Abstract
CD8+ T lymphocytes and class I major histocompatibility complex (MHC-I) molecules profoundly influence the severity of neuronal herpes simplex virus (HSV) infection in experimentally infected mice. Paradoxically, neurons are classically regarded as MHC-I deficient. However, it is shown here that H2-encoded heavy chains (αCs) and their associated light chain, β2 microglobulin, are present on the surfaces of primary sensory neurons recovered from sensory ganglia within 1 to 2 weeks of HSV infection. During this time, some neurons are found to be tightly associated with T cells in vivo. Prior data showed that termination of productive HSV infection in the peripheral nervous system is not dependent on cell-mediated lysis of infected neurons. Consistent with these data, immunogold electron microscopy showed that the density of cell surface H2 on neurons is an order of magnitude lower than on satellite glia, which is predicted to favor a noncytolytic CD8 cell response.
Herpes simplex virus (HSV) infection has high community impact as a result of the high prevalence of genital herpes and its ability to cause life-threatening infections in immunocompromised hosts and sporadic cases of rapidly fatal encephalitis (35). Consequently, the pathobiology of HSV infection is an object of intensive study. During initial infection, the virus spreads by retrograde axonal transport from the skin to primary sensory neurons, creating the potential for lethal spread of the virus to the brain (36). Fortunately, neuronal infection is usually controlled rapidly by timely development of an adaptive immune response (28). However, after recovery from productive infection, clearance of virus from the host is not complete. Rather, viral genomes persist in a nonreplicating state in neuronal nuclei, creating a reservoir of infection that periodically gives rise to reactivations of disease (33).
Considerable progress has been made towards identifying key components of the host response that terminate the potentially lethal productive neuronal infection associated with primary herpes simplex. In experimentally infected mice, genes linked to class I major histocompatibility complex (MHC-I) loci profoundly influence the severity of acute infection in sensory nerve ganglia (26). Further, we showed previously that transcription of MHC-I genes is rapidly upregulated in virtually all resident cells of an HSV-infected ganglion, including neurons (20). These data strongly suggest that CD8+ T lymphocytes, which recognize antigenic peptides in the context of MHC-I molecules (12), play an important protective role. In direct support of this proposal, it has been shown that mice treated with anti-CD8 fail to clear the virus from the nervous system (27).
Paradoxically, detection of H2 complexes on the surfaces of neurons in HSV-infected ganglia was found previously to be problematic (20). This finding reflects the conventional view that neuronal MHC-I expression is blocked in order to protect this vital cell type against attack by cytotoxic CD8+ T cells (6). However, CD8+ cells can mediate their effector functions via cytokine release rather than cytolysis (29). Further, cytokine-mediated, noncytolytic responses may be associated with low-density antigen recognition (1). Significantly, termination of productive ganglionic HSV infection is not dependent on destruction of infected neurons (27), leading to the hypothesis that prior difficulties in demonstrating neuronal MHC-I expression might be a result of an unusually low density, rather than an absence, of H2 molecules at the cell surface.
Several features of the experimental system used to address this hypothesis require introduction. First, mice were infected by inoculation of flank skin (25), which results in acute ganglionic infection by centripetal spread of virus along sensory nerve axons, resembling the spread of virus to human ganglia. This process causes minimal disruption to the physical integrity of the peripheral nervous system. Second, to distinguish clearly between neuronal and glial cell surfaces, ganglia were enzymatically dissociated prior to labeling. To prevent loss of putative H2 expression ex vivo, cells were not cultured prior to analysis. Third, three different techniques for MHC-I detection were adapted for the present task, including dual-label flow cytometry and a rosetting procedure shown to be up to 100 times more sensitive than cytotoxicity for detection of cell surface MHC-I molecules (19, 23). Finally, immunoelectron microscopy was used to obtain independent confirmation that neuronal membranes were fully dissociated from satellite glia and to compare the densities of MHC-I molecules induced on different cell types.
It has been shown that H2-encoded heavy chains (αCs) and the associated light chain, β2 microglobulin (β2m), are present on the surfaces of primary sensory neurons recovered from sensory ganglia at times concurrent with, and several days after, virus clearance. In contrast, neurons obtained from latently infected ganglia were MHC-I negative. Induction was widespread and outlasted detectable productive infection in the vast majority of cells. Finally, the density of cell surface H2 was approximately 10-fold lower on neurons than on satellite glial cells. Nonetheless, T cells and neurons were detected in close physical contact in vivo.
