Abstract
Acute kidney injury (AKI) is defined as sudden loss of renal function characterized by increased serum creatinine levels and reduced urinary output with a duration of 7 days. Ferroptosis, an iron-dependent regulated necrotic pathway, has been implicated in the progression of AKI, while ferrostatin-1 (Fer-1), a selective inhibitor of ferroptosis, inhibited renal damage, oxidative stress and tubular cell death in AKI mouse models. However, the clinical translation of Fer-1 is limited due to its lack of efficacy and metabolic instability. In this study we designed and synthesized four Fer-1 analogs (Cpd-A1, Cpd-B1, Cpd-B2, Cpd-B3) with superior plasma stability, and evaluated their therapeutic potential in the treatment of AKI. Compared with Fer-1, all the four analogs displayed a higher distribution in mouse renal tissue in a pharmacokinetic assay and a more effective ferroptosis inhibition in erastin-treated mouse tubular epithelial cells (mTECs) with Cpd-A1 (N-methyl-substituted-tetrazole-Fer-1 analog) being the most efficacious one. In hypoxia/reoxygenation (H/R)- or LPS-treated mTECs, treatment with Cpd-A1 (0.25 μM) effectively attenuated cell damage, reduced inflammatory responses, and inhibited ferroptosis. In ischemia/reperfusion (I/R)- or cecal ligation and puncture (CLP)-induced AKI mouse models, pre-injection of Cpd-A1 (1.25, 2.5, 5 mg·kg−1·d−1, i.p.) dose-dependently improved kidney function, mitigated renal tubular injury, and abrogated inflammation. We conclude that Cpd-A1 may serve as a promising therapeutic agent for the treatment of AKI.
Keywords: AKI, ferroptosis, ferrostatin-1, Cpd-A1, tissue distribution, lipid peroxidation
Introduction
Acute kidney injury (AKI) has emerged as a major public health problem and is now considered a systemic disease characterized by a rapid decline in renal function, a high incidence rate, and mortality [1]. The most common etiological and pathophysiological mechanisms underlying AKI include nephrotoxin exposure, ischemia/reperfusion (I/R) and sepsis [2–4]. Severe or recurrent AKI may progress to chronic kidney disease or end-stage renal disease [5, 6]. Adequate hydration, adequate blood volume circulation and prevention of nephrotoxin are effective treatments for AKI [6]. However, there are currently no effective pharmacological strategies to prevent AKI [7]. Therefore, further investigation into the pathophysiology of AKI and effective therapeutic drugs is urgently needed.
Ferroptosis is an iron-dependent form of non-apoptotic cell death initiated by the accumulation of lipid reactive oxygen species (ROS) and lipid peroxidation [8]. Emerging evidence has indicated that ferroptosis is involved in various diseases including cancers [9], neurodegenerative diseases [10], cardiovascular diseases [11], AKI [12], and autoimmune diseases [13]. Emerging evidence has demonstrated the relationship between ferroptosis and AKI in vivo and in vitro. Previous studies indicated in I/R and oxalate crystal-induced AKI models, ferroptosis leads to simultaneous tubular necrosis [14]. In addition, Friedmann [15] found that upregulation of the ferroptosis marker protein GPX4 can inhibit AKI in mouse renal tubular epithelial cells. In the mouse I/R model, Müller [16] pointed out that upregulation of the key enzyme ACSL4 of ferroptosis lipid peroxidation exacerbates I/R, while knocking out ACSL4 in mouse and human cells can prevent ferroptosis induced by erastin and RSL3, thereby reducing renal tubular cell death. Ferrostatin-1(Fer-1) has been reported as a selective inhibitor of ferroptosis [8]. Fer-1 significantly inhibited renal damage, oxidative stress, and tubular cell death in mice with I/R- and cecal ligation and puncture (CLP)-induced AKI [17, 18].
However, the clinical translation of Fer-1 is limited because of its lack of efficacy and metabolic instability [17–19]. Therefore, we optimized the structure of Fer-1 to design four novel ferroptosis inhibitors. Based on the preliminary pharmacokinetic and pharmacodynamic studies, Cpd-A1 replaced the ester group with a tetrazole, was expected to be more stable in plasma than Fer-1. In this study, we demonstrated that Cpd-A1 inhibits ferroptosis more effectively than Fer-1, and possesses the protective effects against AKI in vitro and in vivo. Together, these findings suggest that Cpd-A1 could be considered a novel agent for the treatment of AKI.
Materials and methods
Reagents and materials
The primary antibodies used were: KIM1 (Abcam, ab302932, Cambridge, UK), GPX4 (HUABIO, ET1706-45, Hangzhou, China), ACSL4 (HUABIO, ET7111-43, Hangzhou, China), TfRC (HUABIO, ER1702-06, Hangzhou, China), Fth1 (HUABIO, ET1705-55, Hangzhou, China), Ftl (HUABIO, ET1705-54, Hangzhou, China), RIPK3 (HUABIO, ER1901-27, Hangzhou, China), pRIPK3 (HUABIO, HA500330, Hangzhou, China), MLKL (HUABIO, ET1601-25, Hangzhou, China), pMLKL (HUABIO, ET1705-51, Hangzhou, China), Bax (HUABIO, ET1603-34, Hangzhou, China), Bcl-2 (WANLEIBIO, WL01556, Shenyang, China), cleaved-caspase3 (Abcam, ab32042, Cambridge, MA, USA) and β-Actin (Servicebio, GB12001, Wuhan, China). Periodic acid-Schiff (PAS), malondialdehyde (MDA) assay kit (Colorimetric method), glutathione (GSH) assay kit, lipid peroxidation assay kit (LPO), creatinine (Cr) assay kit and blood urea nitrogen (BUN) assay kit were purchased from JianCheng Bioengineering Institute (Nanjing, China). Total iron content colorimetric assay kit was purchased from Applygen Institute of Biotechnology (Beijing, China). ROS assay (DCF Assay) kit, Dihydroethidium (DHE), and Calcein AM were purchased from Beyotime Institute of Biotechnology (Nantong, China). BODIPY-C11 dye was obtained from Thermo Fisher Scientific (Waltham, MA). Fer-1 and erastin (Era) were purchased from Med Chem Express (Monmouth Junction, NJ, USA). Fer-1 derivatives were synthesized from our laboratory. The synthesis method was detailed in supplemental information files (Supplementary Methods). The structures of compounds are shown in Fig. 1a.
