Abstract
In a previous study, we had found that the extent of T-cell dysfunctions induced by a T-tropic strain of human immunodeficiency virus type 1 (HIV-1) in SCID mice reconstituted with human peripheral blood lymphocytes (hu-PBLs) (hu-PBL-SCID mice) was related to the in vivo state of activation of the human lymphocytes. In this article, we compared the effect of infection of hu-PBL-SCID mice with either T-tropic (X4) or M-tropic (R5) strains of HIV-1 by performing virus inoculation at either 2 h or 2 weeks after the hu-PBL transfer, when the human T cells exhibited a marked activation state or a predominant memory phenotype, respectively. A comparable level of infection was found when hu-PBL-SCID mice were challenged with either the SF162 R5 or the IIIB X4 strain of HIV at 2 h postreconstitution, while at 2 weeks, the R5 virus infection resulted in a higher level of HIV replication than the X4 virus. The R5 strain induced a marked human CD4+ T-cell depletion along with a drop in levels of human immunoglobulin M in serum and release of soluble factors at both infection times, while the X4 virus induced severe immune dysfunctions only at 2 h. Of interest, injection of hu-PBLs into SCID mice resulted in a marked up-regulation of CCR5 on human CD4+ T cells. The percentage of CXCR4+ cells did not change after transplantation, even though a significant decrease in antigen expression was observed. Comparative experiments with two molecular clones of HIV-1 (X4 SF2 and R5 SF162) and two envelope recombinant viruses generated from these viruses showed that R5 viruses (SF162 and the chimeric env-SF162-SF2) caused an extensive depletion of human CD4+ T cells in SCID mice at both 2 h and 2 weeks after reconstitution, while the X4 viruses (SF2 and the chimeric env-SF2-SF162) induced CD4 T-cell depletion only when infection was performed at the 2-h reconstitution time. These results emphasize the importance of the state of activation/differentiation of human CD4+ T cells and gp120-coreceptor interactions at the time of primary infection in determining HIV-1 pathogenicity in the hu-PBL-SCID mouse model.
Human immunodeficiency virus type 1 (HIV-1) replication is a dynamic process influenced by a combination of viral and host factors, whose interactions may shape the natural history of HIV-1 infection in AIDS patients (11). Viral characteristics sustaining viral replication within a patient include replicative fitness and cell tropism. Cell tropism is strongly linked to the ability of different HIV-1 envelopes to utilize CC or CXC chemokine receptors as coreceptors for initiating viral fusion and entry into target cells (1, 7, 9, 24, 32, 51). CXCR4 has been shown to mediate the entry of T-cell-line-adapted SI HIV-1 strains (namely, X4 strains), while CCR5 has been identified as the coreceptor for macrophage-tropic NSI strains (denominated as R5 strains) (3). R5 strains are most frequently transmitted during primary HIV-1 infection and persist throughout the course of infection, while expanded coreceptor usage and evolution to T-tropic viruses are closely linked with disease progression (51). Further studies have shown that CXCR4 is primarily expressed on naive CD4+ T cells, whereas CCR5 is mainly expressed on memory CD4+ T cells (4). Notably, memory CD4+ T cells have been shown to be highly permissive to HIV-1 infection (38, 40, 43, 50).
An in vivo acute or chronic state of activation of the immune system may be an important factor in rendering the host more receptive to HIV-1 infection and more susceptible to virus-induced pathological effects. In fact, several in vitro data indicate that a particular cellular activation state is required for the establishment of a productive HIV infection (31, 41, 44). Moreover, studies of African populations suggest that in vivo immune activation, due to endemic parasitic infections, may be an important cofactor in susceptibility to progressive HIV infection and disease (2, 36). Thus, the state of activation/differentiation of the immune system at the moment of primary infection may be a crucial factor in determining the extent of early viral replication (21) and the establishment of a pool of latently infected cells (6, 12) that have been shown to represent a long-lasting reservoir for HIV-1.
Small animal models represented by SCID mice engrafted with human peripheral blood lymphocytes (25, 48), lymphoid cells (20), or tissues (22, 28) have been largely employed to investigate the mechanisms underlying HIV-1 infection and AIDS pathogenesis (16, 19, 20, 23, 26, 27, 29, 35, 37). In particular, previous studies with the hu-PBL-SCID mouse model had shown that X4 HIV-1 strains, which are highly cytopathic for T cells in vitro (5), caused little CD4+ T-cell depletion in SCID mice reconstituted with human peripheral blood lymphocytes (hu-PBLs), whereas noncytopathic, macrophage-tropic R5 strains induced extensive CD4+ T-cell depletion, at an equivalent viral burden (27). Therefore, in vitro assays did not predict CD4 T-cell depletion in the hu-PBL-SCID model. Further studies suggested that the envelope gene determines properties important for in vivo pathogenesis as well as for cell tropism (14, 34, 35). In a previous study (37), we had described a modified protocol of HIV-1 infection in which virus challenge was performed shortly after hu-PBL transfer, when the human immune system proved highly activated. Thus, we could show that infection of hu-PBL-SCID mice with the IIIB X4 strain of HIV-1 shortly after reconstitution induced an impressive CD4+ T-cell depletion and immune dysfunctions that were not observed by infecting SCID mice at 2 weeks after human peripheral blood mononuclear cell (PBMC) transfer, when virtually all of the CD4+ T cells expressed the memory phenotype (37). More recently, we demonstrated that the transfer of a human lymphoblastoid CD4+ T-cell line into SCID mice induced differentiation toward the CD45RO phenotype (20). This in vivo-induced differentiation was associated with an acquired permissiveness to R5 HIV-1 strains, as a consequence of a CCR5 up-regulation (20), as well as with a marked susceptibility to an early and massive autocrine Fas-mediated suicide of uninfected cells through apoptosis (33). These findings highly supported the concept that the state of differentiation/activation of human cells at the moment of primary infection is a key factor in influencing HIV-1 pathogenesis. In the present article, we have extended these studies to the hu-PBL-SCID mouse model by comparing virus replication and immune dysfunctions following primary in vivo infection with the HIV-1 X4 or R5 strain at times when the human immune system is highly activated (2 h after hu-PBL transfer) or predominantly in a memory state of differentiation (2 weeks after reconstitution) (37). We found that primary infection of hu-PBL-SCID mice with R5 HIV-1 strains led to marked immune dysfunctions independent from the state of activation of the human immune system at the moment of viral infection. This effect was associated with the up-regulation of CCR5 expression on human CD4+ T cells occurring in SCID mice reconstituted with hu-PBLs.