MATERIALS AND METHODS
Virus and infection of mice.
HSV-1, strain SC16 (4), was grown and titrated in Vero cells and stored at −70°C until required. SC16 is a well-characterized low-passage oral isolate that is neuroinvasive and neurovirulent when inoculated into mouse flanks (25). Female C57BL/10, C3H, CBA, BALB/c, and BALB/k mice (Specific Pathogen Free Facility, Animal Resource Center, Perth, Western Australia, Australia) were infected when they were more than 8 weeks old.
Experiments were done with a well-characterized murine model of HSV infection that is described in detail elsewhere (25). Briefly, 2 × 105 PFU of SC16 was introduced into the peripheral nervous system by retrograde axonal transport along spinal nerves after scarification of left mid-flank skin with a 27 gauge needle through a 10-μl drop of virus suspension (2 × 107/ml).
Quantification of virus in ganglia.
Ganglia (T8-T13) were homogenized in 1 ml of cell culture maintenance medium, and 10-fold dilutions of homogenate were tested for infectious virus with a standard plaque assay (21), as previously described (25).
Enzymatic dissociation of ganglia and enrichment of neurons.
Thoracic ganglia (T8-T13) from which spinal nerves had been removed were dissociated by incubation for 3 h at 37°C with collagenase and dispase (1 mg/ml in phosphate-buffered saline [PBS] [Boehringer Mannheim]). Prior to rosetting and flow cytometry, dissociated ganglionic cells were separated from axonal debris by centrifugation (800 × g, 10 min, 4°C) in a 25 to 40% Percoll gradient. A two-step procedure was used to enrich dissociated cells for neurons prior to electron microscopy procedures: after removal of axonal debris, cells were fractionated according to size by rate-zonal centrifugation (100 × g, 8 min, 4°C) in 30% Percoll.
Antibodies.
For flow cytometry, cells were labeled with 34-1-2S (anti-Kd/b/Dd; ATCC HB79) and/or rabbit anti-human PGP9.5 (Ultraclone, Isle of Wight, United Kingdom), followed by a cocktail of phycoerythrin (PE)-conjugated anti-mouse immunoglobulin G2b (IgG2b) (Caltag, South San Francisco, Calif.) and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit Ig (Zymed, South San Francisco, Calif.). PGP9.5 (protein gene product 9.5) is a marker for all neurons in the central and peripheral nervous systems. In rosetting reactions, the following primary antibodies were used to detect αCs: 34-1-2S, SF1-1.1 (anti-Kd; Pharmingen, San Diego, Calif.), and 36-7-5 (anti-Kk; Pharmingen). S19.8 (34) was used to detect β2m. Primary antibodies used in immunohistochemistry were as follows: to detect T cells, a rabbit anti-human serum to the teliologically conserved cytoplasmic region of the CD3 epsilon chain (Dakopatts, Glostrup, Denmark); to stain neurons, polyclonal rabbit anti-human PGP9.5 (Ultraclone).
Flow cytometry.
After removal of axonal debris, dissociated ganglionic cells were fixed in 2% paraformaldehyde and permeabilized with 0.1% saponin (22) prior to labeling. Preparations were analyzed with a Coulter EPICS XL-MCL flow cytometer (Coulter, Hialeah, Fla.).
Rosetting.
The proportion of neurons expressing β2m and αCs on their surfaces was determined with a monoclonal antibody (MAb)-based rosetting procedure, using protein A-coated sheep erythrocytes (sRBC) as described (23). Specificity was stringently controlled by studying the mouse strain distribution pattern of reactivity between allele-specific antibodies and their targets. The number of rosette-forming cells (RFCs) counted in order to ensure statistically defensible accuracy was determined by the frequency of rosette formation.
Immunoelectron microscopy.
Neurons were labeled with 34-1-2S (1 h, 4°C) followed by protein A-gold (15-nm gold particles, 1 hr, 4°C) and fixed overnight in 4% paraformaldehyde–0.25% glutaraldehyde in PBS containing 4% sucrose. Preparations were processed according to standard methods for transmission or scanning electron microscopy (TEM and SEM, respectively). For detection of gold particles by SEM a back-scatter detector was used in conjunction with a Philips XL 30 microscope.