Fig. 1. Effect of Cpd-A1 on erastin-induced ferroptosis and H/R-induced cell injury in mTECs.
a Molecular structural formula of Fer-1, Cpd-A1, Cpd-B1, Cpd-B2, and Cpd-B3. b The blood concentrations of Fer-1, Cpd-A1, Cpd-B1, Cpd-B2, and Cpd-B3 in mice. Mice were injected with Fer-1, Cpd-A1, Cpd-B1, Cpd-B2 or Cpd-B3 and sacrificed after 0.083, 0.25, 0.5, 1, 3, 8, and 12 h. Plasma were collected for in vivo-based pharmacokinetic assays by LC-MS/MS (n = 3). c The kidney concentrations of Fer-1, Cpd-A1, Cpd-B1, Cpd-B2, and Cpd-B3 in mice. Mice were injected with Fer-1, Cpd-A1, Cpd-B1, Cpd-B2 or Cpd-B3 and sacrificed after 0.083, 0.25, 0.5, 1, 3, 8, and 12 h. Kidney tissues were collected for in vivo-based pharmacokinetic assays by LC-MS/MS (n = 3). d Fer-1, Cpd-A1, Cpd-B1, Cpd-B2, and Cpd-B3 restored cell viability in erastin-treated mTECs as assessed using MTT assay (n = 6). e Effect of different concentrations of Cpd-A1 on viability of mTECs and Cpd-A1 restored cell viability in erastin-treated mTECs as assessed using MTT assay (n = 6). f Western blot analysis of KIM1 in H/R-treated mTECs (n = 3). g IF and quantification of KIM1 in H/R-treated mTECs (n = 3). h Real-time PCR analyses of KIM1 in H/R-treated mTECs (n = 3). i Real-time PCR analyses of renal MCP-1, TNF-α, IL-1β, and IL-6 mRNA levels in H/R-treated mTECs (n = 3). Data represents the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to erastin or H/R-treated group. Scale bar = 50 μm.
Models of AKI
Male C57BL/6J mice (aged 6–8 weeks, 20–22 g) were provided by the Experimental Animal Center, Anhui Medical University. All animal experiments were conducted following the International Guiding Principles for Biomedical Research Involving Animals and approved by the Animal Experimentation Ethics Committee of the Anhui Medical University. All mice were accessed to food and water freely and housed on a 12/12 h light/dark cycle. Three AKI mouse models were adopted in this study. In protocol I, all mice were randomly divided into 7 groups of 6 mice each group. The groups are as following: sham group, sham + Cpd-A1 5 mg/kg group, I/R group, I/R + Cpd-A1 1.25 mg/kg group, I/R + Cpd-A1 2.5 mg/kg group, I/R + Cpd-A1 5 mg/kg group and I/R + Fer-1 5 mg/kg group. Cpd-A1 or Fer-1 was injected intraperitoneally 12 h before I/R surgery for AKI prevention study in protocol I. In protocol II, all mice were randomly divided into 3 groups of 6 mice each group. The groups are as following: sham group, I/R group and I/R + Cpd-A1 5 mg/kg group. Cpd-A1 was injected intraperitoneally 2 h after I/R surgery for AKI treatment study in protocol II. AKI treatment study in I/R-induced AKI mouse model involved intraperitoneally injection of Cpd-A1, 2 h after I/R surgery. For I/R-induced AKI model, microaneurysm clamps were used to clip both renal pedicles for 40 min. After 40 min, the clamps were removed for 24 h reperfusion [20]. In protocol III, all mice were randomly divided into 5 groups of 6 mice each group. The groups are as following: sham group, sham + Cpd-A1 5 mg/kg group, CLP group, CLP + Cpd-A1 5 mg/kg group and CLP + Fer-1 5 mg/kg group. Cpd-A1 or Fer-1 was injected intraperitoneally 12 h before CLP surgery for AKI prevention study in protocol III. In CLP-induced AKI model, mice were fasted for 12 h before surgery. After anesthetized, a small incision was made in the abdomen to expose the abdominal cavity. A sterile cotton swab was used to find and separate the cecum on the left side of the abdomen of the mice, and then the cecum was ligated with a sterile surgical thread 1 cm distal to the cecum. A puncture was made between the ligation site and the distal end of the cecum in the direction of the cecum ligation. We squeezed out a small amount of feces and stuffed back the cecum into the abdominal cavity. After 24 h, all the mice were sacrificed [21]. Blood samples and kidney tissues were harvested for further study.
Cell culture
Mouse tubular epithelial cells (mTECs) were provided by Prof Hui-yao Lan (Chinese University of Hong Kong) as previously reported [20]. Cells were cultured in DMEM-F12 containing 5% fetal bovine serum (FBS) in an incubator at 37 °C. For hypoxia/reoxygenation (H/R) cell model, mTECs were cultured in 0.5% low glucose medium in a hypoxic incubator (94% N2, 5% CO2, and 1% O2) for 12 h. Then, the cells were taken to normal growth conditions for 6 h for reoxygenation. For lipopolysaccharide (LPS) cell model, mTECs were pretreated with Cpd-A1 (0.25 μM) or Fer-1 (1 μM) in DMEM-F12 containing 0.5% FBS for 12 h and then exposed to LPS for 24 h.
Cell viability assay
Cell viability was detected by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Cells were seeded in 96-well plates in DMEM-F12 (5% FBS). Then the cells were pre-treated with a set of concentrations of Cpd-A1 (arranged from 0.0625 to 8 μM) for 12 h, followed by incubation with Era for 24 h. Then, under 37 °C and dark conditions, 15 μL 5 mg/mL MTT solution was added to each well to incubate for 4 h. After removing the supernatant, 150 μL DMSO was added to dissolve formazan crystals. Optical density at 492 nm was measured using a microplate reader (Multiskan MK3, Thermo, USA) [22].
Reactive oxygen species determination
Fluorescent dyes 2,7-dichlorofluorescein diacetate (DCFH-DA) and DHE were used to measure intracellular ROS level. After treatment, the cells were incubated with DCFH-DA (10 μM) or DHE (10 μM) in an incubator at 37 °C in the dark for 30 min. Cells were washed 2–3 times with serum-free medium to fully remove probes that have not entered the cell. Cells are imaged under a fluorescence microscope (Leica, Bensheim, Germany) [23].
Detection of lipid ROS levels
The lipid ROS levels were measured using previously described methods [24, 25]. BODIPY 581/591 C11 was purchased from Thermo Fisher Scientific. After treatment, the cells were incubated with BODIPY 581/591 C11 (10 μM) in an incubator at 37 °C in the dark for 60 min. Then the cells were washed three times with serum-free medium and evaluated lipid ROS levels using a fluorescence inverted microscope (Leica, Bensheim, Germany) and the CytoFLEX Flow Cytometer (Beckman Coulter, USA).
