Abstract
Objective
We retrospectively compared the dose, cost, and safety of vadadustat and daprodustat for the treatment of renal anemia in patients with chronic kidney diseases who were not undergoing dialysis.
Methods
The primary outcome of this study was the change in dose and cost from the initiation of vadadustat and daprodustat treatment. The secondary outcome was the drug safety.
Patients
We treated 30 patients each with the hypoxia-inducible factor prolyl-hydroxylase inhibitors (HIF-PHIs) daprodustat and vadadustat. The hemoglobin (Hb) concentration was targeted at 11-13 g/dL, and transferrin saturation was maintained at ≥20%, as per the 2018 Japanese guidelines for the diagnosis and treatment of chronic kidney disease.
Results
Hb levels increased from 10.7 to 11.5 g/dL after the first month of daprodustat administration, whereas those for vadadustat patients remained relatively stable, going from 10.7 to 10.6 g/dL. After six months, the Hb level reached 12.1 g/dL and 11.3 g/dL for daprodustat and vadadustat, respectively. The dosage of vadadustat was significantly increased by 46% and 70% after 3 and 12 months, respectively, compared with the initial doses, whereas that of daprodustat did not change substantially. The average cost of vadadustat also increased in the first 3 months and remained over 500 yen/day after 3 months, while that of daprodustat showed little change from the initial cost of 360 yen/day.
Conclusion
These results suggest that heterogeneity exists in the drug potency and dosage required for treatment between daprodustat and vadadustat. Serious adverse events [death, cardiovascular disease, end stage renal disease (ESRD), and malignancy] occurred in more than 20% of participants with both HIF-PHIs. Further studies are required to confirm the safety of HIF-PHIs.
Keywords: renal anemia, HIF-PH inhibitor, dose, cost, safety
Introduction
Renal anemia is a universal comorbidity of advanced chronic kidney disease (CKD) and is associated with a reduced quality of life in patients afflicted by the disease. Renal anemia also triggers the progression of chronic heart failure and CKD, known as cardiorenal anemia syndrome (1). Treatment of anemia improves the physical capacity and reduces fatigue in patients with CKD, as demonstrated by the amelioration of the cardiorespiratory function in patients with CKD undergoing hemodialysis (2).
Hypoxia-inducible factor prolyl-hydroxylase inhibitors (HIF-PHIs) are novel drugs that activate the HIF oxygen-sensing pathway and promote erythropoiesis by activating endogenous erythropoietin and inhibiting hepcidin gene expression (3,4). HIF-PHIs and erythropoiesis-stimulating agents (ESAs) are used clinically for the treatment of renal anemia. Japan is a leader in the treatment of renal anemia via five HIF-PHIs (roxadustat, daprodustat, vadadustat, enarodustat, and molidustat), all of which are commercially available and approved by the Japan Pharmaceuticals and Medical Devices Agency (PMDA). Daprodustat was approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (USFDA/FDA). However, the FDA has approved it only for patients undergoing dialysis.
The clinical doses and drug prices of the five HIF-PHIs varied. The initial doses of roxadustat, daprodustat, vadadustat, enarodustat, and molidustat were 50, 2, 4, 2, 300, and 25 mg, respectively, with initial doses costing 777.30, 179.70, 316.80, 366, 270.50, and 163.80 yen, respectively. Roxadustat was administered three times per week, whereas the other treatments were administered daily.
However, physicians with sufficient knowledge and experience in the use of ESAs are doubtful of the safety of HIF-PHIs, expressing concern about the theoretical HIF-PHI-induced induction of vascular endothelial growth factor (VEGF), which can cause angiogenesis and vascular permeability (3,4). These genes are associated with the growth of existing tumors and retinal neovascularization. Furthermore, thrombotic events increase with the use of HIF-PHIs and ESAs (5-7). In a pooled analysis, the incidence of major adverse cardiac events (MACE) was significantly higher with vadadustat than with darbepoetin alfa (6,8). Daprodustat has not been approved by the FDA for patients not undergoing dialysis because of the cardiovascular risk in the US (United States) population (9).
