Abstract
Background:
Postoperative atrial fibrillation (AF) has a complex etiology, and beta-blockers are commonly recommended for its pharmacological prevention. This study aims to assess the impact of beta-blocker therapy on postoperative AF occurrence in patients undergoing aortic valve replacement, mitral valve replacement, surgical revascularization of the myocardium, or a combination of these procedures.
Methods:
The study encompassed 472 patients who received aortic valve replacement, mitral valve replacement, surgical revascularization, or their combination. We evaluated the efficacy of preoperative and one-month postoperative beta-blocker administration in preventing postoperative AF, and the associated risk factors involved in the development of postoperative AF.
Results:
Of the total patient population, 36% experienced postoperative AF. Our study demonstrated a significant reduction in postoperative AF incidence among patients receiving beta-blocker treatment (all p-values 0.05). Additionally, one-month post-surgery, beta-blocker treatment exerted a protective effect by maintaining the sinus rhythm (p = 0.0001). Regarding the risk factors involved in the development of postoperative AF, both age and left atrium (LA) sizeassessed pre-and postoperatively—were positively correlated with the occurrence of postoperative AF (p = 0.006). No relationship was found between leukocyte counts and AF incidence. Notably, C-reactive protein (CRP) levels were significantly elevated on the fifth postoperative day in patients with AF (p 0.007). The duration of ischemia was significantly longer in patients with AF (p = 0.009).
Conclusions:
This study establishes the efficacy of perioperative beta-blocker treatment in mitigating postoperative AF. One month post-surgery, most patients under beta-blocker therapy maintained sinus rhythm, suggesting a potential long-term protective effect of beta-blockers against late-onset AF.
Keywords: postoperative atrial fibrillation, supraventricular arrhythmia, cardiac surgery, beta-blocker
1. Introduction
Postoperative atrial fibrillation (POAF) is the most prevalent supraventricular arrhythmia observed after cardiac surgery, affecting roughly one-third of such patients [1]. This condition elevates the risk for stroke and heart failure [2]. Moreover, it is linked with a higher rate of complications, including cardiovascular, cerebral, renal failure, and infections [3, 4]. It’s important to note that while re-intervention for bleeding has been associated with POAF, it may not be the causative agent but rather an exacerbating factor [3, 4]. POAF can be considered the final outcome of multiple predisposing factors, which seem to involve certain pathologies that contribute to the onset of arrhythmia. Furthermore, POAF pathogenesis involves many factors: advanced age, left ventricular dysfunction, dilation of the left atrium, cardiac injury induced by surgical manipulation of the heart, myocardial ischemia, and systemic inflammatory response syndrome caused by cardiopulmonary bypass are may lead to the development of postoperative fibrillation [5, 6]. In addition, patients who develop POAF have significantly higher morbidity and mortality, extended hospitalization days, and higher hospitalization costs [7, 8].
In clinical presentation, POAF often manifests as a tachyarrhythmia that disrupts hemodynamics by shortening the ventricular filling in diastole and coronary flow, thus leading to myocardial ischemia [7, 9]. This is particularly detrimental, especially in patients undergoing surgical myocardial revascularization [7]. Atrial fibrillation (AF) reduces cardiac output by approximately 30%, mainly due to the loss of atrial systole and subsequent suboptimal ventricular filling, factors that can precipitate heart failure [10]. Over time, ventricular remodeling may ensue, significantly elevating the risk of thromboembolic events [9, 10].
The majority of patients undergoing cardiovascular surgery receive chronic drug treatment with beta-blockers, and reintroducing these medications early in the postoperative period has been shown to mitigate adverse events in the sympathetic nervous system, reducing the onset of POAF [11, 12]. The 2020 ESC Guidelines [13] recommend perioperative administration of beta-blockers to patients undergoing cardiac surgery to prevent POAF, as a Class I of Recommendation, Level of Evidence A. Despite these recommendations, the clinical implementation of beta-blocker therapy remains suboptimal, marked by high rates of postoperative discontinuation [14, 15].
In this study, we analyzed the occurrence of POAF and the betablocker prophylaxis in patients undergoing both myocardial revascularization surgery and mitral and/or aortic valve replacement.