Determination of MDA and GSH
The levels of MDA and GSH in cells or in mouse tissues were measured with commercial kits according to the manufacturer’s instructions. MDA levels and GSH levels were determined at 450 and 405 nm using a microplate reader (Multiskan MK3, Thermo, USA). The protein concentration was determined with the BCA protein concentration assay kit to facilitate subsequent calculation of MDA or GSH content in tissue or cells per unit protein weight [23].
Iron measurements
The levels of iron in samples were measured with an iron assay kit according to the manufacturer’s instructions (APPLYGEN, ET1050) [26]. After treatment, samples were rapidly homogenized in an iron assay buffer on ice. Samples are mixed with iron reagent and incubated for 30 min. The supernatant of the solution was centrifuged and measured at 550 nm using a microplate reader (Multiskan MK3, Thermo, USA). Intracellular iron levels were measured using a labile iron pool assay. After treatment, mTECs were incubated with calcein-acetoxymethyl ester (5 μM) in an incubator at 37 °C in the dark for 30 min. Next, mTECs were washed 2–3 times with PBS and then the serum-free cell culture medium was added. Then the fluorescence was analyzed using with a fluorescence inverted microscope (Leica, Bensheim, Germany) [27].
Western blot
Total proteins were extracted from mTECs or ground kidney tissues with RIPA lysis buffer and protease inhibitor PMSF (100:1) on ice. Western blot was performed as described previously [28]. Signals were determined using a Licor/Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA) and protein bands were quantified using Image J software (NIH, Bethesda, MD, USA).
RNA extraction and real-time PCR
Total RNA from mTECs or tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) as previously described [29]. Complementary DNA (cDNA) was synthesized using RealMasterMix (TOYOBO, Japan). Real-time PCR was performed in a CFX96 real-time PCR detection system (Bio-Rad, USA). The primer sequences are listed in Supplementary Table 1.
Immunofluorescence
The kidney sections (4 μm) were placed in a 65 °C oven for 2 h for dewaxing, and then treated with EDTA buffer for antigen repair. After blocking with 10% BSA for 30 min, kidney sections were incubated with primary antibodies against KIM1 (1:200), TNF-α (1:200), and F4/80 (1:200) at 4 °C overnight. After the kidney sections was restored to room temperature, they were incubated with a secondary antibody for 1.5 h in the dark. After treatment, cells were washed with PBS for three times, fixed with paraformaldehyde for 10 min, blocked with 10% BSA for 30 min and then incubated with the primary antibody KIM1 (1:200) overnight at 4 °C. Cells were washed with PBS for three times and then incubated with goat anti-rabbit IgG-rhodamine (Servicebio, Wuhan, China) for 1.5 h in the dark at room temperature. The cells were counterstained using DAPI. Images were visualized and captured by an inverted fluorescence microscope (Leica, Bensheim, Germany). The IF images were quantified using IPWIN software as previously described [22].
Renal function and histology
Serum creatinine and BUN levels were measured to evaluate renal function as previously described. PAS assay kit was used to conduct PAS staining to evaluate histological damage [27]. Hematoxylin and eosin (H&E) staining was performed according to the manufacturer’s instructions [28]. Images were captured using a microscope (Olympus, Center Valley, PA, USA). PAS staining was performed in accordance with industry standards. The percentage of renal tubules that had celllysis, the loss of the brush border, and the production of casts were used to classify the degree of tissue damage (0, no damage; 1, 25%; 2, 26%–50%; 3, 51%–75%; 4, >75%).
Transmission electron microscopy
Kidney tissues were immersed in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate (pH 7.4) at 4 °C overnight, and postfixed for 2 h in buffered 1% osmium tetroxide on ice. After staining and fixation with 2% uranium acetate (pH 7.4) for 2 h, specimens were dehydrated in a graded series of ethanol (50%–70%–90%–100%) and 100% acetone, and then embedded in EPON 812 resin. Polymerization was achieved in gelatin capsules at 60 °C for 48 h and then sliced at the thickness of 70–100 nm. The samples were then detected by transmission electron microscopy (Thermo scientific Talos L120C G2).
In vivo-based pharmacokinetic model
Mice were intraperitoneally injected with 5 mg/kg Cpd-A1 and sacrificed at 0.083, 0.25, 0.5, 1, 3, 8, 12 h after injection. Whole blood and kidney were collected for the experiment. The concentration of Cpd-A1 was determined by LC-MS/MS method, and loratadine was used as the internal standard compound. Whole blood samples were centrifuged at 5000 rpm for 15 min at 4 °C to obtain plasma samples. For tissue samples, homogenate was prepared by homogenizing the tissue with a triploid volume of homogenate solution (MeOH/15 mM PBS = 1:2). 50 µL of the sample solution was precipitated with 200 µL acetonitrile, vortex-mixed, and centrifuged at 12,000 r/min for 15 min at 4 °C. Then 5 μL of the supernatant was injected directly for LC-MS/MS analysis. The separation column was the C18 column (5 cm × 2.1 mm, 3.5 μm). The mobile phases used on HPLC were 0.1% formic acid in purified water (A) and acetonitrile (B). The gradient (B) was held at 10% (0–1 min), 50% (1–3 min), increased to 95% at 4 min, then kept at isocratic 95% B for 5 min, and then immediately stepped back down to 10% for 4 min re-equilibration. The flow rate was set at 0.5 mL/min. PK parameters were calculated using DAS2.1.
Statistical analysis
Data obtained were analyzed by using a two-sample t-test or one-way analysis of variance (ANOVA) using GraphPad Prism 8 software (GraphPad Software, Inc, CA, USA). Data were expressed as the mean ± SEM for at least 6 mice in vivo or at least 3 independent in vitro experiments. P < 0.05 can be considered statistically significant.