We were interested in the differences in the efficacy and cost of renal anemia treatment among HIF-PHIs. We therefore retrospectively compared the doses and costs of vadadustat and daprodustat for the treatment of renal anemia in patients with CKD who were not undergoing dialysis. We also studied the incidence of the adverse effects of vadadustat and daprodustat.
Materials and Methods
Study protocol
This was a retrospective, observational study. The Nakayamadera Imai Clinic treats approximately 1,700 patients per month, 95% of whom have chronic non-communicable diseases (mainly diabetes and CKD). The number of patients with CKD stages G3b, G4, and G5 was 235, 140, and 80, respectively. All patients treated with vadadustat or daprodustat between August 2020 and December 2022 were enrolled in this study. The first six patients were treated with daprodustat, and the next five patients were treated with vadadustat in August and September 2020. We then accepted 10 cases of post-marketing surveillance for daprodustat. Subsequently, 25 cases of post-marketing surveillance for vadadustat were performed. After completing the vadadustat study, three additional cases of post-marketing surveillance of daprodustat were accepted. Ultimately, 11 patients treated with daprodustat were enrolled in this study.
Clinically, we treated patients with HIF-PHIs by targeting a hemoglobin (Hb) level of 11-13 g/dL and a transferrin saturation (TSAT) of ≥20%, according to the 2018 Guidelines for the Diagnosis and Treatment of Chronic Kidney Disease (as published by the Japanese Society of Nephrology) (10).
The study protocol was approved by the Ethics Committee of Hyogo Medical Association (R4-005). As this study was retrospective, we used an opt-out approach, and the requirement for written informed consent was waived. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Patients' follow-up and study outcomes
Patients visit every month for the treatment of CKD, including renal anemia. Clinical data were obtained from the patients' clinical records. We compared the values of Hb, ferritin, transferrin, TSAT, systolic blood pressure, serum potassium, HIF-PHI dose, and daily cost of HIF-PHIs between patients treated with vadadustat and daprodustat. The primary outcome of this study was the difference in dose and cost between vadadustat and daprodustat after the initiation of treatment. The secondary outcomes were the drug safety, brain natriuretic peptide, serum potassium, and blood pressure, all of which were measured before initiating HIF-PHI treatment and at six months post-treatment.
Statistical analyses
A t-test or Mann-Whitney U test was used to evaluate significant differences between the two groups. Multiple group comparisons were performed using Dunnett's test. A one-way analysis of variance was performed, as indicated. Statistical significance was set at p<0.05 to indicate statistical significance. Statistical analyses were performed using the GraphPad Prism 8.0 software program (GraphPad Software, San Diego, USA) or R 4.0.3. Data are expressed as the mean±standard deviation (SD) or median (interquartile range).
Results
Participants
We enrolled all the patients treated with either vadadustat or daprodustat between August 2020 and December 2022. Sixty patients were administered either vadadustat or daprodustat during this period. The mean age of all participants was 75.5 years old, and 61.6% of them were men. Clinical characteristics relevant to renal anemia were similar between the groups (Table 1). The participants had advanced CKD with a mean estimated glomerular filtration rate (eGFR) of 15 mL/min/1.73 m2. At baseline, the mean TSAT was 31%, with a mean serum ferritin level of 159 ng/mL, indicating that the iron status for erythropoiesis was adequate.
Table 1.
Baseline Characteristics of Patients.
