Abstract
“Athlete’s heart” is a spectrum of morphological, functional, and regulatory changes that occur in people who practice regular and long-term intense physical activity. The morphological characteristics of the athlete’s heart may overlap with some structural and electrical cardiac diseases that may predispose to sudden cardiac death, including inherited and acquired cardiomyopathies, aortopathies and channelopathies. Overdiagnosis should be avoided, while an early identification of underlying cardiac life-threatening disorders is essential to reduce the potential for sudden cardiac death. A step-by-step multimodality approach, including a first-line evaluation with personal and family history, clinical evaluation, 12-lead resting electrocardiography (ECG), followed by second and third-line investigations, as appropriate, including exercise testing, resting and exercise echocardiography, 24-hour ECG Holter monitoring, cardiac magnetic resonance, computed tomography, nuclear scintigraphy, or genetic testing, can be determinant to differentiate between extreme physiology adaptations and cardiac pathology. In this context, cardiovascular imaging plays a key role in detecting structural abnormalities in athletes who fall into the grey zone between physiological adaptations and a covert or early phenotype of cardiovascular disease.
Keywords: athlete's heart, cardiovascular imaging, pre-participation screening, sports activity, sports cardiology, sudden cardiac death
1. Introduction
Physical activity, defined as any body movement resulting from the contraction of skeletal muscle that raises energy expenditure above the resting metabolic rate [1], if carried out regularly and for long periods, can result in substantial adaptations of the cardiovascular (CV) system to improve athletic performance. The athlete’s heart results from these morphological, functional and regulatory adaptations and may be characterized by increased mass, cavity dimensions, and wall thickness with at least normal systolic and diastolic function [2, 3, 4, 5, 6]. The physiological factors of this remodeling are various and not fully known, but they depend on many non-modifiable properties of the athletes and the type of exercise, including type and duration of physical activity, other than environmental and genetic factors.
Sometimes, there may be some overlap (the so-called “grey zone”) between the physiological adaptation of the athlete’s heart and some pathological conditions, such as hypertrophic cardiomyopathy (HCM) or arrhythmogenic cardiomyopathy (ACM), that may pose an athlete at risk of dying suddenly. Therefore, the differentiation between physiological and pathological cardiac anomalies in athletes may be challenging, but it is mandatory because the incorrect diagnosis may have important consequences, such as exclusion from competitive sport, false reassurance, and missed opportunities for effective therapeutic interventions. Sudden cardiac death (SCD) in young athletes is usually caused by genetic or congenital structural cardiac disorders [7, 8], such as HCM, ACM, or an anomalous coronary artery origin. In athletes 35 years of age, most of all SCDs are due to atherosclerotic coronary artery disease (CAD) [9].
For this reason, pre-participation cardiovascular screening (PPS) aims to identify pathological conditions in athletes to prevent morbidity and SCD [10, 11, 12]. However, the best strategies remain controversial [12]: while European [13] guidelines recommend performing a 12-lead resting electrocardiogram (ECG) and Italian [14] guidelines even also a mandatory exercise stress test (EST) as the initial screening of competitive athletes, the United States [10] and American Heart Association [2] positions do not support a systematic national screening based on resting ECG in competitive athletes. However, both agree that further evaluations should be recommended in symptomatic (syncope, chest pain, exercise dyspnea, palpitations) and/or high CV-risk patients [8, 15, 16]. Therefore, the PPS of asymptomatic competitive or leisure athletes must be distinguished from the assessment of athletes reporting specific symptoms or conditions or conditions that may fall into grey zones [3].
To date, many cardiovascular diagnostic techniques have been tested on athletes, but the best strategies to highlight the main features of the athlete’s heart remain unknown [17]. Therefore, the present paper summarizes evidence about a step-by-step CV multimodality approach to diagnosing the athletes’ heart.
2. Physiological and Pathological Cardiac Adaptations to Physical Activity
Systematic training leads to CV changes that markedly increase cardiorespiratory fitness, enabling the athlete to improve performance and achieve higher sports results. The CV system can significantly adapt to changes in the hemodynamic conditions of the body [18]. The perfect efficiency of the CV system is therefore crucial for physical performance: the greater supply of oxygen to the muscles is ensured by increased district blood flow and an increased oxygen extraction from blood [19]. Maximal oxygen uptake (max) is a physiological characteristic determined by the product of maximal cardiac output (the product of heart rate and left ventricle stroke volume) and maximal arteriovenous oxygen content difference [20].
Also, sports activity is associated with variations in the overall hemodynamic state. Endurance and strength training lead the athlete’s heart to different types of adaptations, even though most disciplines cause mixed adaptation scenarios. The persistence of such modifications in athletes depends on various factors, such as sex, age, ethnicity and physiological characteristics of the subject [21, 22], which are largely genetically determined: indeed female [21] and pediatric [23, 24] athlete’s hearts are growing topic in current literature. Furthermore, these adaptations vary on the duration, type and intensity of the sports activity practiced by the subject [20, 25]. Studies suggest that at least 3 hours of training per week for at least 3 months could be sufficient to see some initial morpho-functional adaptations of the heart [20], but identifying an athlete’s heart requires much more training. Endurance activity can be defined as aerobic isotonic dynamic exercise: it involves large muscle groups working thanks to aerobic metabolism and includes sporting disciplines such as long and middle-distance running, swimming or cycling [8]. Strength activity can be defined as 30% maximal voluntary contraction and includes sporting disciplines performed at high intensity unsustainable by oxygen delivery alone and requiring metabolism of stored energy to be processed largely by glycolysis: examples are martial arts, short running distance, wind-surfing and weight-lifting. It is important to note that many sporting disciplines involve a combination of strength and endurance exercises (football, basketball, volleyball) and, therefore, there is likely to be an overlap in ranges [26]. In 1975, Morganroth et al. [27] introduced the concept that endurance and strength forms of exercise lead to different adaptations in cardiac structure [28]. Specifically, athletes exposed to endurance training demonstrate eccentric left ventricle (LV) hypertrophy, often accompanied by a right ventricle (RV) dilatation, due to an increased LV volume that increases diastolic wall stress [20]. Athletes exposed to strength training instead demonstrate concentric LV hypertrophy, characterized by normal LV cavity dimensions, but increased wall thickness and mass because of a pressure overload and increased systolic wall stress [20]. This hypothesis, called the “Morganroth Hypothesis” from the name of the scientist who developed it, has some limitations because many sports, such as rowing or cycling, imply both endurance and strength exercise, and hypertrophy results in an intermediate phenotype [8]. Moreover, this hypothesis has been challenged by recent studies suggesting that the increase in LV mass is proportional to the increase in LV volume (balanced remodeling) irrespective of the sports discipline [29], and normal LV geometry can be frequently observed also in top-level athletes [30].
Autonomic nervous system adjustments to the heart and blood vessels are necessary for mediating the CV responses required to meet the metabolic demands of working skeletal muscle during exercise; these demands are met by precise exercise intensity-dependent alterations in sympathetic and parasympathetic nerve activity [31]. Endurance training increases parasympathetic activity and decreases sympathetic activity in the heart at rest. These two training-induced autonomic effects, coupled with a possible reduction in intrinsic heart rate, decrease resting heart rate. Long-term endurance training also decreases submaximal exercise heart rate by reducing sympathetic activity to the heart [32]. However, the athlete’s heart is also a proarrhythmic heart, which may explain the prevalence of atrial fibrillation, ventricular arrhythmias and conduction tissue disease in athletes: dilatation of atria and ventricles, hypertrophy, bradycardia, vagal tone at rest, ionic changes, early repolarization, sympathetic tone during exercise and high wall stress are all possible underlying mechanisms [33].
Furthermore, heat [34] and cold [35] adaptation, as well as high [36] and low [37] atmospheric pressure exposure during exercise [38], are equally responsible for different CV behaviour to increase athletic performance. Also, some drugs, approved for therapeutic use in some pathologies but used by athletes not only for their capacity to improve selective aspects of physical performance [39], but also as doping substances, lead to heart alterations which can have serious side effects, especially when used at high doses and for long duration: it is the case of anabolic androgenic steroids [40, 41, 42].
3. The Step-By-Step Approach to Athlete’s Heart
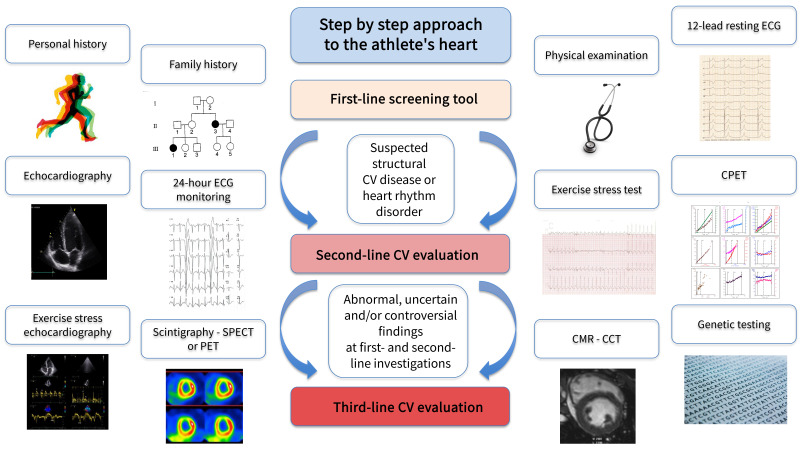
To discriminate between extreme physiology adaptations or an early-stage structural cardiac disease is a crucial task for the physician evaluating an athlete. It is important to point out that the physician performing and/or interpreting an athlete’s PPS should possess a basic knowledge of fundamental exercise physiology and exercise-induced cardiac remodeling features, to avoid the misinterpretation of data [43]. We therefore propose a systematic approach to conducting the CV evaluation of an athlete. The optimal way to begin the PPS should include family and personal history collection, physical examination and 12-lead resting ECG, as proposed by several scientific societies and as shown as a first-line evaluation in our step-by-step approach (Fig. 1). Only if in the presence of clinical suspicion or ECG abnormalities, it may be necessary to request other examinations, as indicated in the International Recommendations for Electrocardiographic Interpretation in athletes [44]. In that sense, the most common, accessible and cost-effective exams as a second-line examination are echocardiography, EST, 24-hours Holter ECG monitoring and cardiopulmonary exercise testing (CPET). If the results of one or more of these second-line evaluations are highly suspicious or fall in the grey zone, a third-line evaluation is needed, which is represented by less accessible or more costly diagnostic techniques such as exercise stress echocardiography (ESE), cardiovascular magnetic resonance (CMR), coronary computer tomography (CCT), genetic testing, single photon emission computed tomography (SPECT) and positron emission tomography (PET).
