Abstract
Background
Dyslipidemia is an important and modifiable risk factor for CVD in children with CKD.
Methods
In a cross-sectional study of baseline serum lipid levels in a large prospective cohort study of children with stage 3–5 (predialysis) CKD, frequencies of abnormal lipid levels and types of dyslipidemia were analyzed in the entire cohort and in subpopulations defined by fasting status or by the presence of nephrotic range proteinuria. Associated clinical and laboratory characteristics were determined by multivariable linear regression analysis.
Results
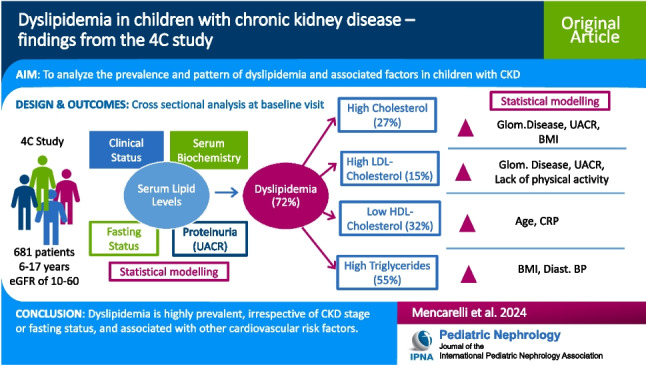
A total of 681 patients aged 12.2 ± 3.3 years with a mean eGFR of 26.9 ± 11.6 ml/min/1.73 m2 were included. Kidney diagnosis was classified as CAKUT in 69%, glomerulopathy in 8.4%, and other disorders in 22.6% of patients. Nephrotic range proteinuria (defined by a urinary albumin/creatinine ratio > 1.1 g/g) was present in 26.9%. Dyslipidemia was found in 71.8%, and high triglyceride (TG) levels were the most common abnormality (54.7%). Fasting status (38.9%) had no effect on dyslipidemia status. Except for a significant increase in TG in more advanced CKD, lipid levels and frequencies of dyslipidemia were not significantly different between CKD stages. Hypertriglyceridemia was associated with younger age, lower eGFR, shorter duration of CKD, higher body mass index (BMI-SDS), lower serum albumin, and higher diastolic blood pressure.
Conclusions
Dyslipidemia involving all lipid fractions, but mainly TG, is present in the majority of patients with CKD irrespective of CKD stage or fasting status and is significantly associated with other cardiovascular risk factors.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-024-06389-3.
Keywords: Children, Chronic kidney disease, Dyslipidemia, Fasting, Albuminuria
Introduction
Dyslipidemia is a common metabolic complication of chronic kidney disease (CKD). Prior to dialysis or transplantation, CKD is typically associated with increased levels of triglycerides (TGs) and a decrease in high-density lipoprotein cholesterol (HDL-C), while levels of total cholesterol (CHOL) and low-density lipoprotein cholesterol (LDL-C) are variable [1–3]. A variety of factors, such as primary kidney disease, CKD stage, degree of proteinuria, medications, diet, the presence of malnutrition or obesity, and gender effects, have further modifying effects on the lipid profile [4]. Marked hyperlipidemia with highly elevated levels of CHOL and LDL-C may be found in patients with CKD and nephrotic syndrome or nephrotic range proteinuria [2, 5].
Dyslipidemia is a traditional risk factor for cardiovascular disease (CVD), the leading cause of morbidity and mortality in patients with CKD, including children [6]. Cross-sectional data from large cohort studies in children with CKD show a high prevalence of dyslipidemia (35–65%), significantly associated with the degree of proteinuria, obesity, and diminishing kidney function [5, 7]. Thus, dyslipidemia is an important and modifiable risk factor for CVD in children with CKD, a pediatric population with an extremely high cardiovascular risk [8].
Pediatric cohort studies provide the unique opportunity to study the impact of CKD in a population without diabetes or age- or lifestyle-related comorbidities. Here, we performed a cross-sectional investigation to analyze the prevalence and pattern of dyslipidemia, as well as associated patient-related and CKD-related factors, in a large cohort of children enrolled in the prospective Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study.
Methods
Study setting and design
The prospective 4C study (ClinicalTrials.gov NCT01046448) observed 704 children aged 6–17 years with predialysis CKD stage 3–5 and a baseline eGFR of 10–60 ml/min/1.73 m2, who were enrolled between 2010 and 2012 at 55 pediatric nephrology units in 12 European countries and followed until the end of 2018. The study was approved by the Ethics Committee of the University of Heidelberg (S-032/2009) and the Institutional Review Boards of each participating center. Written informed consent was obtained from all parents and participants, where appropriate. Patients underwent standardized biannual clinical evaluations including the collection of blood and urine samples and an annual cardiovascular examination; a detailed study protocol and an analysis of the cardiovascular phenotype of this patient cohort have been published [9, 10].
Patients with at least one serum lipid value available at baseline were included in the present study. Patients taking lipid-lowering drugs were excluded. Fasting status was recorded at each visit. The baseline data of the different lipid fractions (TG, CHOL, LDL-C, HDL-C) was analyzed for associations with clinical and laboratory variables. Subgroup analyses were performed in children with or without nephrotic range proteinuria and in fasting patients.
Definitions
Dyslipidemia was defined as the presence of at least one abnormal serum lipid level (CHOL, LDL-C, HDL-C, TG) according to published age-dependent cut-off values (Supplemental Table 1) [11].
The various forms of dyslipidemia were categorized according to their baseline serum levels as (a) no dyslipidemia, (b) isolated dyslipidemia (one abnormal), and (c) multiple dyslipidemia (multiple abnormal measurements, i.e., at least 2 lipids abnormal).
Primary kidney diseases were classified as CAKUT (congenital abnormalities of the kidney and urinary tract), glomerular diseases, and others.
Body mass index (BMI), height, and office blood pressure (median of three oscillometric measurements) were normalized by the calculation of standard deviation scores (SDSs) based on reference values for healthy children [12, 13]. To analyze the impact of nutritional status, baseline lipid levels were stratified by BMI categories (underweight, BMI-SDS < − 2; normal, − 2 ≤ BMI-SDS < 1; overweight, 1 ≤ BMI-SDS < 2; obese, BMI-SDS ≥ 2) [15].
The albumin/creatinine ratio in spot urine was used to quantify proteinuria as recommended by KDIGO guidelines [14]. There is currently no standard definition for nephrotic range albuminuria in children [15]. A urinary protein excretion of > 1 g/m2/d and > 0.5 g/d is the accepted cut-off for nephrotic range proteinuria in children and adults, respectively. It has been shown that a urine total protein excretion of 3.5 g/d translates to 2.2 g/g for the urine albumin/creatinine ratio (UACR) in adults [16, 17]. Since 3.5 g/d for the average adult body surface area of 1.73 m2 amounts to 2 g/m2/d, we adjusted the adult definition (UACR of 2.2 g/g) to the pediatric definition of 1 g/m2/d, arriving at a UACR of 1.1 g/g, which was used as the cut-off to define nephrotic range albuminuria.
Laboratory methods
Estimated GFR (eGFR) was calculated using the updated Schwartz equation [18]. All blood and urine samples were collected at a regular visit in the outpatient clinic and measured by fully automated methods at a central laboratory (Synlab, Heidelberg) using stored serum samples collected at each 6-monthly visit. Urinary albumin and creatinine were measured in spot urine samples. All lipids were measured directly by enzymatic test on a Siemens Advia 2400 analyzer, including LDL-C (and not calculated with the widely used Friedewald formula, which should not be used in non-fasting patients).