| Daprodustat (n=30) | Vadadustat (n=30) | p value | ||||
|---|---|---|---|---|---|---|
| Age (y.o) | 76±8 | 75±11 | 0.70 | |||
| Sex (n(%)) | 19 (63.3) | 18 (60) | 1.00 | |||
| Follow-up period (months) | 14 [13, 22.75] | 16 [9, 16.75] | 0.39 | |||
| sBP (mmHg) | 133±15 | 131±17 | 0.61 | |||
| Cre (mg/dL) | 2.96 [2.01, 4.47] | 2.91 [1.71, 4.71] | 0.87 | |||
| eGFR (mL/min/1.73m2) | 15.0 [11.1, 24.4] | 15.6 [9.5, 30.7] | 0.94 | |||
| CKD stage | ||||||
| 1 (n(%)) | 0 (0) | 0 (0) | ||||
| 2 (n(%)) | 0 (0) | 0 (0) | ||||
| 3a (n(%)) | 2 (6.7) | 0 (0) | ||||
| 3b (n(%)) | 2 (6.7) | 9 (30) | ||||
| 4 (n(%)) | 11 (36.7) | 6 (20) | ||||
| 5 (n(%)) | 15 (50) | 15 (50) | ||||
| Hb (g/dL) | 10.7±1.1 | 10.7±0.8 | 0.93 | |||
| Fe (μg/dL) | 87.7±36.8 | 85.3±22.2 | 0.76 | |||
| TIBC (μg/dL) | 286.0±53.4 | 278.4±50.8 | 0.57 | |||
| TSAT (%) | 30.9±12.2 | 31.1±8.5 | 0.97 | |||
| Ferritin (ng/mL) | 164±107 | 154±90 | 0.67 | |||
| Albumin (g/dL) | 3.7±0.6 | 3.7±0.5 | 0.98 | |||
| BNP (pg/mL) | 48 [31, 124] | 44 [20, 76] | 0.44 | |||
| K (mEq/L) | 4.7±0.5 | 4.6±0.6 | 0.53 | |||
| Cause of kidney disease | ||||||
| Diabetes (n(%)) | 6 (20.0) | 9 (33.3) | 0.55 | |||
| Nephrosclerosis (n(%)) | 14 (46.7) | 14 (46.7) | 1.00 | |||
| CGN (n(%)) | 4 (13.3) | 1 (3.3) | 0.35 | |||
| ADPKD (n(%)) | 0 (0) | 3 (10) | 0.24 | |||
| Other (n(%)) | 6 (20) | 3 (10) | 0.47 | |||
| Patients on peritoneal dialysis (n(%)) | 4 (13.3) | 4 (13.3) | 1.00 | |||
| Previous treatment for renal anemia | ||||||
| Epoetin beta pegol (n(%)) | 16 (53.3) | 21 (70) | 0.29 | |||
| Other HIF-PHIs (n(%)) | 5 (16.7) | 3 (10) | 0.71 | |||
| No treatment (n(%)) | 9 (30) | 6 (20) | 0.55 |
Mean±SD, median [interquartile range], or n (%)
Outcomes
The mean Hb level increased from a baseline of 10.7 to 11.5 g/dL at 1 month in the daprodustat group but showed no marked difference at the same point in the vadaustat group (10.7 to 10.6 g/dL). However, the mean Hb level increased gradually and reached 12.1 g/dL in the daprodustat group and as high as 11.3 g/dL in the vadadustat group at 6 months post-commencement of the study (Fig. 1).
Figure 1.
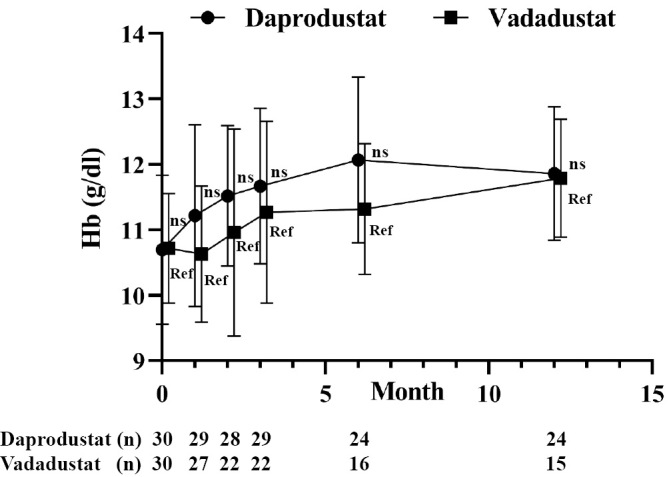
Change in Hb levels after treatment with HIF-PHI. Patients with advanced CKD who were not on dialysis were treated with daprodustat (n=30) or vadadustat (n=30) and followed for 12 months. All results are presented as the mean±SDs. ns: not significant, Ref: reference. The multiple t-test was applied with Bonferroni’ correction, and a p value <0.01 indicates statistical significance.