Immunohistochemistry.
Paraffin-embedded sections (5 μm thick) were stained for the presence of lymphocytes with rabbit anti-human CD3. Prior to incubation with antiserum, antigen was retrieved by a standard microwave procedure (10). Binding of anti-CD3 was detected with biotinylated goat anti-rabbit Ig, followed by streptavidin-peroxidase complex and 3,3′-diaminobenzidine containing 0.1% H2O2 (all serological reagents were from Dakopatts). Antibody reactions were 45 min at room temperature, and sections were washed twice for 10 min in PBS between steps. Slides were lightly counterstained with hematoxylin.
RESULTS
Termination of productive infection.
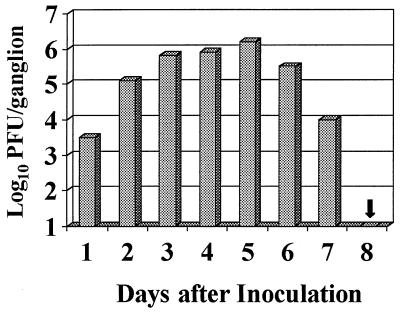
Prior data indicated that ganglionic infection in BALB/c mice infected with 2 × 105 PFU of HSV-1, strain SC16, resolves 8 days after virus inoculation. Productive infection of spinal ganglia is confined to neurons between the 8th and 13th thoracic segments (T8-T13), ipsilateral to the site of cutaneous inoculation (30). At the peak of infection (day 5), viral antigens can be detected in up to 13% of neurons, located mainly at T8 and T9 (30). After termination of productive infection, HSV DNA is not eliminated from the PNS; rather, viral genomes persist in a latent nonreplicating state in approximately 1% of thoracic ganglionic neurons (28). This is typical of infection in several experimental models. Clearance kinetics were confirmed in the present mouse colony by recovering infectious virus from spinal ganglia removed daily from groups of 10 infected mice (Fig. 1). In terms of ganglionic virus load, infection peaked 5 days after inoculation and was terminated in all mice by day 8.
FIG. 1.
Mean daily virus recovery from ganglia (T8-T13) of BALB/c mice inoculated (day 0) with 2 × 105 PFU of HSV-1, strain SC16. No virus was recovered on day 8.
Colocalisation of H2Kd and a neuronal antigen, PGP9.5.
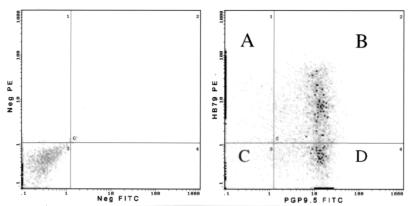
Seven days after inoculation, density gradient and rate zonal centrifugation steps were used to enrich 150 enzymatically dissociated BALB/c (H2d) ganglia for cells with a diameter of ∼20 μm or greater which, on the basis of their large size, were presumed to be neurons. To confirm the identity of these cells and determine whether H2 antigens could be detected on their surfaces, flow cytometry was used to detect simultaneously a neuron-specific cytoplasmic antigen, PGP9.5, and cell surface H2Kd (Fig. 2). Of the PGP9.5-positive cells, 62.9% were found to coexpress H2Kd. The mean PE (H2) fluorescence of PGP9.5-positive cells (i.e., neurons) was 3.6 times lower than that of PGP9.5-negative cells (i.e., principally glia), implying that the density of H2 molecules on neuronal surfaces is lower than on satellite cells.
FIG. 2.
Analysis of ganglionic cells by dual-label flow cytometry at 7 days after inoculation. Conjugate controls are shown in the lefthand panel. In the righthand panel, quadrants A and D represent cells stained with either HB79-FITC (anti-H2) or PGP9.5-PE (a neuronal marker) alone. The intensity of PE fluorescence of the majority of cells in quadrant A is very low, such that the signal lies on the y axis. Heavy points represent 10 or more cells. Quadrant C shows double-negative cells, whereas quadrant B shows HB79 and PGP9.5 double-positive cells, i.e., H2-positive neurons. The mean HB79-PE fluorescence (H2) of cells in quadrant A is 3.6 times that of those in quadrant B.
Enumeration and characterization of MHC-I-specific RFCs.
