Abstract
The study of genetic resistance to retroviral diseases provides insights into the mechanisms by which organisms overcome potentially lethal infections. Fv-2 resistance to Friend virus-induced erythroleukemia acts through nonimmunological mechanisms to prevent early virus spread, but it does not completely block infection. The current experiments were done to determine whether Fv-2 alone could provide resistance or whether immunological mechanisms were also required to bring infection under control. Fv-2-resistant mice that were CD4+ T-cell deficient were able to restrict early virus replication and spread as well as normal Fv-2-resistant mice, but they could not maintain control and developed severe Friend virus-induced splenomegaly and erythroleukemia by 6 to 8 weeks postinfection. Mice deficient in CD8+ T cells and, to a lesser extent, B cells were also susceptible to late Friend virus-induced disease. Thus, Fv-2 resistance does not independently prevent FV-induced erythroleukemia but works in concert with the immune system by limiting early infection long enough to allow virus-specific immunity time to develop and facilitate recovery.
Understanding mechanisms of genetic resistance to retroviral infections may lead to new ideas and methods for preventing or treating human diseases caused by agents such as human immunodeficiency virus or human T-cell leukemia virus type 1. One of the best-studied models for investigating such resistance is the Friend virus (FV) model in mice. Four major histocompatibility complex (MHC) genes (H-2 in the mouse) (6, 33, 38, 45) and one non-MHC gene, Rfv-3 (5), operate through immunological mechanisms to provide resistance. Such immunological resistance does not prevent infection, but it is extremely important in recovery from infection. In addition, there are six genes (Fv-1 through Fv-6) which confer various degrees of resistance through nonimmunological mechanisms (see references 2, 4, and 18 for reviews). This study examines how immunological deficiencies impact the potent resistance conferred by the nonimmunological gene, Fv-2.
FV is a complex of two retroviruses, a replication-competent helper virus and a replication-defective virus. The helper virus is Friend murine leukemia virus (F-MuLV), which encodes the structural proteins necessary for virus particle formation (25). The F-MuLV proteins are important in the recognition of FV by the immune system (9, 11, 14, 21, 22, 37). The defective component is spleen focus-forming virus (SFFV) (25), which is packaged in virions only if the host cells are coinfected with helper virus. SFFV is closely related to endogenous mouse retroviruses, and these viruses do not elicit protective immune responses (17), probably because of immunological tolerance. When adult mice of Fv-2-susceptible strains (Fv-2s/s or Fv-2r/s) are infected with FV complex their spleens rapidly enlarge, increasing in weight up to 10-fold by 2 weeks postinfection (14, 34, 52). This enlargement is due to an inappropriate mitotic signal caused by the binding of SFFV gp55 envelope glycoproteins to erythropoietin receptors (epoR) on erythroid precursor cells in the spleen and does not occur in Fv-2-resistant (Fv-2r/r) mice (13, 18). The binding of gp55 to epoR in Fv-2-sensitive mice stimulates uncontrolled erythroblast proliferation and increases the migration of erythroid precursors from the bone marrow to the spleen (12, 19, 32). Such expansion of mitotically active target cells is thought to be essential for FV-induced malignant transformations because of the increased probability of proviral integrations at the Spi-1 (ets) c-oncogene locus (39–41, 44, 48) and at the p53 tumor suppressor gene (23, 24, 31, 43).
The exact mechanism of Fv-2 resistance is not understood, but several studies have indicated that the resistance is an intrinsic property of the erythroblast targets, probably related to their mitogenic status (1, 49, 51). Fv-2-resistant mice, such as B6 mice, become infected by FV and synthesize SFFV glycoproteins (34), so the effect is a reduction but not a complete prevention of FV infection. Thus, the immune system may be involved in the resistant phenotype through the elimination of virus and virus-infected cells. Immunological mechanisms have been implicated in Fv-2 resistance by reports that nude Fv-2-resistant mice are susceptible to FV-induced erythroleukemia when infected at very high doses of FV (27). In addition, specific T-cell immunosuppression with anti-Thy-1.2 antibody in Fv-2-resistant mice has been shown to increase susceptibility to FV infection (53). In the current experiments, the role of the immune system in the resistant phenotype of Fv-2r/r mice is more carefully examined by studying FV infections in mice that are immunocompromised due to specific gene inactivations which disrupt development of B cells, CD4+ T cells, or CD8+ T cells.