Doses of daprodustat did not substantially change throughout the study period, while the doses of vadadustat significantly increased by 31% (394 mg) after 1 month, 46% (436 mg) after 3 months, and 70% (510 mg) after 12 months (Fig. 2).
Figure 2.
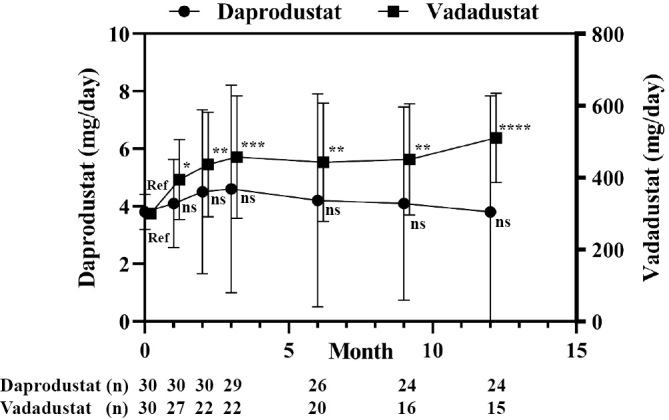
Changes in the HIF-PHI dose for treating renal anemia. Patients with advanced CKD who were not on dialysis were treated with daprodustat (n=30) or vadadustat (n=30) and followed for 12 months. All results are presented as the mean±SDs. ns: not significant, Ref: reference. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ANOVA followed by Dunnett’s post hoc test.
The cost of treatment for renal anemia was significantly higher in patients receiving vadadustat than in those receiving daprodustat at all time points (Fig. 3). The price of vadadustat at an initial dose of 300 mg was 366 yen, which was higher than that of daprodustat. The initial doses of daprodustat 2 mg (Hb ≥9 g/dL) and 4 mg (Hb <9 g/dL) were 180 and 317 yen, respectively. The average cost of vadadustat at 3 months was 565 yen, which was significantly higher than that of daprodustat (361 yen after 3 months). At 6 months, the costs of vadadustat and daprodustat were 551 and 327 yen, respectively. At 12 months, the average cost of vadadustat was 634 yen, which was more than double that of daprodustat (316 yen) (Fig. 4).
Figure 3.
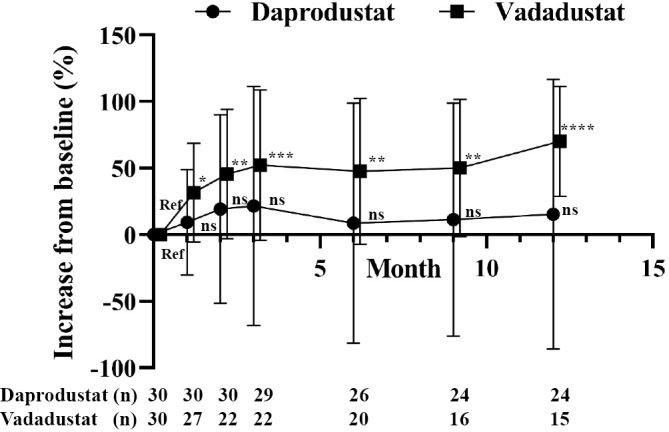
The mean percent change in the HIF-PHI dose after treatment of renal anemia. Patients with advanced CKD who were not on dialysis were treated with daprodustat (n=30) or vadadustat (n=30) and followed for 12 months. All results are presented as the mean±SDs. ns: not significant, Ref: reference. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ANOVA followed by Dunnett’s post hoc test.
Figure 4.