Statistical analysis
Clinical and laboratory characteristics for the study population are described using mean (SD), median (IQR), or frequencies for continuous and categorical variables, respectively. Differences between non-nephrotic vs. nephrotic range proteinuria groups were analyzed using a t-test, Mann–Whitney U test, or chi-squared test, respectively. Abnormal lipid levels at baseline and multiple dyslipidemia based on all four lipids are described by frequencies in the total sample and stratified by CKD stage where differences between stages were analyzed by the chi-squared test. Additionally, differences in means between CKD stage categories or between BMI categories were analyzed by analysis of variance (ANOVA) for each lipid. Frequencies of TG vs. HDL dyslipidemia are shown in four-fold tables with p-values based on chi-squared tests to compare the distribution of TG and HDL dyslipidemia. To explore linear relationships, Pearson’s correlation coefficients were calculated among baseline lipids or among two clinical variables of interest (e.g., albumin and UACR) where skewed distributed variables were log-transformed.
Multivariable linear regression analysis was performed to identify clinical and laboratory characteristics associated with baseline lipid levels. In the full model, all candidate variables included were considered to be biologically relevant. Missing values were not imputed, and an available case analysis was performed. To reduce the model, variable selection was applied with backward selection based on the Akaike information criterion (AIC). Regression coefficients with 95% confidence intervals (CI) and p-values are shown.
Statistical analysis was performed using SAS Version 9.4. p-values < 0.05 were considered statistically significant and were interpreted descriptively due to the exploratory nature of all analyses.
Results
Baseline characteristics
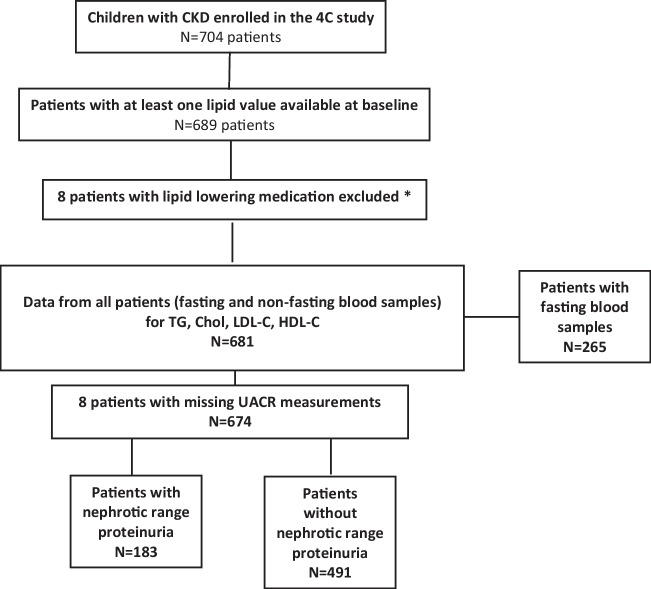
A total of 689 patients had at least one lipid measurement at enrollment; eight patients taking lipid-lowering medication were excluded. Thus, 681 patients (611 Caucasian = 89.7%; 448 boys = 65.8%) aged 12.2 ± 3.3 years meeting the eligibility criteria, with a median eGFR of 26.9 ± 11.6 ml/min/1.73 m2, were included in the analysis (Fig. 1). Baseline characteristics of all patients are summarized in Table 1. Primary kidney diagnosis was CAKUT in 69% (n = 470), glomerulopathy in 8.4% (n = 57), and others in 22.6% (n = 154) patients. The mean BMI was 18.4 ± 3.9 kg/m2, 18.4% (n = 125) were overweight, and 5.3% (n = 36) patients were obese. Of all patients, 265 (38.9%) were fasting at the time of examination, and 416 (61.1%) were not fasting.
Fig. 1.
Patient and observation selection flow chart. The albumin/creatinine ratio (UACR) in spot urine was used to quantify proteinuria. A UACR of 1.1 g/g was defined as the cut-off for nephrotic range proteinuria in children. *Lipid-lowering medication: statins (n = 7; in 2 cases in combination with ezetimibe), fibrate (n = 1)
Table 1.
Clinical characteristics of the study population and subgroups with or without nephrotic range proteinuria
| All (n = 681) | Nephrotic range proteinuria (n = 183) | Non-nephrotic range proteinuria (n = 491) | P | |
|---|---|---|---|---|
| Age (yrs) | 12.2 (3.3) | 12.8 (3.2) | 11.9 (3.4) | 0.003 |
| Sex (% boys) | 448 (65.8%) | 116 (63.4%) | 329 (67.0%) | 0.378 |
| Caucasian ethnicity | 611 (89.7%) | 163 (89.1%) | 441 (89.8%) | 0.778 |
| Primary kidney diagnosis | < 0.001 | |||
| CAKUT | 470 (69.0%) | 113 (63.7%) | 354 (72.1%) | |
| Glomerular | 57 (8.4%) | 33 (18.0%) | 24 (4.9%) | |
| Other | 154 (22.6%) | 37 (20.2%) | 113 (23.0%) | |
| Pubertal status-pubertal | 303 (44.9%) | 94 (51.6%) | 204 (42.0%) | 0.025 |
| Height (cm) | 140.9 (20.2) | 142.9 (19.5) | 140.1 (20.4) | 0.110 |
| SDS | − 1.35 (1.36) | − 1.51 (1.25) | − 1.29 (1.39) | 0.061 |
| BMI (kg/m2) | 18.4 (3.9) | 18.6 (3.6) | 18.4 (4.0) | 0.552 |
| SDS | 0.10 (1.28) | 0.15 (1.24) | 0.09 (1.29) | 0.621 |
| Obese (%) | 36 (5.3%) | 10 (5.5%) | 26 (5.3%) | 0.940 |
| Overweight (%) | 125 (18.4%) | 33 (18.0%) | 91 (18.5%) | 0.881 |
| Waist-to-hip ratio | 0.92 (0.12) | 0.90 (0.11) | 0.93 (0.12) | 0.005 |
| Waist-to-height ratio | 0.48 (0.07) | 0.48 (0.07) | 0.49 (0.07) | 0.259 |
| eGFR (ml/min/1.73 m2) | 26.9 (11.6) | 22.1 (9.5) | 28.7 (11.8) | < 0.001 |
| Time since CKD stage II (years) | 6.1 (4.6) | 5.3 (4.1) | 6.4 (4.7) | 0.010 |
| Bicarbonate (mmol/l) | 21.3 (3.6) | 20.7 (4.2) | 21.5 (3.4) | 0.010 |
| CRP (mg/dl) | 0.55 (1.99) | 0.60 (2.6) | 0.54 (2.0) | 0.400 |
| Hemoglobin (g/dl) | 11.7 (1.6) | 11.5 (1.7) | 11.8 (1.6) | 0.025 |
| Serum albumin (g/l) | 40.0 (5.5) | 35.0 (6.9) | 40.5 (3.9 g) | < 0.001 |
| UACR (g/g) | 0.33 (1.14) | 2.29 (1.93) | 0.17 (0.38) | < 0.001 |
| Systolic BP SDS | 0.80 (1.35) | 1.07 (1.50) | 0.71 (1.29) | 0.002 |
| Diastolic BP SDS | 0.69 (1.08) | 1.0 (1.17) | 0.58 (1.03) | < 0.001 |
| Physical activity | < 0.001 | |||
| < 1 h per week | 159 (23.8%) | 61 (33.7%) | 95 (19.8%) | |
| 1–4 h per week | 228 (34.2%) | 52 (28.7%) | 172 (35.9%) | |
| > 4 h per week | 280 (42.0%) | 68 (37.6%) | 212 (44.3%) |
Nephrotic range proteinuria was defined as urinary albumin/creatinine ratio > 1.1 g/g (UACR; measurements available in N = 674). CRP and UACR expressed as median and IQR; all other measurements as mean and SD. UACR (g/g) = 10*ualb (mg/dl)/ucrea (mg/l). There were 7 patients with missing UACR measurements (not permitting classification for proteinuria). Obesity: BMI-SDS ≥ 2. Overweight: 1 ≤ BMI-SDS < 2. P denotes significance of differences between subgroups with or without nephrotic range proteinuria.