MATERIALS AND METHODS
Mice.
The mice used in this study were age- and sex-matched mice of 3 to 6 months of age at experimental onset. (B10 × A.BY)F1 mice were bred at Rocky Mountain Laboratories from Jackson Laboratories stock animals. C57BL/6 mice were also obtained from Jackson Laboratories. B-cell-deficient mice were C57BL/6-Igh-6tm1Cgn (28) (N8 generation) and were obtained from Klaus Rajewsky through Jackson Laboratories. The animals were confirmed to be homozygous knockouts by flow cytometric analysis showing <0.2% positive staining for B220 antigen and cell surface immunoglobulin. As controls for the experiment in Fig. 5, nine Fv-2r/s B-cell-deficient mice were obtained by back-crossing (B6μMT × A.BY)F1 to B6μMT, typing the offspring for lack of cell surface immunoglobulin and B220 antigens and for development of rapid FV-induced splenomegaly. CD4-deficient mice were C57BL/6CD4m1 (N6 generation) and were generously provided by Dan Littman (26, 35). Fewer than 0.2% of the peripheral blood nucleated cells from these mice stained positive for CD4 by flow cytometry. CD8-deficient mice were C57BL/6B2m (N6 generation) and were generously provided by Maarten Zjilstra (54). In these mice, fewer than 0.3% of the peripheral blood nucleated cells stained positive for CD8 by flow cytometry. All animals were treated in accordance with the regulations of The National Institutes of Health and the Animal Care and Use Committee of Rocky Mountain Laboratories.
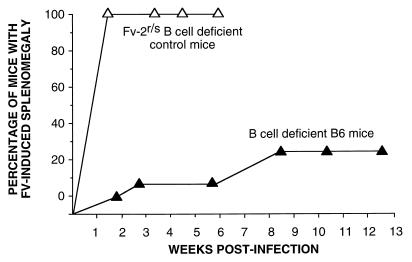
FIG. 5.
Effect of B-cell deficiency on FV-induced splenomegaly. All mice were infected with 1,500 SFFU of FV complex at time zero. Symbols (number of mice in each group): ▴, B-cell-deficient B6 mice (n = 24); ▵, B-cell-deficient Fv-2r/s control mice, B6μMT × (B6μMT × A.BY)B1 (n = 9). These control mice had to be euthanized at 6 weeks postinfection because of severe FV-induced splenomegaly.
CD8+ T-cell depletions.
T-cell depletions were performed as described earlier (7, 16). Briefly, mice were inoculated intraperitoneally with 0.5 ml of supernatant fluid obtained from rat hybridoma 169.4 producing immunoglobulin G2b anti-mouse CD8 monoclonal antibodies. Mice were inoculated three times per week for 2 weeks after infection with FV.
Virus challenge and splenomegaly.
Mice were challenged by an intravenous injection of 1,500 spleen focus-forming units (SFFU) of B-tropic, polycythemia-inducing FV complex (stock number FV-B 38-30) propagated as described previously (14). The standard procedure for monitoring the progression of Friend disease is palpation of splenomegaly (8, 11, 46), and this method was used in a blinded fashion as recently described (14). In the experiment testing for disease induction by helper virus alone, the mice were injected intravenously with 104 focus-forming units of B-tropic F-MuLV (stock number LLV-B 25-37).
Virus-neutralizing antibody assays.