2. Materials and Methods
A cohort of 472 patients, undergoing mitral valve replacement, aortic valve replacement, surgical revascularization of the myocardium, or a combination of these procedures were prospectively followed at the Emergency Institute for Cardiovascular Diseases and Transplantation Targu Mures, Romania. The study period spanned from October 2020 to May 2023 (Fig. 1). Patients with a history of AF and those who needed a postoperative pacemaker were excluded from the study. Study approval was obtained from the Ethics Committee of the Emergency Institute for Cardiovascular Diseases and Transplantation, Targu Mures, Romania.
Fig. 1.
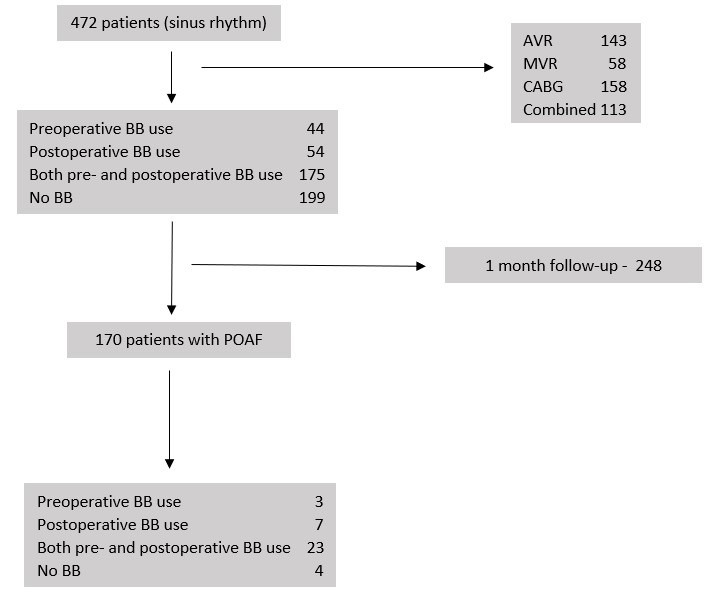
A flowchart of the patients enrolled in the study. BB, beta-blockers; AVR, aortic valve replacement; MVR, mitral valve replacement; CABG, coronary artery bypass grafting; POAF, post-operative atrial fibrillation.
The primary objective was to evaluate the efficacy of beta-blockers in preventing POAF. Beta-blockers were used as preoperative (n = 44), postoperative (n = 54) and both pre- and postoperative (n = 175) treatment. We evaluated the incidence of POAF, comparing the patients receiving beta-blockers therapy to a control group that received neither pre- or postoperative beta-blocker therapy (n = 199). Patients were monitored throughout their hospital stay and one month following surgery. Following cardiovascular surgery, oral beta-blocker therapy was initiated during the immediate postoperative period, unless contraindicated due to factors such as hypotension, bradycardia, sinus node disease, grade II/III atrioventricular blocks, or inotropic support). Heart rate was monitored daily through electrocardiography during hospitalization.
The secondary endpoints for assessing POAF risk included cardiac chamber size and ejection fraction (EF), both evaluated using transthoracic echocardiography both pre-and postoperatively. Echocardiographic evaluations were performed one day before surgery and 3–7 days postoperatively. During the examination, the patients were hemodynamically stable, and there was no need for inotropic agents. The levels of potassium, and inflammatory factors (C-reactive protein [CRP] and leukocytes) in the patients were monitored during the postoperative period. The influence of myocardial ischemia time on the occurrence of AF was also evaluated.
All statistical analyses were performed using GraphPad Prism version 8.0.2 (GraphPad Software; San Diego, CA, USA). Statistical significance was set at a p-value 0.05. Chi-square test was used to compare the frequencies of the nominal variables. Quantitative variables were compared according to the data distribution using the t-test, and the results were reported as mean standard deviation, Mann–Whitney test, and the results were reported as median and range.
3. Results
POAF occurred in 36% of patients, with a mean occurrence on postoperative day 2. Patients over 60 years of age were the most affected. There was no statistically significant difference in the gender distribution. The occurrence of AF was not influenced by the type of surgery performed (Table 1).
Table 1.