Data were further analyzed in the entire cohort and in the subgroups of patients with (n = 183; 26.8%) or without (n = 491; 72.2%) nephrotic range proteinuria as defined by a UACR of > 1.1 g/g. Serum lipid levels at baseline in all patients and in the subgroups are shown in Table 2. The pattern of “uremic dyslipidemia” characterized by high TG, low-normal HDL-C, and normal CHOL and LDL-C levels was seen in patients with or without nephrotic range proteinuria. Lipid levels showed several significant intercorrelations (Supplemental Table 2). Furthermore, lipids, with the exception of TG, were not significantly different between fasting and non-fasting patients (Supplemental Table 3). The TG and HDL-C levels were significantly different between BMI categories, with a steady increase in TG and a decrease in HDL-C with increasing BMI, whereas CHOL and LDL-C levels were similar (Supplemental Table 4).
Table 2.
Baseline lipid levels (all in mg/dl) of the study population and subgroups
| A. All patients | ||||
| All (n = 681) | Nephrotic range proteinuria (n = 183) | Non-nephrotic range proteinuria (n = 491) | p-value | |
| Total cholesterol | ||||
| Mean (SD) | 180 (48) | 199 (62) | 172 (37) | < 0.001 |
| Median (IQR) | 173 (55) | 185 (72) | 171 (50) | |
| HDL cholesterol | ||||
| Mean (SD) | 48 (14) | 49 (16) | 48(14) | 0.676 |
| Median (IQR) | 46 (19) | 46 (20) | 46 (18) | |
| LDL cholesterol | ||||
| Mean (SD) | 98 (39) | 113 (51) | 92 (30) | < 0.001 |
| Median (IQR) | 93 (46) | 100 (53) | 91 (42) | |
| Triglycerides | ||||
| Mean (SD) | 147 (86) | 170 (101) | 138 (78) | < 0.001 |
| Median (IQR) | 126 (84) | 142 (107) | 119 (78) | |
| B. Fasting patients | ||||
| All (n = 265) | Nephrotic-range proteinuria (n = 85) | Non-nephrotic range proteinuria (n = 177) | p-value | |
| Total cholesterol | ||||
| Mean (SD) | 180 (54) | 198 (68) | 169 (38) | < 0.001 |
| Median (IQR) | 172 (58) | 182 (67) | 169 (52) | |
| HDL cholesterol | ||||
| Mean (SD) | 48 (15) | 48 (16) | 47 (14) | 0.749 |
| Median (IQR) | 46 (19) | 45 (22) | 46 (18) | |
| LDL cholesterol | ||||
| Mean (SD) | 100 (44) | 115 (55) | 91 (31) | < 0.001 |
| Median (IQR) | 94 (47) | 102 (50) | 90 (40) | |
| Triglycerides | ||||
| Mean (SD) | 137 (75) | 154 (83) | 129 (70) | 0.015 |
| Median (IQR) | 123 (80) | 135 (96) | 115 (74) | |
n = 674 (panel A) and n = 262 (panel B) patients in the subgroups (nephrotic vs. non-nephrotic) due to 7 (panel A) (3 for panel B) with missing data for albuminuria. P-values are based on a two-sample t-test.
Abnormal lipid levels, types, and frequency of dyslipidemia
Hypertriglyceridemia was the most frequent abnormality, found in 54.7% of the study population. Abnormal serum concentrations of HDL-C (31.9%), CHOL (27.1%), and LDL-C (15.2%) were also frequent (Table 3).
Table 3.
Lipids and frequency of abnormal levels at baseline stratified by CKD stage
| All | CKD stage 3 | CKD stage 4 | CKD stage 5 | *p-value | |
|---|---|---|---|---|---|
| Total cholesterol (n) | 679 | 249 | 322 | 108 | |
| Mean (± SD; mg/dl) | 180 (48) | 177 (45) | 183 (49) | 178 (52) | 0.294 |
| Normal (n, %) | 495 (72.9%) | 181 (72.7%) | 233 (72.4%) | 81 (75.03%) | 0.863 |
| Abnormal (n, %) | 184 (27.1%) | 68 (27.3%) | 89 (27.6%) | 27 (25.0%) | |
| HDL cholesterol | 681 | 249 | 324 | 108 | |
| Mean (± SD; mg/dl) | 48 (14) | 49 (14) | 47 (15) | 46 (16) | 0.088 |
| Normal | 464 (68.1%) | 188 (75.5%) | 207 (63.9%) | 69 (63.9%) | 0.007 |
| Abnormal | 217 (31.9%) | 61 (24.5%) | 117 (36.1%) | 39 (36.1%) | |
| LDL cholesterol | 678 | 249 | 322 | 107 | |
| Mean (± SD; mg/dl) | 98 (39) | 96 (37) | 101 (40) | 96 (41) | 0.217 |
| Normal | 575 (84.8%) | 211 (84.7%) | 268 (83.2%) | 96 (89.7%) | 0.269 |
| Abnormal | 103 (15.2%) | 38 (15.3%) | 54 (16.8%) | 11 (10.3%) | |
| Triglycerides | 680 | 249 | 324 | 107 | |
| Mean (± SD; mg/dl) | 147 (86) | 134 (78) | 154 (89) | 154 (92) | 0.015 |
| Normal | 308 (45.3%) | 130 (52.2%) | 135 (41.7%) | 43 (40.2%) | 0.022 |
| Abnormal | 372 (54.7%) | 119 (47.8%) | 189 (58.3%) | 64 (59.8%) |
CKD stage was modeled as a categorical variable
*p-value is based on the chi-squared test for dichotomized lipid variables (normal vs. abnormal) and on ANOVA for continuous lipid variables
In the entire cohort and in the subgroup of fasting patients, those with nephrotic range proteinuria had significantly elevated levels of CHOL, TG, and LDL-C, while HDL-C levels were similar. Serum albumin levels were significantly lower in patients with nephrotic range proteinuria, but largely within the normal range and only weakly correlated with log-transformed UACR (r = − 0.40; p < 0.0001).
Except for a significant increase in TG in more advanced CKD, lipid levels and frequencies of dyslipidemia were not significantly different between CKD stages in the entire cohort (Table 3). In fasting patients, all lipid levels including TG were not significantly different between CKD stages (Supplemental Table 5).
Increased TG levels were frequently accompanied by low levels of HDL-C, especially in patients with nephrotic range proteinuria (Table 4).
Table 4.
Prevalence of normal and abnormal TG and HDL-C levels
| All | HDL-C low | HDL-C normal | p-value | |
|---|---|---|---|---|
| A. Total study population | ||||
| High vs. normal TG (n; %) | ||||
| Normal | 308 ( 45.3%) | 64 ( 29.5%) | 244 ( 52.7%) | < 0.001 |
| Abnormal | 372 ( 54.7%) | 153 ( 70.5%) | 219 ( 47.3%) | |
| Missing | 1 | 0 | 1 | |
| B. Patients with nephrotic range proteinuria | ||||
| High vs. normal TG (n; %) | ||||
| Normal | 65 ( 35.5%) | 14 ( 23.0%) | 51 ( 41.8%) | 0.012 |
| Abnormal | 118 ( 64.5%) | 47 ( 77.0%) | 71 ( 58.2%) | |
| C. Patients without nephrotic range proteinuria | ||||
| High vs. normal TG (n; %) | ||||
| Normal | 240 ( 49.0%) | 50 ( 32.5%) | 190 ( 56.5%) | < 0.001 |
| Abnormal | 250 ( 51.0%) | 104 ( 67.5%) | 146 ( 43.5%) | |
| Missing | 1 | 0 | 1 | |
The frequencies of different types of dyslipidemia at the various CKD stages were not significantly different (Table 5) in the entire cohort as well as in fasting patients (Supplemental Table 6). Irrespective of the CKD stage, the predominant lipid abnormality was high TG levels, followed by low HDL-C, high CHOL, and high LDL-C.