For the virus-neutralizing antibody assays, freshly frozen plasma samples were heat inactivated (56°C, 10 min), and serial twofold dilutions were incubated with virus stock in the presence of complement at 37°C as previously described (42). The samples were then added to cultures of Mus dunni cells (30) that were pretreated with 4 μg of polybrene per ml; the cells were then cultivated for 5 days, fixed with ethanol, and stained with F-MuLV envelope-specific monoclonal antibody 720 (47), followed by the addition of goat anti-mouse peroxidase conjugate (Cappel, West Chester, Pa.) and development with 3-amino-9-ethylcarbazole substrate to detect foci. The titer was defined as the highest plasma dilution giving 75% neutralization of input virus.
Infectious center assays.
Single-cell suspensions from infected mouse spleens were cocultivated with M. dunni cells at 10-fold dilutions ranging up to 107 spleen cells per well of a 6-well tissue culture dish, and the cultures were treated to detect infectious centers as described above for the virus-neutralizing antibody assay.
Flow cytometry.
Single-cell suspensions from infected mouse spleens with erythrocytes lysed by ammonium chloride-Tris were stained and analyzed by using a FACStar flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) modified for five-parameter analysis. Ter-119 was used to stain erythroid lineage cells (20), followed by the use of fluorescein isothiocyanate (FITC)-labeled goat anti-rat immunoglobulin (Pharmingen, San Diego, Calif.). FITC and phycoerythrin-labeled antibodies specific for CD4, CD8, and B220 for staining of blood cells were also obtained from Pharmingen.
RESULTS
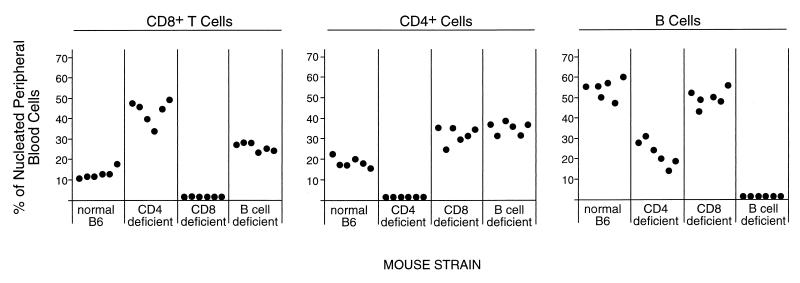
To determine whether specific subsets of lymphocytes were involved in the resistance of B6 mice (Fv-2r/r) to FV-induced erythroleukemia, B6 mice with deficiencies in CD8+ T cells, CD4+ T cells, and B cells were analyzed. The normal levels of each lymphocyte subset in the peripheral blood of each of the B6 strains was determined by flow cytometry to confirm the phenotype. All strains had <1% expression of the deficient cell type (Fig. 1). It should be noted that the specific gene inactivations affected the percentages of lymphocytes other than the targeted ones. For example, the CD4-deficient mice had significantly higher percentages of CD8+ cells and significantly lower percentages of B cells. In both the CD8- and B-cell-deficient strains, there were compensatory increases in the numbers of the lymphocyte subsets not targeted for inactivation (Fig. 1).
FIG. 1.
Effects of gene inactivations on circulating lymphocyte percentages. Fresh blood samples with erythrocytes lysed and removed were analyzed by flow cytometry for the cell surface antigens CD8, CD4, and B220. B-cell percentages were determined by cells which stained positive for B220 and negative for CD4 and CD8. Each dot represents the determination from a single mouse.
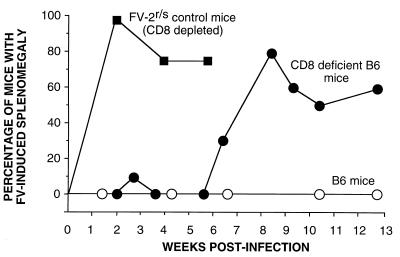
CD8-deficient mice were infected with FV and monitored for disease induction by spleen palpation. Fv-2-susceptible control mice that had been depleted of CD8+ T cells were also infected. As expected, all of these control mice had grossly enlarged spleens by 2 weeks postinfection (Fig. 2). In contrast, only 1 of 10 CD8-deficient Fv-2r/r mice had mild and transient splenomegaly at the 2-week time point (“early time point”). However, beginning at 6 weeks postinfection, increasing numbers of CD8-deficient mice became splenomegalic, and 80% were positive by 8 weeks postinfection (“late timepoint”). Interestingly, two of these mice later recovered from splenomegaly. The remaining splenomegalic animals had to be euthanized due to severely enlarged spleens and clinical signs of terminal erythroleukemia. None of the normal B6 mice that were infected with FV became splenomegalic or showed other clinical signs of illness. Thus, CD8+ T cells were not necessary for Fv-2-mediated control of early splenomegaly but were required in most animals to prevent late splenomegaly.