Fig. 1.
The step-by-step approach in the management of athlete’s heart. CV, cardiovascular; ECG, electrocardiogram; CPET, cardiopulmonary exercise test; CMR, cardiac magnetic resonance; CCT, cardiac computer tomography; SPECT, single photon emission computer tomography; PET, positron emission tomography.
3.1 First-Line Evaluation
Even if today there are numerous advanced modalities to assess CV health, the backbones of the athlete’s screening process are family and personal history, including sports history and potential assessment of CV effect of doping substances or ergogenic aids, and physical examination. The American Heart Association recommends these as the only tools in PPS [2]. Several questionnaires exist about the family and personal history of the athlete, and they are all based on the detection of congenital or personal CV diseases that may pose the athletes at risk of SCD [12], while the physical examination of the athletes aims at identifying heart and vessels’ congenital abnormalities (i.e., cardiac murmur, peripheral pulses), and features associated with genetic conditions such as the Marfan syndrome [45]. However, if used alone, their false positive rate is high [46, 47], especially if compared with to the PPS with 12-lead ECG, that remains still the most stand-alone and recommended screening method for athletes. Therefore, the simultaneous use of history, physical examination and ECG as first-line screening tools in athletes is highly recommended.
Electrocardiogram
ECG is a simple, quick, cheap and non-invasive diagnostic technique [48], that provides a graphic recording of the electrical cardiac activity. It is nowadays widely used for CV screening, given its important role in reducing SCD rate [49], but its cost effectiveness, the need for experienced physicians to correctly interpret it and a high false positive rate are criticisms often moved about it [12, 50, 51]. ECG changes in athletes are common and usually reflect adaptive structural and electrical remodeling of the heart in response to regular training [52, 53, 54, 55, 56]. Furthermore, ECG adaptations may vary according to demographic characteristics, such as age, sex and ethnicity, as well as the type of sport and level of training. Based on the International Recommendations for ECG interpretation in athletes, which should be applied only to those exercising vigorously for at least 4–8 hours per week [44], ECG findings in athletes are classified as normal, abnormal and borderline (Table 1, Ref. [44]): if one abnormal or two borderline findings together are detected, further evaluation must be performed. However, some of these adaptive changes overlap with patterns reflective of underlying pathology. Accurate interpretation of the ECG in asymptomatic athletes is of paramount importance to avoid unnecessary further investigations (given the possibility of false positive findings of this technique [50, 51]) or sport disqualification, and prevent serious consequences, including SCD, in case of high-risk cardiovascular conditions. A proposal of a modified algorithm for ECG interpretation in children athletes has been recently hypothesized [57].
Table 1.
ECG findings in athletes based on the international criteria [44].
| Normal ECG findings | Borderline ECG findings | Abnormal ECG findings |
| Sinus bradycardia or sinus arrhythmia | Left axis deviation | ST-T repolarization abnormalities (T-wave inversion, ST-segment depression) |
| First-degree AV block, Mobitz type 1 second-degree AV block | Left atrial enlargement | Pathological Q waves |
| Ectopic atrial or junctional escape rhythm | Right axis deviation | QRS 140 ms duration |
| Incomplete RBBB | Right atrial enlargement | Epsilon wave |
| Early repolarization/ST-segment elevation | Complete RBBB | Complete LBBB |
| Increased QRS voltage criteria for left or right ventricular hypertrophy | QT Abnormalities (Long and Short) | |
| ST elevation followed by T-wave inversion V1–V4 in black athletes | Ventricular pre-excitation | |
| T wave inversion V1–V3 in age 16 years | Brugada type 1 pattern | |
| Profound sinus bradycardia 30 bpm | ||
| PR interval 400 ms | ||
| Mobitz type 2 second-degree AV block, third-degree AV block | ||
| 2 PVCs at rest | ||
| Atrial tachyarrhythmias | ||
| Ventricular arrhythmias |
ECG, electrocardiogram; AV, atrioventricular; LBBB, left bundle branch block; PVC, premature ventricular contraction; RBBB, right bundle branch block.
3.2 Second-Line Evaluation
Most scientific societies worldwide do not recommend the echocardiogram as a screening modality in athletes, even if its use in the initial PPS is growing [15, 16], given its potential role in identifying CV abnormalities that can be undetected by ECG [58, 59]. However, nowadays, echocardiography is a very useful second-line diagnostic modality [15], when a suspicion of a structural CV disease is raised. On the other side, when a heart rhythm disorder is suspected, exercise-related CV diagnostic modalities are recommended: first, an EST, often followed by a 24-hours ECG Holter monitoring, allows for the investigation of the athlete’s CV system during physical effort [60]. Therefore, echocardiography, EST and 24-hours ECG Holter monitoring are often used together as second-line investigation tools, given their wide availability and low cost. When it is necessary to follow up with an athlete, the three examinations or a combination of them are very effective in identifying subtle changes over time [61, 62]. Nevertheless, also CPET can have an important role in the diagnostic process of athlete’s heart [63, 64], but it requires experienced personnel, it is expensive and time-consuming, limiting its wide dissemination and use in athletes to only some selected cases.
3.2.1 Echocardiogram
Due to its ability to provide information on cardiac morphology, function and hemodynamics, its low cost and wide and easy availability, the use of echocardiography in athlete’s evaluation is increasing [58, 65, 66, 67], also given that the low acoustic chest impedance of the athletic population makes it possible to obtain high-quality images [16].
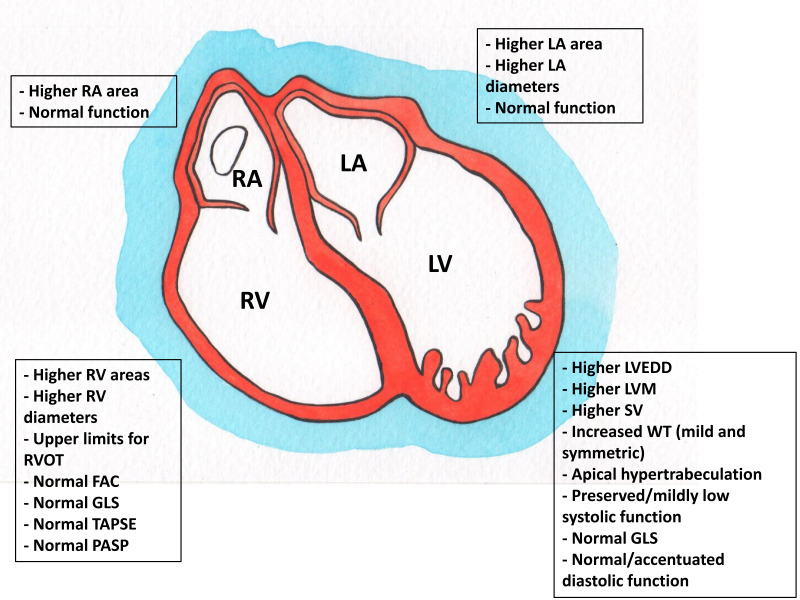
Cardiovascular adaptations of an athlete’s heart include balanced increases in all heart chambers. While interest has largely focused on the LV in the past, attention has recently been directed to other structures such as the RV, the atria, and the aorta [68, 69]: adaptations to physical activity include a proportional increase in the left and right cardiac cavity sizes, increased LV wall thickness and LV mass, and supra-normal indices of systolic and diastolic function [70, 71, 72, 73] (Fig. 2). These adaptations, strictly dependent upon the duration, type and intensity of training, are often benign and physiological but may sometimes predispose to pathological conditions [74].
Fig. 2.
Echocardiographic cardiovascular adaptations in the athlete’s heart. RA, right atrium; LA, left atrium; RV, right ventricle; RVOT, right ventricle outflow tract; FAC, fractional area change; GLS, global longitudinal strain; TAPSE, tricuspid area plane systolic excursion; PASP, pulmonary artery systolic pressure; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVM, left ventricular mass; SV, stroke volume; WT, wall thickness.
Several reference values about age, gender, ethnicity, and sports disciplines have been published in the literature by different study groups (Table 2, Ref. [75, 76, 77, 78, 79, 80]; Table 3, Ref. [80, 81]). However, we currently lack universally accepted cut-offs for basic echocardiographic measurements [75, 82], and therefore there are no unanimous recommendations about the use of echocardiographic cut-offs to distinguish between physiological and pathological adaptations. Indeed, comprehensive nomograms including sufficient sample size (of both genders), evaluating different ages (including master athletes) and ethnicities and various sports, evaluating a complete dataset of 2D (and new 3D and strain analysis indexes) echocardiographic measures, and built using a rigorous statistical approach (uniform normalization and way to express normalized data—preferably as Z-scores) are still missing in current literature [83]. Therefore, care is needed when interpreting this exam.
Table 2.