Table 5.
Types of dyslipidemia and frequency at baseline stratified by CKD stage
| All (n; %) | CKD stage 3 | CKD stage 4 | CKD stage 5 | p-value | |
|---|---|---|---|---|---|
| Dyslipidemia | |||||
| No dyslipidemia | 190 (27.6%) | 81 (32.5%) | 77 (23.8%) | 32 (29.4%) | 0.102 |
| Dyslipidemia | 489 (71.8%) | 168 (67.5%) | 246 (75.9%) | 75 (64.4%) | |
| Isolated vs. multiple dyslipidemia | |||||
| No dyslipidemia | 190 (27.9%) | 81 (32.5%) | 77 (23.8%) | 32 (29.6%) | 0.312 |
| Isolated dyslipidemia | 220 (32.3%) | 79 (31.7%) | 110 (34.0%) | 31 (28.7%) | |
| Abnormal lipid | |||||
| Isolated high Chol | 28 (12.7%) | 14 (17.7%) | 12 (10.9%) | 2 (6.5%) | 0.242 |
| Isolated low HDL-C | 59 (26.8%) | 19 (24.1%) | 34 (30.9%) | 6 (19.4%) | |
| Isolated high TG | 133 (60.5%) | 46 (58.2%) | 64 (58.2%) | 23 (74.2%) | |
| Isolated high LDL-C | 0 | ||||
| Multiple dyslipidemia | 268 (39.4%) | 89 (35.7%) | 135 (41.7%) | 44 (40.7%) | |
| Number of abnormal lipids: multiple dyslipidemia | |||||
| 2 | 170 (63.4%) | 63 (70.8%) | 80 (59.3%) | 27 (61.4%) | 0.264 |
| 3 | 77 (28.7%) | 23 (25.8%) | 42 (31.1%) | 12 (27.3%) | |
| 4 | 21 (7.8%) | 3 (3.4%) | 13 (9.6%) | 5 (11.4%) | |
Dyslipidemia could not be classified due to missing values in 3 patients. CKD stage was modeled as a categorical variable. p-values are based on the chi-squared test
Factors associated with lipid levels and dyslipidemia at baseline
To further analyze factors associated with lipid levels at baseline, multiple linear regression models were constructed for each lipid with covariates, including the fasting status (Table 6). Fasting status had a significant effect only for TG. Serum albumin was the only variable significantly associated with all lipid levels. Glomerular disease and the BMI-SDS had a strong impact on CHOL, LDL-C, and TG levels. To investigate whether the same factors were associated with dyslipidemia, multivariable logistic regression models were constructed for the status of dyslipidemia for each lipid (Table 7). Fasting status had no significant effect on dyslipidemia status. The risk for low HDL-C levels was much lower for girls than for boys. Proteinuria was associated with increased levels of CHOL, LDL-C, and HDL-C, whereas hsCRP had an inverse association with CHOL and HDL-C. The BMI-SDS was associated with abnormal CHOL and TG levels and the presence of glomerular disease with increased levels of CHOL and LDL-C. Hypertriglyceridemia was associated with younger age, lower eGFR, shorter duration of CKD, higher body mass index (BMI-SDS), lower serum albumin, and higher diastolic blood pressure.
Table 6.
Baseline correlates of lipid levels
| Cholesterol (n = 630) | LDL cholesterol (n = 629) | HDL cholesterol (n = 632) | Triglycerides (n = 631) | |||||
|---|---|---|---|---|---|---|---|---|
| Beta [95%CI] | p-value | Beta [95%CI] | p-value | Beta [95%CI] | p-value | Beta[95%CI] | p-value | |
| Age (yrs) | − 1.77 [− 2.79, − 0.76] | 0.0007 | − 1.21 [− 2.06, − 0.38] | 0.0046 | − 0.77 [− 1.03, − 0.44] | < 0.0001 | n.i | |
| Sex (ref male) | n.i | n.i | 3.82 [1.50, 6.11] | 0.0013 | − 14.02 [− 27.60, − 0.45 | 0.0429 | ||
| Glomerular disease (ref CAKUT) | 38.19 [25.34, 51.05] | < 0.0001 | 28.27 [17.67, 38.85] | < 0.0001 | n.i | 37.92 [14.07, 61.79] | 0.0019 | |
| Other diagnosis (ref CAKUT) | 12.13 [3.98, 20.28] | 0.0036 | 8.83 [2.12, 15.54] | 0.0100 | n.i | 2.18 [− 13.49, 17.86] | 0.7846 | |
| eGFR (ml/min/1.73 m2) | n.i | n.i | 0.15 [0.05, 0.25] | 0.0040 | − 0.74 [− 1.28, − 0.20] | 0.0075 | ||
| Time since CKD2 (years) | n.i | n.i | 0.54 [0.29, 0.79] | < 0.0001 | − 1.30 [− 2.78, 0.18] | 0.0861 | ||
| Log UACR | 6.20 [4.11, 8.29] | < 0.0001 | 4.39 [2.67, 2.11] | < 0.0001 | 1.41 [0.70, 2.12] | 0.0001 | n.i | |
| Log hsCRP | − 2.74 [− 4.87, − 0.60] | 0.0120 | − 1.38 [− 3.14, 0.38] | 0.1232 | − 1.34 [− 2.02, − 0.66] | 0.0001 | − 5.52 [− 9.52, − 1.53] | 0.0068 |
| BMI-SDS | 3.89 [1.26, 6.52] | 0.0038 | 2.21 [− 0.05, 4.39] | 0.0449 | n.i | 10.59 [5.61, 15.57] | < 0.0001 | |
| Serum albumin | − 1.22 [− 1.93, − 0.50] | 0.0009 | − 1.05 [− 1.64, − 0.47] | 0.0005 | 0.49 [0.27, 0.71] | < 0.0001 | − 3.68 [− 4.92, − 2.45] | < 0.0001 |
| Diastolic BP SDS | n.i | n.i | n.i | 8.31 [2.50, 14.12] | 0.0052 | |||
| Lack of physical activity (ref at least 1 h per week) | 9.89 [1.88, 17.89] | 0.0156 | 7.46 [0.87, 14.06] | 0.0267 | n.i | n.i | ||
| Serum bicarbonate | 1.66 [0.71, 2.60] | 0.0006 | 1.35 [0.56, 2.14] | 0.0008 | 0.26 [− 0.05, 0.56] | 0.0966 | n.i | |
| Serum hemoglobin | n.i | n.i | n.i | n.i | ||||
| Non-fasting status (ref fasting) | − 0.52 [− 7.47, 6.43] | 0.8831 | − 3.02 [− 8.75, 2.72] | 0.3020 | − 0.90 [− 3.12, 1.31] | 0.4238 | 17.78 [4.87, 30.68] | 0.0070 |
Multivariable linear regression for lipids (continuous). Biologically relevant variables were considered candidate variables; all candidate variables are shown in the table with “n.i.” indicating “not included.” Since the variables age and puberty as well as waist-to-height ratio and BMI-SDS are correlated, only one of these variables (age, BMI-SDS) was included in the model. Final models after variable selection; backward selection based on AIC. Fasting status forced in the model
n.i. not included in the final model, CI confidence interval, Beta regression coefficient, ref reference category, UACR urinary albumin/creatinine ratio
Table 7.