Results
Cpd-A1 attenuated H/R-induced cell damage and inflammatory response in mTECs
The molecular structures of Fer-1, Cpd-A1, Cpd-B1, Cpd-B2, and Cpd-B3 are shown in Fig. 1a. Fer-1, a selective inhibitor of ferroptosis, inhibits lipid peroxidation and iron-dependent programmed cell death by blocking cysteine transport and glutathione production. It has been shown that Fer-1 has poor metabolic stability in vivo, leading to its poor in vivo inhibition. Structural analysis revealed that the ethyl benzoate in Fer-1 is easily hydrolyzed by proteases and other enzymes present in vivo. Therefore, we optimized the structure of Fer-1 to design four novel ferroptosis inhibitors. First, N-methyl-substituted tetrazoles were used to replace the carboxyl groups. Tetrazoles, as bioelectronic equivalents of carboxyl groups, have a pKa of 5.4–7.2, which is similar to that of carboxyl groups (4.76, formic acid), as well as a lipophilicity that is 10-fold higher than that of carboxyl groups under physiological conditions, which improves membrane permeability. Moreover, tetrazole derivatives obstruct metabolic degradation pathways. Therefore, the replacement of the ethyl formate group in Fer-1 by N-methyl-substituted tetrazoles was expected to improve the in vivo metabolic properties of Fer-1 in the present study. The results showed a significant prolongation of the T1/2 of Cpd-A1 compared to Fer-1, reaching a Cmax of 524.7 ng/mL and an AUC of 1628.5 ng/mL*h in plasma (Supplementary Table S2). Without changing the antioxidant moiety, 2,4-diaminophenyl, we aimed to enhance the enrichment of Cpd-A1 in tissues by further increasing its esterophilicity. The cyclohexane of Cpd-A1 was replaced with cycloheptane, pyrimidine, and 4-tert-butyl-phenyl to obtain Cpd-B1, Cpd-B2, and Cpd-B3 (Fig. 1a). Unfortunately, these compounds had no elevated plasma concentration, and there was a significant decrease in kidney enrichment (Fig. 1b, c). According to the pharmacokinetic data, we determined that among these five compounds, Cpd-A1 had the highest blood concentration and affinity for the kidneys.
Preliminary erastin-induced ferroptosis inhibition experiments on the modified compounds revealed that all compounds showed favorable efficacy in inhibiting ferroptosis in mTECs except Cpd-B2 (Fig. 1d). Based on the preliminary pharmacokinetic and pharmacodynamic studies, we showed that Cpd-A1 was the most promising molecule for AKI treatment. Therefore, compound Cpd-A1 was selected for further experiments. The MTT assay was used to evaluate the effect of Cpd-A1 on cell viability. We determined that Cpd-A1 has little effect on the growth of mTECs at concentrations lower than 4 μM (Fig. 1e). We treated mTECs with erastin for 24 h to induce ferroptosis. MTT results showed that erastin prevented cell growth, but treatment with Cpd-A1 at concentrations of 0.0625 μM restored cell viability in a concentration-dependent manner in mTECs (Fig. 1e). Cpd-A1 significantly altered the expression of the ferroptosis-related proteins ACSL4 and GPX4 compared to that in the erastin group without Cpd-A1(Supplementary Fig. S1b). We also analyzed the expression of KIM1, a marker of tubular damage. The results showed that erastin upregulated KIM1 levels, whereas Cpd-A1 suppressed KIM1 expression in mTECs (Supplementary Fig. S1c).
According to previous reports, ferroptosis in the renal tubular cells contributes significantly to AKI progression. Therefore, we investigated the potential role of Cpd-A1 in AKI. We analyzed the protein expression and mRNA levels of KIM1 in H/R-induced cell damage. Western blot and real-time PCR results showed that H/R up-regulated KIM1 level; however, Cpd-A1 (0.0625, 0.125, and 0.25 μM) suppressed KIM1 expression in mTECs. Pretreatment with Cpd-A1 (0.25 μM) had better suppressive effects on KIM1 expression than Fer-1 (1 μM) (Fig. 1f, h). The suppressive effect of Cpd-A1 on KIM1 expression was further confirmed using immunofluorescence (Fig. 1g).
Ferroptosis is a proinflammatory process that recruits macrophages, which cause inflammation in AKI. A dramatic increase in inflammatory cytokine levels is a major clinical feature of AKI. We examined the levels of pro-inflammatory cytokines in mTECs to investigate whether Cpd-A1 affected the inflammatory response. As shown in Fig. 1i, H/R treatment greatly increased the mRNA levels of the MCP-1, IL-1β, IL-6, and TNF-α. However, Cpd-A1 treatment mitigated this upregulation (Fig. 1i).
Collectively, these results suggest that Cpd-A1 exhibits a better therapeutic effect than Fer-1 in H/R-treated mTECs.
Cpd-A1 suppressed ferroptosis in mTECs with H/R-induced AKI
Given that lipid peroxidation caused by excessive intracellular ROS is a key event in ferroptosis, we investigated the effect of H/R on ROS production. Intracellular ROS in mTECs was evaluated by ROS formation (DHE staining and DCF staining), and lipid ROS generation was measured using BODIPY 581/591 C11. H/R treatment upregulates intracellular and lipid ROS levels. Compared with the anti-ferroptotic effect of Fer-1, Cpd-A1 exerted better inhibition of the overproduction of ROS and lipid ROS (Fig. 2a–c). We investigated the effect of Cpd-A1 on the expression of key ferroptosis-related proteins (GPX4 and ACSL4). ACSL4 is an essential enzyme that synthesizes phospholipids containing polyunsaturated fatty acids, which are the main substrates for lipid peroxidation. GPX4 is an essential phospholipid peroxidase that is responsible for the reduction of lipid peroxides. The data showed that the expression of the pro-ferroptotic protein ACSL4 increased, whereas that of the anti-ferroptotic protein GPX4 decreased with H/R treatment. However, these changes were suppressed upon treatment with Cpd-A1 or Fer-1 (Fig. 2f). These findings supported the hypothesis that Cpd-A1 protects mTECs from H/R-induced ferroptosis.
Fig. 2. Cpd-A1 suppressed ferroptosis in mTECs with H/R-induced AKI.
a DCF Assay and DHE staining of intracellular ROS levels. b Lipid ROS level was assayed using C11-BODIPY. c Representative images of the fluorescent probe C11-BODIPY 581/591. d Intracellular iron levels in mTECs. e Calcein staining and its quantification of intracellular iron level. f Western blot of ferroptosis-related proteins ACSL4 and GPX4 expression. g Mitochondrial membrane potential was measured using JC-1. h Western blot of the expression of TfRC, Fth1, and Ftl in mTECs. i Real-time PCR analyses of renal TfRC, Fth1, and Ftl mRNA levels. j The level of MDA. k The level of GSH. Data represent the mean ± SEM for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to H/R-treated group. Scale bar = 100 μm.
Iron is required for the accumulation of lipid peroxides and ferroptosis. Iron is of great significance in the process of AKI and initiation of ferroptosis. We used an iron kit to measure the intracellular iron levels in mTECs and elucidated that H/R significantly increased the iron concentration in mTECs (Fig. 2d). Treatment with Cpd-A1 significantly reversed these effects. In addition, we used calcein-AM to further investigate the change in cellular labile iron, and determined the results to be consistent with previous results (Fig. 2e). Iron homeostasis is strictly regulated by iron metabolism-related proteins. The expression of TfRC, a membrane protein important for iron uptake, was upregulated by H/R treatment, and Cpd-A1 supressed its expression. The expression levels of the iron-binding proteins Fth1 and Ftl were upregulated in H/R-treated mTECs. Treatment with Cpd-A1 substantially reversed the increase in Fth1 and Ftl protein levels (Fig. 2h). Moreover, Cpd-A1 significantly suppressed the alterations in the mRNA levels of Fth1 and Ftl. However, the mRNA level of TfRC has not changed significantly (Fig. 2i). Thus, iron homeostasis was disrupted and iron accumulation was induced in mTECs following H/R treatment, and Cpd-A1 treatment significantly restored iron metabolism.