FIG. 2.
Effect of CD8 deficiency on FV-induced splenomegaly. All mice were infected with 1,500 SFFU of FV complex at time zero. Symbols (number of mice in each group): ○, normal B6 (n = 24); ●, CD8-deficient B6 (n = 10); ■, Fv-2r/s (B10 × A.BY)F1 CD8-depleted mice (n = 8; the F1 mice had to be euthanized at 6 weeks postinfection due to severe FV-induced splenomegaly). The difference between the CD8-deficient B6 group and the (B10 × A.BY)F1 CD8-depleted group was highly significant (P = 0.0004 by the Fisher’s exact test).
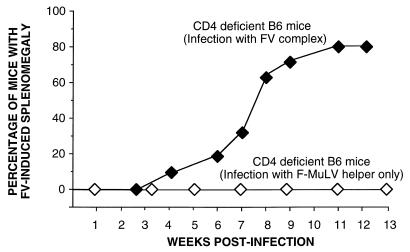
Next, CD4-deficient mice were used to determine whether CD4+ T cells were also necessary to control FV-induced splenomegaly. These mice had no palpable splenomegaly at the early time point, but by between 6 and 11 weeks postinfection 80% of the mice became severely splenomegalic (Fig. 3). In contrast to the CD8-deficient mice, none of the CD4-deficient mice recovered from FV-induced splenomegaly. The splenomegaly became progressively more severe, and all of the mice had to be euthanized. Thus, deficiencies in the CD4+ or CD8+ T-cell subsets did not significantly alter host susceptibility to early splenomegaly, but deficiencies in either subset resulted in a late-onset splenomegaly induced by FV infection.
FIG. 3.
Effect of CD4 deficiency on FV-induced splenomegaly. All mice were infected with 1,500 SFFU of FV complex at time zero. Symbols (number of mice in each group): ⧫, CD4-deficient B6 mice infected with FV complex (n = 20); ◊, CD4-deficient mice infected with 104 focus-forming units of B-tropic F-MuLV helper virus (n = 14).
Infectious center (IC) assays were performed on the CD4-deficient mice to determine the effect of this deficiency on the spread of virus in the spleen. CD4 deficiency did not increase the number of spleen ICs compared to normal B6 mice by 1 week postinfection (Table 1). In contrast, Fv-2r/s-susceptible control mice at the 1-week time point had over 10 times as many infected spleen cells as did the B6 mice. By 30 days postinfection, the situation had changed dramatically. While the normal B6 mice had a >10-fold decrease in their mean IC number, the CD4-deficient mice showed a 100-fold increase. These results were consistent with the splenomegaly data and indicated that the ability of normal B6 mice to bring down the level of infected cells in the spleen was dependent on CD4+ T cells.
TABLE 1.
Effect of CD4+ T-cell deficiency on F-MuLV helper virus replication
| Mouse strain | Fv-2 type | Mean IC titer/spleen ata:
|
|
|---|---|---|---|
| 8 d.p.i. | 30 d.p.i. | ||
| B6 | r/r | 4.1 × 105 | 1.1 × 104 |
| CD4-deficient B6 | r/r | 1.3 × 105 | 1.4 × 107 |
| (B10 × A.BY)F1 | r/s | 4.9 × 106 | ND |
The numbers of mice in each group used to determine log10 geometric mean titers were as follows: for B6 mice, n = 5; for B6 CD4 KO mice, n = 5; for (B10 × A.BY)F1 mice, n = 7. ND, not determined; d.p.i., days postinfection. Hematocrits in the B6- and CD4-deficient B6 mice were both in the normal range at 1 week and 4 weeks postinfection. Hematocrits became abnormally high coincident with gross splenomegaly in the CD4-deficient B6 mice (data not shown).