Athlete’s left heart echocardiography evaluation.
| Cardiac chamber | Parameter | Study | Mean value ( SD) |
| LV | EDD (mm) (BSA 1.8 M, BSA 1.5 F) | Boraita et al. [75], Spain – 2022 (3282 elite athletes, mean age 23 6 years) | 55 4 M |
| 49 4 F | |||
| Magalski et al. [76], USA – 2011 (964 competitive athletes, ages 18–21 years) | 52 4 M | ||
| 46 4 F | |||
| 49 5 white | |||
| 50 5 black | |||
| Pelliccia et al. [77], Italy – 1991 (1309 elite athletes, mean age 22 years) | 54.6 3.5 high impact M (BSA 1.8) | ||
| 48 3.6 high impact F (BSA 1.5) | |||
| 51.2 3 low impact M (BSA 1.8) | |||
| 45.3 2.8 low impact F (BSA 1.5) | |||
| IVS (mm) | Boraita et al. [75], Spain – 2022 (3282 elite athletes, mean age 23 6 years) | 9 1 M | |
| 8 1 F | |||
| Magalski et al. [76], USA – 2011 (964 competitive athletes, aged 18–21 years) | 9 1 M | ||
| 8 1 F | |||
| 9 1 white | |||
| 9 1 black | |||
| D’Andrea et al. [78], Italy – 2010 (615 elite athletes, mean age 28.4 10 years) | 9.7 3.1 endurance | ||
| 9.2 2.1 strength | |||
| Systolic function (EF%) | Boraita et al. [75], Spain – 2022 (3282 elite athletes, mean age 23 6 years) | 61 7 M and F | |
| D’Andrea et al. [78], Italy – 2010 (615 elite athletes, mean age 28.4 10 years) | 69.7 4.7 endurance | ||
| 67.1 3.8 strength | |||
| Diastolic function (E/A) | Boraita et al. [75], Spain – 2022 (3282 elite athletes, mean age 23 6 years) | 85 14/43 11 M | |
| 92 14/45 13 F | |||
| LA | Antero-posterior diameter (mm) | Boraita et al. [75], Spain – 2022 (3282 elite athletes, mean age 23 6 years) | 35.9 4.7 M |
| 32.1 4.2 F | |||
| Magalski et al. [76], USA – 2011 (964 competitive athletes, ages 18–21 years) | 34 4 M | ||
| 30 4 F | |||
| 32 4 white | |||
| 33 4 black | |||
| D’Andrea et al. [78], Italy – 2010 (615 elite athletes, mean age 28.4 10 years) | 34.5 5.5 | ||
| Longitudinal diameter (mm) | Boraita et al. [79], Spain – 2016 (3281 elite athletes, mean age 23.1 5.7 years) | 52.6 5.9 M | |
| 48.1 5.5 F | |||
| Area (cm) | Gjerdalen et al. [80], Norway – 2015 (595 elite athletes, mean age 25.1 4.6 years) | 20.7 4.4 | |
| Volume index (mL/) | D’Andrea et al. [78], Italy – 2010 (615 elite athletes, mean age 28.4 10 years) | 28.2 9.2 M | |
| 26.5 7.2 F |
LV, left ventricle; LA, left atrium; BSA, body surface area; EF, ejection fraction; F, female; LA, left atrium; LAVI, left atrial volume index; LV, left ventricle; EDD, left ventricular end-diastolic diameter; M, male; IVS, interventricular septum; E/A, early (E) to late (A) diastolic filling velocity.
Table 3.
Athlete’s right heart echocardiography evaluation.
| Cardiac chamber | Parameter | Mean value (95% CI) | Mean value (95% CI) | Mean ( SD) F athletes | Study |
| M endurance athletes | M strength athletes | ||||
| RV | RVOT PLAX (mm) | 29 (26–33) | 29 (26–33) | 28 2 | D’Ascenzi et al. [81], Italy – 2017 (6806 competitive athletes, aged 18–39 years) |
| RVOT PSAX (mm) | 34 (32–35) | 34 (32–35) | 30 1 | ||
| Basal diameter (mm) | 40 (38–42) | 38 (31–45) | 35.7 0.2 | ||
| Midcavity diameter (mm) | 29 (27–30) | 26 (23–29) | 29.1 0.3 | ||
| RV wall thickness (mm) | 4.2 (3.9–4.4) | 4.0 (3.5) | |||
| End-diastolic area () | 23 (20–27) | 21 (17–25) | 23.0 0.1 | ||
| End-systolic area () | 13 (10–15) | 10 (8–13) | |||
| TAPSE (mm) | 25 (22–28) | 25 (22–28) | |||
| FAC (%) | 35 (32–38) | 41 (32–49) | 39 4 | ||
| RA | Antero-posterior diameter (mean SD, mm) | 45.1 5.8 | Gjerdalen et al. [80], Norway – 2015 (595 elite athletes, mean age 25.1 4.6 years) | ||
| Area () | 18 (14–23) | 18 (14–23) | 16 1 | D’Ascenzi et al. [81], Italy – 2017 (6806 competitive athletes, aged 18–39 years) |
F, female; FAC, fractional area change; M, male; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; TAPSE, tricuspid annulus peak systolic excursion; PLAX, parasternal long axis; PSAX, parasternal short axis.
While a mild dilatation of the aorta can sometimes be observed, particularly in some categories of athletes (i.e., master endurance athletes), a more-than-mild dilatation is not part of the athlete’s heart and should warrant further investigations in case of an aortic root more than 40 mm in males (an indexed values of 20 mm/ for allometric scale) and 34 mm in females and more than 34 mm for the proximal ascending aorta [65, 73].
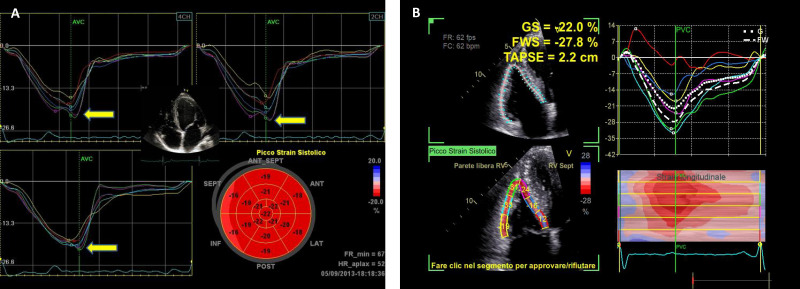
In the last decades, advances in ultrasound technology have evolved echocardiography from simple M-mode to 2-dimensional imaging, Doppler assessments, 3-dimensional (3D) anatomical imaging, and dimensional analysis of myocardial deformation [84]. Speckle-tracking echocardiography is very useful in evaluating the athletes’ heart [85], especially in identifying regional wall motion abnormalities and pre-clinical impairment in HCM and DCM. The mean value of global longitudinal strain in athletes is –18.1 2.2% in LV and –27 6% in RV [65] (Fig. 3). Three-dimensional echocardiography has added quantitative information to assess the athlete’s heart; cardiac volumes and mass can be estimated more precisely than 2D echo without geometric assumptions [86]. Moreover, myocardial work, calculated by adjusting myocardial deformation to the instantaneous LV pressure, has been recently proposed to have a role in the athlete’s heart diagnostic process, due to its less dependency on loading contraction than global longitudinal strain [87].
Fig. 3.
Global longitudinal strain (GLS) values of the left ventricle (A) and right ventricle (B) in a professional athlete: the bull’s eye is within normal values despite left ventricular hypertrophy (A) and right ventricular dilation (B). GS, global strain; FWS, free-wall strain; TAPSE, tricuspid annular plane excursion.
3.2.2 Exercise-Stress Test
EST is the most widely available functional test. The continuous ECG and blood pressure (BP) monitoring of the subject during a treadmill or cycle-ergometer incremental test, provides information on exercise capacity, heart rate and BP response to exercise, other than exercise-induced abnormalities, including arrhythmias [88]. It can be used for diagnostic, prognostic, or functional evaluation purposes [89], and it can also be adopted using different protocols based, for instance, on the type of sports practiced by the athlete [65]. Indeed, both treadmill and cycle ergometer protocols have their strengths and disadvantages and should be used with a precise aim in athletes [90]. However, some contraindications to EST must be considered [91]. In addition, EST requires specific care because of the wide range of normal findings, the use of different stress-inducing protocols, and the lack of generally accepted reference values [92].
In athletes over 35 years of age, EST investigates the presence of a silent ischemic cardiovascular disease through specific alterations in ST-segment and T-wave. However, it is less specific for myocardial ischemia than other functional tests, especially in asymptomatic and low-risk individuals [8]: for example, an asymptomatic upsloping ST-segment depression with normalization in the early (1 min) phase of recovery should not be considered pathological [93].
EST also permits assessing BP changes during exercise. An exaggerated BP response to exercise should lead to starting or optimizing antihypertensive medical therapy and performing a cardiologic evaluation, even if the athlete is normotensive at rest since it predicts incident and early hypertension in athletes [94]. In a large cohort of elite athletes undergoing EST, the 95th percentile of BP values was 220/85 mmHg in males and 200/80 mmHg in females [95]. Also, a decrease in BP during the test is not normal and should be further investigated.
Even if ventricular arrhythmias may be unrelated to heart diseases, some of them could be a marker for an arrhythmogenic condition in athletes with no relevant history, normal physical examination, and resting ECG, and therefore EST plays a pivotal role in describing its effort-related characteristics [96]. Other arrhythmias that can be studied through EST are atrial fibrillation, first- or second-degree AV block, and asymptomatic pre-excitation [65]. Also, QT interval adaptation to exercise and recovery phase is an important phenomenon to consider when evaluating an EST.
3.2.3 24-Hours ECG Holter Monitoring
ECG continuous monitoring is a method that provides more information for the detection of cardiac rhythm alterations than resting 12-lead ECG recording. ECG Holter monitoring recording with 12-leads configuration should always be preferred to determine the origin of ventricular arrhythmias (morphology and axis) and the presence of ischemia [97]. The monitoring period is usually 24 hours, even if it may be longer in specific cases, and should always include a training session, to reproduce as much as possible the “natural” physical effort of the athlete: this allows to study the response of the arrhythmias to exercise and to elicit arrhythmias that are in relation with the effort. However, ECG Holter monitoring in athletes is often rich in motion artifacts; therefore, an experienced physician is required to interpret it.