The type of surgery and the occurrence of postoperative atrial fibrillation.
| AF | No AF | Total | AF (%) | No AF (%) | |
| AVR | 49 | 94 | 143 | 34.26 | 65.73 |
| MVR | 24 | 34 | 58 | 41.37 | 58.62 |
| CABG | 51 | 107 | 158 | 32.27 | 67.72 |
| Combined | 46 | 67 | 113 | 40.7 | 59.29 |
AF, atrial fibrillation; AVR, aortic valve replacement; MVR, mitral valve replacement; CABG, coronary artery bypass grafting.
Our analysis showed that -blockers significantly reduce the incidence of POAF when compared to the control group (all p-values 0.05; Table 2). Beta-blockers were typically reintroduced on the second postoperative day. One-month postoperative evaluations were available for 248 of the 472 patients. At this follow-up, only 3.6% (9) of patients exhibited fibrillation, underscoring the protective effect of beta-blocker treatment in maintaining sinus rhythm (p = 0.0001).
Table 2.
Occurrence of postoperative atrial fibrillation in patients with or without beta-blocker.
| POAF | No POAF | p-value vs. no beta-blocker | OR | |
| Preoperative beta-blocker | 7 (15.9%) | 37 (84.09%) | 0.0006 | 4.36 |
| Postoperative beta-blocker | 15 (27.77%) | 39 (72.22%) | 0.03 | 2.14 |
| Preoperative and postoperative beta-blocker | 58 (33.14%) | 117 (66.85%) | 0.02 | 1.66 |
| No beta-blocker | 90 (45.22%) | 109 (54.77%) |
POAF, Postoperative atrial fibrillation.
We applied the Student’s t-test to independently compare the difference between the means, and we analyzed the association between the dimensions of the left atrium (LA), left ventricle (LV), right ventricle (RV), EF, and cardiac chambers pre-and postoperatively. The size of the LA pre-and postoperative was positively associated with the occurrence of AF. In contrast, pre-and postoperative LV, and RV size did not correlate with the occurrence of AF. The same results were observed for pre-and postoperative EF, without statistical significance (Table 3).
Table 3.
Correlations between atrial fibrillation and cardiac chamber size, and EF evaluated pre and postoperatively.
| Preoperative | POAF | No POAF | p-value | |
| LA (mm) | 45.15 6.96 | 43.31 6.9 | 0.006 | |
| LV (mm) | 52.45 7.49 | 51.61 6.68 | 0.21 | |
| RV (mm) | 33.55 5.89 | 32.98 5.31 | 0.28 | |
| LVEF (%) | 51.38 8 | 51.85 8 | 0.54 | |
| Postoperative | ||||
| LA (mm) | 44.5 6.58 | 42.61 6.83 | 0.007 | |
| LV (mm) | 49.92 7 | 49 6.53 | 0.17 | |
| RV (mm) | 31.87 4.84 | 31.82 4.76 | 0.9 | |
| LVEF (%) | 49 5.99 | 49.43 6.55 | 0.5 | |
All data were expressed as mean and standard deviation. LA, left atrium; LV, left ventricle; RV, right ventricle; LVEF, left ventricle ejection fraction; POAF, Postoperative atrial fibrillation; EF, ejection fraction.
Our analysis showed no significant differences in serum potassium concentrations between patients with and without POAF (all p 0.05; Table 4). The Mann–Whitney test was performed to search for any potential influence of leukocyte levels on the incidence of AF, however there was no correlation between this inflammatory marker and the occurrence of AF. Interestingly, CRP values were significantly higher on the fifth postoperative day in patients with AF (Table 5).
Table 4.
Correlations between serum potassium levels and atrial fibrillation.
| Serum potassium concentration | Postoperative day | Postoperative atrial fibrillation | ||
| Yes | No | p-value | ||
| Day 0 | 3.79 0.47 | 3.79 0.49 | 0.91 | |
| Day 1 | 4 0.34 | 4 0.37 | 0.21 | |
| Day 2 | 3.88 0.38 | 3.88 0.35 | 0.97 | |
| Day 3 | 3.81 0.35 | 3.85 0.38 | 0.24 | |
| Day 4 | 3.79 0.44 | 3.81 0.4 | 0.68 | |
| Day 5 | 3.82 0.43 | 3.91 0.45 | 0.09 | |
All data were expressed as mean and standard deviation.
Table 5.