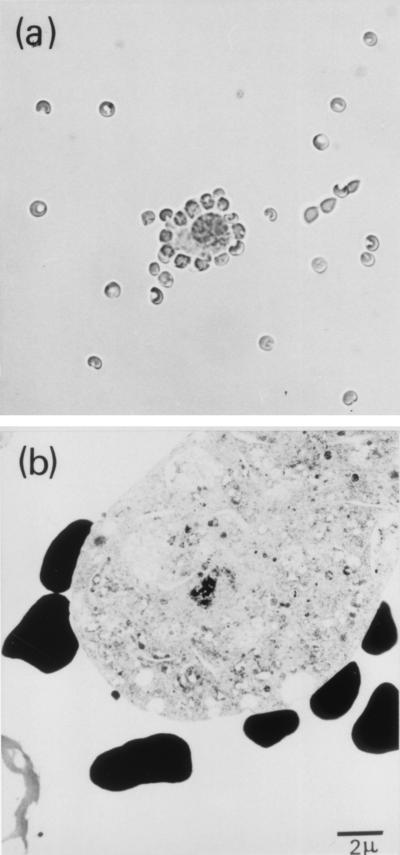
Neuronal H2 expression was further characterized by rosetting, which allowed us to confirm that H2 molecules were present on neuronal membranes rather than on contaminating fragments of glial cells. First, αC-specific neuronal rosettes were formed by sequential incubation of dissociated ganglionic cells with anti-H2 MAbs and protein A-coated sRBC. In the first instance, cell suspensions were prepared from a group of five BALB/c (H2Kd) mice killed 8 days after HSV infection; 30.8% of neurons reacted (Fig. 3a) with MAb 34-1-2S (anti-H2Kd/b/Dd), in contrast with neurons from a matched group of uninfected mice, which were H2 negative in this test. To check the specificity of rosetting, we determined the mouse strain distribution pattern of reactivity between neurons and a panel of reagents comprising 34-1-2S and two other allele-specific anti-H2 antibodies, namely SF1-1.1 (anti-Kd) and 36-7-5 (anti-Kk). Each antibody reacted only as expected, i.e., with cells of the corresponding mouse haplotype (Table 1).
FIG. 3.
(a) Light micrograph of a neuronal rosette viewed under a coverslip (diameter of sRBC, 4.4 μm). RFCs were enumerated with a Neubauer counting chamber. (b) TEM illustrating direct contact between membranes of sRBC (black cells) and a rosette-forming neuron.
TABLE 1.
Mouse strain distribution pattern of reactivity between anti-H2 antibodies and primary sensory neurons from HSV-infected mice
| Mouse strain | H2 type | Antibody specificitya
|
||
|---|---|---|---|---|
| Kd/b/Dd | Kd | Kk | ||
| C57BL/10 | Kb/Db | + | NT | − |
| BALB/c | Kd/Dd | + | + | − |
| CBA | Kk/Dk | − | − | + |
| C3H | Kk/Dk | − | − | + |
| BALB/k | Kk/Dk | − | NT | + |
+, 15 to 30% specific rosette formation on neurons isolated from pooled thoracic ganglia at 8 days after infection; −, no specific rosette formation; NT, not tested.
β2m is an integral component of the molecular complex responsible for presenting antigens to CD8+ T cells (5) and is expressed at the cell surface only in association with MHC-I heavy chains and antigenic peptides. Detection of cell surface β2m was regarded as a surrogate marker of expression of potentially functional MHC-I complexes.
To determine whether β2m was present on neuronal surfaces, groups of 10 C57BL/10 mice were killed 5, 8, and 13 days after HSV infection and ganglionic cell preparations were reacted with MAb S19.8. The specificity of the reaction was stringently controlled by exploiting the fact that S19.8 identifies a unique allele of β2m expressed only by mice with the C57BL genetic background (34).
Five days after inoculation, corresponding with the peak of infection (Fig. 1) (25), β2m was not detected on neuronal surfaces. By day 8 after inoculation, 30.1% of neurons from C57BL/10 thoracic ganglia were β2m positive, closely resembling the proportion of neurons on which αCs were detected by rosetting at this time. By day 13, the proportion of ganglionic neurons (T8-T13) which formed β2m-specific rosettes had risen to 60%, approaching the proportion which had been found to be αC positive by FACS on day 7. On this basis, FACS appeared to be the more sensitive technique. In terms of the specificity of rosetting, S19.8 did not react with neurons from CBA, C3H, BALB/c, or BALB/k animals, from which it was concluded that the rosetting reaction was specific for β2m. These data were confirmed in several independently infected groups of mice (data not shown).
