cell wall components, TLR4 recognizes Gram-negative bacterial cell wall components, and other TLRs recognize bacterial DNA or RNA[28]. Once activated, TLR signaling cascades can promote hepatic inflammation by activating nuclear factor (NF)-κB and the production of inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β by Kupffer cells and hepatic fibrosis by activating transforming growth factor (TGF)-β in hepatic stellate cells. Translocation of viable bacteria can also occur once the protective intestinal barrier is disrupted in chronic alcohol use[29, 30]. This may contribute to decompensation of liver disease, as Enterobacteriaceae are the most common bacteria detected in ascites fluid samples from patients with cirrhosis[24].
Some gut microbes such as Akkermansia muciniphila directly promote intestinal barrier function by maintaining the mucus layer through mucus production. The abundance of A. muciniphila was found to be reduced with increasing severity of ALD, with lowest abundance in patients with severe alcoholic hepatitis[31]. Ethanol-fed mice supplemented with A. muciniphila demonstrated decreased intestinal permeability and enhanced mucus thickness and tight-junction expression.
Other gut microbes produce microbial-specific metabolites that are critical to maintaining an intact intestinal barrier and are disrupted by alcohol use. Decreased abundance of SCFA-producing gut microbiota in ALD leads to a decrease in butyrate and propionate, which are vital for colonic cell health[6]. In fact, supplementation of tributyrin, an ester composed of butyric acid and glycerol, to ethanol-fed mice ameliorated the intestinal tight junction disruption, intestinal permeability, and liver injury caused by ethanol[32]. The tryptophan metabolite indoles are another group of microbial metabolites decreased by alcohol use. Indoles stimulate the secretion of interleukin (IL)-22 by T-cells, which induces production of antimicrobial peptides such as regenerating islet derived factor 3 (REG3) into the mucus layer to reduce bacterial translocation[33]. Ethanol-fed mice treated with indole-3-acetic acid or a strain of Lactobacillus reuteri engineered to produce IL-22 were protected from liver damage, inflammation, and bacterial translocation to the liver.
Alcohol-associated gut microbial dysbiosis also induces liver damage due to an increased abundance of pathogenic microorganisms capable of producing exotoxins. For example, cytolysin, an exotoxin secreted by Enterococcus faecalis, can directly induce hepatocyte damage by forming pores in the cell membrane. Increased abundance of cytolysin-positive E. faecalis is associated with higher severity of liver disease and increased mortality in patients with alcoholic hepatitis[34]. Germ-free mice colonized with cytolysin-positive E. faecalis and treated with bacteriophages specifically targeted towards cytolytic E. faecalis were protected from ethanol-induced liver disease.
Lastly, ALD is associated with cholestasis and the accumulation of bile acids, perpetuated in a feedback loop. ALD-induced dysbiosis results in an increase in the gut bacteria that deconjugates bile acids in the intestine, leading to increased exposure of hepatocytes to the more toxic deconjugated secondary bile acids. Additionally, the increase in intestinal secondary bile acids decreases farnesoid X receptor (FXR) signaling, which is a crucial regulator of bile acid homeostasis and lipid and glucose metabolism. Because FXR activation plays an anti-inflammatory role during liver injury, suppresses the development of hepatic fibrosis, and helps to maintain the gut-vascular barrier [35, 36], decreased FXR signaling further exacerbates liver disease.
A few clinical trials have attempted to modulate the gut microbiomes of patients with alcohol-associated liver disease with encouraging results. One trial of 66 men with alcohol-associated liver injury randomized to receiving a probiotic of Bifidobacterium bifidum and Lactobacillus plantarum versus standard therapy of abstinence and vitamins found that patients treated with probiotics had significantly lower AST and ALT activity at the end of treatment than those treated with standard therapy alone[37]. Another clinical trial using probiotics in patients with alcoholic hepatitis found that probiotic treatment significantly decreased levels of LPS and TNF-α[38]. Other clinical trials have used fecal microbiota transplantation (FMT) to modulate the gut microbiomes of patients with alcoholic hepatitis. Two small studies in patients with severe alcoholic hepatitis who received either healthy donor FMT or standard of care found that those who received FMT demonstrated lower rates of alcohol relapse, higher rates of hepatic encephalopathy and ascites resolution, and improved survival at 28 and 90 days in one trial compared with those who received standard of care[39, 40]. Another study that randomized 120 patients with steroid-eligible severe alcoholic hepatitis to prednisolone or healthy donor FMT demonstrated improved 90-day survival and reduced incidence of infection in the patients receiving FMT compared with prednisolone[41]. Though only a few clinical trials targeting the gut microbiota of patients with ALD have been conducted, with relatively small sample sizes, this is a promising area for further research.