Correlations between CRP level, leukocytes and the occurrence of postoperative atrial fibrillation.
| CRP level | Postoperative day | Postoperative atrial fibrillation | p-value | |
| Yes | No | |||
| Day 1 | 5.1 (0.64–26.95) | 5.58 (0.38–38.81) | 0.12 | |
| Day 2 | 13.58 (2.8–30.91) | 15.48 (0–31.84) | 0.25 | |
| Day 3 | 15.15 (1.56–30.98) | 15.71 (1.27–38.67) | 0.34 | |
| Day 4 | 12 (1–31.97) | 10.32 (0.36–45) | 0.31 | |
| Day 5 | 8.23 (0.89–28) | 6.55 (0.36–29.7) | 0.007 | |
| White blood cell count | Postoperative day | Postoperative atrial fibrillation | p-value | |
| Yes | No | |||
| Day 0 | 10.85 3.82 | 10.72 3.71 | 0.83 | |
| Day 1 | 11.1 3.27 | 11 3.39 | 0.91 | |
| Day 2 | 12.58 3.93 | 12.12 3.85 | 0.22 | |
| Day 3 | 11.35 3.86 | 10.77 3.91 | 0.19 | |
| Day 4 | 9.81 3.22 | 9.4 3.65 | 0.33 | |
| Day 5 | 9.42 3.29 | 9.54 3.86 | 0.79 | |
All data were expressed as median and interquartile range. CRP, C-reactive protein.
Our data revealed that the mean ischemia time was notably longer in patients with AF compared to those without, with mean durations of 85.89 35.22 minutes and 77.67 31 minutes, respectively (p = 0.009). Additionally, hospitalization durations were significantly extended in patients who developed AF, with a median stay of 10 days (ranging from 5 to 34 days), compared to a median of 8 days (ranging from 5 to 67 days) for those without AF (p = 0.0001).
4. Discussion
The underlying mechanisms of AF, including both intra- and postoperative phenomena, are not completely understood. These mechanisms, which include inflammation, sympathetic nervous system activation, and myocardial ischemia, often interact with pre-existing risk factors like advanced age and atrial dilation to induce AF. Therefore, strategies to prevent AF are only partially effective, with no significant reduction in the incidence of arrhythmias [16, 17].
Adrenergic overstimulation or activation of the sympathetic nervous system can act as a catalyst for AF [18]. The 2020 EACTS Guidelines recommend initiating beta-blocker treatment as soon as possible to mitigate the risk of AF [13]. While ceasing beta-blocker treatment in the immediate aftermath of surgery increases the risk of AF development, timely reintroduction or even new treatment initiation is associated with a lower incidence of such arrhythmic complications [19, 20, 21, 22]. Despite this, the protective benefits of perioperative beta-blocker use against AF appear inconsistent. Although the administration of beta-blocker has increased over time, it hasn’t led to a corresponding decline in the occurrence of AF [23]. A possible explanation could be a rebound phenomenon caused by discontinuation of beta-blocker treatment, which can increase the risk of AF due to the hyperadrenergic status [24, 25, 26]. To avoid this rebound effect, patients on preoperative beta-blocker therapy should receive beta-blockers on the day of surgery and should continue the treatment on the first postoperative day, unless contraindicated [27].
Some studies that evaluated drug strategies, including the administration of beta-blockers as prophylaxis for AF, have shown mixed results regarding their protective efficacy [28]. A study by Kim et al. [29] observed administering beta-blockers prior to aortic valve replacement surgery (on the occurrence of AF), did not yield protective effects. However, when beta-blocker therapy was initiated immediately after surgery, a protective effect was observed. Trials avoiding beta-blocker withdrawal still found the treatment to be effective, but less so compared to studies where the treatment was discontinued [30, 31]. Our study corroborates these findings: initiating or continuing beta-blocker treatment reduced both the occurrence of AF and the length of the hospital stay. These findings are consistent with the data from randomized and non-randomized trials from the literature, that are showing lower rates of POAF in those receiving perioperative beta-blocker therapy [32, 33].
At the 1-month follow-up visit, most patients on beta-blocker therapy were in sinus rhythm, and this could suggest a long-term protective effect in maintaining sinus rhythm.
Hypokalemia, which increases myocardial automatism and excitability, can lead to supraventricular and ventricular arrhythmias; therefore, it is considered a risk factor that contributes to AF occurrence [34, 35]. Some studies report that maintaining a serum potassium level of above 4.5 mmol/L reduces the occurrence of AF [36]. Although the involvement of hypokalemia in the pathology of POAF has been recognized, the relationship between potassium levels and AF after cardiac surgery has not been well defined.