Life-threatening arrhythmias are infrequent among young athletes who require ECG monitoring, whereas their presence may suggest an underlying cardiac disease according to some specific characteristics [98] (Table 4, Ref. [96, 97]). A diary in which the patient reports the main daily activities (i.e., exercise sessions, sleeping times, etc.), any drug therapy taken or symptoms experienced should always be part of the assessment.
Table 4.
Features of uncommon premature ventricular beats in athletes that should raise suspicious of underlying disease requiring further investigations [96, 97].
| Characteristics of uncommon PVBs | |
| Ectopic QRS morphology | RBBB and wide QRS (130 ms) |
| LBBB with intermediate or superior axis | |
| Response to exercise testing | Persistence/increase |
| Complexity of PVBs | Couplets, triplets or NSVT |
| Polymorphic | |
| Short coupling interval* | Yes |
*: PVBs are superimposed on the preceding T-wave peak or earlier (i.e., R on T). LBBB, left bundle branch block; PVBs, premature ventricular beats; RBBB, right bundle branch block; NSVT, non-sustained ventricular tachycardia.
Progress in science and technology has led to the development of numerous devices, such as the external loop recorder, event recorders or wearables, for assessing cardiac arrhythmias, that are nowadays available for patients and should be used in selected cases (i.e., symptomatic athletes with infrequent symptoms) and with careful interpretation [99, 100].
3.2.4 Cardiopulmonary Exercise Test
CPET is a valuable tool to evaluate the responses of the cardiac, pulmonary, vascular, and musculoskeletal systems to exercise [101, 102, 103, 104]. Although still underutilized, its high reproducibility offers important prognostic and diagnostic information [105] and can be integrated with other imaging techniques [106]. Different from an EST, CPET involves measurements of respiratory oxygen uptake, carbon dioxide production, and ventilatory measures during a symptom-limited exercise test. CPET indications in athletes are manifold, including cardiorespiratory fitness estimation, evaluation of symptoms of unexplained origin and exercise prescription [107]. It is known that highly trained athletes have higher cardiorespiratory fitness compared to untrained individuals or low-trained athletes [108]. Therefore, cardiorespiratory fitness considered in the normal predicted ranges may mask latent disorders or physiological impairments in athletes. For this reason, interpreting CPET results requires caution within the clinical context, as predicted gas exchange parameters have been derived in the general population [109]. Moreover, athletes show further differences in exercise hemodynamic response and gas exchange parameters compared to non-athletes, including higher cardiac output, faster heart rate recovery, higher prevalence of exercise-induced arterial hypoxemia, and lower breathing reserve [107, 110] (Table 5, Ref. [107, 110, 111, 112]), even if reference values have yet to be determined [113]. Knowing these parameters in the context of the athlete’s physiological response to exercise could help guide the differential diagnosis between the athlete’s heart and underlying CV diseases [114].
Table 5.
Expected CPET parameters in healthy individuals and their response to exercise in athletes.
| CPET Parameter | In healthy subjects [111, 112] | In elite Athletes [107, 110] |
| HR max | 85% age-predicted HR max | Equal or more |
| HR increase 10 bpm per every 3.5 mL/kg/min of | ||
| HR recovery | 12 beats at first-minute recovery | More |
| Blood pressure | SBP increase 10 mmHg per every 3.5 mL/kg/min of | More |
| DBP stable or fall | ||
| 95% (rest and exercise) | Equal or less | |
| should not decrease below 95% | ||
| peak | Percent predicted values should be about 100% | Quite more |
| Predictive equations for endurance athletes [110] | ||
| ➢ Treadmill: peak (L/min) – 0.83(sex) + 0.033(height) – 0.017(age) – 1.15 | ||
| ➢ Cycle: peak (L/min) – 0.72(sex) + 0.048(height) – 0.00019() – 4.30 | ||
| at VT | Not mentioned | Quite more |
| Oxygen pulse | Percent predicted values should be about 100% | Quite more |
| Continual linear rise throughout the exercise with possible plateau approaching maximal exertion | ||
| Breathing reserve | 20% | Less |
| VE/ slope | 30 throughout the exercise | No differences |
| Not mentioned for apparently healthy individuals (usually resting is between 36 and 42 mmHg) | No differences | |
| OUES | Not mentioned | No differences |
CPET, cardiopulmonary exercise test; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; , peripheral oxygen saturation; peak, oxygen consumption at peak exercise; at VT, oxygen consumption at the ventilatory threshold; VE/ slope, minute ventilation/carbon dioxide production slope; , partial pressure of end-tidal carbon dioxide; OUES, oxygen uptake efficiency slope.
In clinical settings, CPET is generally used to evaluate the etiology of unexplained symptoms such as exertional dyspnea, chest discomfort, and fatigue. Moreover, excessive training load without an adequate recovery period exposes athletes to decreased performance and sometimes even to overtraining syndrome. CPET may be particularly useful in this condition.
Finally, CPET can be used for a tailored exercise prescription, not only to improve the performance of elite endurance athletes but also in patients at risk of and with CV disease, especially those who are older and engage for the first time in moderate to vigorous physical activity [111, 115]. Through the identification of ventilatory thresholds, the physician may draw out a personally tailored program with the appropriate level of intensity associated with possible enhancements for healthy athletes and proven benefits for patients with chronic diseases [116, 117]. Moreover, CPET should be part of the routine assessment of patients with cardiomyopathies who wish to exercise to obtain information about functional capacity and risk stratification [97, 106, 118, 119].
3.3 Third-Line Evaluation
In the presence of abnormal, uncertain, and/or controversial findings from the upstream diagnostic work-up (first- and second-line evaluation), other CV diagnostic modalities can be useful to differentiate between physiological and pathological adaptation of the athlete’s heart. However, due to their high cost and limited availability, these are not routinely recommended, but must be guided by a precise clinical suspicion, carefully considering each indication (Table 6). While CMR is the contemporary gold standard for defining myocardial structure and myocardial tissue architecture and is increasingly applied both for the study and clinical management of athlete’s heart, stress imaging represents a useful tool to unmask reduced cardiac functional reserve and covert pathological changes that are not evident at rest, especially in athletes in whom arrhythmias and/or early-stage cardiomyopathies are suspected [3]. In that sense, ESE represents the first choice, but also CCT and nuclear CV imaging techniques have pivotal diagnostic importance, especially in specific populations, such as master athletes. Finally, the use of genetic testing in athletes is increasing because genetic studies have identified many genetic variants that underpin cardiac disorders and technological advances have transformed genetic testing into a more readily available and affordable clinical tool [120].
Table 6.
Details of some third-line cardiac diagnostic techniques in athletes.
| Diagnostic techniques | Pros | Cons |
| ESE | - Assessment of biventricular function during exercise | - Require specific and expensive equipment |
| - Unmask pathologies not apparent at rest | - Motion artefacts | |
| - Physiological activation of the cardiovascular system | - Limiting skeletal muscle fatigue in individuals not accustomed to cycling | |
| - Diastolic stress testing | ||
| - Ability to characterize valve function and morphology | ||
| - Non-radiation imaging modality | ||
| - Low cost | ||
| CMR | - Non-radiation imaging modality | - Costs |
| - High spatial and temporal resolution | - Limited access | |
| - No blind spots | ||
| - Not limited by the thoracic wall, pulmonary parenchyma or wall thickness evaluation | ||
| - Accurate evaluation of cardiac function, flow, volumes and perfusion | ||
| - Excellent evaluation of wall motion abnormalities | ||
| - Multiparametric tissue characterization (LGE, mapping techniques) | ||
| CCT | - High spatial resolution | - Costs |
| - Obtain high-quality multiplanar reconstructions in any desired image orientation | - Limited access | |
| - Low contrast volume and low radiation dose | - Radiation dose | |
| - Evaluate morphological patterns and global and regional kinetic functions | - Low temporal resolution | |
| - Short examination time |
CMR, cardiac magnetic resonance; ESE, exercise stress echocardiography; LGE, late gadolinium enhancement; CCT, cardiac computed tomography.
3.3.1 Exercise-Stress Echocardiography
ESE is a reliable, safe, non-invasive imaging test that provides a dynamic cardiac function evaluation. Combined with clinical and ECG data, ESE helps detect cardiac abnormalities that may not occur at rest, such as exercise-induced ischemia in athletes with suspected coronary artery disease or congenital coronary artery anomalies [121]. Furthermore, ESE can assess contractile reserve during exercise in endurance athletes with LV and/or RV dilatation and mildly reduced ejection fraction at rest: an increase of LV EF of at least 15% during exercise may support the diagnosis of athlete’s heart [122]. Finally, ESE may be useful in athletes with valvular heart disease, providing information about exercise tolerance, biventricular contractile reserve, changes in hemodynamics (LV filling pressure, pulmonary pressure), and valvular functional parameters (transvalvular gradients, regurgitation entity—i.e., bicuspid aortic valve) [78].
Pharmacological stress is generally not indicated in athletes and an exercise test is usually performed through a bed cycle ergometer. Since the time for image acquisition is limited, the echocardiographic protocol is usually tailored to the clinical indication [65]. However, even if some limitations to the use of ESE exist, improvements in imaging equipment and technology, and allowing the movement towards more robust quantitative analysis, have led ESE to become a valuable tool in the diagnostic process of athlete’s heart.
3.3.2 Cardiovascular Magnetic Resonance
CMR is an established imaging modality for the cardiovascular assessment of athletes. It is a third-tier diagnostic tool that helps to discriminate between physiology and pathology [65], and it is superior to echocardiography in differentiating athlete’s heart from structural and functional change [123]. The limitations of CMR include, among others, high cost, limited accessibility and claustrophobia, other than untested or low interobserver variability [124].