MATERIALS AND METHODS
Subjects.
hu-PBLs were obtained from the peripheral blood of healthy donors. All donors were screened for HIV-1 and hepatitis prior to donation. The hu-PBLs were obtained by Ficoll-Paque density gradient centrifugation. Thirty million cells were resuspended in 0.5 ml of RPMI 1640 and injected intraperitoneally into the recipient mice.
Animals.
CB17 scid/scid female mice (Harlan, Italy) were used at 4 weeks of age and were kept under specific-pathogen-free conditions. SCID mice were housed in microisolator cages and all food, water, and bedding were autoclaved prior to use.
HIV-1 infection.
hu-PBL-SCID mice were injected intraperitoneally 2 h and 2 weeks after reconstitution with 105 50% tissue culture infective doses (TCID50s) of either the X4 T-tropic IIIB or the R5 M-tropic SF162 strain of HIV-1.
In a second set of experiments, the xenochimeras were injected with 105 TCID50s of envelope recombinant viruses generated between two molecular clones of HIV-1, T-tropic SF2 and M-tropic SF162 (14, 42).
Cell recovery from peritoneal cavity and organs of the SCID mice.
hu-PBL-SCID mice were sacrificed at 4 weeks after HIV infection, and cells were collected from the peritoneal cavity, spleen, and lymph nodes. At each time, a two-step peritoneal lavage was done. The first washing was performed with 1 ml of cold RPMI 1640 medium. The recovered volume was centrifuged, and the supernatant was stored at −20°C, while the cells were pooled with those obtained with a second 4-ml washing. Spleen and lymph nodes were disrupted with the blunt end of a 5-ml syringe plunger. Connective tissue and debris were allowed to settle, and the single-cell suspensions were washed twice in RPMI 1640 medium (37).
ELISA for soluble human factors and Igs.
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used for determination of soluble ICAM-1 (sICAM-1) in peritoneal lavage fluids and of soluble interleukin-2 receptor (sIL-2R) (Genzyme) in sera of hu-PBL-SCID mice, as described elsewhere (37). An ELISA system was used to quantitate human immunoglobulin G (IgG), IgM, and IgA in sera of the chimeras by using a goat antihuman F(ab′)2 Ig antibody and peroxidase-coupled goat anti-human IgG, IgM, and IgA (Cappel-Cooper Biomedical, West Chester, Pa.) as previously described (37). All ELISAs were performed in duplicate, and laboratory standards were included on each plate. Sera and peritoneal lavage fluids from nonreconstituted SCID mice and C.B17 mice were used as negative controls of all ELISA determinations.
Flow cytometric analysis.
Cells recovered from the peritoneum of the hu-PBL-SCID mice were resuspended in phosphate-buffered saline (PBS) and incubated with the appropriate fluorochrome-conjugated monoclonal antibodies (MAbs) for 30 min. The cells were then washed with a mixture of 2% PBS, 0.1% fetal calf serum, and sodium azide and fixed with 2.5% paraformaldehyde. Two-color flow cytometry was performed with a FACSort fluorescence-activated cell-sorter (FACS) cytometer (Becton Dickinson, San Jose, Calif.), and stained cells were analyzed with LYSIS II (Becton Dickinson) software. A total of 5,000 events/sample were collected. Cells were analyzed according to forward and side scatter properties to gate the live cell population, allowing for the exclusion of erythrocytes, dead cells, and cell and tissue debris. The MAbs used were antihuman leukocyte antigen (CD45) (Becton-Dickinson); antihuman CD4, CD8, CD69, HLA-DR, CD45RA, and CD45RO (Becton-Dickinson); and anti-CXCR4-PE and anti-CCR5-PE (Pharmingen, San Diego, Calif.). CXCR4 and CCR5 analyses were performed with gated live CD4+ cells.
Detection of viral infection.