Some studies investigating potassium replacement protocols did not detect a decreased risk of atrial tachyarrhythmias [37]. For example, while Lancaster et al. [38] found no protective benefits of postoperative potassium supplementation against AF, patients with AF were found to have higher potassium levels. Our study found no link between serum potassium levels and AF occurrence. Patients with and without AF had approximately the same levels of potassium 1–5 days postoperatively. Old age remains a strong risk factor for AF. The process of atrial remodeling, with fibrosis, results in slower atrial conduction, thus increasing the risk of AF [39, 40]. The results of this study are in accordance with those of previous studies.
Atrial dilation, which is considered to be a marker of increased filling pressure, is associated with inflammatory and degenerative changes that cause alterations in atrial electrophysiological properties [41]. Thus constituting a substrate in the development of AF, as confirmed in our study [41]. Both advanced age and left atrial dilation are related to the onset of AF and can be used to stratify the risk of AF [42, 43]. Inflammatory changes induced by the use of cardiopulmonary bypass and myocardial ischemia have also been recognized as factors involved in the pathogenesis of AF, as they are capable of triggering an inflammatory cascade that can induce fibrotic changes in the myocardium, which could stimulate arrhythmogenesis [44, 45]. Some studies suggest that a pronounced increase in leukocyte and CRP levels due to the inflammatory response caused by cardiopulmonary bypass and myocardial ischemia could be considered as predictive factors for the occurrence of AF [46, 47].
In this study, we found no significant correlations between leukocytosis and the incidence of AF, potentially due to the absence of inflammatory syndrome features among the patients. In contrast, CRP levels were significantly higher in patients with AF on the fifth postoperative day. Generally, C-reactive protein reaches a maximum value approximately 2–3 days postoperatively, corresponding to the most frequent onset of AF [48]. While our study demonstrated maximum CRP values on postoperative days 2–3 (in both groups), there was no statistically significant difference between the groups, as both showed similar values. By the fifth postoperative day, patients with AF had significantly higher CRP levels compared to patients without AF, in whom CRP values had progressively decreased. Additionally, our study identified a significant link between myocardial ischemia and AF; specifically patients with AF experienced longer durations of ischemia.
There are several limitations to be noticed in this study. First, the reintroduction of beta-blockers could be better optimized in terms of dosage, titration, drug type, timing to minimize patient variability. Second, further research involving a larger patient cohort and extended follow-up periods are needed to solidify preventative drug strategies. Lastly, additional studies are required to determine whether potassium replacement serves as a protective factor against postoperative POAF and to better understand the role of the systemic inflammatory response syndrome in the development of this condition.
5. Conclusions
Current prevention strategies are only partially effective against POAF. Our study demonstrate the efficacy of preoperative or postoperative beta-blocker treatment in the prevention of early POAF. Regarding late-onset AF, most patients at 1 month postoperative mark were maintaining sinus rhythm while on beta-blocker therapy, suggesting long-term protection. While the systemic inflammatory response syndrome is acknowledged as a contributor to the onset or maintenance of AF, the connection between them is not well defined. This study only partially elucidated the involvement of these risk factors to AF development.
In conclusion, given the complex interplay of factors contributing to the onset and maintenance of POAF, beta-blocker-based prevention strategies are only partially effective. This is further compounded by suboptimal adherence to beta-blocker therapy and high rate of postoperative discontinuation.
Acknowledgment
Not applicable.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Availability of Data and Materials
All the data can be found in the archive of Emergency Institute for Cardiovascular Diseases and Transplantation Târgu Mureș, Romania.