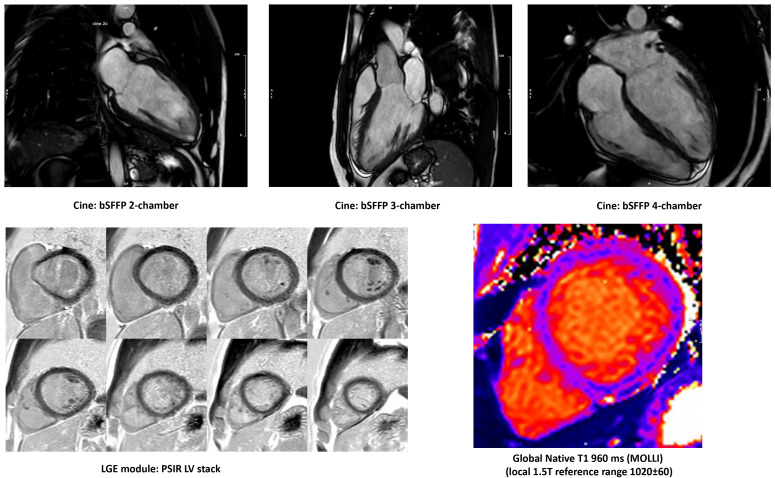
CMR is the gold standard for defining biventricular volumes and mass, and quantification of volumes and flow (Table 7, Ref. [125]) [126], providing advanced myocardial tissue characterization with excellent accuracy and precision. CMR has the incremental benefit of allowing tissue characterization by identifying myocardial inflammation and fat infiltration through T1 and T2 weighted images and mapping. CMR allows the detection of replacement fibrosis by late gadolinium enhancement (LGE) imaging, also pointing to the description of ischemic vs nonischemic patterns of myocardial damage [127]. As such, CMR supports the diagnosis of myocarditis and cardiomyopathies [128], such as HCM [129] and ACM [130, 131, 132, 133]. To differentiate between pathologic modification and physiologic remodeling, cardiac volumes and masses should always be compared to reference ranges deriving from CMR studies on healthy athletes [134], and adjusted to several factors, including type of sport, static and dynamic component, training hours per week, body surface area, age, gender, and ethnicity [135] (Fig. 4).
Table 7.
Normative CMR values for male endurance athletes.
| Cardiac chamber | Parameter | Mean (95% CI) | Study |
| LV | EDV (mL) | 208 (195–220) | D’Ascenzi et al. [125], Italy – 2019 (1053 competitive athletes, aged 18–55 years) |
| EDV index (mL/) | 111 (104–121) | ||
| ESV (mL) | 74 (68–79) | ||
| ESV index (mL/) | 49 (45–55) | ||
| SV (mL) | 125 (116–135) | ||
| SV index (mL/) | 63 (45–79) | ||
| EF (%) | 59 (58–61) | ||
| RV | EDV (mL) | 230 (214–245) | |
| EDV index (mL/) | 120 (113–126) | ||
| ESV (mL) | 101 (91–110) | ||
| ESV index (mL/) | 55 (49–61) | ||
| SV (mL) | 123 (112–134) | ||
| SV index (mL/) | 65 (59–71) | ||
| EF (%) | 54 (52–56) |
CMR, cardiac magnetic resonance; LV, left ventricle; RV, right ventricle; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction.
Fig. 4.
Cardiovascular magnetic resonance of an endurance athlete, investigated for premature ventricular beats and left ventricle (LV) dilatation and demonstrating balanced LV dilatation, borderline-low normal LV ejection fraction, no regional wall motion abnormalities, high-normal stroke volume, no late gadolinium enhancement, low-normal native myocardial T1, normal extracellular volume (25%).
In the last years, it is spreading the use of stress CMR that has the advantages to assess biventricular function, wall motion and valve function during exercise, even if it requires high levels of training, dedicated devices, and long-scan times [136].
3.3.3 Coronary Computed Tomography
CCT shows high accuracy in evaluating coronary atherosclerosis and coronary origin and course [121]. The assessment of coronary arteries by CCT is non-invasively performed and requires a very low radiation dose, thanks to the latest generation scanners (from 0.7 to 1 mSv with optimized acquisition parameters and protocols [136]). Therefore, depending on the local availability and expertise, CCT may be considered in athletes with symptoms suggestive of CAD and in older, asymptomatic athletes with risk factors for CV disease or equivocal exercise stress test. Indeed, it has been recently theorized the use of CCT in the screening process of mature athletes increases the negative predictive value for excluding coronary artery disease [137]. Moreover, CCT should be considered when a precise definition of proximal coronary anatomy or characterization of great vessel morphology is indicated [136]. When dilatation of the aortic root or ascending aorta is suspected, at least one comprehensive aortic tomographic assessment by CT angiography or angio-MR should be performed [73]. Cardiac CT well visualizes pericardial thickening and calcification, and CT attenuation values may differentiate pericardial fluid contents. Once anatomical abnormalities have been detected, a CV functional assessment performed during exercise is required to evaluate their functional clinical impact.
However, due to ionizing radiation exposure and high costs, this imaging modality is not recommended as the first-line technique for young athletes.
3.3.4 Nuclear Imaging Techniques
Myocardial perfusion scintigraphy techniques are generally considered a valuable diagnostic and prognostic modality and often used for further diagnostic evaluation in athletes with electrocardiographic findings indicative of myocardial ischemia in the PPS [138]. SPECT or PET can research exercise-induced ischemia and stratify the risk of athletes with suspected or known CAD, anomalous origin, or course of coronary arteries (e.g., myocardial bridging) [139]. The accuracy of both PET and SPECT in detecting CAD is excellent [140]. However, PET may be preferred in balanced 3-vessel disease since it permits absolute quantification of myocardial blood flow. Conversely, SPECT can only provide semi-quantitative values (normalized to the maximum value), failing to detect relative perfusion differences [141, 142]. Moreover, even SPECT specificity in competitive athletes has to be considered reduced, given that myocardial perfusion defects can be present also in healthy young male athletes, and they are associated with LV hypertrophy and no wall motion abnormalities on echocardiography [142]. Thus, cardiac nuclear imaging in the athlete’s setting is more suitable for research purposes than for a clinical application and should not be recommended as a first-line test in competitive athletes [123]. According to European guidelines [8], nuclear imaging may also be considered an alternative or complementary exam to ESE or CCT for evaluating asymptomatic individuals aged 35 years with CV risk factors before engaging in high or very high-intensity sports.
3.3.5 Genetic Testing in Athletes
Genetic testing is a valuable tool for diagnosing several inherited cardiac disorders [120, 143]. In athletes, it can be beneficial in terms of diagnosis, management, decisions relating to sports participation, and prognosis [144]. Moreover, identifying disease-causing mutations allows cascade screening in first-degree family members due to the autosomal dominant pattern in most inherited cardiac disorders [145].
The diagnostic yield of genetic testing is significantly different according to the clinical phenotype of the athlete. From a general point of view, genetic testing in diagnosing an inherited cardiac disorder is useful in individuals with clear phenotypes. In recent years, attention has been given to the diagnostic role of genetic testing in an individual who exhibits an overlapping phenotype between inherited cardiac disease and athlete’s heart [146, 147]. In selected cases, when a comprehensive clinical evaluation is suspicious but fails to reach a definitive diagnosis of inherited cardiac disease, genetic testing may be considered, keeping in mind the specific diagnostic yield for each disease and that it can be even lower in athletes. The benefit of genetic testing should always be weighed with potential harm. The genetic testing panel should only include genes with supporting solid evidence to cause the athletes’ clinical phenotype to minimize the identification of a variant of uncertain significance or allelic variants associated with the different clinical phenotype [148], which increases the difficulties inherent to the interpretation of genetic testing results.
Since the diagnostic yield of genetic testing is significantly different according to the clinical phenotype of the athlete, physicians involved in the athlete’s management should have a solid understanding of the indications, strengths and limitations of genetic testing (Table 8, Ref. [120]).
Table 8.
Indication of genetic testing in competitive athletes [120].
| Genetic test recommendations | Pre-test probability | ECG abnormalities |
| Recommended | High | HCM |
| DCM | ||
| ACM | ||
| LQTS | ||
| CPVT | ||
| May be recommended | Intermediate | LVH + additional features |
| LV + additional features | ||
| RV dilatation + additional features | ||
| QTC 480 ms + additional features | ||
| NSVT or polymorphic PVC + additional features | ||
| Not recommended | Low | Isolated LVH |
| Isolated LV dilatation | ||
| Isolated RV dilatation | ||
| Isolated QT prolongation | ||
| Isolated monomorphic PVC | ||
| Isolated T-wave inversion |
ECG, electrocardiography; LVH, left ventricle hypertrophy; LV, left ventricle; RV, right ventricle; PVC, premature ventricular contraction; NSVT, non-sustained ventricular tachycardia; HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; ACM, arrhythmogenic cardiomyopathy; LQTS, long QT syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia.
4. The Grey Zones in the Athlete’s Heart
Athlete’s heart is characterized by cardiac remodeling features that can resemble those found in pathological conditions. Distinguishing athletic cardiac remodeling from cardiomyopathy is a frequent clinical dilemma for physicians evaluating an athlete [3, 72]. There are, in fact, several “grey zones” in which physiology and pathology overlap, and therefore it is essential to relate the degree of cardio-circulatory adaptations of athletes to the biomechanical characteristics of the practiced sport [122, 149]: LV wall thickening, LV dilatation, RV dilatation and LV hypertrabeculation (Table 9). It is, therefore, necessary to have a precise definition of the features of the athlete’s heart and stringent criteria to optimize the clinical management of these subjects to be able to make a differential diagnosis with HCM [150], DCM [151], left ventricular noncompaction (LVNC) and ACM.
Table 9.