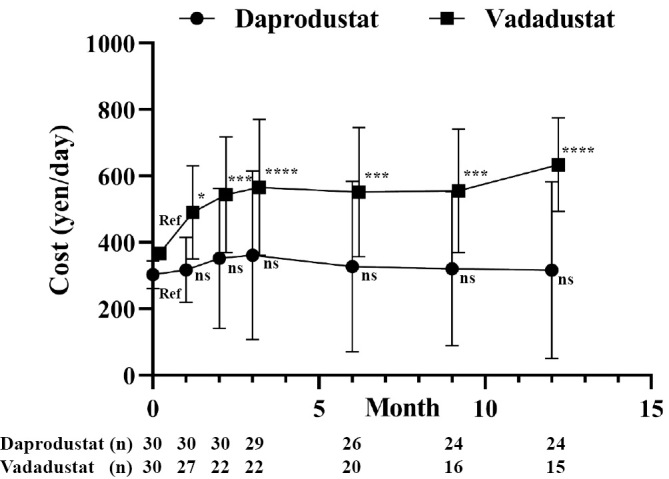
Daily cost of HIF-PHI after treatment of renal anemia. Patients with advanced CKD who were not on dialysis were treated with daprodustat (n=30) or vadadustat (n=30) and followed for 12 months. All results are presented as the mean±SDs. ns: not significant, Ref: reference. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ANOVA followed by Dunnett’s post hoc test.
Safety
The safety profiles of the two HIF-PHIs are listed in Table 2. Two patients administered daprodustat and three patients administered vadadustat died during the study period. The cause of death for those receiving daprodustat was acute heart failure due to aortic valve stenosis and cerebral hemorrhaging. The causes of death in the patients receiving vadadustat were pancreatic cancer and chronic renal failure (one patient on peritoneal dialysis and one patient undergoing conservative kidney management).
Table 2.
Adverse Effects.
| Daprodustat (n=30) | Vadadustat (n=30) | |||
|---|---|---|---|---|
| Death [n (%)] | 2 (6.6%) | 3 (10%) | ||
| Acute heart failure | 1 (3.3%) | 0 (0%) | ||
| Cerebral hemorrhage | 1 (3.3%) | 0 (0%) | ||
| Chronic renal failure | 0 (0%) | 2 (6.6%) | ||
| Cancer | 0 (0%) | 1 (3.3%) | ||
| Heart failure (n (%)) | 2 (6.6%) | 0 (0%) | ||
| ESRD (n (%)) | 6 (20%) | 4 (13.3%) | ||
| HD | 5 (16.7%) | 2 (6.6%) | ||
| PD | 1 (3.3%) | 2 (6.6%) | ||
| Malignancy [n (%)] | 0 (0%) | 3 (10%) | ||
| Pancreas cancer | 0 (0%) | 1 (3.3%) | ||
| Colon cancer | 0 (0%) | 1 (3.3%) | ||
| Myeloma | 0 (0%) | 1 (3.3%) | ||
| Medication discontinuation (Exc cancer) (n (%)) | 0 (0%) | 7 (23.3%) | ||
| Allergy (drug eruption) | 0 (0%) | 3 (10%) | ||
| Diarrhea | 0 (0%) | 3 (10%) | ||
| Dizziness | 0 (0%) | 1 (3.3%) |
Two patients who received daprodustat experienced heart failure (one died, as mentioned above). Three patients receiving vadadustat had malignancies [colon cancer, pancreatic cancer (having died, as mentioned above), and myeloma].
Six patients treated with daprodustat and four patients treated with vadadustat developed end stage renal disease (ESRD). Their mean eGFR at baseline was 10.0±2.9 mL/min/1.73 m2. Three patients discontinued vadadustat owing to allergic reactions. Three patients discontinued vadadustat because of persistent diarrhea. The diarrhea was exacerbated by increasing the dose of vadadustat. No adverse gastrointestinal effects were observed after daprodustat treatment.
Discussion
We observed slow and mild erythropoiesis with vadadustat during the first three months of treatment compared to daprodustat. The mean Hb level of vadadustat did not increase at 1 month and increased to 11.3 g/dL at 3 months, which was 1 g/dL lower than that of daprodustat. Because of the slow erythropoiesis associated with vadadustat, its dose was increased by 46% at 3 months and reached 70% higher than the initial dose at 12 months. The daily cost of vadadustat was significantly higher than that of daprodustat at all the time points during the study period.