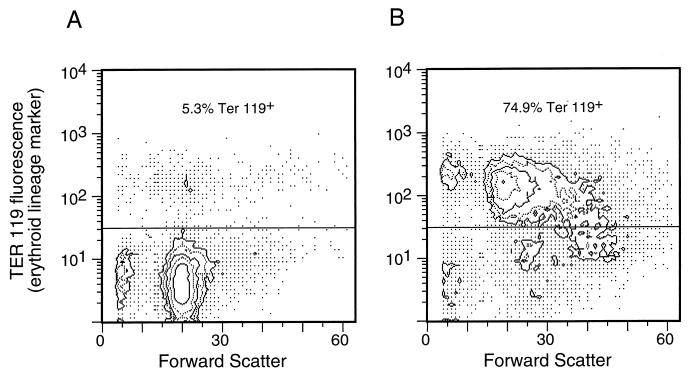
To determine which types of cells were proliferating to cause splenomegaly in the CD4-deficient mice, spleen cell suspensions were stained with lineage-specific antibodies for analysis by flow cytometry. Figure 4 shows staining with an erythroid cell marker (Ter-119) comparing spleen cells from an uninfected CD4-deficient mouse to those from an infected mouse with late-onset splenomegaly. The infection with FV caused a shift from 5% Ter-119-positive cells in the spleen to 75% positive. This finding demonstrated that the splenomegaly in this mouse was due to proliferation of cells in the erythroid lineage.
FIG. 4.
Flow cytometric analysis of erythroid cells in CD4 deficient mice. (A) Nucleated spleen cells from an uninfected CD4-deficient B6 mouse stained with the erythroid cell surface marker Ter-119 (20) in the vertical direction. This mouse spleen contained 5.3% Ter-119+ cells. (B) Ter-119 staining of spleen cells from an FV-infected CD4-deficient mouse harvested at 7 weeks postinfection. This mouse had a grossly enlarged spleen with 1.1 × 109 total cells and 2.2 × 106 F-MuLV-positive ICs per spleen. A total of 74.9% of the cells stained positive for the Ter-119. The percentages of the cells with surface markers for other cell lineages were also markedly distorted. There were as follows: <1%, CD4+ cells; 1.8%, CD8+ cells; 3.5%, Mac-1+ cells; and 3.5%, B220+ cells. The shift in forward scatter in the infected cells reflects a shift to a larger size.
The early splenomegaly induced by FV infection of Fv-2-susceptible animals is due to SFFV-stimulated proliferation of erythroid precursor cells which was not seen in the CD4- and CD8-deficient mice. The late timing of the splenomegaly in the CD4- and CD8-deficient mice suggested that SFFV might not be involved. However, infection of CD4-deficient mice with F-MuLV helper virus only (no SFFV) did not produce any clinical signs in the 14 mice tested (Fig. 3). This indicated that SFFV was a necessary component of the FV complex for the induction of pathogenesis in Fv-2-resistant mice.
It was previously shown that FV-neutralizing antibody was necessary for the control of virus in Fv-2-susceptible mice (3, 5, 15). Since the FV-specific antibody response is T-cell dependent (50), part of the susceptibility of the CD4-deficient mice might have been due to lack of help for B cells. In addition, the diminished number of circulating B cells in the CD4-deficient mice (Fig. 1) could have contributed to their susceptibility. Therefore, it was of interest to determine whether B-cell deficiencies would also affect disease in Fv-2-resistant mice. Like the T-cell-deficient mice, most B-cell-deficient mice were also resistant to early splenomegaly (Fig. 5). By comparison, B-cell deficiency on an Fv-2-susceptible background resulted in very rapid and severe splenomegaly with no recovery (Fig. 5). By 9 weeks postinfection, 25% of the B6 B-cell-deficient mice developed severe splenomegaly; significantly more than was observed in normal B6 mice (P = 0.0219 by Fisher’s exact test). Thus, B-cell deficiency had a demonstrable but relatively weak effect on the development of splenomegaly. As expected, no virus-neutralizing antibodies were detectable in any of the B-cell-deficient mice tested at 30 days postinfection. In contrast, all nine immunocompetent mice tested had detectable virus-neutralizing antibody titers, with a geometric mean titer of 5.4 doubling dilutions (data not shown). Since the B-cell-deficient mice were less susceptible than the CD4-deficient mice, the high incidence of late splenomegaly in the CD4-deficient mice was probably not entirely due to a lack of virus-neutralizing antibody production.