In order to address the concern that detection of MHC-I molecules on neuronal surfaces, whether by flow cytometry or rosetting, could be an artifact resulting from failure to enzymatically disrupt the tight association between neurons and the plasma membranes of surrounding satellite glia (18), RFCs were fixed and their ultrastructure was examined at high resolution by electron microscopy (Fig. 3b). Rosettes were found to be formed by direct interaction between neuronal surfaces and sRBC, from which it was concluded that primary sensory neurons are capable of expressing MHC-I molecules at the cell surface.
Direct contact between neurons and T cells.
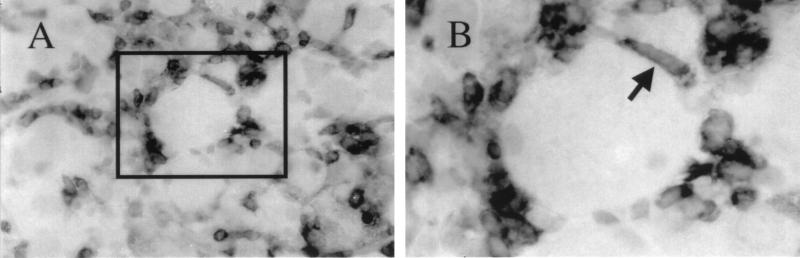
To determine whether T cells in HSV-infected ganglia make direct contact with neurons, ganglia were removed 7 days after inoculation (five mice) and tissue sections were stained with anti-CD3 antibody. T cells were abundant, comprising the majority of infiltrating inflammatory cells at this time (Fig. 4A). Unexpectedly, some cells which would have been regarded in hematoxylin-stained sections as satellite glia on the basis of their proximity to neurons and flattened appearance were CD3 positive (e.g., Fig. 4B). It was concluded that T cells were tightly associated with neurons concurrent with termination of infection. At earlier times after inoculation, T cells comprised only a minor fraction of infiltrating inflammatory cells, and contact between T cells and neurons could not be detected.
FIG. 4.
HSV-infected ganglion stained for CD3-positive cells, showing close direct contact between a primary sensory neuron and a flattened T cell (arrow), disclosed by the presence of cytoplasmic CD3. The section (5 μm thick) was lightly counterstained with hematoxylin.
Neuronal MHC-I expression is not maintained in latently infected ganglia.
To determine whether cell surface MHC-I expression is maintained by the restricted viral transcription associated with HSV latency (33), five mice were killed 64 weeks after infection and fractionated ganglionic neurons were tested by rosetting for the presence of cell surface β2m. In concordance with the previous failure to detect MHC-I transcripts by in situ hybridization during latency (20), β2m molecules were not detected on neurons from latently infected mice or uninfected controls. From these data, it was concluded that latent infection does not stimulate continued expression of MHC-I molecules on neuronal surfaces. It remains to be shown whether this is related to the host’s inability to eradicate the virus.
Surface density of MHC-I is lower on neurons than on satellite glia.
FACS analysis had implied that satellite glia express a greater number of MHC-I molecules per unit of surface area than do neurons. However, direct assessment of the relative amounts of H2 on each cell type was not felt to be possible by this technique, as a result to the perceived potential problem of artifacts created by a small proportion of neurons to which glial membranes remain attached.