Slow erythropoiesis with vadadustat, particularly in patients previously treated with ESA, was also observed in phase III trials when compared with darbepoetin alfa (8,11). In a Japanese phase III trial of vadadustat, Hb levels did not increase in the initial six weeks in patients who had previously used ESAs (11). In patients previously treated with ESAs, the dose of vadadustat was increased to 450 mg after 8 weeks. These results suggest that slow erythropoiesis is characteristic of vadadustat at the initiation of treatment. Slow erythropoiesis has not been reported with daprodustat in patients with CKD or in those on dialysis (12,13). The reason for slow erythropoiesis remains unclear. The initial dose of vadadustat (300 mg) was approximately 100 times larger than that of daprodustat. Differences in drug potency may contribute to differences in responses to erythropoiesis. The initial dose of vadadustat may have been too low to increase Hb levels in the first month because Hb levels increased after administration of higher doses of vadadustat (450 mg or 600 mg). In a phase II study, vadadustat was initiated at 450 mg, and by the second week, the mean Hb levels had increased significantly from baseline, further increasing by 0.5 g/dL by the fourth week (14).
Differences in potency between vadadustat and daprodustat were observed, with several possible causes. First, absorption and distribution differ among drugs. The maximum plasma concentration (Cmax) of 300 mg of vadadustat and 5 mg of daprodustat after repeated administration represent an approximate 425 times difference (vadadustat 39.6 μg/mL vs daprodustat 93.0 ng/mL). Second, the inhibition of prolyl hydroxylase domain (PHD)3 was stronger than that of PHD2 by vadadustat, whereas inhibition by daprodustat was equal between PHD2 and PHD3. Consequently, the stabilization of HIF-2a by vadadustat is greater than that by HIF-1a, whereas daprodustat stabilizes both HIF-1a and HIF-2a equally (15). Interestingly, the target Hb levels for the treatment of anemia differ between countries. In the PRO2TECT study, the risk of cardiovascular events in non-US countries was significantly higher than that in the US (8). In that study, the target Hb level was 10-11 g/dL in the US and 10-12 g/dL in other countries. The target Hb level was therefore not consistent among countries, although the initial dose of vadadustat was 300 mg, which is similar to the initial dose in Japan. The lower target Hb level allowed the use of less vadadustat in the US, as there might be fewer recorded cardiovascular adverse events there than in non-US countries (8). In the present study, the target Hb level was 11-13 g/dL, set according to the 2018 Japanese CKD guidelines (10); this required a relatively high dose of vadadustat. A high dose of vadadustat may cause a high incidence of adverse events, particularly cardiovascular disease. Furthermore, our results suggest that the initial dose of 300 mg vadadustat may have been too low to target and maintain Hb levels of 11-13 g/dL. The Japanese Guidelines for the Diagnosis and Treatment of Chronic Kidney Disease were revised in June 2023 (16), and the target Hb level for the treatment of renal anemia was reduced to 10-13 g/dL. This may improve the gap between guideline recommendations and real-world clinical practice. Furthermore, it may reduce the adverse events caused by vadadustat.
Serious adverse events (death, cardiovascular disease, ESRD, and malignancy) occurred in >20% of the participants in our study. HIF-PHIs and ESA potentially cause thromboembolic diseases, such as myocardial infarction, deep vein thrombosis, and cerebral infarction. It was reported that vadadustat did not meet its pre-specified non-inferiority margin with respect to MACE (hazard ratio=1.17, 95% confidence interval=1.01-1.36) compared to darbepoetin alfa, suggesting a higher risk of MACE in vadadustat cases than darbepoetin alfa (8). The incidence of thromboembolic diseases was, however, reported to be <1% for both vadadustat and daprodustat (8,10). In addition, recent meta-analyses did not show a statistically significant difference between HIF-PHIs and placebo agents in terms of all-cause mortality and MACE (17).