DISCUSSION
The Fv-2 gene has not yet been identified, and its mechanism of action is still not known. The current results support the idea that Fv-2 is not an immunological gene but that immunological functions certainly influence the resistant phenotype of B6 mice. Although both CD4 and CD8 T-cell deficiencies markedly increased the incidence of FV-induced splenomegaly, the timing of the splenomegaly and the delay of virus spread through the spleen indicated that this was not a direct effect by Fv-2. Instead, it appeared that Fv-2 exerted normal control over early FV replication and induction of splenomegaly but that the mice were generally unable to maintain control and eliminate infection in the absence of T cells. Thus, Fv-2 and the immune system appear to act in concert to provide resistance, with Fv-2 delaying early virus replication long enough to allow development of a strong T-cell-mediated immune response.
B-cell-deficient mice also showed decreased resistance to FV-induced splenomegaly, but the incidence was lower than that observed in the T-cell-deficient mice. This lower incidence in B-cell-deficient mice not only indicates that the major role for CD4+ T cells is not to provide help for B cells but also indicates that antigen presentation to T cells is not a critical function of the B-cell compartment in these mice. Furthermore, it also appears that the B-cell function of antibody production is less important in Fv-2-resistant mice than in Fv-2-susceptible mice, which always develop erythroleukemia in the absence of an antibody response (5, 10, 15). The data indicate that Fv-2 resistance can at least partially compensate for the lack of a virus-neutralizing antibody response. Since Fv-2 resistance operates primarily in the early phase of infection, it follows that the effects of virus-neutralizing antibodies may also be most important during the early phase of infection.
The requirement for SFFV in the induction of erythroleukemia in the T-cell-deficient, Fv-2-resistant mice is interesting since there was no early expansion of target cells induced by SFFV gp55 (Fig. 2, 3, and 5 and Table 1). The relatively long latency before erythroleukemia induction could reflect the extra time required to acquire integrations into oncogenic sites such as Spi-1 (41) and p53 (43) in the absence of an expanded target population of susceptible cells. Alternatively, the latency time might be required for the selection of virus variants that can overcome Fv-2 resistance (18, 36).
Since the B6 lymphocyte knockout mice were originally derived from the 129 mouse strain, the question arises as to whether 129 genes might contribute to the effects seen in the knockouts. Each of the knockout strains was back-crossed to B6 to at least generation N6, which statistically produces 97% B6 genes (29). The failure of the knockout strains to become splenomegalic (Fig. 2) and their control of early virus replication (Table 1) indicate that their Fv-2 genes were derived from the resistant B6 parent. While it cannot be ruled out that the late leukemias seen in the knockout mice were due to effects from residual 129 genes, the most likely explanation is that they were due to the specific lymphocyte deficiencies of the mice.
Almost all strains of mice are highly susceptible to FV-induced erythroleukemia, but the B6 mouse has a unique combination of resistance and recovery genes that act in concert to provide a highly resistant phenotype. One implication of these results is that therapeutics or prophylactics designed to confer resistance through mimicking or modulating single genetic mechanisms might prove unsuccessful. Thus, a combination of drugs and vaccination may afford much better protection than either treatment alone.