Lipid peroxidation is a direct result of the execution phase of ferroptosis and can be assessed by measuring the levels of MDA and GSH. Cpd-A1 significantly increased the GSH levels and decreased the MDA levels (Fig. 2j, k). Collectively, these results indicate that Cpd-A1 alleviates H/R-induced AKI by targeting ferroptosis.
In addition, ferroptosis is associated with mitochondrial function, with a significant reduction in MMP levels. The control group showed red JC-1 fluorescence, whereas green fluorescence was observed after H/R treatment. Cpd-A1 and Fer-1 pretreatment normalized MMP in H/R-treated cells, which exhibited red fluorescent signals (Fig. 2g).
In addition, Cpd-A1 has little effect on apoptosis or necroptosis related proteins, which indicates Cpd-A1 was not involved in apoptosis or necroptosis pathways. Cpd-A1 mainly exerts renal protective effects by inhibiting ferroptosis (Supplementary Fig. S2a, b).
Collectively, these results revealed that Cpd-A1 suppresses ROS generation, downregulates ferroptosis-related protein expression, and mitigates iron accumulation, lipid peroxidation, and mitochondrial dysfunction in mTECs with H/R-induced AKI.
Cpd-A1 attenuated LPS-induced cell damage, inflammatory response, and ferroptosis in mTECs
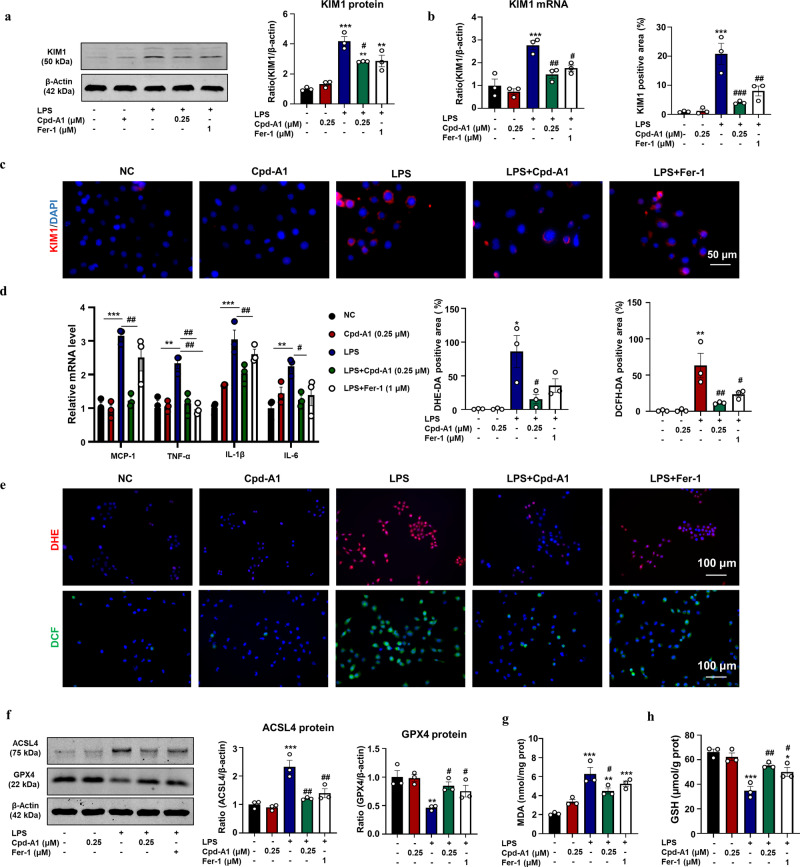
AKI is a common complication of sepsis, and its onset involves ferroptosis. A cell model was established by treating mTECs with LPS to induce ferroptosis. We confirmed the therapeutic role of Cpd-A1 in the LPS-induced cell damage model in vitro. Cpd-A1 significantly downregulated KIM1 levels, as determined by Western blot, real-time PCR, and immunofluorescence (Fig. 3a–c). In addition, Cpd-A1 greatly decreased the mRNA levels of pro-inflammatory cytokines MCP-1, IL-1β, IL-6, and TNF-α (Fig. 3d). Thus, Cpd-A1 exhibited a potent effect in attenuating LPS-induced cell damage and showed a better protective effect than Fer-1.
Fig. 3. Cpd-A1 attenuated LPS-induced cell damage, inflammatory response and ferroptosis in mTECs.
a Western blot analysis of KIM1 in mTECs. b Real-time PCR analyses of renal KIM1 level. c IF and quantification of KIM1 in mTECs. d Real-time PCR analyses of renal MCP-1, TNF-α, IL-1β, and IL-6 mRNA levels. e DHE staining and DCF Assay of intracellular ROS levels. f Western blot of ferroptosis-related proteins GPX4 and ACSL4 expression. g The level of MDA. h The level of GSH. Data represent the mean ± SEM for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to LPS -treated group. Scale bar = 100μm.
Compared to the anti-ferroptotic effect of Fer-1, Cpd-A1 exerted better inhibition of ROS overproduction (Fig. 3e). We investigated the effect of Cpd-A1 on the expression of key ferroptosis-related proteins. The data showed that the expression of the proferroptotic protein ACSL4 increased, whereas that of the anti-ferroptotic protein GPX4 was reduced by LPS treatment. However, these changes were suppressed upon treatment with Cpd-A1 or Fer-1 (Fig. 3f). In addition, Cpd-A1 reduced the levels of the lipid peroxidation marker MDA and increased the levels of GSH (Fig. 3g, h). These findings suggested that Cpd-A1 attenuated LPS-induced ferroptosis in mTECs.
Cpd-A1 attenuated I/R-induced AKI, inflammation, and ferroptosis in mice
We evaluated the therapeutic effects of Cpd-A1 on I/R-induced AKI. Male mice were pretreated with Cpd-A1 (1.25, 2.5, or 5 mg/kg) or 5 mg/kg Fer-1 (positive control), 12 h before I/R (Fig. 4a). Serum creatinine and BUN levels are commonly used parameters for clinical detection of AKI. Serum creatinine and BUN levels indicated the renoprotective effect of Cpd-A1 (Fig. 4b, c). Periodic acid-Schiff (PAS) staining showed severe pathological damage after I/R, which was characterized by the dilation of renal tubules, loss of brush border, and cytoplasmic vacuoles. Interestingly, kidney damage was mitigated by I/R injury after pretreatment with Cpd-A1 and Fer-1. Notably, Cpd-A1 exhibited better protective effects than Fer-1 at the same concentration (5 mg/kg) (Fig. 4d). Additionally, Western blot and real-time PCR analysis showed that Cpd-A1 treatment reduced KIM1 expression in I/R AKI mice, which was consistent with the results of the immunofluorescence studies (Fig. 4e–g).