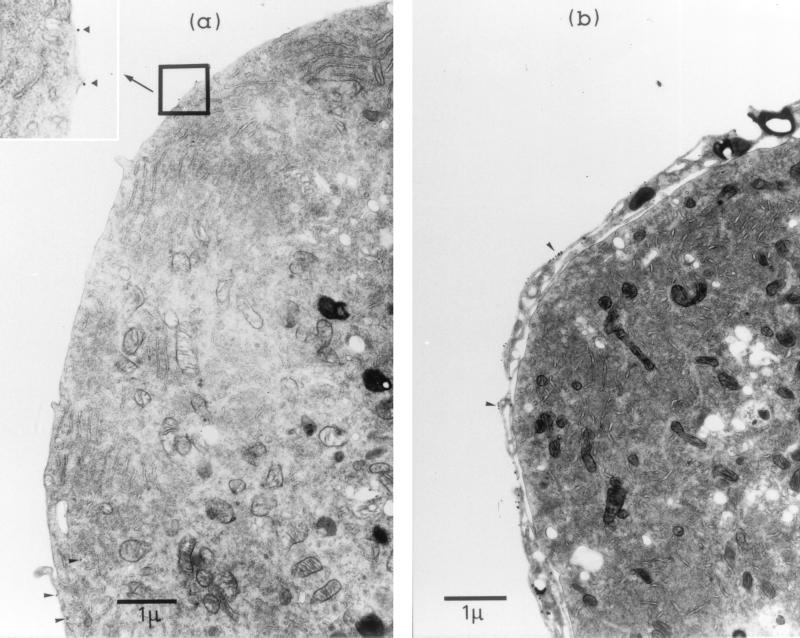
Therefore, to quantify the density of H2 on the surfaces of neurons (Fig. 5a) and satellite glia (Fig. 5b), immunogold TEM was used to localize 34-1-2S (H2Kd) epitopes on the surfaces of cells dissociated from BALB/c ganglia removed 7 days after infection. In some preparations, ganglia were dissociated partially by shorter exposure to collagenase and dispase (e.g., Fig. 5b), allowing positive identification of satellite glia by their attachment to neurons. The number of gold grains per unit of length of the cell membrane was 17 ± 2.1 (mean ± standard deviation) for satellite cells and 1.8 ± 0.2 for neurons. The surface density of H2 molecules on neuronal surfaces was therefore estimated to be ∼10 times lower than on surrounding satellite glia.
FIG. 5.
(a) Immunoelectron micrograph showing part of a primary sensory neuron isolated from a preparation of BALB/c ganglia at 7 days after HSV inoculation and stained with HB79 (anti-H2d) and 15-nm protein A-gold. Gold particles (arrowheads and enlarged inset) mark the locations of H2d molecules on the neuronal surface, which were enumerated per unit of length of plasma membrane. (b) Part of a neuron incompletely dissociated from surrounding glia, demonstrating the relatively high density of H2 molecules (clusters of gold particles [arrowheads]) on satellite cells.
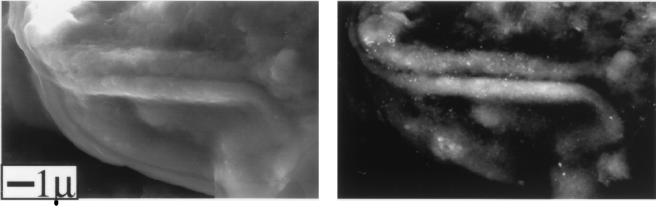
Immunogold SEM with back-scatter showed that epitopes identified by 34-1-2S were distributed evenly over the neuronal surface (Fig. 6). On random cuts of the SEM images, the density of gold grains detected by SEM correlated well with that measured by TEM (data not shown).
FIG. 6.
Immunogold SEM showing part of the surface of a single neuron recovered from a ganglion at 7 days after HSV inoculation. (Left) Conventional image to confirm that the cell surface was devoid of glial debris. (Right) Back-scatter image to detect the presence of gold on the plasma membrane. There is widespread random distribution of gold-tagged H2 molecules (bright spots) over the cell surface. The diameter of the cell was ∼60 μm.
We estimate that neurons have on average a surface area ∼10 times that of satellite glia, based on their diameters when dissociated. On this basis, it is likely that the numbers of MHC-I molecules synthesized by each cell type are similar.
DISCUSSION
Under normal conditions, lack of neuronal MHC-I expression is primarily the result of constraints on the transcription of genes encoding αCs, β2m, and the transporter associated with antigen processing (TAP) (6). Previously, we showed that these constraints are relaxed in response to HSV infection (20); here, we show that class I molecules are expressed on neuronal surfaces. These data address a long-standing controversy regarding the ability of neurons to express MHC-I molecules. CD8+ cells, which must be presented with antigens by MHC-I molecules (12), were shown previously to play a critical role in terminating productive ganglionic infection (27). This finding, in conjunction with the present observations, suggests strongly that HSV infection restores the capacity of primary sensory neurons to interact directly with lymphocytes.