REFERENCES
- 1.Behringer R R, Dewey M J. Cellular site and mode of Fv-2 gene action. Cell. 1985;40:441–447. doi: 10.1016/0092-8674(85)90158-8. [DOI] [PubMed] [Google Scholar]
- 2.Ben-David Y, Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991;66:831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- 3.Britt W J, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–2367. [PubMed] [Google Scholar]
- 4.Chesebro B, Miyazawa M, Britt W J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 5.Chesebro B, Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci USA. 1979;76:425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro B, Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978;120:1081–1085. [PubMed] [Google Scholar]
- 7.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 8.Corbin A, Sitbon M. Protection against retroviral diseases after vaccination is conferred by interference to superinfection with attenuated murine viruses. J Virol. 1993;65:2539–2544. doi: 10.1128/jvi.67.9.5146-5152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dittmer U, Brooks D M, Hasenkrug K J. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol. 1998;72:6554–6558. doi: 10.1128/jvi.72.8.6554-6558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doig D, Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell surface antigens in leukemic mice: Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979;150:10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl P L, Moss B, Morrison R P, Wehrly K, Nishio J, Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986;234:728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- 12.Ferro F E, Jr, Kozak S L, Hoatlin M E, Kabat D. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1993;268:5741–5747. [PubMed] [Google Scholar]
- 13.Geib R W, Dizik M, Anand R, Lilly F. Infection and transformation of Fv-2rr erythroprogenitor cells with Friend virus. Virus Res. 1987;8:327–333. doi: 10.1016/0168-1702(87)90005-0. [DOI] [PubMed] [Google Scholar]
- 14.Hasenkrug K J, Brooks D M, Robertson M N, Srinivas R V, Chesebro B. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology. 1998;248:66–73. doi: 10.1006/viro.1998.9264. [DOI] [PubMed] [Google Scholar]
- 15.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasenkrug K J, Brooks D M, Dittmer U. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenkrug K J, Brooks D M, Nishio J, Chesebro B. Differing T-cell requirements for recombinant retrovirus vaccines. J Virol. 1996;70:368–372. doi: 10.1128/jvi.70.1.368-372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoatlin M E, Kabat D. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 1995;3:51–57. doi: 10.1016/s0966-842x(00)88875-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoatlin M E, Kozak S L, Lilly F, Chakraborti A, Kozak C A, Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and downmodulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci USA. 1990;87:9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y H, Weissman I L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara C, Miyazawa M, Nishio J, Chesebro B. Induction of protective immunity to Friend murine leukemia virus in genetic nonresponders to virus envelope protein. J Immunol. 1991;146:3958–3963. [PubMed] [Google Scholar]
- 22.Iwashiro M, Kondo T, Shimizu T, Yamagishi H, Takahashi K, Matsubayashi Y, Masuda T, Otaka A, Fujii N, Ishimoto A, Miyazawa M, Robertson M J, Chesebro B, Kuribayashi K. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J Virol. 1993;67:4533–4542. doi: 10.1128/jvi.67.8.4533-4542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson P, Benchimol S. Friend virus-induced murine erythroleukaemia: the p53 locus. Cancer Surv. 1992;12:137–151. [PubMed] [Google Scholar]
- 24.Johnson P, Chung S, Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 1993;13:1456–1463. doi: 10.1128/mcb.13.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 26.Killeen N, Sawada S, Littman D R. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa M, Matsubara O, Kasuga T. Dynamics of lymphocytic subpopulations in Friend leukemia virus-induced leukemia. Cancer Res. 1986;46:3034–3039. [PubMed] [Google Scholar]
- 28.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B-cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 29.Klein J. Biology of the mouse histocompatibility complex. New York, N.Y: Springer-Verlag; 1975. [Google Scholar]
- 30.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell forus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavigueur A, Bernstein A. p53 transgenic mice: accelerated erythroleukemia induction by Friend virus. Oncogene. 1991;6:2197–2201. [PubMed] [Google Scholar]