Baseline correlates of dyslipidemia
| High cholesterol (n = 630) | High LDL cholesterol (n = 629) | Low HDL cholesterol (n = 632) | High triglycerides (n = 631) | |||||
|---|---|---|---|---|---|---|---|---|
| OR [95%CI] | p-value | OR [95%CI] | p-value | OR [95%CI] | p-value | OR [95%CI] | p-value | |
| Age (yrs) | 0.93 [0.88, 0.99] | 0.0137 | 0.91 [0.84, 0.98] | 0.0100 | 1.09 [1.03, 1.15] | 0.0025 | 0.87 [0.82, 0.91] | < 0.0001 |
| Sex (ref male) | n.i | n.i | 0.43 [0.29, 0.65] | < 0.0001 | n.i | |||
| Glomerular disease (ref CAKUT) | 5.02 [2.65, 9.49] | < 0.0001 | 5.07 [2.47, 10.42] | < 0.0001 | n.i | 1.80 [0.90, 3.59] | 0.0956 | |
| Other diagnosis (ref CAKUT) | 1.43 [0.90, 2.26.] | 0.1262 | 1.36 [0.76, 2.45] | 0.3056 | n.i | 0.75 [0.49, 1.14] | 0.1790 | |
| eGFR (ml/min/1.73 m2) | 1.02 [1.00, 1.04] | 0.0595 | n.i | 0.97 [0.95, 0.99] | 0.0007 | 0.98 [0.97, 0.99] | 0.0293 | |
| Time since CKD2 (years) | n.i | n.i | 0.92 [0.88, 0.96] | 0.0002 | 0.94 [0.91, 0.99] | 0.0076 | ||
| Log UACR | 1.38 [1.22 1.57] | < 0.0001 | 1.22 [1.04, 1.43] | 0.0159 | 0.84 [0.74, 0.94] | 0.0032 | n.i | |
| Log hsCRP | 0.85[0.74, 0.96] | 0.0109 | n.i | 1.24[1.11, 1.39] | 0.0002 | n.i | ||
| BMI-SDS | 1.26 [1.07, 1.48] | 0.0057 | 1.21 [0.99, 1.49] | 0.0577 | n.i | 1.20 [1.05, 1.37] | 0.0090 | |
| Serum albumin | n.i | 0.95 [0.91, 0.99] | 0.0274 | 0.93 [0.89, 0.96] | 0.0001 | 0.95 [0.92, 0.99] | 0.0078 | |
| Diastolic BP -SDS | n.i | n.i | n.i | 1.25 [1.06, 1.48] | 0.0069 | |||
| Lack of physical activity (ref ≥ 1 h per week) | n.i | 1.84 [1.00, 3.36] | 0.0487 | n.i | 1.44 [0.95, 2.18] | 0.0825 | ||
| Serum bicarbonate | 1.05 [1.00, 1.11] | 0.0702 | n.i | 0.94 [0.89, 0.99] | 0.0172 | n.i | ||
| Serum hemoglobin | n.i | n.i | n.i | n.i | ||||
| Non-fasting status (ref fasting) | 1.19 [0.80, 1.78] | 0.3884 | 1.28 [0.79, 2.07] | 0.3223 | 0.94 [0.65, 1.37] | 0.7611 | 0.90 [0.64, 1.28] | 0.5555 |
Multivariable logistic regression for lipids (binary). Variables remaining significant at the 0.1 level after backward selection included, fasting status forced in the model
n.i. not included, OR odds ratio, CI confidence interval, ref reference category, UACR urinary albumin/creatinine ratio
Discussion
In this largest pediatric CKD cohort investigated to date, a characteristic pattern of dyslipidemia involving all lipid fractions, but mainly TG, was highly prevalent irrespective of CKD stage or fasting status and significantly associated with other cardiovascular risk factors.
Prevalence and type of dyslipidemia
Dyslipidemia was observed in 72% of patients. This prevalence is considerably higher than reported in two other large pediatric cohort studies: 45.2% in the CKiD study (n = 391; USA) [4] and 61.5% in the KNOW-Ped CKD study (n = 437; South Korea) [5].
However, a smaller cross-sectional study from India (n = 80) found a similar prevalence of 73.8% [19]. Many patient characteristics are similar in these cohorts: age (about 11–12 years), a preponderance of boys (60–70% boys), and primary kidney disease CAKUT (about 70%); however, eGFR was lower in our study (26 vs. 43 and 55 ml/min/1.73 m2) and the percentage of patients with nephrotic range proteinuria was higher (27% vs. 12% and 15.7%) when compared to the U.S. and Korean studies, respectively. Therefore, a higher degree of proteinuria and a lower eGFR are likely important factors contributing to the high prevalence of dyslipidemia observed in our study. Indeed, proteinuria and eGFR were among the variables significantly associated with dyslipidemia in the multivariate analysis.
As in the other cohort studies, high TG levels were the most common abnormality, but the prevalence was much higher in the present study (54.7%) than in the CKiD study (32%) and the KNOW-Ped CKD study (15.2%).
There were no significant differences between CKD stages in the prevalence of abnormal lipid levels and prevalence and type of dyslipidemia. Isolated dyslipidemia (32.3%), most often in the form of hypertriglyceridemia, was less common than multiple dyslipidemia (39.4%).
Factors associated with lipid levels and dyslipidemia
The baseline TG levels were independently associated with male sex, eGFR, BMI-SDS, a diagnosis of glomerular disease, a shorter time of CKD, diastolic blood pressure, serum hsCRP and serum albumin levels, and, in contrast to all other measured lipids, the fasting status.
The logistics of implementing fasting conditions in pediatric studies can be challenging. In our study, patients were asked to be fasting, and fasting status was documented at all clinic visits. In the Korean KNOW-Ped CKD study, children were apparently not fasting [5], and in the CKiD study, fasting status was unknown in 29% of the children [4]. However, in a study from India reporting a similar prevalence of dyslipidemia, all children were investigated in the fasting status. As expected, we found that triglycerides, but not other lipids, were affected by fasting; however, fasting status had no significant effect on dyslipidemia status in the multivariate analysis. Of note, the non-fasting state may in fact better reflect lipid metabolism under real-world conditions. For practical reasons and for cardiovascular risk prediction [20], several European Medical Societies have recommended that non-fasting blood samples be routinely used for the assessment of plasma lipid profiles in adult patients [21]. While unmeasured variables such as differences in genetic factors or dietary habits between fasting and non-fasting patients may have played a role, our findings suggest that lipid measurements in the fasting state have only limited relevance for identifying dyslipidemia in children with CKD.
Multivariate analysis showed that the serum albumin level was the only variable significantly associated with all lipid measurements and thus, in the case of hypoalbuminemia, with an atherogenic constellation of lipids, i.e., higher levels of CHOL, LDL-C, and TG and lower HDL-C. This cannot be explained by malnutrition, inflammation, or proteinuria since UACR, hsCRP, and BMI-SDS were included as covariates. While to our knowledge no studies have been performed in humans to investigate the pathophysiology of this association in non-nephrotic CKD, animal experiments have shown that hypoalbuminemia has a strong inhibiting effect on lipoprotein lipase, independent of proteinuria [22]. It has been shown that lipoprotein lipase activity is reduced in hypoalbuminemia in the absence of proteinuria due to defective endothelial binding [23]. Diminished lipolysis of very low-density lipoprotein (VLDL) with an increase in relatively cholesterol-rich VLDL remnants and diminished formation of HDL cholesterol has been demonstrated as a characteristic disturbance of lipoprotein metabolism in CKD [2]. Hence, an inhibiting effect of lower serum albumin levels on lipolysis of TG-rich lipoproteins would increase the serum levels of TG-rich lipoproteins and explain the associations found in our study, i.e., strong impact on TG and HDL-C and a weak association with CHOL and LDL-C.