Daprodustat has only been approved by the FDA for use in CKD patients on dialysis, not for those not on dialysis (9). This is because the hazard ratio of cardiovascular endpoints was higher than 1.0 in daprodustat compared with darbepoetin alfa in the ACEND-ND trial (10). The US population showed significantly greater hazard ratios for all-cause mortality, hospitalization for heart failure, and thromboembolic events in cases of daprodustat use than in those of darbepoetin alfa use compared to the non-US population, although the target Hb level was the same (10-11 g/dL) in both the US and non-US populations (9). In a meta-analysis, the overall effect of daprodustat in increasing the risk of hospitalization for heart failure was similar between daprodustat and ESAs, and the results were consistent between the ASCEND-ND and ASCEND-D subgroups (20). In a pooled analysis of Japanese patients (n=369), the incidence rate of heart failure events was nominally lower in the daprodustat group than in the ESA group (20). These results suggest a difference in susceptibility to cardiovascular diseases between US and non-US populations. The incidence of cardiovascular diseases, particularly myocardial infarction and heart failure, in the CKD population differed significantly between the US and Japan (21). In our study, 2 of the 30 patients treated with daprodustat developed heart failure that required hospitalization. Further studies are needed to clarify concerns regarding heart failure.
Malignancy was observed after vadadustat treatment in the present study. As mentioned above, the HIF pathway potentially activates tumor growth (3,4). In ASCEND-ND, cancer-related death, tumor progression, and recurrence were more commonly observed with daprodustat than with darbepoetin alfa, with a relative risk of 1.47 (95% confidence interval=1.03-2.01) (12,18). However, the increased risk of cancer-related adverse events was attributed to differences in dosing intervals between randomized treatment arms (19). Increases in malignancy-related risks have not been observed with vadadustat (8,11).
Diarrhea is a common adverse event in HIF-PHI and is observed more frequently with HIF-PHIs than with ESAs (17). The incidence rate of diarrhea was higher in the vadadustat group in the present study and was aggravated by dose escalation. Elevated serum potassium levels and blood pressure have been reported in patients with HIF-PHI and ESA treatment. Hyperkalemia and hypertension were not observed in the present study.
In the present study, the cost of vadadustat for the treatment of renal anemia was significantly higher than that of daprodustat alone. Regarding the reasons for this, the cost of vadadustat in the first month was higher than that of daprodustat, as the cost of the initial dose of vadadustat was 366 yen higher than that of daprodustat [180 yen (2 mg) or 317 yen (4 mg)]. The dosage then had to be increased in the second month for 48.1% of patients receiving vadadustat because their Hb levels remained unchanged after the first month. The dosage of vadadustat was further increased in 78.3% of the patients after the third month because slow erythropoiesis continued throughout the second month. The maximum dose of vadadustat is often required to maintain an Hb level of 11-13 g/dL. In fact, the maximum dose of vadadustat [600 mg (732 yen)] was required by 45.5% and 53.3% of the participants after 6 and 12 months, respectively. In contrast, 70% of the patients treated with daprodustat were administered an initial dose of 4 mg (317 yen) or less after 3 months. Taken together, these findings indicate that daprodustat had a lower cost of treatment for renal anemia than vadadustat.
Several limitations associated with the present study warrant mention. First, this was a retrospective observational study conducted at a single center. Therefore, randomization could not be performed. Second, the sample size of this study was small. Subtle changes in the clinical parameters may not be detected in some patients; therefore, a real-world prospective multicenter study is warranted.