The chimeras were sacrificed after 4 weeks and analyzed for HIV-1 infection by (i) cocultivation of cell suspensions (30) from peritoneal lavage and spleen with human phytohemagglutinin (PHA)–IL-2-stimulated PBMCs for 7 days, with positivity of cocultivation determined by detection of p24 antigen by ELISA (Dupont, B-1130 Brussels, Belgium) in culture supernatants; (ii) DNA-PCR for virus-specific sequences as described elsewhere (37); (amplification and detection of the HLA-DQα gene fragment performed with the GH26-GH27 primer); and (iii) reverse transcription-PCR with specific primers to detect all viral RNAs (total RNA isolated with RNAzol B [Biotecx, Houston, Tex.]), which were treated with RNase-free DNase (Boehringer, Mannheim, Germany) and processed as previously described (19).
Statistical analysis.
Student’s t test and the nonparametric two-tailed Wilcoxon’s rank-sum test were used as appropriate for the statistical analysis of the data.
RESULTS
HIV-1 infection of hu-PBL-SCID mice at 2 h and 2 weeks after reconstitution.
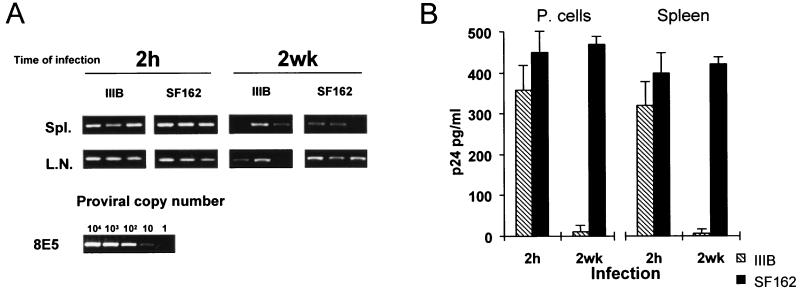
We infected xenochimeric mice with the HIV-1 X4 (T-tropic) IIIB or the R5 (M-tropic) SF162 strain at either 2 h or 2 weeks after transfer of hu-PBLs into SCID mice. Four weeks after infection, the xenochimeras were sacrificed and intensively studied for evaluation of the levels of HIV-1 infection. First, we assessed proviral load in organs from infected mice by PCR. Figure 1A shows the HIV PCR results obtained by testing DNA extracted from spleens or lymph nodes from three representative mice in each infection group. HIV DNA could generally be detected in the spleen and/or lymph nodes from mice infected under both conditions. The evaluation of the proviral copy number revealed the presence of a lower level of HIV-1 copies in organs of SCID mice infected at 2 weeks postreconstitution compared to those of animals infected at 2 h, particularly when the HIV-1 IIIB strain was used (Table 1). Peritoneal cells and splenocytes from infected mice, showing detectable proviral copies in the organs examined, were cocultured with PHA–IL-2-activated autologous human PBMCs, and HIV-1 replication was evaluated by measuring p24 antigen production. As shown in Fig. 1B, comparable levels of p24 antigen were detected in coculture supernatants of cells from hu-PBL-SCID mice infected with either the SF162 strain or the IIIB strain at 2 h after engraftment, although p24 was produced in smaller amounts in cocultures of cells isolated from IIIB-infected xenochimeras. At 2 weeks after reconstitution, although detectable proviral DNA copies were found in infected organs from all groups of infected mice, high levels of p24 antigen were only observed in coculture supernatants from mice infected with SF162, while very low levels of p24 were found in supernatants of cells from xenochimeras infected with the HIV-1 IIIB strain (Fig. 1B). Notably, coculture of cells from hu-PBL-SCID mice infected with the HIV-1 SF162 strain at 2 h and 2 weeks after engraftment exhibited comparable levels of p24 production (Fig. 1B). The comparative PCR analysis of xenochimeric mouse organs and p24 antigen levels in cocultivations showed that (i) both X4 and R5 viruses infected 100% of hu-PBL-SCID mice when HIV-1 challenge was performed at 2 h postreconstitution, and (ii) the R5 HIV-1 strain infected hu-PBL-SCID mice at 2 weeks postreconstitution with no significant differences with respect to the 2-h infection (90 to 100% of infected animals), while the X4 virus infected 60 to 70% of xenochimeras in the 2-week protocol of infection, with low levels of proviral copies (Table 1) and viral production (Fig. 1B).
FIG. 1.
Detection of HIV-1 proviral sequences in spleens (Spl.) and lymph nodes (L.N.) (A) and p24 release in the cell supernatants of cocultures with peritoneal cells (P. cells) or splenocytes (B) from hu-PBL-SCID mice infected with HIV-1 at 2 h or 2 weeks after reconstitution. (A) DNA was extracted by standard procedures and amplified for gag-specific sequences as previously described (37). The sensitivity of the assay was tested by amplifying DNA, prepared from the 8E5 T-cell line (13), which was serially diluted into SCID mouse cell DNA. hu-PBL-SCID mice were infected with either the IIIB or SF162 strain of HIV-1 at 2 h or at 2 weeks postreconstitution. Mice were sacrificed at 4 weeks postinfection, and the spleen and the lymph nodes were analyzed for the presence of HIV-1 proviral copies by DNA PCR. The proviral copy number is indicated. Three mice per group were analyzed in five different experiments. The figure shows the results of one representative experiment. (B) p24 antigen was measured by ELISA in supernatants of peritoneal cells and spleen cells isolated from hu-PBL-SCID mice infected at 2 h or at 2 weeks with either the IIIB or the SF162 strain of HIV-1 and cocultivated with donor autologous PBMCs. The cocultivation assay was performed by using cells from IIIB- and SF162-infected hu-PBL-SCID mice that showed detectable proviral copies in the organs examined. Six mice for each time point were analyzed. Each point in the diagram represents the mean ± standard error. Similar results were obtained in two additional experiments.