Fig. 4. Cpd-A1 attenuated I/R-induced AKI, inflammation and ferroptosis in mice.
a Scheme of the experiment. b BUN assay. c Serum Cr assay. d Representative images of PAS-stained kidney sections. e Western blot analysis of KIM1. f Real-time PCR analyses of renal KIM1 mRNA level. g IF and quantification of KIM1. h IF and quantification of TNF-α. i IF and quantification of F4/80. j Real-time PCR analyses of MCP-1, TNF-α, IL-1β and IL-6 mRNA levels. k The level of MDA. l The level of LPO. m Western blot analysis and quantification of GPX4 and ACSL4. n The level of GSH. o Iron levels in the kidneys were measured. p Western blot of the expression of TfRC, Fth1, and Ftl in mTECs. Data represent the mean ± SEM for six independent experiments. **P < 0.01, ***P < 0.001 as compared with the sham. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to I/R-treated group. Scale bar = 100 μm.
We also evaluated the anti-inflammatory effects of Cpd-A1 on I/R-induced AKI in mice. The mRNA levels of MCP-1 TNF-α, IL-1β, and IL-6 were down-regulated with Cpd-A1 pretreatment (Fig. 4j). Consistently, the immunofluorescence studies demonstrated that Cpd-A1 can reduce the levels of TNF-α and F4/80 in I/R-induced AKI (Fig. 4h, i).
Lipid peroxidation is the key driver of ferroptosis. Therefore, we investigated the effects of Cpd-A1 on ferroptosis during I/R-induced kidney damage. Detection of the lipid peroxidation markers MDA and lipid peroxide LPOs revealed that I/R increased the levels of MDA and LPOs in the kidneys. Cpd-A1 substantially mitigated the alterations in MDA and LPO levels, indicating that Cpd-A1 suppressed the lipid peroxidation process occurring during I/R-induced AKI. (Fig. 4k, l) Additionally, renal GSH levels decreased and Cpd-A1 treatment effectively inhibited the reduction in GSH (Fig. 4m). To clarify the role of ferroptosis in AKI, we examined the expression of ferroptosis-related proteins. I/R treatment obviously reduced the expression of the GPX4 and increased ACSL expression. These changes were resistant to Cpd-A1 treatment (Fig. 4n). In addition, Cpd-A1 exerted a stronger beneficial effect on I/R-induced AKI than Fer-1 in vivo. Besides, we investigated the change in iron levels and found that the I/R increased the renal iron concentration. However, Cpd-A1 prominently reversed this effect (Fig. 4o). The expression of iron metabolism related proteins TfRC, Fth1 and Ftl was upregulated by I/R treatment, and Cpd-A1 substantially reduced the increase in the levels of the TfRC, Fth1 and Ftl proteins (Fig. 4p). Thus, Cpd-A1 treatment could significantly restore iron metabolism.
Cpd-A1 attenuated kidney injury and ferroptosis in an established AKI mouse model
Next, we determined whether Cpd-A1 exerts therapeutic effects in an established AKI mouse model. Male mice were treated with Cpd-A1 at a concentration of 5 mg/kg 2 h after I/R (Fig. 5a). Serum creatinine and BUN levels were lower in the Cpd-A1 treated mice as compared with the untreated mice (Fig. 5b, c). PAS staining showed that Cpd-A1 markedly mitigated tubule damage compared to that in the I/R-induced AKI group untreated with Cpd-A1(Fig. 5d). Cpd-A1 markedly downregulated KIM1 levels as determined by Western blotting, real-time PCR, and immunofluorescence (Fig. 5h, j, f). Cpd-A1 also led to the down-regulation of pro-inflammatory cytokines MCP-1 and TNF-α in the mice with established AKI (Fig. 5e, g). Cpd-A1 inhibited ferroptosis in an established AKI mouse model. Cpd-A1 reduced the ACSL4 levels and increased the GPX4 levels after I/R (Fig. 5i). In addition, Cpd-A1 reduced the levels of LPO and MDA while increasing the level of GSH (Fig. 5k–m).
Fig. 5. Cpd-A1 suppressed kidney injury and ferroptosis in an established AKI mouse model.
a Scheme of the experiment. b BUN assay. c Serum Cr assay. d Representative image of PAS-stained kidney sections. e Real-time PCR analyses of MCP-1 mRNA level. f IF and quantification of KIM1. g IF and quantification of TNF-α. h Western blot analysis of KIM1. i Western blot analysis and quantification of ACSL4 and GPX4. j Real-time PCR analyses of renal KIM1 mRNA level. k The level of MDA. l The level of LPO. m The level of GSH. Data represents the mean ± SEM. for six independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the sham. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to I/R-treated group. Scale bar = 100 μm.
Cpd-A1 attenuated CLP-induced AKI, inflammation, and ferroptosis in mice
To further confirm the effect of Cpd-A1 on SA-AKI, male mice were pretreated with 5 mg/kg Cpd-A1 and 5 mg/kg Fer-1 as positive controls 12 h before CLP (Fig. 6a). Consequently, PAS staining and quantitative analysis of the kidney tissue sections of mice with CLP-induced AKI showed that Cpd-A1 treatment abrogated CLP-induced renal injury while decreasing serum creatinine and BUN levels in the blood samples of the Cpd-A1 treated mice as compared to the untreated mice (Fig. 6b–d). Administration of Cpd-A1 significantly decreased the expression as KIM1 by Western blotting, real-time PCR, and immunofluorescence (Fig. 6e–j). In addition, the immunofluorescence studies demonstrated that Cpd-A1 can reduce the levels of TNF-α and F4/80 in CLP-induced AKI (Fig. 6h, i). Additionally, we determined that Cpd-A1 greatly decreased the mRNA levels of pro-inflammatory cytokines MCP-1, IL-1β, IL-6, and TNF-α (Fig. 6j).