In conclusion, we retrospectively compared changes in the dose and cost over 12 months between vadadustat and daprodustat in patients with CKD not currently undergoing dialysis. The dose of vadadustat was significantly increased from the initial dose after the first three months and persisted after nine months, whereas that of daprodustat did not change substantially. The average cost of vadadustat also increased in the first 3 months and remained over 500 yen/day after 3 months, while that of daprodustat did not change markedly compared with the initial cost of 360 yen/day. These results show that heterogeneity was present among HIF-PHIs. The difference in costs between drugs should be considered when treating renal anemia. Serious adverse events (death, cardiovascular disease, and malignancy) were observed with both daprodustat and vadadustat. Further studies will be required to confirm the safety of HIF-PHIs.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure - the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol 38: 295-310, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Macdougall IC, Lewis NP, Saunders MJ, et al. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet 335: 489-493, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Sugahara M, Tanaka T, Nangaku M. Future perspectives of anemia management in chronic kidney disease using hypoxia-inducible factor-prolyl hydroxylase inhibitors. Pharmacol Ther 239: 108272, 2022. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Del Vecchio L. Hypoxia-inducible factor-prolyl hydroxyl domain inhibitors: from theoretical superiority to clinical noninferiority compared with current ESAs? J Am Soc Nephrol 33: 1966-1979, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzano R, Szczech L, Leong R, et al. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin J Am Soc Nephrol 16: 1190-1200, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Roxadustat for the treatment of anemia due to chronic kidney disease in adult patients not on dialysis and on dialysis. FDA Presentation: Cardiovascular and Renal Drugs Advisory Committee Meeting [Internet]. [updated 2021 Jul 15; cited 2023 May 27]. Available from:https://www.fda.gov/media/150756/download
- 7.Akebia Therapeutics. Press release. Akebia therapeutics receives complete response letter from the FDA for vadadustat for the treatment of anemia due to chronic kidney disease in adult patients [Internet]. [updated 2022 Mar 30; cited 2023 May 27]. Available from: https://ir.akebia.com/news-releases/news-release-details/akebia-therapeutics-receives-complete-response-letter-fda
- 8.Chertow GM, Pergola PE, Farag YMK, et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med 384: 1589-1600, 2021. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Updated contact information: October 26, 2022 meeting of the Cardiovascular and Renal Drugs Advisory Committee meeting announcement [Internet]. [updated 2022 Oct 26; cited 2023 May 31]. Available from: https://www.fda.gov/advisory-committees/advisory-committee-calendar/updated-contact-information-october-26-2022-meeting-cardiovascular-and-renal-drugs-advisory.
- 10.Japanese Society of Nephrology. The Guideline for the Diagnosis and Treatment of Chronic Kidney Disease 2018. Tokyo Igakusha, Tokyo, 2018. [Google Scholar]
- 11.Nangaku M, Kondo K, Kokado Y, et al. Phase 3 randomized study comparing vadadustat with darbepoetin alfa for anemia in Japanese patients with nondialysis-dependent CKD. J Am Soc Nephrol 32: 1779-1790, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AK, Carroll K, McMurray JJV, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med 385: 2313-2324, 2021. [DOI] [PubMed] [Google Scholar]
- 13.Nangaku M, Hamano T, Akizawa T, et al. Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: a 52-week randomized open-label phase 3 trial. Am J Nephrol 52: 26-35, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 90: 1115-1122, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int 21: S110-S124, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japanese Society of Nephrology . The guideline for the diagnosis and treatment of chronic kidney disease. Tokyo Igakusya, Tokyo, 2023. [Google Scholar]
- 17.Mohamed MMG, Oyenuga M, Shaikh S, Oyenuga A, Kheiri B, Nwankwo C. Hypoxia inducible factorprolyl hydroxylase inhibitors in anemic patients with non-dialysis dependent chronic kidney disease: a meta-analysis of randomized clinical trials. Int Urol Nephrol 55: 167-171, 2023. [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Causland FRM, Claggett BL, et al. Analysis of on-treatment cancer safety events with daprodustat versus conventional erythropoiesis-stimulating agents - post hoc analyses of the ASCEND-ND and ASCEND-D trials. Nephrol Dial Transplant 38: 1890-1897, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatima K, Ahmed W, Fatimi AS, et al. Evaluating the safety and efficacy of daprodustat for anemia of chronic kidney disease: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol 78: 1867-1875, 2022. [DOI] [PubMed] [Google Scholar]
- 20.Nangaku M, Akizawa T, Nagakubo T, et al. Safety of daprodustat in patients with anemia of chronic kidney disease: a pooled analysis of phase 3 studies in Japan. Ther Apher Dial 26: 1065-1078, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Watanabe T, Takeuchi A, et al. ; CKD-JAC Investigators . Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int 91: 227-234, 2017. [DOI] [PubMed] [Google Scholar]