TABLE 1.
HIV-1 proviral copy numbers in hu-PBL-SCID mice infected with HIV-1 IIIB or SF162 at 2 h or 2 weeks after reconstitutiona
| Tissue | Mean proviral copy no./106 cells at time of infection:
|
|||
|---|---|---|---|---|
| 2 h
|
2 wk
|
|||
| IIIB | SF162 | IIIB | SF162 | |
| Spleen | 620 ± 105 | 870 ± 240 | 125 ± 50 | 325 ± 75 |
| Lymph nodes | 605 ± 135 | 440 ± 80 | 230 ± 75 | 450 ± 70 |
Results are expressed as mean proviral copy number/106 cells ± standard error calculated for 9 mice/group. The sensitivity of the assay was tested by amplifying 8E5 T-cell DNA, which was serially diluted into SCID mouse DNA, as described in Materials and Methods.
These results suggested that HIV-1 infection with X4 HIV-1 was more efficient at 2 h than at 2 weeks after reconstitution with hu-PBLs, while R5 viruses efficiently infected hu-PBL-SCID mice independent of the in vivo state of activation of human cells.
HIV-1-induced CD4+ T-cell depletion and IgM production impairment.
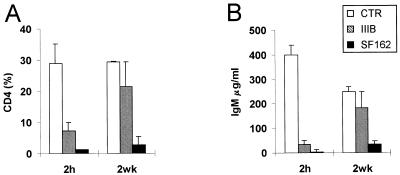
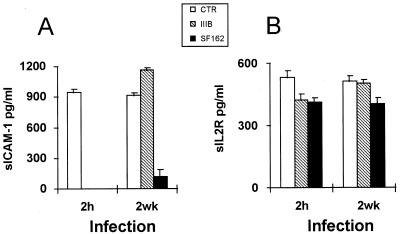
HIV-1 R5 and X4 strains were also compared for the rate of CD4 T-cell depletion and alteration of human immune parameters in hu-PBL-SCID mice. When hu-PBL-SCID mice were infected at 2 h after human cell transfer, both viruses induced a marked CD4+ T-cell depletion (Fig. 2A). When the xenochimeras were infected at 2 weeks postengraftment, HIV-1 SF162 induced a CD4+ T-cell depletion comparable to that of 2-h infection, while the levels CD4+ T cells in IIIB-infected mice did not significantly differ from those of uninfected controls (Fig. 2A), even though some CD4+ T-cell depletion was occasionally observed in single animals. Because IgM production is known to be driven by CD4+ T helper cells in hu-PBL-SCID mice (39), we also measured IgM levels in the sera of infected and uninfected animals. The results showed that SF162 infection induced a dramatic drop in the levels of human IgM in serum at both 2 h and 2 weeks postreconstitution, while HIV-1 IIIB induced a significant decrease in the IgM levels only when hu-PBL-SCID mice were infected at 2 h after the engraftment (Fig. 2B). Of interest, the levels of human IgM in serum in infected and uninfected xenochimeras significantly correlated with the levels of CD4+ T cells in all of the experiments (P < 0.001). To further explore the rate of immune dysfunction induced by HIV-1 infection at the two different time points, we measured by ELISA the levels of human sICAM-1 in the peritoneal lavage fluid and the levels of human sIL-2R in serum in the infected xenochimeras and compared them to those in the uninfected controls. Consistent with the percentage of CD4+ T cells and the serum IgM levels, both the sICAM-1 levels in the peritoneal lavage (Fig. 3A) and, to a lesser extent, the serum sIL-2R levels (Fig. 3B) were significantly decreased in hu-PBL-SCID mice infected with SF162 independently from the time of primary infection, while the HIV-1 IIIB infection led to a drop in both sICAM-1 and sIL-2R levels only when xenochimeras were infected at 2 h postreconstitution (Fig. 3). Notably, a significant correlation was found between the proviral copy number and the extent of CD4+ T-cell depletion in mice infected at 2 h postreconstitution with both viruses (P < 0.01), while in mice infected at 2 weeks, a correlation was observed only with the HIV-1 SF162 strain (P < 0.01). These results suggested that HIV-1 R5 strains induced CD4+ T-cell depletion and triggered immune dysfunctions independently from the state of activation of the immune system at the moment of primary infection. In contrast, the state of activation of the immune system appeared to be a crucial factor in determining the extent of immune derangement induced by HIV-1 X4 strains.
FIG. 2.