Hypoalbuminemia is a marker of inflammation in several disease states and is further aggravated by urinary losses of albumin in nephrotic patients; it has been confirmed as a principal risk factor for the progression of CKD and cardiovascular disease in CKD patients [24, 25].
The degree of proteinuria was also significantly associated with lipid levels and the presence of dyslipidemia, respectively. For reliable quantification of proteinuria, measurement of the albumin/creatinine ratio in spot urine is recommended in current guidelines, acknowledging the greater sensitivity, less variability, and better standardization of this method compared to the protein/creatinine ratio [24]. Especially, low concentrations of urine albumin are better detected with the UACR, and large cohort studies have shown that it accurately predicts kidney and cardiovascular risks [14]. Accordingly, we have used the albumin/creatinine ratio in spot urine for proteinuria quantification and used a cut-off for nephrotic range proteinuria in children (1.1 g/g), which should be validated in future studies of children with proteinuria.
Serum bicarbonate levels were associated with higher levels of CHOL and LDL-C. This was also noted in previous studies in adults [25, 26]. Higher levels of total and LDL cholesterol could reflect better nutrition with correction of metabolic acidosis [27]. However, a potential increase in serum cholesterol, i.e., atherogenic lipoproteins (presumably VLDL remnants and modified LDL), adds to the uncertainties of bicarbonate supplementation [28]. Gender differences in CKD patients have not been much investigated [29]. In our study, the female sex had a significant association with lower TG and higher HDL-C levels. In contrast, high TG levels were more often found in boys in the CKiD study [30].
Time since the diagnosis of CKD (stage 2), a variable reflecting CKD duration, is likely a measure of progression or alternatively, late referral to tertiary centers, although the latter is not common in European children; it was associated with lower TG and higher HDL-C levels. The association of diastolic (but not systolic) blood pressure with TG levels (but not other lipids) remains unclear and should be further investigated in clinical studies.
We found significant associations of BMI with CHOL, LDL-C, and TG levels at baseline and with dyslipidemia status for CHOL and TG. The strong association of BMI with elevated TG levels was also found in all previous cohort studies, although BMI distribution was different: the CKiD study and the study from India had a much higher percentage of obese patients (both 28%), whereas the percentage of overweight patients (18%, 20%) was like ours. The study from Korea had only 7% overweight patients and, like our study, 5.1% obese patients while reporting a similar high prevalence of hypertriglyceridemia as in our study. Mean TG levels were clearly elevated across all categories of BMI and highest in overweight and obese children. These data suggest that TG levels are elevated in most CKD patients regardless of BMI category but further increase in overweight and obese individuals.
Although overweight/obesity is associated with inflammation and lack of physical activity, it should be noted that these variables showed independent significant associations with serum lipid levels and dyslipidemia. Physical activity was much lower in patients with nephrotic range proteinuria, but the BMI-SDS was similar to other patients, further suggesting an independent effect of physical activity, which was associated with hypercholesterolemia and hypertriglyceridemia in this study. Also, in contrast to the positive association with BMI, hsCRP had a negative impact on all baseline lipid levels; it was found to be associated with a lower risk for hypercholesterolemia and a higher risk for low HDL-C levels. This is in line with a large cross-sectional analysis of adults with mild to moderate CKD that found lower levels of CHOL and LDL-C (but not TG or HDL-C) in patients with increased hsCRP (or IL-6) levels [31]. Although not many patients were underweight in our study, these associations could reflect malnutrition, which may not be tightly linked to BMI [32].
Pathogenesis
Previous studies have revealed a multifactorial pathogenesis of dyslipidemia in patients with CKD [2]. High TG levels, the hallmark of CKD-induced dyslipidemia, are caused by increased hepatic production of VLDL accompanied by diminished catabolism by lipoprotein lipase and hepatic lipase [33]. Additional mechanisms have been demonstrated in nephrotic syndrome, where pronounced hyperlipidemia is driven by massive proteinuria. It could be shown that circulating glycoprotein angiopoietin-like 4 (Angptl4), in response to nephrotic range proteinuria, reduces proteinuria and also inactivates endothelium-bound lipoprotein lipase resulting in impaired lipolysis and hypertriglyceridemia [34]. Thus, Angptl4 provides a molecular link between proteinuria and hypertriglyceridemia and could explain the presence of dyslipidemia in patients with near-normal serum albumin levels (as in the present study). In addition, there is emerging evidence for the role of inflammatory cytokines in the pathogenesis of dyslipidemia in glomerular disease [35]. It could be shown that interleukin-13 (IL-13) induced changes in hepatic cholesterol metabolism in a rat model of minimal change disease leading to hypercholesterolemia preceding the onset of proteinuria [36]. Accumulation of TG-rich lipoproteins in CKD leads to enrichment of all lipoprotein fractions with TG [37] and increased plasma levels of bioactive lipid components, many of which are subject to oxidation, resulting in profound changes in free fatty acid profiles and complex lipids [38, 39]. In fact, modifications in all components of lipoproteins induced by the uremic milieu can promote pro-inflammatory and pro-atherogenic processes and oxidative stress in the kidney (reviewed in [40]). Emerging data from prospective cohort studies confirm the atherogenic potential of dyslipidemia in children with CKD: data from the CKiD study shows that dyslipidemia is associated with increased cIMT in children with CKD at 12 months of follow-up [41], and in the 4C study, pulse wave velocity over time was associated with LDL-C among other risk factors [42].
It is a limitation of our study that more qualitative and detailed analytics such as measuring lipoprotein composition and subclasses (such as VLDL remnants) or exploratory studies (lipolytic activity, free fatty acids, Angplt4, oxidative modifications, etc.) were not performed. Most of our study population was Caucasian, limiting the relevance of our findings for other populations. The potential effect of some antihypertensive drugs (diuretics, beta-blockers) on the lipid profile could not be analyzed due to the small number of patients treated with these medications.