Fig. 6. Cpd-A1 attenuated CLP-induced AKI, inflammation and ferroptosis in mice.
a Scheme of the experiment. b BUN assay. c Serum Cr assay. d Representative image of PAS-stained kidney sections. e Western blot analysis of KIM1. f Real-time PCR analyses of renal KIM1 mRNA level. g IF of KIM1. h IF of TNF-α. i IF of F4/80. j Real-time PCR analyses of MCP-1, TNF-α, IL-1β, and IL-6 mRNA levels. k Representative pictures acquired by transmission electron microscopy (Scale bar = 2 μm or 500 nm). l Western blot analysis and quantification of GPX4 and ACSL4. m The level of MDA. n The level of LPO. o The level of GSH. Data represent the mean ± S.E.M. for six mice. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the sham. #P < 0.05, ##P < 0.01, ###P < 0.001 as compared to CLP -treated group.
Using TEM to study the ultrastructure of tubular epithelial cells, we determined that CLP drastically damaged the mitochondria, which showed reduced volume, incomplete membranes, loss of mitochondrial cristae, and decreased mitochondrial content in renal tubular epithelial cells. Cpd-A1 treatment significantly decreased the proportion of damaged mitochondria (Fig. 6k). Cpd-A1 substantially downregulated the alteration of MDA and LPO levels while increasing the level of GSH in response to CLP-induced renal injury in vivo (Fig. 6m–o). Next, we investigated the effects of Cpd-A1 on the expression of ferroptosis-related target molecules. The expression of GPX4 was down-regulated following CLP in mice, whereas the expression of ACSL4 was up-regulated. However, treatment with Cpd-A1 significantly inhibited these changes (Fig. 6l). Collectively, these findings supported the hypothesis that Cpd-A1 protects against renal damage, inflammation, and ferroptosis during CLP-induced AKI.
Cpd-A1 displayed safety in other organs at therapeutic dose
Mice were given either saline (vehicle control) or Cpd-A1 (5 mg/kg) to test the toxin effects on other organs. Between the Cpd-A1- and saline-treated groups, there were no discernible variations in ALT and AST levels (Fig. 7a, b). H&E staining was used to investigate various other organs, including the heart, liver, spleen, and lungs. The outcomes demonstrated that there were no anomalies in the organs of the Cpd-A1-treated mice compared to the vehicle group, demonstrating the safety of Cpd-A1 at the therapeutic dose (5 mg/kg) (Fig. 7c).
Fig. 7. The safety of Cpd-A1 at therapeutic dose.
a ALT level. b AST level. c H&E staining. Data represent the mean ± SEM for six independent experiments. Scale bar = 100 μm. ns not significant.
Discussion
In this study, we designed and synthesized a novel ferroptosis inhibitor, Cpd-A1. Moreover, Cpd-A1 exhibited a more effective protective effect than Fer-1. The present study demonstrated that Cpd-A1 has a protective effect against renal injury, as evidenced by the reduction in tubular cell damage and amelioration of renal inflammation. At the molecular level, Cpd-A1, similar to the positive control, Fer-1, exerted a reno-protective effect by inhibiting ferroptosis, as evidenced by limiting iron accumulation, restoring renal iron homeostasis, and inhibiting lipid peroxidation and oxidative stress. These results provide sufficient evidence to prove that Cpd-A1 is an effective therapeutic agent for AKI which is better than the positive control agent, Fer-1. In our study, 0.0625–0.25 μM Cpd-A1 was used for in vitro assays and 1.25–5 mg/kg was used for in vivo assays. Treatment with Cpd-A1 alone exerted no adverse effects on other organs compared to saline-treated mice, and did not reduce the viability of mTECs at the maximal concentration used in this study.
AKI is caused by numerous factors such as renal I/R, septicemia, or renal toxin [2–4]. There are several well-studied pathways of regulated necrosis, including apoptosis [30], necroptosis [2], and pyroptosis [31], which has been well studied. However, AKI remains no specific tool has been developed for treatment so far. Ferroptosis, a new type of regulated cell death, has attracted attention since Dixon first proposed its concept in 2012 [8]. Emerging evidence supports the concept that ferroptosis, among all the types of cell death, plays a critical role in the pathophysiology of AKI. Ferroptosis can directly cause necrosis of renal tubules in a model of I/R-induced AKI [18] and has been proven to be of great significance in nephrotoxic folic acid (FA)- and LPS-induced AKI [32]. Based on our results, ferroptosis is a promising target for AKI treatment. The ferroptosis inhibitor Fer-1 prevented AKI [8]. However, the clinical application of Fer-1 is limited because of its potential metabolic and plasma instability [18], and a more stable agent is urgently needed. Therefore, we modified the structure of Fer-1 and synthesized four new compounds. After preliminary pharmacokinetic and pharmacodynamic studies, we screened Cpd-A1 as a potential molecule for the treatment of AKI that may be superior to the positive control agent, Fer-1. Compound Cpd-A1 was synthesized by replacing the ester group with a tetrazole in the basic structure of Fer-1. As expected, Cpd-A1 significantly inhibited erastin-induced ferroptosis and increased the cell viability. Cpd-A1 also attenuated kidney injury in two cell models of AKI and two AKI mouse models. According to our experimental results, compared with Fer-1, Cpd-A1 has a better therapeutic effect on I/R- and CLP-induced AKI, which indicates Cpd-A1 is more stable and has a better bioavailability than Fer-1. In addition, Cpd-A1 not only prevents AKI but also has a therapeutic effect on AKI. We verified that Cpd-A1 had little effect on apoptosis or necroptosis related proteins, which indicates Cpd-A1 was not involved in apoptosis or necroptosis pathways. We proved that Cpd-A1 significantly ameliorated AKI by inhibiting ferroptosis. Ferroptosis is characterized by iron accumulation [33], lipid peroxidation [34–36], and ROS generation [37]. In addition, mitochondria can amplify ferroptosis; mitochondrial function is an indicator of ferroptosis [38]. Our results indicated that Cpd-A1 suppresses ROS generation, regulates key ferroptosis-related proteins, and mitigates iron accumulation, lipid peroxidation, and mitochondrial dysfunction in AKI.
Iron is filtered by glomeruli and reabsorbed in the renal tubules [39, 40]. Fe2+ is partially transferred by ferritin and is stored in the LIP. The excessive accumulation of free iron (Fe2+) is essential for lipid peroxidation. Typically, iron can be loaded onto transferrin (TF), which enters the cell through endocytosis by binding to TfRC on the cell membrane. Excess iron is stored as ferritin, which is encoded by Fth1(heavy chain) and Ftl (light chain). Here, Cpd-A1 reduced the H/R-induced iron accumulation in mTECs. Cpd-A1 directly binds to free iron and regulates the expression of key iron metabolism-related proteins including TfRC, Fth1, and Ftl, thereby restoring intracellular iron metabolism.
It is well known that inflammation is an important feature of AKI [40]. Dead cells release inflammatory factors that exacerbate tissue damage. Numerous studies have shown that ferroptosis is immunogenic and contributes to inflammation. However, the mechanism by which ferroptosis causes inflammation remains unclear. Our findings validated that Cpd-A1 reduced inflammation in AKI and identified important cellular and molecular mediators that may be effective targets for AKI.