Effects of HIV-1 infection on human CD4+ T cells and IgM levels in hu-PBL-SCID mice. (A) FACS analysis of CD4+ cells in the peritoneal washings of hu-PBL-SCID mice infected with either the IIIB or SF162 strain of HIV-1 at 2 h or 2 weeks after hu-PBL injection compared to that of uninfected controls. The histograms represent the mean percentages ± standard error of human CD4+ T cells normalized to the total number of human T cells in peritoneal washings. A total of 18 mice for each group in six different experiments were analyzed. (B) Levels of human IgM (measured by ELISA) in sera of hu-PBL-SCID mice infected with either the IIIB or the SF162 strain of HIV-1 at 2 h and 2 weeks after hu-PBL injection compared to those of uninfected controls (CTR). The histograms represent the mean percentages ± standard error for levels of human IgM in sera from 18 mice for each group in six different experiments.
FIG. 3.
Effects of HIV-1 infection on the levels of human sICAM-1 and sIL-2R in hu-PBL-SCID mice. Values represent levels of human sICAM-1 in peritoneal washings (A) and levels of sIL-2R in serum (B) in hu-PBL-SCID mice infected with either the HIV-1 IIIB or SF162 strain at 2 h or 2 weeks postreconstitution compared to those in uninfected controls (CTR). The histograms represent the mean ± standard error for 18 animals in six different experiments.
Expression of HIV-1 coreceptors and role of envelope proteins in HIV-1 pathogenesis in the hu-PBL-SCID mouse model.
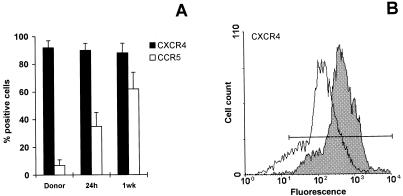
We examined the expression of CCR5 and CXCR4 on human CD4+ T cells shortly after injection into SCID mice compared to that of the autologous PBLs before injection. FACS analysis showed that the percentage of human CD4+ T cells expressing CCR5 was significantly increased as early as 24 h after injection into SCID mice, compared to the donor’s CD4+ T cells before injection (Fig. 4A). At 1 week postreconstitution, up to 60% of human CD4+ T cells expressed CCR5 (Fig. 4A). The percentage of CD4+ T cells expressing CXCR4 did not change at the different times after transplantation (Fig. 4A). However, FACS analysis revealed that the intensity of CXCR4 expression was significantly reduced on human CD4+ T cells at 1 week postreconstitution (Fig. 4B). At 2 weeks, the patterns of CCR5 and CXCR4 expression on human CD4+ T cells were very similar to those observed at 1 week after hu-PBL transfer into SCID mice (data not shown).
FIG. 4.
FACS analysis of CXCR4 and CCR5 chemokine receptor expression on human lymphocytes recovered from uninfected hu-PBL-SCID mice. (A) Histograms represent percentages of CD4+ T cells positive for CCR5 (□) or CXCR4 (■) before hu-PBL injection in SCID mice (time 0) and in the peritoneal washings of hu-PBL-SCID mice 24 h and 1 week after the hu-PBL inoculation. Cells were stained with an anti-CD4 MAb and with either an anti-CCR5 or an anti-CXCR4 MAb; only CD4+ T cells were analyzed by flow cytometry. Percentages were calculated over the total number of CD4+ T cells. Values represent the mean ± standard error for 12 animals in three different experiments. (B) CXCR4 expression on human CD4+ T cells recovered from the peritoneal cavity of hu-PBL-SCID mice 24 h (shaded histograms) and 1 week (open histograms) postreconstitution. Four mice per group were analyzed in three different experiments. The figure shows the results of one representative experiment.
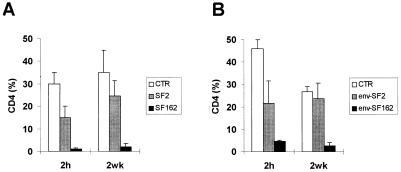
To clearly assess whether the in vivo differential pathogenicity of the T-tropic versus the M-tropic strain of HIV-1 was related to different coreceptor usage, we performed a second set of experiments aimed at comparing the effects of parental HIV-1 X4 SF2 and R5 SF162 strains with those induced by the injection of two recombinant viruses exhibiting a reciprocal substitution of the env gene on the backbone of the parental SF2 and SF162 genomes (14, 42). hu-PBL-SCID mice were challenged with the different viruses either at 2 h or at 2 weeks postreconstitution. The data reported in Fig. 5A confirmed the effectiveness of SF162 in depleting CD4+ T cells independently from the time point at which virus injection was performed, while SF2 determined significant CD4 T-cell depletion only when injected at 2 h after reconstitution of SCID mice, even through some decrease in the levels of CD4+ T cells was occasionally observed in single animals infected at 2 weeks postreconstitution (data not shown). The analysis of the mice challenged with the recombinant SF2 and SF162 viruses containing the envelope gene of the HIV-1 SF162 and SF2 strains, respectively, showed that the env gene played a major role in determining the pattern of in vivo CD4+ T-cell depletion. In fact, the env-SF162-SF2 chimeric virus behaved like the parental SF162 virus and induced an impressive rate of CD4 T-cell depletion at both times of infection, while the env-SF2-SF162 virus behaved like the parental SF2 strain (Fig. 5B and A, respectively).
FIG. 5.