Prior data indicating that neurons are able to express MHC-I molecules are limited, and many reports indicate that they do not (e.g., references 2, 6, 13, and, a review, 16). However, it has been reported that peripheral sensory neurons are susceptible, at very high effector-to-target ratios (ca. 100:1), to lysis by alloreactive T cells (7). Paradoxically, cell surface MHC-I molecules were not detected directly, perhaps, as suggested by the present study, because their density is unusually low. In addition, MHC-I expression by central nervous system motor neurons has been reported after peripheral nerve section (11). More recently, electrically silent neurons in culture were shown to express MHC-I in a gamma interferon (IFN-γ)-dependent manner (14), although there is no reason to suppose that HSV causes widespread down-regulation of neuronal activity in an infected ganglion. The molecule responsible for neuronal MHC-I induction in response to HSV is therefore not known, but prior studies have suggested that it arises from infected neurons (20). A neuronally derived molecule that is similar, but not identical, to IFN-γ has been reported by two groups (8, 17) and remains a strong candidate. It has also been suggested that normal sensory neurons may synthesize conventional IFN-γ (15), and IFN-γ has recently been shown to regulate the phenotype of HSV in vivo (9).
The time course of MHC-I induction merits discussion. H2 antigens were first detected 7 days after infection, coincident with the disappearance of infectious virus. Therefore, the great majority of the neurons on which MHC-I molecules could be detected in the present study were not, at the time analyzed, productively infected. There are two possible implications of these data. First, neuronal infection may be terminated rapidly as a direct result of the appearance of MHC-I molecules on the surfaces of infected neurons. In support of this hypothesis, it was recently shown that termination of HSV infection in spinal ganglia of C57BL/10 mice, judged by the disappearance of antigen-positive neurons, is precipitous, taking only 8 to 16 h (32). Perhaps productively infected neurons are sought out and destroyed by MHC-I-restricted cytotoxic T cells, but at least three observations do not support this hypothesis: (i) termination of productive infection is not dependent on neuronal destruction (27); (ii) expression of HSV α-47 is known to turn off the TAP, particularly in human fibroblasts (3); and (iii) the relatively low density of H2 on neurons compared with glia is predicted to favor a noncytolytic response. A second possibility is that virus structural antigens are displayed at the neuronal surface for perusal by CD8+ T cells immediately following virus uptake, allowing immune intervention prior to the onset of virus gene expression. This may serve a purpose unrelated to clearance of HSV from productively infected ganglia; specifically, it might inhibit virus spread and even enhance establishment of latency in neurons adjacent to those infected productively (31).
Ganglionic infection peaked 5 days after inoculation, and clearance of infectious virus commenced 6 to 7 days after inoculation and was complete by day 8. At the peak of infection, three or four of the six spinal ganglia studied (T8-T13) contain productively infected neurons, judged by the presence of infectious virus or viral antigens (30). In the current work, H2 molecules could be detected by rosetting on the surfaces of 60% of neurons 13 days after inoculation, which most likely comprises virtually all neurons recovered from productively infected ganglia in the sample. In support of this proposal, upregulation of MHC-I transcription was demonstrated previously in all neurons in approximately two-thirds of ganglionic profiles studied by in situ hybridization (20).
In summary, we have shown that sensory neurons not only transiently express MHC-I molecules at the cell surface in response to viral infection but also make close direct contact with T cells in vivo. The major implication of these data is that neurons are not as invisible to the immune system as previously thought.
ACKNOWLEDGMENTS
We thank Peter Smith for helpful advice and assistance with TEM.