Using in vivo pharmacokinetic data, we determined that Cpd-A1 showed a significant prolongation of the T1/2 of Cpd-A1 compared to Fer-1, reached a Cmax of 524.7 ng/mL and an AUC of 1628.5 ng/mL*h in plasma. Further structural modifications did not increase the plasma concentration, and there was a significant decrease in kidney enrichment. Among the five compounds, Cpd-A1 showed the highest blood concentration and affinity for the kidney, which could account for its superior therapeutic effects in in vivo AKI models. These results suggest that Cpd-A1 is a valuable ferroptosis inhibitor for kidney diseases with superior plasma stability and high affinity for the kidney.
In conclusion, the present study demonstrated that Cpd-A1, a novel ferroptosis inhibitor, displayed significant anti-ferroptosis and anti-inflammatory effects in different AKI models. Indeed, our study indicates that Cpd-A1 is a clinical candidate targeting ferroptosis in AKI therapy.
Supplementary information
Acknowledgements
We acknowledge the Center for Scientific Research of Anhui Medical University and the Inflammation and Immune Mediated Diseases Laboratory of Anhui Province for their valuable help. This work was supported by the National Natural Science Foundation of China (No. 81970584), the promotion plan of basic and clinical cooperative research in Anhui Medical University (No. 2019xkjT014), the Project of Collaborative Innovation for Colleges of Anhui Province (No. GXXT-2021-070), the Major Projects of Science and Technology in Anhui Province (202103a07020013), the Natural Science Foundation of Anhui (2208085QH240), and the Graduate Research and Practice Innovation Project of Anhui Medical University (YJS20230059).
Author contributions
YC designed the study, performed the cell experiments, analyzed the data and wrote the manuscript. MFW performed the cell experiments, analyzed the data and wrote the manuscript. MMX and YL. performed the cell experiments analyzed the data. CL, SSX, and WXM performed part of the animal experiments and histological analysis. MLJ, RH, ZHD, RBH, MMZ, HL, and LG performed part of the cellular experiments and histological analysis. JGW, JJ, and XWD provided a series of experimental instructions and help. JXC and XMM provided a series of experimental instructions and wrote the manuscript. All authors have revised and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ying Chen, Ming-fei Wu, Man-man Xie, Yang Lu.
Contributor Information
Jin-xin Che, Email: chejx@zju.edu.cn.
Xiao-ming Meng, Email: mengxiaoming@ahmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-024-01277-w.
References
- 1.Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Prim. 2021;7:51. 10.1038/s41572-021-00291-0 [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015;26:2647–58. 10.1681/ASN.2014080741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. 10.1038/nrneph.2017.119 [DOI] [PubMed] [Google Scholar]
- 4.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–99. 10.1016/j.kint.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–72. 10.1016/j.kint.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 6.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. 2016;67:872–80. 10.1053/j.ajkd.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushal GP, Shah SV. Challenges and advances in the treatment of AKI. J Am Soc Nephrol. 2014;25:877–83. 10.1681/ASN.2013070780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu ZH, Tang Y, Yu H, Li HD. The role of ferroptosis in breast cancer patients: a comprehensive analysis. Cell Death Discov. 2021;7:93. 10.1038/s41420-021-00473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62. 10.1038/s41418-020-00685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052–9. 10.7150/thno.54113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12:65. 10.1038/s41419-020-03362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol 2021;22:1107–17. 10.1038/s41590-021-00993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni L, Yuan C, Wu X. Targeting ferroptosis in acute kidney injury. Cell Death Dis. 2022;13:182. 10.1038/s41419-022-04628-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74:3631–45. 10.1007/s00018-017-2547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiongyue Z, Xin Y, Meng P, Sulin M, Yanlin W, Xinyi L, et al. Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front Pharmacol. 2022;13:857067. 10.3389/fphar.2022.857067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Nat Acad Sci USA. 2014;111:16836–41. 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–6. 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JN, Wang F, Ke J, Li Z, Xu CH, Yang Q, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14:eabk2709. 10.1126/scitranslmed.abk2709 [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–53. 10.1097/00024382-199704000-00002 [DOI] [PubMed] [Google Scholar]
- 22.Xie SS, Dong ZH, He Y, Chen ZW, Yang Q, Ma WX, et al. Cpd-0225 attenuates renal fibrosis via inhibiting ALK5. Biochem Pharmacol. 2022;204:115240. 10.1016/j.bcp.2022.115240 [DOI] [PubMed] [Google Scholar]
- 23.Wang JN, Liu MM, Wang F, Wei B, Yang Q, Cai YT, et al. RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin Sci. 2019;133:1609–27. 10.1042/CS20190599 [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Chen XB, Hong YC, Zhu H, He QJ, Yang B, et al. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol Sin. 2019;40:1334–442. 10.1038/s41401-019-0233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- 26.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1phosphorylation promotes ferroptosis by directly blocking system Xc(-) activity. Curr Biol. 2018;28:2388–99. 10.1016/j.cub.2018.05.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–60. 10.1136/gut.2006.094060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, Zang HM, Xing T, Zhang SF, Li C, Zhang Y, et al. Gypenoside XLIX protects against acute kidney injury by suppressing IGFBP7/IGF1R-mediated programmed cell death and inflammation. Phytomedicine. 2021;85:153541. 10.1016/j.phymed.2021.153541 [DOI] [PubMed] [Google Scholar]
- 29.Meng XM, Li HD, Wu WF, Ming-Kuen Tang P, Ren GL, Gao L, et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab Invest. 2018;98:79–94. 10.1038/labinvest.2017.115 [DOI] [PubMed] [Google Scholar]
- 30.Schumer M, Colombel MC, Sawczuk IS, Gobé G, Connor J, O’Toole KM, et al. Buttyan R: Morphologic, bio chemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;40:831–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Xia W, Li Y, Wu M, Jin Q, Wang Q, Li S, et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12:139. 10.1038/s41419-021-03431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2020;28:231–43. 10.1016/j.jare.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–76. 10.1016/j.tcb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017;13:81–90. 10.1038/nchembio.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. 10.1038/s41419-019-2064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–21. 10.1016/j.cell.2022.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front Pharmacol 2014;5:124. 10.3389/fphar.2014.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol 2007;69:69–85. 10.1146/annurev.physiol.69.031905.164337 [DOI] [PubMed] [Google Scholar]
- 40.Mapuskar KA, Wen H, Holanda DG, Rastogi P, Steinbach E, Han R, et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019;20:98–106. 10.1016/j.redox.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.