Author Contributions
Conceptualization, AP, HS and MMH; methodology, HuAH; software, SF; validation, CG and CB; formal analysis, SF, KB, SV; investigation, RB; resources, HS; data curation, CO, HaAH; writing—original draft preparation, AP, SF, CG, CB, HaAH; writing—review and editing, MMH, SV, KB, HS, CO, HuAH, RB; visualization, CO; supervision, KB, HS; project administration, HS. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Emergency Institute for Cardiovascular Diseases and Transplantation Targu Mures, Romania (protocol code 5023, June 26th 2021). Informed consent was obtained from all subjects involved in the study.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Melduni RM, Schaff HV, Bailey KR, Cha SS, Ammash NM, Seward JB, et al. Implications of New-onset Atrial Fibrillation after Cardiac Surgery on Long-term Prognosis: A Community-based Study. American Heart Journal . 2015;170:659–668. doi: 10.1016/j.ahj.2015.06.015. [DOI] [PubMed] [Google Scholar]
- [2].Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event. Annals of Surgery . 1997;226:501–513. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Villareal RP, Hariharan R, Liu BC, Kar B, Lee V, Elayda M, et al. Postoperative Atrial Fibrillation and Mortality after Coronary Artery Bypass Surgery. Journal of the American College of Cardiology . 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- [4].Frendl G, Sodickson AC, Chung MK, Waldo AL, Gersh BJ, Tisdale JE, et al. 2014 AATS Guidelines for the Prevention and Management of Perioperative Atrial Fibrillation and Flutter for Thoracic Surgical Procedures. The Journal of Thoracic and Cardiovascular Surgery . 2014;148:e153–e193. doi: 10.1016/j.jtcvs.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS. Inflammation, Oxidative Stress and Postoperative Atrial Fibrillation in Cardiac Surgery. Pharmacology & Therapeutics . 2015;154:13–20. doi: 10.1016/j.pharmthera.2015.06.009. [DOI] [PubMed] [Google Scholar]
- [6].Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative Atrial Fibrillation Following Cardiac Surgery: A Persistent Complication. European Journal of Cardio-Thoracic Surgery . 2017;52:665–672. doi: 10.1093/ejcts/ezx039. [DOI] [PubMed] [Google Scholar]
- [7].Bazarbashi N, Miller M. Icosapent Ethyl: Drug Profile and Evidence of Reduced Residual Cardiovascular Risk in Patients with Statin-managed LDL-C Cholesterol. Expert Review of Cardiovascular Therapy . 2020;18:175–180. doi: 10.1080/14779072.2020.1749596. [DOI] [PubMed] [Google Scholar]
- [8].Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based Study of the Long-term Risks Associated with Atrial Fibrillation: 20-year follow-up of the Renfrew/Paisley Study. The American Journal of Medicine . 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- [9].Issa Z, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. 1st edn . Elsevier; The Netherlands: 2009. pp. 210–216. [Google Scholar]
- [10].Iwasaki Y, Nishida K, Kato T, Nattel S. Atrial Fibrillation Pathophysiology. Circulation . 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- [11].Couffignal C, Amour J, Ait-Hamou N, Cholley B, Fellahi J, Duval X, et al. Timing of β-Blocker Reintroduction and the Occurrence of Postoperative Atrial Fibrillation after Cardiac Surgery: A Prospective Cohort Study. Anesthesiology . 2020;132:267–279. doi: 10.1097/ALN.0000000000003064. [DOI] [PubMed] [Google Scholar]
- [12].Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, Prevention, and Treatment of Atrial Fibrillation after Cardiac Surgery. Journal of the American College of Cardiology . 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- [13].Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. European Heart Journal . 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- [14].Mathew JP. A Multicenter Risk Index for Atrial Fibrillation after Cardiac Surgery. JAMA . 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- [15].Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative Atrial Fibrillation: From Mechanisms to Treatment. European Heart Journal . 2023;44:1020–1039. doi: 10.1093/eurheartj/ehad019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dobrev D, Aguilar M, Heijman J, Guichard J, Nattel S. Postoperative Atrial Fibrillation: Mechanisms, Manifestations and Management. Nature Reviews Cardiology . 2019;16:417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- [17].Hogue CW, Jr, Creswell LL, Gutterman DD, Fleisher LA, American College of Chest Physicians Epidemiology, Mechanisms, and Risks: American College of Chest Physicians Guidelines for the Prevention and Management of Postoperative Atrial Fibrillation after Cardiac Surgery. Chest . 2005;128:9S–16S. doi: 10.1378/chest.128.2_suppl.9s. [DOI] [PubMed] [Google Scholar]