Differential diagnosis between athlete’s heart and SCD-related cardiomyopathies in diagnostic grey zones.
| LV wall thickening | LV dilatation | RV dilatation | LV hypertrabeculation | |
| Athlete’s heart findings | Strength and mixed disciplines (more common) | Endurance athletes (typically) | Endurance and mixed disciplines (more common) | Afro-Caribbean ethnicity |
| Afro-Caribbean ethnicity (more common) | Asymptomatic | Asymptomatic | Asymptomatic | |
| Male gender (more common) | Unremarkable family history | Unremarkable family history | Unremarkable family history | |
| Normal SBP | Normal ECG | Normal ECG | Normal ECG | |
| Asymptomatic | Sometimes association with mild reduction in LVEF with normal function during the exercise | |||
| Unremarkable family history | ||||
| Normal ECG | ||||
| Mild symmetric and balanced LVH (often reversible after detraining) | Concomitant RV dilatation and/or mild LVH | RV dilatation often reversible after detraining | Normal LV systolic function | |
| Concomitant absence of a small LV cavity size | Preserved/mildly reduced LVEF with normal function during the exercise | Absence of wall motion abnormalities | Normal LV GLS | |
| Normal/supranormal LV diastolic function | Normal LV GLS | Concomitant LV dilatation | Normal/supranormal LV diastolic function | |
| Preserved LV systolic function | Normal/supranormal LV diastolic function | Normal RV morphology | Normal or increased compacted LV wall thickness | |
| Normal LV GLS | Normal/mildly enlarged LA and RA | Preserved or mildly reduced LVEF with normal function during the exercise | ||
| Normal aortic and mitral valves | Normal LV GLS | |||
| Normal/supranormal LV diastolic function | ||||
| Normal/mildly enlarged LA and RA | ||||
| Normal RV systolic function | ||||
| Normal RV GLS | ||||
| Normal sPAP or eventually upper limits | ||||
| Normal tricuspid and pulmonary valve | ||||
| Suspicious findings | Isolated/asymmetric LVH (not reversible with detraining) | Reduced LV systolic function | RV dilatation not reversable with detraining | Compacted layer 5 mm |
| LV diastolic disfunction | LV diastolic disfunction | Reduced LV systolic function | Reduced LV systolic function | |
| Other anatomic abnormalities (mitral valve leaflet elongation, anomalous papillary muscle insertion, myocardial crypts or recesses) | Presence of wall motion abnormalities | Reduced RV function | LGE on CMR | |
| Exercise-induced LVOT or mid-cavity obstruction on ESE | Impaired contractile reserve during ESE or stress CMR | RV morphology abnormalities (sacculations, aneurysms, and focal thinning) | ||
| LGE/fibrosis on CMR | Presence of wall motion abnormalities | |||
| Impaired contractile reserve during ESE or stress CMR | ||||
| Differential diagnosis | HCM | DCM | ACM | LVNC |
| Hypertensive heart | Toxic CMP | Toxic CMP | Recent pregnancy | |
| Anabolic steroid abuse | Myocarditis | Pulmonary hypertension | Sickle cell disease | |
| Infiltrative heart disease | Nutritional deficiency | CHD | Aortic/mitral regurgitation | |
| Valvulopathy | Tachyarrhythmias-mediated CMP | Valvulopathy | ||
| Valvulopathy |
HCM, Hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; LVNC, left ventricular noncompaction; ACM, arrhythmogenic cardiomyopathy; LVEF, left ventricle ejection fraction; LV, left ventricle; LA, left atrial; GLS, global longitudinal strain; RV, right ventricle; LGE, late gadolinium enhancement; CMR, cardiac magnetic resonance; CMP, cardiomyopathy; LVH, left ventricle hypertrophy; CHD, congenital heart disease; sPAP, systolic pulmonary artery pressure; ESE, exercise stress echocardiography; LVOT, left ventricle outflow tract; SBP, systolic blood pressure; SCD, sudden cardiac death; ECG, electrocardiography.
It must be emphasized that the effective use of clinical imaging data requires integration with other aspects of the clinical presentation, including the presence or absence of symptoms, a family history of genetic heart disease or SCD, the 12 lead ECG and maximal exercise testing. Therefore, the choice between the proposed step-by-step approach must always be guided by the clinical suspicions, considering the entire clinical scenario the entire spectrum of CV diseases that can afflict the athlete (Table 10).
Table 10.
Practical approach to the athlete’s heart diagnosis.
| 1st-line screening | 2nd-line screening | Clinical suspicious | 3rd-line screening | |
| History + physical examination + ECG | Echocardiography + EST/CPET + 24-hours ECG Holter | First choice | Second choice | |
| Cardiomyopathies | CMR | Genetic testing | ||
| CAD | Echo-stress | CCT, SPECT or PET | ||
| Valvulopathies | CMR | Echo-stress | ||
| Myocarditis, pericarditis | CMR | |||
| Coronary artery abnormalities | CCT | |||
| Aorthopathies | CCT | |||
| Channelopathies | Genetic testing | |||
CAD, coronary artery disease; ECG, electrocardiogram; EST, exercise stress test; CPET, cardiopulmonary exercise test; CMR, cardiac magnetic resonance; CCT, cardiac computer tomography; SPECT, single photon emission computer tomography; PET, positron emission tomography.
5. Conclusions
Discriminating the athlete’s heart from the differential diagnosis of early-phenotype cardiomyopathy or a concealed cardiovascular pathology requires a comprehensive diagnostic work-up based on morphologic, electrical, structural, and functional evaluations. Since the wide availability and the indications of several multimodality techniques, a practical step-by-step approach is helpful to systematically proceed in the evaluation, if indicated after the first-line screening PPS, only if second- and third-line diagnostic modalities are needed.
Despite reducing the false-positive rate, many athletes inevitably fall into the grey zone with multiple layers of overlap between pathology and physiologic remodeling. A multimodality cardiovascular diagnostic approach can play a central role in supporting an appropriate final diagnosis.
Acknowledgment
Vectors image courtesy of FreeDigitalPhotos.net.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
SP, EC, FD, SC, and FR designed the research study. MV, AS, EB, LC, GL, and ABif performed the research. EM analyzed the data. ALG, ABag, and AD conceived the study and wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All author greed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Elena Cavarretta is serving as one of the Guest editors and Giuseppe Limongelli is serving as one of the Editorial Board members of this journal. We declare that Elena Cavarretta and Giuseppe Limongelli had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Zhonghua Sun.
References
- [1].Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports . 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- [2].Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 2: Preparticipation Screening for Cardiovascular Disease in Competitive Athletes: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation . 2015;132:e267–e272. doi: 10.1161/CIR.0000000000000238. [DOI] [PubMed] [Google Scholar]
- [3].De Innocentiis C, Ricci F, Khanji MY, Aung N, Tana C, Verrengia E, et al. Athlete’s Heart: Diagnostic Challenges and Future Perspectives. Sports Medicine . 2018;48:2463–2477. doi: 10.1007/s40279-018-0985-2. [DOI] [PubMed] [Google Scholar]
- [4].Prior DL, La Gerche A. The athlete’s heart. Heart . 2012;98:947–955. doi: 10.1136/heartjnl-2011-301329. [DOI] [PubMed] [Google Scholar]
- [5].Fagard R. Athlete’s heart. Heart . 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pavlik G, Major Z, Varga-Pintér B, Jeserich M, Kneffel Z. The athlete’s heart Part I (Review) Acta Physiologica Hungarica . 2010;97:337–353. doi: 10.1556/APhysiol.97.2010.4.1. [DOI] [PubMed] [Google Scholar]
- [7].Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults. Journal of the American College of Cardiology . 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- [8].Sharma S, Pelliccia A, Gati S. The ‘Ten Commandments’ for the 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. European Heart Journal . 2021;42:6–7. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]
- [9].D’Ascenzi F, Valentini F, Pistoresi S, Frascaro F, Piu P, Cavigli L, et al. Causes of sudden cardiac death in young athletes and non-athletes: systematic review and meta-analysis: Sudden cardiac death in the young. Trends in Cardiovascular Medicine . 2022;32:299–308. doi: 10.1016/j.tcm.2021.06.001. [DOI] [PubMed] [Google Scholar]
- [10].Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. Journal of the American College of Cardiology . 2014;64:1479–1514. doi: 10.1016/j.jacc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- [11].Drezner JA, O’Connor FG, Harmon KG, Fields KB, Asplund CA, Asif IM, et al. AMSSM Position Statement on Cardiovascular Preparticipation Screening in Athletes: Current evidence, knowledge gaps, recommendations and future directions. British Journal of Sports Medicine . 2017;51:153–167. doi: 10.1136/bjsports-2016-096781. [DOI] [PubMed] [Google Scholar]
- [12].Palermi S, Sirico F, Fernando F, Gregori G, Belviso I, Ricci F, et al. Limited diagnostic value of questionnaire-based pre-participation screening algorithms: a “risk-exposed” approach to sports activity. Journal of Basic and Clinical Physiology and Pharmacology . 2022;33:655–663. doi: 10.1515/jbcpp-2022-0109. [DOI] [PubMed] [Google Scholar]
- [13].Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Brugada Terradellas J, et al. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. European Journal of Preventive Cardiology . 2017;24:41–69. doi: 10.1177/2047487316676042. [DOI] [PubMed] [Google Scholar]
- [14].Delise P, Mos L, Sciarra L, Basso C, Biffi A, Cecchi F, et al. Italian Cardiological Guidelines (COCIS) for Competitive Sport Eligibility in athletes with heart disease: update 2020. Journal of Cardiovascular Medicine . 2021;22:874–891. doi: 10.2459/JCM.0000000000001186. [DOI] [PubMed] [Google Scholar]
- [15].Palermi S, Serio A, Vecchiato M, Sirico F, Gambardella F, Ricci F, et al. Potential role of an athlete-focused echocardiogram in sports eligibility. World Journal of Cardiology . 2021;13:271–297. doi: 10.4330/wjc.v13.i8.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ricci F, Sutton R, Palermi S, Tana C, Renda G, Gallina S, et al. Prognostic significance of noncardiac syncope in the general population: A systematic review and meta-analysis. Journal of Cardiovascular Electrophysiology . 2018;29:1641–1647. doi: 10.1111/jce.13715. [DOI] [PubMed] [Google Scholar]
- [17].Lee L, Addetia K, Singh A. Echocardiographic Evaluation of the Athlete’s Heart: Focused Review and Update. Current Cardiology Reports . 2022;24:1907–1916. doi: 10.1007/s11886-022-01812-3. [DOI] [PubMed] [Google Scholar]