Recovery of human CD4+ T cells in hu-PBL-SCID mice infected with the parental SF2 and SF162 HIV-1 molecular clones (A) or recombinant HIV-1 viruses exhibiting reciprocal substitutions in the V3 region of gp120 (env-SF2 and env-SF162) (B). The hu-PBL-SCID mice were infected with each virus (as indicated) at 2 h or 2 weeks postreconstitution. At 4 weeks after HIV infection, the percentage of CD4+ T cells was calculated relative to the total number of human CD3+ T cells recovered in peritoneal washings compared to that in the uninfected controls (CTR). All of the animals were infected with 105 TCID50s of cell-free virus. The histograms represent the mean ± standard error for nine animals in three different experiments.
DISCUSSION
The hu-PBL-SCID mouse model (25) proved useful for in vivo studies of human immune functions under normal (39, 45–48) and pathologic (10, 17) conditions, including HIV infection (26, 27, 34, 35, 37). In the large majority of studies involving HIV-1 infection in hu-PBL-SCID mice, virus challenge had been performed at 2 weeks after reconstitution. In this regard, it is worth pointing out that, at this time after transplantation of hu-PBLs into SCID mice, the majority of CD4+ T lymphocytes exhibit a CD45RO memory phenotype (47). We have recently confirmed the appearance of a memory phenotype at 1 to 2 weeks after the transfer of human cells into SCID mice and we have described a modified infection model of the xenochimeras suitable for the evaluation of the in vivo effects of primary HIV infection on highly activated human immune cells (37). In fact, soon after their injection into SCID mice, hu-PBLs underwent an impressive activation expressing the early activation marker CD69 and, subsequently, HLA-DR and CD25 antigens, while at 2 weeks, virtually all human lymphocytes proved to be quiescent CD45RO+ cells (37). Thus, we could find that primary infection of hu-PBL-SCID mice with a T-tropic strain of HIV at 2 h postreconstitution (when the majority of human T cells were highly activated) led to marked CD4+ T-cell depletion and immune dysregulation, whereas no or marginal CD4+ T-cell depletion or immune dysfunctions were observed when mice were infected at 2 weeks (37). In this article, we have shown that infection of hu-PBL-SCID mice with X4 T-tropic or R5 M-tropic strains of HIV may lead to a distinctive pattern of CD4+ T-cell depletion and immune dysregulation, depending on the state of activation/differentiation of human T lymphocytes at the moment of in vivo infection. Consistent with our previous results (37), T-tropic HIV-1 X4 strains proved to be highly virulent when injected at a time the transferred human T cells were highly activated (i.e., 2 h postreconstitution), showing they were less infective and poorly cytopathic when the great majority of T lymphocytes at the moment of viral infection were quiescent or memory cells. In contrast, the infection with M-tropic HIV-1 R5 strains always caused impressive CD4+ T-cell depletion and immune dysfunctions, independent from the time of virus challenge (i.e., the state of activation of target cells in the SCID mouse environment). Notably, the high efficiency of infection and the virus-induced T-cell dysfunctions detected in the xenochimeras infected with R5 strains of HIV were associated with the CCR5 expression on human CD4+ T cells, which, even though it occurred as early as 24 to 48 h, was considerably increased at 1 to 2 weeks after the hu-PBL transfer into SCID mice. Of interest, these results are strongly reminiscent of recent data obtained with a model of SCID mice transplanted with a human lymphoblastoid CD4+ T-cell line (CEM cells) (20). In this model, we showed that CEM cells acquired a memory phenotype, when inoculated in SCID mice, and became permissive to R5 M-tropic strains and clinical isolates of HIV-1 through an up-regulation of CCR5 (20). In this regard, it is worth mentioning that recent data show that CXCR4 is mostly expressed on naive CD4+ T cells, while CCR5 is mainly present on the cell surface of memory T cells (4). Thus, the progressive differentiation of human CD4+ T cells toward a memory-CCR5+ phenotype during their persistence in SCID mice may explain the different behaviors of the HIV-1 X4 and R5 strains in hu-PBL-SCID mice. Notably, the decrease in the intensity of CXCR4 expression on CD4+ T cells observed at 1 to 2 weeks postreconstitution could explain, at least in part, the limited spread of infection by X4 viruses when HIV-1 is injected at 2 weeks.
R5 strains are commonly implicated in the transmission of HIV infection and are prevalent during the asymptomatic stages of HIV infection (3, 49). In contrast, X4 strains are generally associated with a decline in peripheral CD4+ T-cell levels and the onset of clinical symptoms and are frequently assumed to be more pathogenic (3, 51). However, the differential pathogenicity may be related to the pool of different target cells present in vivo at the infection site, as well as to a different chemokine receptor expression, rather than to intrinsic viral properties. As an example of the importance of direct relationships between CCR5 expression and the memory phenotype of the target cells in determining the pattern of infection by R5 viruses, we mention recent findings obtained in our laboratory with human intestinal lymphocytes (18), showing that (i) these cells, the vast majority of which are activated or memory T lymphocytes (8), are naturally permissive to infection with a wide spectrum of HIV-1 strains, including R5 viruses, in the absence of exogenous stimuli; and (ii) they normally express a high level of CCR5, and infection with R5 HIV-1 strains is entirely blocked by Rantes (18).