- [18].Lopes LA, Agrawal DK. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. Journal of Surgery and Research . 2022;5:159–172. doi: 10.26502/jsr.10020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Blessberger H, Lewis SR, Pritchard MW, Fawcett LJ, Domanovits H, Schlager O, et al. Perioperative Beta-blockers for Preventing Surgery-related Mortality and Morbidity in Adults Undergoing Non-cardiac Surgery. The Cochrane Database of Systematic Reviews . 2019;9:CD013435. doi: 10.1002/14651858.CD013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coleman CI, Perkerson KA, Gillespie EL, Kluger J, Gallagher R, Horowitz S, et al. Impact of Prophylactic Postoperative Beta-blockade on Post-cardiothoracic Surgery Length of Stay and Atrial Fibrillation. The Annals of Pharmacotherapy . 2004;38:2012–2016. doi: 10.1345/aph.1E310. [DOI] [PubMed] [Google Scholar]
- [21].Ferguson TB, Jr, Coombs LP, Peterson ED, Society of Thoracic Surgeons National Adult Cardiac Surgery Database Preoperative β-Blocker Use and Mortality and Morbidity Following CABG Surgery in North America. JAMA . 2002;287:2221–2227. doi: 10.1001/jama.287.17.2221. [DOI] [PubMed] [Google Scholar]
- [22].Connolly SJ, Cybulsky I, Lamy A, Roberts RS, O’brien B, Carroll S, et al. Double-blind, Placebo-controlled, Randomized Trial of Prophylactic Metoprolol for Reduction of Hospital Length of Stay after Heart Surgery: The Beta-Blocker Length of Stay (BLOS) study. American Heart Journal . 2003;145:226–232. doi: 10.1067/mhj.2003.147. [DOI] [PubMed] [Google Scholar]
- [23].Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Schuessler RB. The Persistent Problem of New-onset Postoperative Atrial Fibrillation: A Single-institution Experience over Two Decades. The Journal of Thoracic and Cardiovascular Surgery . 2011;141:559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Expert Consensus Document on Beta-adrenergic Receptor Blockers. Revista Espanola de Cardiologia . 2005;58:65–90. doi: 10.1157/13070510. (In Spanish) [DOI] [PubMed] [Google Scholar]
- [25].London MJ. Perioperative Beta-blockade, Discontinuation, and Complications: Do you really know it when you see it. Anesthesiology . 2009;111:690–694. doi: 10.1097/ALN.0b013e3181b6a79f. [DOI] [PubMed] [Google Scholar]
- [26].Alawami M, Chatfield A, Ghashi R, Walker L. Atrial fibrillation after Cardiac Surgery: Prevention and Management: the Australasian Experience. Journal of the Saudi Heart Association . 2018;30:40–46. doi: 10.1016/j.jsha.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sousa-Uva M, Milojevic M, Head SJ, Jeppsson A. The 2017 EACTS Guidelines on Perioperative Medication in Adult Cardiac Surgery and Patient Blood Management. European Journal of Cardio-Thoracic Surgery . 2018;53:1–2. doi: 10.1093/ejcts/ezx448. [DOI] [PubMed] [Google Scholar]
- [28].Kim MH, Deeb GM, Morady F, Bruckman D, Hallock LR, Smith KA, et al. Effect of Postoperative Atrial Fibrillation on Length of Stay After Cardiac Surgery (The Postoperative Atrial Fibrillation in Cardiac Surgery Study [PACS2]) The American Journal of Cardiology . 2001;87:881–885. doi: 10.1016/s0002-9149(00)01530-7. [DOI] [PubMed] [Google Scholar]
- [29].Kim SH, Hwang HY, Choi JW, Jang M, Kim KH, Kim K. The Impact of Beta-blocker use on Postoperative Atrial Fibrillation after Aortic Valve Replacement. Journal of Thoracic Disease . 2020;12:2545–2552. doi: 10.21037/jtd.2020.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, et al. Interventions for Preventing Post-operative Atrial Fibrillation in Patients Undergoing Heart Surgery. The Cochrane Database of Systematic Reviews . 2013;2013:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burgess DC, Kilborn MJ, Keech AC. Interventions for Prevention of Post-operative Atrial Fibrillation and its Complications after Cardiac Surgery: A Meta-analysis. European Heart Journal . 2006;27:2846–2857. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- [32].Kim SH, Jang M, Hwang HY. Perioperative Beta-Blocker for Atrial Fibrillation after Cardiac Surgery: A Meta-Analysis. The Thoracic and Cardiovascular Surgeon . 2021;69:133–140. doi: 10.1055/s-0040-1708472. [DOI] [PubMed] [Google Scholar]