Conclusions and outlook
We found a high prevalence of dyslipidemia with an established atherogenic potential and could identify significant associations with several other cardiovascular risk factors, which raises the question of treatment options. At present, pharmacological treatment of hyperlipidemia in children with CKD is hardly considered. The current KDIGO guidelines recommend the assessment of lipid profiles; however, lipid-lowering medications are not recommended for this age group, and only lifestyle changes are advised [43]. In contrast, statin treatment is recommended in adult patients [43], where large-scale evidence supports aggressive treatment of dyslipidemia in all stages of CKD (reviewed in [44]). Statin therapy has also been recommended by an expert panel (American Heart Association) for all children with a very high cardiovascular risk > 10 years of age with abnormal LDL-C, in addition to dietary advice and lifestyle modifications [45]. While therapeutic experience in children is limited [46], the anti-inflammatory effect of statins might be reconsidered in treatment decisions in view of the harmful effects of modified lipoproteins as part of the chronic inflammatory process in CKD [47]. The large CANTOS trial has recently shown that in adult CKD patients—in contrast to patients without CKD—already treated with statins, markers of inflammation (hsCRP, IL-6), but not lipid levels, were predictive of major adverse cardiovascular events, suggesting the need for additional anti-inflammatory therapeutic options [48]. At present, further prospective data are needed to support a rationale for choosing and evaluating lipid-lowering medications in children with CKD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 4C Study Collaborators
The following principal investigators contributed to the 4C Study:
Austria: G. Cortina, Children’s Hospital, Innsbruck; K. Arbeiter, University Children’s Hospital, Vienna. Czech Republic: J. Dusek, University Hospital Motol, Prague. France: J. Harambat, Hôpital des Enfants, Bordeaux; B. Ranchin, Hôpital Femme Mère Enfant et Université de Lyon; M. Fischbach, A.Zalosczyk, Hôpital de Hautepierre, Strasbourg. Germany: U. Querfeld, Charité Children’s Hospital, Berlin; S.Habbig, University Children’s Hospital, Cologne; M. Galiano, University Children’s Hospital, Erlangen; R. Büscher, University Children’s Hospital, Essen; C. Gimpel, Center for Pediatrics and Adolescent Medicine, Freiburg; M. Kemper, UKE University Children’s Hospital, Hamburg; A. Melk, D. Thurn, Hannover Medical School, Hannover; F. Schaefer, A. Doyon, E. Wühl, Center for Pediatrics and Adolescent Medicine, Heidelberg; M. Pohl, Center for Pediatrics and Adolescent Medicine, Jena; S. Wygoda, City Hospital St. Georg, Leipzig; N. Jeck, KfH Kidney Center for Children, Marburg; B. Kranz, University Children’s Hospital, Münster; M. Wigger, Children’s Hospital, Rostock. Italy: G. Montini, S. Orsola-Malpighi Hospital, Bologna; F. Lugani, Istituto Giannina Gaslini, Genova; S. Testa, Fondazione Ospedale Maggiore Policlinico, Milano; E. Vidal, Pediatric Nephrology, Dialysis & Transplant Unit, Padova; C. Matteucci, S. Picca, Ospedale Bambino Gesù, Rome. Lithuania: A. Jankauskiene, K. Azukaitis, University Children’s Hospital, Vilnius. Poland: A. Zurowska, Pediatric and Adolescent Nephrology, Gdansk; D. Drodz, University Children’s Hospital, Krakow; M. Tkaczyk, Polish Mothers Memorial Hospital Research Institute, Lodz; T. Urasinski, Clinic of Pediatrics, Szczecin; M. Litwin, A.Niemirska, Children’s Memorial Health Institute, Warsaw; M. Szczepanska, Zabrze. Portugal: A. Texeira, Hospital Sao Joao, Porto; Serbia: A. Peco-Antic, University Children’s Hospital, Belgrade. Switzerland: B.Bucher, Inselspital, Bern; G. Laube, University Children’s Hospital, Zurich. Turkey: A. Anarat, A.K. Bayazit, Cukurova University, Adana; F. Yalcinkaya, University Faculty of Medicine, Ankara; E. Basin, Baskent University Faculty of Medicine, Ankara; N. Cakar, Diskapi Children’s Hospital, Ankara; O. Soylemezoglu, Gazi University Hospital, Ankara; A. Duzova, Y. Bilginer, Hacettepe Medical Faculty, Ankara; H. Erdogan, Dortcelik Children’s Hospital, Bursa; O. Donmez, Uludag University, Bursa; A. Balat, University of Gaziantep; A. Kiyak, Bakirkoy Children’s Hospital, Istanbul; S. Caliskan, N. Canpolat, Istanbul University Cerrahpasa Faculty of Medicine, Istanbul; C. Candan, Goztepe Educational and Research Hospital, Istanbul; M. Civilibal, Haseki Educational and Research Hospital, Istanbul; S. Emre, Istanbul Medical Faculty, Istanbul, H. Alpay, Marmara University Medical Faculty, Istanbul; G. Ozcelik, Sisli Educational and Research Hospital, Istanbul; S. Mir, B. Sözeri, Ege University Medical Faculty; Izmir; O. Yavascan, Tepecik Training and Research Hospital, Izmir; Y. Tabel, Inonu University, Malatya; P. Ertan, Celal Bayar University, Manisa; E. Yilmaz, Children’s Hospital, Sanliurfa. UK: R. Shroff, Great Ormond Street Hospital, London.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was made possible by grants from the European Renal Association (ERA), the Kuratorium für Dialyse und Nierentransplantation (KfH) Foundation for Preventive Medicine, and the German Federal Ministry of Education and Research (reference no. 01EO0802).
Data availability
The data concerning the findings of this study are available from the corresponding author, UQ, upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saland JM, Kupferman JC, Pierce CB, Flynn JT, Mitsnefes MM, Warady BA, Furth SL (2019) Change in dyslipidemia with declining glomerular filtration rate and increasing proteinuria in children with CKD. Clin J Am Soc Nephrol 14:1711–1718 10.2215/CJN.03110319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khurana M, Silverstein DM (2015) Etiology and management of dyslipidemia in children with chronic kidney disease and end-stage renal disease. Pediatr Nephrol 30:2073–2084 10.1007/s00467-015-3075-9 [DOI] [PubMed] [Google Scholar]
- 3.Moradi H, Vaziri ND (2018) Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci (Landmark Ed) 23:146–161 10.2741/4585 [DOI] [PubMed] [Google Scholar]
- 4.Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL, CKiD Investigators (2010) Dyslipidemia in children with chronic kidney disease. Kidney Int 78:1154–1163 10.1038/ki.2010.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek HS, Kim SH, Kang HG, Choi HJ, Cheong HI, Ha IS, Han KH, Cho HY, Shin JI, Park YS, Lee JH, Ahn YH, Park E, Cho MH (2020) Dyslipidemia in pediatric CKD patients: results from KNOW-PedCKD (KoreaN cohort study for outcomes in patients with pediatric CKD). Pediatr Nephrol 35:1455–1461 10.1007/s00467-020-04545-z [DOI] [PubMed] [Google Scholar]
- 6.Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585 10.1681/ASN.2011111115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saland JM, Ginsberg H, Fisher EA (2002) Dyslipidemia in pediatric renal disease: epidemiology, pathophysiology, and management. Curr Opin Pediatr 14:197–204 10.1097/00008480-200204000-00009 [DOI] [PubMed] [Google Scholar]
- 8.de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, Mietus-Snyder M, Mitsnefes MM, Peterson AL, St-Pierre J, Urbina EM, Zachariah JP, Zaidi AN (2019) Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 139:e603–e634 10.1161/CIR.0000000000000618 [DOI] [PubMed] [Google Scholar]
- 9.Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, Caliskan S, Civilibal M, Doyon A, Duzova A, Kracht D, Litwin M, Melk A, Mir S, Sozeri B, Shroff R, Zeller R, Wuhl E, Schaefer F; 4C Study Group (2010) The cardiovascular comorbidity in children with chronic kidney disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol 5:1642–1648 10.2215/CJN.08791209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sozeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wuhl E, Shroff R, Querfeld U, 4C Study Consortium (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28 10.2215/CJN.01090216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute (2011) Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128(Suppl 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonthuis M, van Stralen KJ, Verrina E, Edefonti A, Molchanova EA, Hokken-Koelega AC, Schaefer F, Jager KJ (2012) Use of national and international growth charts for studying height in European children: development of up-to-date European height-for-age charts. PLoS One 7:e42506 10.1371/journal.pone.0042506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576 10.1542/peds.114.S2.555 [DOI] [PubMed] [Google Scholar]