Non-bacterial gut microbial changes in alcohol use and alcohol-associated liver disease
The contribution of fungal dysbiosis and the overgrowth of specific fungal species to alcohol-associated liver disease is a rapidly expanding area of study. Levels of serum anti-Saccharomyces cerevisiae immunoglobulin G antibodies (ASCA), a marker of systemic immune response to fungi and fungal products, are elevated in patients with alcohol use disorder and alcohol-associated liver disease[42], and in patients with alcoholic hepatitis, serum ASCA levels are associated with increased mortality[43]. One mechanism by which gut fungi mediate liver injury is via translocation of beta-glucan, the predominant fungal cell wall polysaccharide, to the liver, where it acts via the C-type lectin–like receptor CLEC7A on Kupffer cells to increase expression of IL-1β, inducing liver inflammation and hepatocyte damage[42]. Additionally, increased relative abundance of specific fungal species such as Candida albicans and Malassezia restricta in the gut are associated with worse liver injury and mortality in patients with alcohol-associated liver disease, though via different molecular mechanisms[44, 45]. Candida albicans secretes the peptide toxin candidalysin, which is directly cytotoxic to hepatocytes, whereas Malassezia restricta induces inflammatory cytokines and chemokines in Kupffer cells via C-type lectin domain family 4, member N signaling.
Changes in the gut viral microbiome (virome) composition are also being recognized as important across the spectrum of alcohol use and alcohol-associated liver disease. For example, the relative abundance of different Lactococcus phages in the gut is increased in patients with alcohol use disorder and higher abundance is associated with more severe alcohol-associated liver disease[46]. In fact, even in patients with metabolic syndrome-associated fatty liver disease (MAFLD), those who consumed low to moderate amounts of alcohol (less than 20 g/day) demonstrated increased relative abundance of Lactoccoccus phages in their gut compared to those who completely abstained from alcohol, and the abundance of several Lactococcus phages in the gut could successfully predict the presence of alcohol use in patients with hepatic steatosis[47]. In fact, even in patients with metabolic syndrome-associated fatty liver disease (MAFLD), those who consumed low to moderate amounts of alcohol (less than 20 g/day) demonstrated increased relative abundance of Lactoccoccus phages in their gut compared to those who completely abstained from alcohol, and the abundance of several Lactococcus phages in the gut could successfully predict the presence of alcohol use in patients with hepatic steatosis[47].
Conclusion
Changes to the gut microbiota with chronic alcohol use are an important risk factor for the development of alcohol use disorder and alcohol-associated liver disease. Gut dysbiosis occurs with chronic alcohol exposure, leading to an increased abundance of pathogenic organisms and a decreased abundance of commensal organisms. The gut microbiota can affect alcohol use by producing or metabolizing neurotransmitters and other metabolites to directly interact with the host nervous system or indirectly modulate neuroinflammation. Gut dysbiosis can also directly result in liver damage via the production of exotoxins, or indirectly via impairment of the intestinal barrier, stimulation of pro-inflammatory pathways, or disruption of bile acid homeostasis. While many pre-clinical trials have successfully implemented gut-microbial directed therapies for alcohol use disorder and alcohol-associated liver disease, only a handful of clinical trials have successfully translated these therapies into humans thus far. As we gain understanding of the effects of alcohol on the gut-brain and gut-liver axes, the potential for impactful therapies will grow.
Funding
This study was supported in part by NIH grants T32 DK007202 and the Southern California Research Center for ALPD and Cirrhosis funded by the National Institute on Alcohol Abuse and Alcoholism (P50AA011999) to C.L.H.
Abbreviations
- ALD
alcohol-associated liver disease
- ASCA
anti-Saccharomyces cerevisiae immunoglobulin G antibodies
- AUD
alcohol use disorder
- BDNF
brain-derived neurotrophic factor (BDNF)
- FOXO
forkhead box class O
- FMT
fecal microbiota transplantation
- FXR
farnesoid X receptor
- HCC
hepatocellular carcinoma
- IL-1β
interleukin-1β
- IL-22
interleukin-22
- LPS
lipopolysaccharide
- MAFLD
metabolic syndrome-associated fatty liver disease
- PAMPs
pathogen-associated molecular patterns
- REG3
regenerating islet derived factor 3
- TGF-β
transforming growth factor β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor α
Footnotes
Conflicts of interest statement
M.H.J. and C.L.H. have no conflicts of interest.