- [18].Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annual Review of Physiology . 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- [19].Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annual Review of Physiology . 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- [20].Martinez MW, Kim JH, Shah AB, Phelan D, Emery MS, Wasfy MM, et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology . 2021;78:1453–1470. doi: 10.1016/j.jacc.2021.08.003. [DOI] [PubMed] [Google Scholar]
- [21].Castelletti S, Gati S. The Female Athlete’s Heart: Overview and Management of Cardiovascular Diseases. European Cardiology . 2021;16:e47. doi: 10.15420/ecr.2021.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pieles GE, Stuart AG. The adolescent athlete’s heart; A miniature adult or grown-up child. Clinical Cardiology . 2020;43:852–862. doi: 10.1002/clc.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McClean G, Riding NR, Ardern CL, Farooq A, Pieles GE, Watt V, et al. Electrical and structural adaptations of the paediatric athlete’s heart: a systematic review with meta-analysis. British Journal of Sports Medicine . 2018;52:230. doi: 10.1136/bjsports-2016-097052. [DOI] [PubMed] [Google Scholar]
- [24].Rodriguez-López AM, Javier G, Carmen P, Esteban P, Luisa GC, Tomas F, et al. Athlete Heart in Children and Young Athletes. Echocardiographic Findings in 331 Cases. Pediatric Cardiology . 2022;43:407–412. doi: 10.1007/s00246-021-02736-5. [DOI] [PubMed] [Google Scholar]
- [25].Palermi S, Bragazzi NL, Cular D, Ardigò LP, Padulo J. How chest press-based exercises can alleviate the burden of cardiovascular diseases. Human Movement . 2022;23:88–98. [Google Scholar]
- [26].Oxborough D, Augustine D, Gati S, George K, Harkness A, Mathew T, et al. A guideline update for the practice of echocardiography in the cardiac screening of sports participants: a joint policy statement from the British Society of Echocardiography and Cardiac Risk in the Young. Echo Research and Practice . 2018;5:G1–G10. doi: 10.1530/ERP-17-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Annals of Internal Medicine . 1975;82:521–524. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- [28].Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography . 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [29].Kooreman Z, Giraldeau G, Finocchiaro G, Kobayashi Y, Wheeler M, Perez M, et al. Athletic Remodeling in Female College Athletes: The “Morganroth Hypothesis” Revisited. Clinical Journal of Sport Medicine . 2019;29:224–231. doi: 10.1097/JSM.0000000000000501. [DOI] [PubMed] [Google Scholar]
- [30].D’Ascenzi F, Biella F, Lemme E, Maestrini V, Di Giacinto B, Pelliccia A. Female Athlete’s Heart: Sex Effects on Electrical and Structural Remodeling. Circulation: Cardiovascular Imaging . 2020;13:e011587. doi: 10.1161/CIRCIMAGING.120.011587. [DOI] [PubMed] [Google Scholar]
- [31].Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Comprehensive Physiology . 2015;5:475–512. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- [32].Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Medicine . 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- [33].Heidbuchel H. The athlete’s heart is a proarrhythmic heart, and what that means for clinical decision making. Europace . 2018;20:1401–1411. doi: 10.1093/europace/eux294. [DOI] [PubMed] [Google Scholar]
- [34].Tyler CJ, Reeve T, Hodges GJ, Cheung SS. The Effects of Heat Adaptation on Physiology, Perception and Exercise Performance in the Heat: A Meta-Analysis. Sports Medicine (Auckland, N.Z.) . 2016;46:1699–1724. doi: 10.1007/s40279-016-0538-5. [DOI] [PubMed] [Google Scholar]
- [35].Veicsteinas A, Ferretti G, Rennie DW. Superficial shell insulation in resting and exercising men in cold water. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology . 1982;52:1557–1564. doi: 10.1152/jappl.1982.52.6.1557. [DOI] [PubMed] [Google Scholar]
- [36].Fitz-Clarke JR. Breath-Hold Diving. Comprehensive Physiology . 2018;8:585–630. doi: 10.1002/cphy.c160008. [DOI] [PubMed] [Google Scholar]
- [37].Siebenmann C, Lundby C. Regulation of cardiac output in hypoxia. Scandinavian Journal of Medicine & Science in Sports . 2015;25 Suppl 4:53–59. doi: 10.1111/sms.12619. [DOI] [PubMed] [Google Scholar]
- [38].Grover RF, Weil JV, Reeves JT. Cardiovascular adaptation to exercise at high altitude. Exercise and Sport Sciences Reviews . 1986;14:269–302. [PubMed] [Google Scholar]
- [39].Strano Rossi S, Botrè F. Prevalence of illicit drug use among the Italian athlete population with special attention on drugs of abuse: a 10-year review. Journal of Sports Sciences . 2011;29:471–476. doi: 10.1080/02640414.2010.543915. [DOI] [PubMed] [Google Scholar]
- [40].D’Andrea A, Radmilovic J, Russo V, Sperlongano S, Carbone A, Di Maio M, et al. Biventricular dysfunction and lung congestion in athletes on anabolic androgenic steroids: a speckle tracking and stress lung echocardiography analysis. European Journal of Preventive Cardiology . 2022;28:1928–1938. doi: 10.1093/eurjpc/zwab086. [DOI] [PubMed] [Google Scholar]
- [41].D’Andrea A, Caso P, Severino S, Galderisi M, Sarubbi B, Limongelli G, et al. Effects of different training protocols on left ventricular myocardial function in competitive athletes: a Doppler tissue imaging study. Italian Heart Journal . 2002;3:34–40. [PubMed] [Google Scholar]
- [42].Adami PE, Koutlianos N, Baggish A, Bermon S, Cavarretta E, Deligiannis A, et al. Cardiovascular effects of doping substances, commonly prescribed medications and ergogenic aids in relation to sports: a position statement of the sport cardiology and exercise nucleus of the European Association of Preventive Cardiology. European Journal of Preventive Cardiology . 2022;29:559–575. doi: 10.1093/eurjpc/zwab198. [DOI] [PubMed] [Google Scholar]
- [43].Wilhelm M, Abreu A, Adami PE, Ambrosetti M, Antonopoulou M, Biffi A, et al. EAPC Core Curriculum for Preventive Cardiology. European Journal of Preventive Cardiology . 2022;29:251–274. doi: 10.1093/eurjpc/zwab017. [DOI] [PubMed] [Google Scholar]
- [44].Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, et al. International Recommendations for Electrocardiographic Interpretation in Athletes. Journal of the American College of Cardiology . 2017;69:1057–1075. doi: 10.1016/j.jacc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- [45].Giese EA, O’Connor FG, Brennan FH, Depenbrock PJ, Oriscello RG. The athletic preparticipation evaluation: cardiovascular assessment. American Family Physician . 2007;75:1008–1014. [PubMed] [Google Scholar]
- [46].Austin AV, Owens DS, Prutkin JM, Salerno JC, Ko B, Pelto HF, et al. Do ‘pathologic’ cardiac murmurs in adolescents identify structural heart disease? An evaluation of 15 141 active adolescents for conditions that put them at risk of sudden cardiac death. British Journal of Sports Medicine . 2022;56:88–94. doi: 10.1136/bjsports-2019-101718. [DOI] [PubMed] [Google Scholar]
- [47].Harmon KG, Zigman M, Drezner JA. The effectiveness of screening history, physical exam, and ECG to detect potentially lethal cardiac disorders in athletes: a systematic review/meta-analysis. Journal of Electrocardiology . 2015;48:329–338. doi: 10.1016/j.jelectrocard.2015.02.001. [DOI] [PubMed] [Google Scholar]
- [48].Myerburg RJ, Vetter VL. Electrocardiograms should be included in preparticipation screening of athletes. Circulation . 2007;116:2616–2626. doi: 10.1161/CIRCULATIONAHA.107.733519. [DOI] [PubMed] [Google Scholar]
- [49].Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. The Journal of the American Medical Association . 2006;296:1593–1601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- [50].Baggish AL, Hutter AM, Jr, Wang F, Yared K, Weiner RB, Kupperman E, et al. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Annals of Internal Medicine . 2010;152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- [51].Pelliccia A, Di Paolo FM, Maron BJ. The athlete’s heart: remodeling, electrocardiogram and preparticipation screening. Cardiology in Review . 2002;10:85–90. doi: 10.1097/00045415-200203000-00006. [DOI] [PubMed] [Google Scholar]
- [52].D’Andrea A, Limongelli G, Caso P, Sarubbi B, Della Pietra A, Brancaccio P, et al. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete’s heart. International Journal of Cardiology . 2002;86:177–184. doi: 10.1016/s0167-5273(02)00194-8. [DOI] [PubMed] [Google Scholar]
- [53].D’Andrea A, Caso P, Severino S, Sarubbi B, Forni A, Cice G, et al. Different involvement of right ventricular myocardial function in either physiologic or pathologic left ventricular hypertrophy: a Doppler tissue study. Journal of the American Society of Echocardiography . 2003;16:154–161. doi: 10.1067/mje.2003.29. [DOI] [PubMed] [Google Scholar]
- [54].D’Andrea A, Caso P, Sarubbi B, Limongelli G, Liccardo B, Cice G, et al. Right ventricular myocardial adaptation to different training protocols in top-level athletes. Echocardiography . 2003;20:329–336. doi: 10.1046/j.1540-8175.2003.03038.x. [DOI] [PubMed] [Google Scholar]
- [55].Abela M, Sharma S. Abnormal ECG Findings in Athletes: Clinical Evaluation and Considerations. Current Treatment Options in Cardiovascular Medicine . 2019;21:95. doi: 10.1007/s11936-019-0794-4. [DOI] [PubMed] [Google Scholar]
- [56].De Castro S, Pelliccia A, Caselli S, Di Angelantonio E, Papetti F, Cavarretta E, et al. Remodelling of the left ventricle in athlete’s heart: a three dimensional echocardiographic and magnetic resonance imaging study. Heart . 2006;92:975–976. doi: 10.1136/hrt.2005.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ragazzoni GL, Cavigli L, Cavarretta E, Maffei S, Mandoli GE, Pastore MC, et al. How to evaluate resting ECG and imaging in children practising sport: a critical review and proposal of an algorithm for ECG interpretation. European Journal of Preventive Cardiology . 2023;30:375–383. doi: 10.1093/eurjpc/zwac218. [DOI] [PubMed] [Google Scholar]
- [58].Donati F, Guicciardi C, Lodi E, Fernando F, Palermi S, Modena MG, et al. Echocardiography in the preparticipation screening: an old topic revisited. Journal of Cardiovascular Medicine . 2023;24:297–301. doi: 10.2459/JCM.0000000000001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Malhotra A, Dhutia H, Finocchiaro G, Gati S, Beasley I, Clift P, et al. Outcomes of Cardiac Screening in Adolescent Soccer Players. The New England Journal of Medicine . 2018;379:524–534. doi: 10.1056/NEJMoa1714719. [DOI] [PubMed] [Google Scholar]
- [60].Riding N, Williams CA, Ryding D, Stuart AG. Cardiac screening and profiling of the elite academy footballer : How new heart imaging methods can help pick up underlying heart disease in young athletes. 2022. [(Accessed: 1 March 2023)]. Available at: https://global.medical.canon/publication/ultrasound/wp_ul_MWPUS0017EA_2022-08.