- 14.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 15.Trautmann A, Boyer O, Hodson E, Bagga A, Gipson DS, Samuel S, Wetzels J, Alhasan K, Banerjee S, Bhimma R, Bonilla-Felix M, Cano F, Christian M, Hahn D, Kang HG, Nakanishi K, Safouh H, Trachtman H, Xu H, Cook W, Vivarelli M, Haffner D, International Pediatric Nephrology Association (2023) IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 38:877–919 10.1007/s00467-022-05739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoycheff N, Stevens LA, Schmid CH, Tighiouart H, Lewis J, Atkins RC, Levey AS (2009) Nephrotic syndrome in diabetic kidney disease: an evaluation and update of the definition. Am J Kidney Dis 54:840–849 10.1053/j.ajkd.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanner C, Cooper ME, Johansen OE, Toto R, Rosenstock J, McGuire DK, Kahn SE, Pfarr E, Schnaidt S, von Eynatten M, George JT, Gollop ND, Marx N, Alexander JH, Zinman B, Perkovic V, CARMELINA investigators (2021) Effect of linagliptin versus placebo on cardiovascular and kidney outcomes in nephrotic range proteinuria and type 2 diabetes: the CARMELINA randomized controlled trial. Clin Kidney J 14:226–236 10.1093/ckj/sfaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandelwal P, Murugan V, Hari S, Lakshmy R, Sinha A, Hari P, Bagga A (2016) Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease. Pediatr Nephrol 31:1313–1320 10.1007/s00467-016-3350-4 [DOI] [PubMed] [Google Scholar]
- 20.Langsted A, Nordestgaard BG (2019) Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 51:131–141 10.1016/j.pathol.2018.09.062 [DOI] [PubMed] [Google Scholar]
- 21.Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Boren J, Chapman MJ, Cobbaert C, Descamps OS, von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M, European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative (2016) Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 37:1944–1958 10.1093/eurheartj/ehw152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer GC, Stevenson FT, Atkinson DN, Jones H, Staprans I, Kaysen GA (2001) Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int 59:179–189 10.1046/j.1523-1755.2001.00478.x [DOI] [PubMed] [Google Scholar]
- 23.Shearer GC, Kaysen GA (2006) Endothelial bound lipoprotein lipase (LpL) depletion in hypoalbuminemia results from decreased endothelial binding, not decreased secretion. Kidney Int 70:647–653 10.1038/sj.ki.5000318 [DOI] [PubMed] [Google Scholar]
- 24.Lamb EJ, MacKenzie F, Stevens PE (2009) How should proteinuria be detected and measured? Ann Clin Biochem 46:205–217 10.1258/acb.2009.009007 [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Kang E, Ryu H, Han M, Lee KB, Kim YS, Sung S, Ahn C, Oh KH (2019) Metabolic acidosis is associated with pulse wave velocity in chronic kidney disease: results from the KNOW-CKD Study. Sci Rep 9:16139 10.1038/s41598-019-52499-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobre M, Yang W, Pan Q, Appel L, Bellovich K, Chen J, Feldman H, Fischer MJ, Ham LL, Hostetter T, Jaar BG, Kallem RR, Rosas SE, Scialla JJ, Wolf M, Rahman M, CRIC Study Investigators (2015) Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): a report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc 4:e001599 10.1161/JAHA.114.001599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM (2009) Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20:2075–2084 10.1681/ASN.2008111205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capusa C, Stefan G, Stancu S, Lipan M, Tsur LD, Mircescu G (2017) Metabolic acidosis of chronic kidney disease and subclinical cardiovascular disease markers: friend or foe? Medicine (Baltimore) 96:e8802 10.1097/MD.0000000000008802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ (2018) Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14:151–164 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 30.Ruebner RL, Ng D, Mitsnefes M, Foster BJ, Meyers K, Warady B, Furth SL (2016) Cardiovascular disease risk factors and left ventricular hypertrophy in girls and boys with CKD. Clin J Am Soc Nephrol 11:1962–1968 10.2215/CJN.01270216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamprea-Montealegre JA, Astor BC, McClelland RL, de Boer IH, Burke GL, Sibley CT, O’Leary D, Sharrett AR, Szklo M (2012) CKD, plasma lipids, and common carotid intima-media thickness: results from the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 7:1777–1785 10.2215/CJN.02090212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mak RH (2024) Protein energy wasting in children with chronic kidney disease: is lean body mass by DEXA a key diagnostic biomarker? Pediatr Nephrol 39:1323–1326 10.1007/s00467-023-06261-w [DOI] [PubMed] [Google Scholar]
- 33.Prinsen BH, de Sain-van der Velden MG, de Koning EJ, Koomans HA, Berger R, Rabelink TJ (2003) Hypertriglyceridemia in patients with chronic renal failure: possible mechanisms. Kidney Int Suppl:S121–124 [DOI] [PubMed]
- 34.Clement LC, Mace C, Avila-Casado C, Joles JA, Kersten S, Chugh SS (2014) Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med 20:37–46 10.1038/nm.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vianna HR, Soares CM, Silveira KD, Elmiro GS, Mendes PM, de Sousa TM, Teixeira MM, Miranda DM, Simoes ESAC (2013) Cytokines in chronic kidney disease: potential link of MCP-1 and dyslipidemia in glomerular diseases. Pediatr Nephrol 28:463–469 10.1007/s00467-012-2363-x [DOI] [PubMed] [Google Scholar]
- 36.Low LD, Lu L, Chan CY, Chen J, Yang HH, Yu H, Lee CGL, Ng KH, Yap HK (2020) IL-13-driven alterations in hepatic cholesterol handling contributes to hypercholesterolemia in a rat model of minimal change disease. Clin Sci (Lond) 134:225–237 10.1042/CS20190961 [DOI] [PubMed] [Google Scholar]
- 37.Attman PO, Alaupovic P, Tavella M, Knight-Gibson C (1996) Abnormal lipid and apolipoprotein composition of major lipoprotein density classes in patients with chronic renal failure. Nephrol Dial Transplant 11:63–69 10.1093/oxfordjournals.ndt.a027068 [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Chen L, Liu D, Chen DQ, Vaziri ND, Yu XY, Zhang L, Su W, Bai X, Zhao YY (2017) Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res 16:1566–1578 10.1021/acs.jproteome.6b00956 [DOI] [PubMed] [Google Scholar]
- 39.Baek J, He C, Afshinnia F, Michailidis G, Pennathur S (2022) Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol 18:38–55 10.1038/s41581-021-00488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noels H, Lehrke M, Vanholder R, Jankowski J (2021) Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol 17:528–542 10.1038/s41581-021-00423-5 [DOI] [PubMed] [Google Scholar]
- 41.Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, White CT, Furth S, Warady BA, Mitsnefes M (2012) Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol 7:1930–1937 10.2215/CJN.03130312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azukaitis K, Kirchner M, Doyon A, Litwin M, Bayazit A, Duzova A, Canpolat N, Jankauskiene A, Shroff R, Melk A, Querfeld U, Schaefer F, 4C Study Investigators (2022) Arterial stiffness and chronic kidney disease progression in children. Clin J Am Soc Nephrol 17:1467–1476 10.2215/CJN.02200222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanner C, Tonelli M, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members (2014) KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 85:1303–1309 10.1038/ki.2014.31 [DOI] [PubMed] [Google Scholar]
- 44.Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM, Malyszko J, Verhaar MC, Ekart R, Vanholder R, London G, Ortiz A, Zoccali C (2018) Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 14:727–749 10.1038/s41581-018-0072-9 [DOI] [PubMed] [Google Scholar]
- 45.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J, American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research (2006) Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert panel on population and prevention science; the councils on cardiovascular disease in the young, epidemiology and prevention, nutrition, physical activity and metabolism, high blood pressure research, cardiovascular nursing, and the kidney in heart disease; and the Interdisciplinary working group on quality of care and outcomes research: endorsed by the American Academy of Pediatrics. Circulation 114:2710–2738 10.1161/CIRCULATIONAHA.106.179568 [DOI] [PubMed] [Google Scholar]
- 46.Ramesh PL, Khandelwal P, Lakshmy R, Sinha A, Bagga A, Hari P (2023) Short-term safety and efficacy of escalating doses of atorvastatin for dyslipidemia in children with predialysis chronic kidney disease stage 2–5. Pediatr Nephrol 38:2763–2770 10.1007/s00467-023-05887-0 [DOI] [PubMed] [Google Scholar]
- 47.German CA, Liao JK (2023) Understanding the molecular mechanisms of statin pleiotropic effects. Arch Toxicol 97:1529–1545 10.1007/s00204-023-03492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadatane SP, Satariano M, Massey M, Mongan K, Raina R (2023) The role of inflammation in CKD. Cells 12:1581 10.3390/cells12121581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data concerning the findings of this study are available from the corresponding author, UQ, upon reasonable request.