References
- 1.Lozupone CA, et al. , Diversity, stability and resilience of the human gut microbiota. Nature, 2012. 489(7415): p. 220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowan-Nash AD, et al. , Cross-Domain and Viral Interactions in the Microbiome. Microbiol Mol Biol Rev, 2019. 83(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson GP, Lee SM, and Mazmanian SK, Gut biogeography of the bacterial microbiota. Nat Rev Microbiol, 2016. 14(1): p. 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project C, Structure, function and diversity of the healthy human microbiome. Nature, 2012. 486(7402): p. 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thursby E and Juge N, Introduction to the human gut microbiota. Biochem J, 2017. 474(11): p. 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan J, et al. , The role of short-chain fatty acids in health and disease. Adv Immunol, 2014. 121: p. 91–119. [DOI] [PubMed] [Google Scholar]
- 7.Lathrop SK, et al. , Peripheral education of the immune system by colonic commensal microbiota. Nature, 2011. 478(7368): p. 250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hild B, et al. , Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nat Metab, 2021. 3(8): p. 1042–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehm J and Shield KD, Global Burden of Alcohol Use Disorders and Alcohol Liver Disease. Biomedicines, 2019. 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam A and Kwo PY, Epidemiology and Disease Burden of Alcohol Associated Liver Disease. J Clin Exp Hepatol, 2023. 13(1): p. 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranzler HR and Soyka M, Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA, 2018. 320(8): p. 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leclercq S, et al. , Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A, 2014. 111(42): p. E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubinkina VB, et al. , Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome, 2017. 5(1): p. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbia C, et al. , The Microbiome-Gut-Brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine, 2023. 89: p. 104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclercq S, et al. , Gut Microbiota-Induced Changes in beta-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep, 2020. 33(2): p. 108238. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, et al. , Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC epsilon levels in mouse. Biofactors, 2020. 46(1): p. 38–54. [DOI] [PubMed] [Google Scholar]
- 17.Leclercq S, et al. , Alterations of kynurenine pathway in alcohol use disorder and abstinence: a link with gut microbiota, peripheral inflammation and psychological symptoms. Transl Psychiatry, 2021. 11(1): p. 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JT, et al. , The Gut Microbiome and Substance Use Disorder. Front Neurosci, 2021. 15: p. 725500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mews P, et al. , Alcohol metabolism contributes to brain histone acetylation. Nature, 2019. 574(7780): p. 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angoa-Perez M and Kuhn DM, Evidence for Modulation of Substance Use Disorders by the Gut Microbiome: Hidden in Plain Sight. Pharmacol Rev, 2021. 73(2): p. 571–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrakis IL, et al. , Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology (Berl), 2019. 236(10): p. 3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, et al. , A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology, 2021. 73(5): p. 1688–1700. [DOI] [PubMed] [Google Scholar]
- 23.Crabb DW, et al. , Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology, 2020. 71(1): p. 306–333. [DOI] [PubMed] [Google Scholar]
- 24.Tuomisto S, et al. , Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol, 2014. 14: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, et al. , Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology, 2011. 54(2): p. 562–72. [DOI] [PubMed] [Google Scholar]
- 26.Mutlu EA, et al. , Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol, 2012. 302(9): p. G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JR, Intestinal mucosal barrier function in health and disease. Nat Rev Immunol, 2009. 9(11): p. 799–809. [DOI] [PubMed] [Google Scholar]
- 28.Yang L and Seki E, Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol, 2012. 3: p. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, et al. , Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe, 2016. 19(2): p. 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CL, et al. , Differences in Bacterial Translocation and Liver Injury in Ethanol Versus Diet-Induced Liver Disease. Dig Dis Sci, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grander C, et al. , Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut, 2018. 67(5): p. 891–901. [DOI] [PubMed] [Google Scholar]
- 32.Cresci GA, et al. , Prophylactic tributyrin treatment mitigates chronic-binge alcohol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrikx T, et al. , Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut, 2019. 68(8): p. 1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnabl B, Update on the Role of the Gut Microbiota on Alcohol-Associated Liver Disease. Gastroenterol Hepatol (N Y), 2021. 17(8): p. 381–383. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YD, et al. , Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology, 2008. 48(5): p. 1632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandl K, et al. , Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol, 2018. 69(2): p. 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirpich IA, et al. , Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol, 2008. 42(8): p. 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han SH, et al. , Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol, 2015. 27(11): p. 1300–6. [DOI] [PubMed] [Google Scholar]
- 39.Sharma A, et al. , Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int, 2022. 16(2): p. 433–446. [DOI] [PubMed] [Google Scholar]
- 40.Philips CA, et al. , Long-term Outcomes of Stool Transplant in Alcohol-associated Hepatitis-Analysis of Clinical Outcomes, Relapse, Gut Microbiota and Comparisons with Standard Care. J Clin Exp Hepatol, 2022. 12(4): p. 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pande A, et al. , Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol Int, 2023. 17(1): p. 249–261. [DOI] [PubMed] [Google Scholar]
- 42.Yang AM, et al. , Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest, 2017. 127(7): p. 2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang S, et al. , Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology, 2020. 71(2): p. 522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu H, et al. , The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol, 2020. 72(3): p. 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng S, et al. , Malassezia restricta promotes alcohol-induced liver injury. Hepatol Commun, 2023. 7(2): p. e0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu CL, et al. , Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol Commun, 2022. 6(8): p. 2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu CL, et al. , Any alcohol use in NAFLD patients is associated with significant changes to the intestinal virome. Hepatology, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]