- [33].Khan MF, Wendel CS, Movahed MR. Prevention of Post-Coronary Artery Bypass Grafting (CABG) Atrial Fibrillation: Efficacy of Prophylactic Beta-Blockers in the Modern Era. Annals of Noninvasive Electrocardiology . 2013;18:58–68. doi: 10.1111/anec.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Howitt SH, Grant SW, Campbell NG, Malagon I, McCollum C. Are Serum Potassium and Magnesium Levels Associated with Atrial Fibrillation after Cardiac Surgery. Journal of Cardiothoracic and Vascular Anesthesia . 2020;34:1152–1159. doi: 10.1053/j.jvca.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schulman M, Narins RG. Hypokalemia and Cardiovascular Disease. The American Journal of Cardiology . 1990;65:E4–E9. doi: 10.1016/0002-9149(90)90244-u. [DOI] [PubMed] [Google Scholar]
- [36].Auer J, Weber T, Berent R, Lamm G, Eber B. Serum Potassium Level and Risk of Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. Journal of the American College of Cardiology . 2004;44:938–939. doi: 10.1016/j.jacc.2004.05.035. [DOI] [PubMed] [Google Scholar]
- [37].Couture J, Létourneau A, Dubuc A, Williamson D. Evaluation of an Electrolyte Repletion Protocol for Cardiac Surgery Intensive Care Patients. The Canadian Journal of Hospital Pharmacy . 2013;66:96–103. doi: 10.4212/cjhp.v66i2.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lancaster TS, Schill MR, Greenberg JW, Moon MR, Schuessler RB, Damiano RJ, et al. Potassium and Magnesium Supplementation do not Protect against Atrial Fibrillation after Cardiac Operation: A Time-Matched Analysis. The Annals of Thoracic Surgery . 2016;102:1181–1188. doi: 10.1016/j.athoracsur.2016.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr, et al. Age-associated Changes in Electrophysiologic Remodeling: A Potential Contributor to Initiation of Atrial Fibrillation. Cardiovascular Research . 2005;66:353–363. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- [40].Pandit SV, Jalife J. Aging and Atrial Fibrillation Research: Where We are and Where we should go. Heart Rhythm . 2007;4:186–187. doi: 10.1016/j.hrthm.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, et al. Left Atrial Volume Predicts the Risk of Atrial Fibrillation after Cardiac Surgery. Journal of the American College of Cardiology . 2006;48:779–786. doi: 10.1016/j.jacc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- [42].Tsang TSM, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. Left atrial Volume: Important Risk Marker of Incident Atrial Fibrillation in 1655 older Men and Women. Mayo Clinic Proceedings . 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- [43].Amar D, Zhang H, Y Leung D, Roistacher N, Kadish A. Older Age is the Strongest Predictor of Postoperative Atrial Fibrillation. Anesthesiology . 2002;96:352–356. doi: 10.1097/00000542-200202000-00021. [DOI] [PubMed] [Google Scholar]
- [44].Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative Atrial Fibrillation: A Maze of Mechanisms. Europace . 2012;14:159–174. doi: 10.1093/europace/eur208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nso N, Bookani KR, Metzl M, Radparvar F. Role of Inflammation in Atrial Fibrillation: A Comprehensive Review of Current Knowledge. Journal of Arrhythmia . 2021;37:1–10. doi: 10.1002/joa3.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative White Blood Cell Count Predicts Atrial Fibrillation after Cardiac Surgery. Journal of Cardiothoracic and Vascular Anesthesia . 2006;20:51–56. doi: 10.1053/j.jvca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [47].Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an Exaggerated Rise in White Blood Cells after Coronary Bypass or Cardiac Valve Surgery to Development of Atrial Fibrillation Postoperatively. The American Journal of Cardiology . 2004;93:1176–1178. doi: 10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- [48].Bruins P, Velthuis HT, Yazdanbakhsh AP, Jansen PGM, van Hardevelt FWJ, de Beaumont EMFH, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation . 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data can be found in the archive of Emergency Institute for Cardiovascular Diseases and Transplantation Târgu Mureș, Romania.