Since HIV-1 cell tropism is strongly dependent on the presence of specific determinants on the viral gp120 Env glycoprotein, in a second set of experiments, we utilized the X4 SF2 and the R5 SF162 viruses along with two chimeric recombinant viruses exhibiting reciprocal substitutions of the env gene on the backbone of parental SF2 and SF162 genomes (14, 42). The results showed that the chimeric viruses behaved as the parental Env phenotype of the virus, in that the SF2-env-SF162 virus induced CD4+ T-cell depletion in hu-PBL-SCID mice independently from the time of infection, while the SF162-env-SF2 virus induced a marked CD4+ T-cell depletion only when infection was performed at 2 h postreconstitution. These results strongly suggested that CD4+ T-cell depletion in infected hu-PBL-SCID mice was dependent on the HIV-1 env genes and that interactions of R5 M-tropic strain envelope determinants with CCR5+ CD4+ T cells expressing the memory phenotype were crucial events in inducing the massive immune dysfunctions observed in animals infected with R5 viruses at 2 weeks after reconstitution. Thus, these results emphasize the importance of the HIV-1 env gene in modulating the pathogenic events occurring in the course of HIV-1 infections and appear to be consistent with a recent report showing that, in SHIV-infected macaques, the efficiency of CD4+ T-cell depletion is determined by single amino acid changes in the env ectodomain, which confer increased chemokine receptor binding and enhanced membrane fusogenic activity (15).
Information obtained with the hu-PBL-SCID mouse model may be important for understanding some events occurring during the natural course of HIV-1 infection, and new knowledge about the in vivo interactions between HIV-1 and target cells can be gained by using these small animal models. As an example with regard to the present study, our data could contribute to envisaging a potential explanation for the predominance of M-tropic isolates throughout most of the course of natural infection with HIV-1, suggesting that the predominance of memory CD4+ T cells in the human body, particularly in mucosal tissues (8), can be a selective factor promoting the maintenance of R5 M-tropic strains of HIV-1, which may prove considerably effective in inducing CD4 T-cell depletion at later stages of the disease, possibly by a bystander effect (5) reminiscent of that recently observed in SCID mouse models (33). On the other hand, the possibility for X4 T-tropic strains of HIV-1 to infect, spread, and induce CD4+ T-cell depletion appears to be directly related to the state of activation of the immune system and to the expression of CXCR4 coreceptor at the moment of primary infection. For instance, in African populations, the in vivo state of chronic immune activation, due to endemic parasitic infections, may favor replication of T-tropic HIV-1 X4 isolates, leading to faster and progressive HIV infection and disease (2). We conclude, therefore, that the hu-PBL-SCID mouse model can represent a very valuable model for exploring the in vivo relationships between HIV-1 and the human immune system during the course of primary infection, especially when specific attention is paid to the changes occurring in the human target cells introduced into the SCID mouse environment in relation to the time of virus exposure.
ACKNOWLEDGMENTS
We are grateful to Jay A. Levy for providing the HIV-1 envelope recombinant viruses R5 (SF2-env-162) and R6 (SF162-env-SF2). We thank Donald Mosier for helpful discussion and comments on the manuscript.
This work was supported by grants from the Italian Ministry of Health (X Progetto di Ricerca sull’AIDS).
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Bentwich Z, Kalinkovich A, Weissman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 3.Berger E A, Doms R W, Fenyo E M, Korber B T M, Littman D, Moore J P, Sattentau Q J, Schuitemaker H, Sodrosky J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casella C R, Finkel T H. Mechanisms of lymphocyte killing by HIV. Curr Opin Hematol. 1997;4:24–31. doi: 10.1097/00062752-199704010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham P R. HIV and chemokines: ligands sharing cell-surface receptors. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 8.De Maria R, Fais S, Silvestri M, Frati L, Pallone F, Santoni A, Testi R. Continuous in vivo activation and transient hyporesponsiveness to TcR/CD3 triggering of human gut lamina propria lymphocytes. Eur J Immunol. 1993;23:3104–3108. doi: 10.1002/eji.1830231209. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza M P, Harden V A. Chemokines and HIV-1 second receptors. Nat Med. 1996;12:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 10.Duchosal M A, McConahey P J, Robinson C A, Dixon F J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990;172:985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauci A S, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanism of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 13.Folks T M, Powell D, Lightfoote M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D, Venkayesan S, Martin M A. Biological and biochemical characterization of a cloned Leu-3− cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulizia R J, Levy J A, Mosier D E. The envelope gp120 gene of human immunodeficiency virus type 1 determines the rate of CD4-positive T-cell depletion in SCID mice engrafted with human peripheral blood leukocytes. J Virol. 1996;70:4184–4187. doi: 10.1128/jvi.70.6.4184-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollmann T R, Pettoello-Mantovani M, Zhuang X, Kim A, Hachamovitch M, Smarnworawong P, Rubinstein A, Goldstein H. Disseminated human immunodeficiency virus-1 (HIV-1) infection in SCID-hu mice after peripheral inoculation with HIV-1. J Exp Med. 1994;179:513–522. doi: 10.1084/jem.179.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krams S H, Dorshkind K, Gershwin M E. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J Exp Med. 1989;170:1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapenta C, Boirivant M, Marini M, Santini S M, Logozzi M, Viora M, Belardelli F, Fais S. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur J Immunol. 1999;29:1202–1208. doi: 10.1002/(SICI)1521-4141(199904)29:04<1202::AID-IMMU1202>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Lapenta C, Fais S, Rizza P, Spada M, Logozzi M, Parlato S, Santini S M, Pirillo M, Belardelli F, Proietti E. U937-SCID mouse xenografts: a new model for acute in vivo HIV-1 infection suitable to test antiviral strategies. Antivir Res. 1997;36:81–90. doi: 10.1016/s0166-3542(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 20.Lapenta C, Parlato S, Spada M, Santini S M, Rizza P, Logozzi M, Proietti E, Belardelli F, Fais S. Human lymphoblastoid CD4+ T cells become permissive to macrophage-tropic strains of human immunodeficiency virus type 1 after passage into severe combined immunodeficient mice through in vivo upregulation of CCR5: in vivo dynamics of CD4+ T-cell differentiation in pathogenesis of AIDS. J Virol. 1998;72:10323–10327. doi: 10.1128/jvi.72.12.10323-10327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune J M, Namikawa R, Kanemisha H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;24:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 23.McCune J M. Animal models of HIV-1 disease. Science. 1997;278:2141–2142. doi: 10.1126/science.278.5346.2141. [DOI] [PubMed] [Google Scholar]
- 24.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 25.Mosier D E, Gulizia R J, Baird S M, Wilson D B. Transfer of functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 26.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human PBL-SCID mice. Science. 1991;25:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 27.Mosier D E, Gulizia R J, MacIsaac P D, Torbett B E, Levy J A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 28.Namikawa R, Weibaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namikawa R, Kanemisha H, Lieberman M, Weissman I L, McCune J M. Infection of the SCID-hu mouse by HIV-1. Science. 1991;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 30.Nara P L, Hatch W C, Dunlop N M, Robey W G, Arthur L O, Gonda M A, Fischinger P J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987;3:283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- 31.Ostrowski M A, Krakauer D C, Li Y, Justement S J, Learn G, Ehler L A, Stanley S K, Nowak M, Fauci A S. Effect of immune activation on the dynamics of human immunodeficiency virus replication and on the distribution of viral quasispecies. J Virol. 1998;72:7772–7784. doi: 10.1128/jvi.72.10.7772-7784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrowski M A, Justement S J, Catanzaro A, Hallahan C A, Ehler L A, Mizell S B, Kumar P N, Mican J A, Chun T W, Fauci A S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 33.Parlato, S., S. M. Santini, C. Lapenta, M. Spada, M. Logozzi, P. Rizza, E. Proietti, F. Belardelli, and S. Fais. Primary HIV-1 infection of human CD4+ T cells passaged into SCID mice leads to selection of latently infected cells through a massive Fas-mediated autocrine suicide of uninfected cells. Importance of CD4+ T cell differentiation in the pathogenesis of HIV-1 infection. Submitted for publication. [DOI] [PubMed]
- 34.Picchio G R, Gulizia R J, Mosier D E. Chemokine receptor CCR5 genotype influences the kinetics of human immunodeficiency virus type 1 infection in human PBL-SCID mice. J Virol. 1997;71:7124–7127. doi: 10.1128/jvi.71.9.7124-7127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picchio G R, Gulizia R J, Wehrly K, Chesebro B, Mosier D E. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn T M, Piot P, McCormick J B, Feinsod F M, Taelman H, Kapita B, Stevens W, Fauci A S. Serologic and immunologic studies in patients with AIDS in North America and Africa. JAMA. 1987;257:2617–2621. [PubMed] [Google Scholar]
- 37.Rizza P, Santini S M, Logozzi M, Lapenta C, Sestili P, Gherardi G, Lande R, Spada M, Parlato S, Belardelli F, Fais S. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J Virol. 1996;70:7958–7964. doi: 10.1128/jvi.70.11.7958-7964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roederer M, Raju P A, Mitra D K, Herzenberg L A, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandhu J, Shipitz B, Gallinger S, Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994;152:3806–3812. [PubMed] [Google Scholar]
- 40.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuitemaker H, Kootstra N A, Fouchier R A, Hooibrink B, Miedema F. Productive HIV-1 infection of macrophages restricted to the cell fraction with proliferative capacity. EMBO J. 1994;13:5929–5936. doi: 10.1002/j.1460-2075.1994.tb06938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shioda T, Levy J A, Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investig. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tary-Lehman M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992;175:503–516. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tary-Lehman M, Lehman P V, Schols D, Roncarolo M G, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–1827. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tary-Lehman M, Saxon A, Lehman P V. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–533. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 48.Torbett B E, Picchio G, Mosier D E. Hu-PBL-SCID mice: a model for human immune function, AIDS, and lymphomagenesis. Immunol Rev. 1991;124:2–26. doi: 10.1111/j.1600-065x.1991.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 49.Wolinsky S, Korber B, Neumann A, Daniels M, Kunstman K, Whetsell A, Furtado M, Cao Y, Ho D, Safrit J, Koup R. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–541. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 50.Wood T C, Roberts B D, Butera S T, Folks T M. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 51.Xiao L, Rudolph D L, Owen S M, Spira T J, Lal R B. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS. 1998;12:F137–F143. doi: 10.1097/00002030-199813000-00001. [DOI] [PubMed] [Google Scholar]