This work was supported by grant 96/0535 from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett P F, Kerr R S C, Bailey K A. Expression of MHC antigens in the central nervous system. Transplant Proc. 1989;21:3163–3165. [PubMed] [Google Scholar]
- 3.Hill A, Jugovic P, York I, Russ I, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature (London) 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 4.Hill T J, Field H J, Blyth W A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975;28:341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- 5.Hood L, Steinmetz M, Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- 6.Joly E, Mucke L, Oldstone M B A. Viral persistence in neurons explained by lack of major histocompatibility complex class I expression. Science (Washington, DC) 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 7.Keane R W, Tallent M W, Poda E R. Resistance and susceptibility of neural cells to lysis by cytotoxic lymphocytes and by cytolytic granules. Transplantation. 1992;54:520–526. doi: 10.1097/00007890-199209000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Kieffer R, Haas C A, Kreutzberg G W. Gamma-interferon like immunoreactive molecule in rat neurons: evidence against a close relationship to gamma interferon. Neuroscience. 1991;45:551–560. doi: 10.1016/0306-4522(91)90270-x. [DOI] [PubMed] [Google Scholar]
- 9.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leong A S W, Milios J. An assessment of the efficacy of the microwave antigen retrieval procedure on a range of tissue antigens. Appl Immunohistochem. 1993;1:267–274. [Google Scholar]
- 11.Maehlen J, Nennesmo I, Olsson A-B, Kristensson K. Peripheral nerve injury causes transient expression of MHC class 1 antigens in rat motor neurons and skeletal muscles. Brain Res. 1989;481:368–372. doi: 10.1016/0006-8993(89)90816-0. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Kappler J. The T-cell receptor. Science (Washington, DC) 1987;238:1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- 13.Massa P T, Ozato K, McFarlin D E. Cell type-specific regulation of major histocompatibility complex (MHC) class 1 gene expression in astrocytes, oligodendrocytes, and neurons. Glia. 1993;8:201–207. doi: 10.1002/glia.440080307. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Cavlié A, Jenne D E, Wekerle H. Induction of MHC class I genes in neurons. Science (Washington, DC) 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 15.Neumann H, Schmidt H, Wilharm E, Behrens L, Werkele H. Interferon γ gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldstone M B. Molecular anatomy of virus persistence. J Virol. 1991;65:6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson T, Kristensson K, Ljungdahl A, Maehlen J, Holmdahl R, Klareskog L. Gamma interferon like immunoreactivity in axotomised rat motor neurons. J Neurosci. 1989;9:3870–3875. doi: 10.1523/JNEUROSCI.09-11-03870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannese E, Bianchi R, Calligaris B, Ventura R, Weibel E R. Quantitative relationships between nerve and satellite cells in spinal ganglia. An electron microscopical study. 1. Mammals Brain Res. 1972;46:215–234. doi: 10.1016/0006-8993(72)90017-0. [DOI] [PubMed] [Google Scholar]
- 19.Parish C R, McKenzie I F C. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- 20.Pereira R A, Tscharke D C, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell W C. A sensitive and precise assay for herpes virus. Nature (London) 1962;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- 22.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 23.Sandrin M S, Potter T A, Morgan G M, McKenzie I F C. Detection of mouse alloantibodies by rosetting with protein A-coated sheep red blood cells. Transplantation. 1978;26:126–130. doi: 10.1097/00007890-197808000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Schachner M G, Hammerling U. The postnatal development of antigens on mouse brain cell surfaces. Brain Res. 1974;73:362–371. doi: 10.1016/0006-8993(74)91058-0. [DOI] [PubMed] [Google Scholar]
- 25.Simmons A, Nash A A. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons A. H2-linked genes influence the severity of herpes simplex virus infection of the peripheral nervous system. J Exp Med. 1989;169:1503–1507. doi: 10.1084/jem.169.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the peripheral nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons A, Tscharke D C, Speck P G. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–55. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 29.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 30.Speck P G, Simmons A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speck P G, Simmons A. Synchronous appearance of antigen positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J Gen Virol. 1992;73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 32.Speck P G, Simmons A. Precipitous clearance of herpes simplex virus from the peripheral nervous systems of experimentally infected C57BL/10 mice. J Gen Virol. 1998;79:561–564. doi: 10.1099/0022-1317-79-3-561. [DOI] [PubMed] [Google Scholar]
- 33.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tada N, Kimura S, Hatzfeld A, Hammerling U. Lym-II: the H2 region of mouse chromosome 2 controls a new surface alloantigen. Immunogenetics. 1980;11:441–449. doi: 10.1007/BF01567813. [DOI] [PubMed] [Google Scholar]
- 35.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. New York, N.Y: Lipincott-Raven; 1996. pp. 2297–2342. [Google Scholar]
- 36.Wildy P, Field H J, Nash A A. Classical herpes latency revisited. In: Mahy B W J, Minson A C, Darby G K, editors. Virus persistence. Cambridge, England: Cambridge University Press; 1982. pp. 133–167. [Google Scholar]