- 32.Li J-P, D’Andrea A D, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature (London) 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 33.Lilly F. The effect of histocompatibility-2 type on response to the Friend leukemia virus in mice. J Exp Med. 1968;127:465–473. doi: 10.1084/jem.127.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970;45:163–169. [PubMed] [Google Scholar]
- 35.Locksley R M, Reiner S L, Hatam F, Littman D R, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar M K, Cho C L, Fox M T, Eckner K L, Kozak S, Kabat D, Geib R W. Mutations in the env gene of Friend spleen focus-forming virus overcome Fv-2r-mediated resistance to Friend virus-induced erythroleukemia. J Virol. 1992;66:3652–3660. doi: 10.1128/jvi.66.6.3652-3660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazawa M, Fujisawa R, Ishihara C, Takei Y A, Shimizu T, Uenishi H, Yamagishi H, Kuribayashi K. Immunization with a single T helper cell epitope abrogates Friend virus-induced early erythroid proliferation and prevents late leukemia development. J Immunol. 1995;155:748–758. [PubMed] [Google Scholar]
- 38.Miyazawa M, Nishio J, Wehrly K, David C S, Chesebro B. Spontaneous recovery from Friend retrovirus-induced leukemia. Mapping of the Rfv-2 gene in the Q/TL region of mouse MHC. J Immunol. 1992;148:1964–7. [PubMed] [Google Scholar]
- 39.Moreau-Gachelin F, Ray D, de Both N J, van der Feltz M J, Tambourin P, Tavitian A. Spi-1 oncogene activation in Rauscher and Friend murine virus-induced acute erythroleukemias. Leukemia. 1990;4:20–23. [PubMed] [Google Scholar]
- 40.Moreau-Gachelin F, Ray D, Mattei M G, Tambourin P, Tavitian A. The putative oncogene Spi-1: murine chromosomal localization and transcriptional activation in murine acute erythroleukemias. Oncogene. 1989;4:1449–1456. . (Erratum, 5:941, 1990.) [PubMed] [Google Scholar]
- 41.Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukemia. Nature (London) 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- 42.Morrison R P, Earl P L, Nishio J, Lodmell D L, Moss B, Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature (London) 1987;329:729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- 43.Munroe D G, Peacock J W, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul R, Schuetze S, Kozak S L, Kozak C A, Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991;65:464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry L L, Miyazawa M, Hasenkrug K, Wehrly K, David C S, Chesebro B. Contrasting effects from a single major histocompatibility complex class II molecule (H-2E) in recovery from Friend virus leukemia. J Virol. 1994;68:4921–4926. doi: 10.1128/jvi.68.8.4921-4926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polsky D, Lilly F. Suppression of H-2b-associated resistance to Friend erythroleukemia virus by a class I gene from the H-2d major histocompatibility complex haplotype. Proc Natl Acad Sci USA. 1991;88:9243–9247. doi: 10.1073/pnas.88.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L H, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 48.Schuetze S, Paul R, Gliniak B C, Kabat D. Role of the PU.1 transcription factor in controlling differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1992;12:2967–2975. doi: 10.1128/mcb.12.7.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silver J, Teich N. Expression of resistance to Friend virus-stimulated erythropoiesis in bone marrow chimeras containing Fv-2rr and Fv-2ss bone marrow. J Exp Med. 1981;154:126–137. doi: 10.1084/jem.154.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Super H J, Brooks D, Hasenkrug K J, Chesebro B. Requirement for CD4+ T cells in the Friend murine retrovirus neutralizing antibody response: evidence for functional T cells in genetic low-recovery mice. J Virol. 1998;72:9400–9403. doi: 10.1128/jvi.72.11.9400-9403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki S, Axelrad A A. FV-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980;19:225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- 52.Tambourin P, Wendling F, Moreau-Gachelin F. Friend leukemia as a multiple-step disease. Blood Cells. 1981;7:133–144. [PubMed] [Google Scholar]
- 53.Van der Gaag H C, Axelrad A A. Friend virus replication in normal and immunosuppressed C57BL/6 mice. Virology. 1990;177:837–839. doi: 10.1016/0042-6822(90)90561-5. [DOI] [PubMed] [Google Scholar]
- 54.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenish R. β2-Microglobulin-deficient mice lack CD4−8+ cytolytic T cells. Nature (London) 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]