- [61].Brunetti G, Graziano F, Cavigli L, Cipriani A, D’Ascenzi F, Bauce B, et al. Reproducibility of ventricular arrhythmias at exercise testing for prediction of non-ischaemic left ventricular scar in athletes. European Journal of Preventive Cardiology . 2023;30:107–116. doi: 10.1093/eurjpc/zwac224. [DOI] [PubMed] [Google Scholar]
- [62].Lotrionte M, Cavarretta E, Abbate A, Mezzaroma E, De Marco E, Di Persio S, et al. Temporal changes in standard and tissue Doppler imaging echocardiographic parameters after anthracycline chemotherapy in women with breast cancer. The American Journal of Cardiology . 2013;112:1005–1012. doi: 10.1016/j.amjcard.2013.05.038. [DOI] [PubMed] [Google Scholar]
- [63].Segreti A, Picarelli F, DI Gioia G, Coletti F, Crispino SP, Fanale V, et al. Athlete’s heart or heart disease in the athlete? Evaluation by cardiopulmonary exercise testing. The Journal of Sports Medicine and Physical Fitness . 2023 doi: 10.23736/S0022-4707.23.14536-1. online ahead of print. [DOI] [PubMed] [Google Scholar]
- [64].Husaini M, Emery MS. Cardiopulmonary Exercise Testing Interpretation in Athletes: What the Cardiologist Should Know. Cardiology Clinics . 2023;41:71–80. doi: 10.1016/j.ccl.2022.08.006. [DOI] [PubMed] [Google Scholar]
- [65].Pelliccia A, Heidbuchel H, Corrado D, Borjesson M, Sharma S. The ESC Textbook of Sports Cardiology. Oxford University Press OUK. editor. 2019. [(Accessed: 6 November 2022)]. Available at: https://global.oup.com/academic/product/the-esc-textbook-of-sports-cardiology-9780191824791?cc=ch&lang=en&.
- [66].Sharma S, Merghani A, Mont L. Exercise and the heart: the good, the bad, and the ugly. European Heart Journal . 2015;36:1445–1453. doi: 10.1093/eurheartj/ehv090. [DOI] [PubMed] [Google Scholar]
- [67].D’Ascenzi F, Anselmi F, Mondillo S, Finocchiaro G, Caselli S, Garza MSDL, et al. The use of cardiac imaging in the evaluation of athletes in the clinical practice: A survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association of Cardiovascular Imaging, the European Heart Rhythm Association and the ESC Working Group on Myocardial and Pericardial Diseases. European Journal of Preventive Cardiology . 2021;28:1071–1077. doi: 10.1177/2047487320932018. [DOI] [PubMed] [Google Scholar]
- [68].Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, McKenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. Journal of the American College of Cardiology . 2002;40:1431–1436. doi: 10.1016/s0735-1097(02)02270-2. [DOI] [PubMed] [Google Scholar]
- [69].Basavarajaiah S, Boraita A, Whyte G, Wilson M, Carby L, Shah A, et al. Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. Journal of the American College of Cardiology . 2008;51:2256–2262. doi: 10.1016/j.jacc.2007.12.061. [DOI] [PubMed] [Google Scholar]
- [70].Zaidi A, Ghani S, Sharma R, Oxborough D, Panoulas VF, Sheikh N, et al. Physiological right ventricular adaptation in elite athletes of African and Afro-Caribbean origin. Circulation . 2013;127:1783–1792. doi: 10.1161/CIRCULATIONAHA.112.000270. [DOI] [PubMed] [Google Scholar]
- [71].Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. Journal of the American College of Cardiology . 2005;46:690–696. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- [72].Carbone A, D’Andrea A, Riegler L, Scarafile R, Pezzullo E, Martone F, et al. Cardiac damage in athlete’s heart: When the “supernormal” heart fails. World Journal of Cardiology . 2017;9:470–480. doi: 10.4330/wjc.v9.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Baggish AL, Battle RW, Beaver TA, Border WL, Douglas PS, Kramer CM, et al. Recommendations on the Use of Multimodality Cardiovascular Imaging in Young Adult Competitive Athletes: A Report from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography . 2020;33:523–549. doi: 10.1016/j.echo.2020.02.009. [DOI] [PubMed] [Google Scholar]
- [74].Niederseer D, Rossi VA, Kissel C, Scherr J, Caselli S, Tanner FC, et al. Role of echocardiography in screening and evaluation of athletes. Heart . 2020;107:262–263. doi: 10.1136/heartjnl-2020-317996. [DOI] [PubMed] [Google Scholar]
- [75].Boraita A, Díaz-Gonzalez L, Valenzuela PL, Heras ME, Morales-Acuna F, Castillo-García A, et al. Normative Values for Sport-Specific Left Ventricular Dimensions and Exercise-Induced Cardiac Remodeling in Elite Spanish Male and Female Athletes. Sports Medicine - Open . 2022;8:116. doi: 10.1186/s40798-022-00510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Magalski A, McCoy M, Zabel M, Magee LM, Goeke J, Main ML, et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. The American Journal of Medicine . 2011;124:511–518. doi: 10.1016/j.amjmed.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [77].Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. The New England Journal of Medicine . 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- [78].D’Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R, et al. Left atrial volume index in highly trained athletes. American Heart Journal . 2010;159:1155–1161. doi: 10.1016/j.ahj.2010.03.036. [DOI] [PubMed] [Google Scholar]
- [79].Boraita A, Heras ME, Morales F, Marina-Breysse M, Canda A, Rabadan M, et al. Reference Values of Aortic Root in Male and Female White Elite Athletes According to Sport. Circulation: Cardiovascular Imaging . 2016;9:e005292. doi: 10.1161/CIRCIMAGING.116.005292. [DOI] [PubMed] [Google Scholar]
- [80].Gjerdalen GF, Hisdal J, Solberg EE, Andersen TE, Radunovic Z, Steine K. Atrial Size and Function in Athletes. International Journal of Sports Medicine . 2015;36:1170–1176. doi: 10.1055/s-0035-1555780. [DOI] [PubMed] [Google Scholar]
- [81].D’Ascenzi F, Pelliccia A, Solari M, Piu P, Loiacono F, Anselmi F, et al. Normative Reference Values of Right Heart in Competitive Athletes: A Systematic Review and Meta-Analysis. Journal of the American Society of Echocardiography . 2017;30:845–858. doi: 10.1016/j.echo.2017.06.013. e2. [DOI] [PubMed] [Google Scholar]
- [82].Cavarretta E, Maffessanti F, Sperandii F, Guerra E, Quaranta F, Nigro A, et al. Reference values of left heart echocardiographic dimensions and mass in male peri-pubertal athletes. European Journal of Preventive Cardiology . 2018;25:1204–1215. doi: 10.1177/2047487318776084. [DOI] [PubMed] [Google Scholar]
- [83].Cantinotti M, Koestenberger M, Santoro G, Assanta N, Franchi E, Paterni M, et al. Normal basic 2D echocardiographic values to screen and follow up the athlete’s heart from juniors to adults: What is known and what is missing. A critical review. European Journal of Preventive Cardiology . 2020;27:1294–1306. doi: 10.1177/2047487319862060. [DOI] [PubMed] [Google Scholar]
- [84].D’Ascenzi F, Caselli S, Solari M, Pelliccia A, Cameli M, Focardi M, et al. Novel echocardiographic techniques for the evaluation of athletes’ heart: A focus on speckle-tracking echocardiography. European Journal of Preventive Cardiology . 2016;23:437–446. doi: 10.1177/2047487315586095. [DOI] [PubMed] [Google Scholar]
- [85].Beaumont A, Grace F, Richards J, Hough J, Oxborough D, Sculthorpe N. Left Ventricular Speckle Tracking-Derived Cardiac Strain and Cardiac Twist Mechanics in Athletes: A Systematic Review and Meta-Analysis of Controlled Studies. Sports Medicine . 2017;47:1145–1170. doi: 10.1007/s40279-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. European Heart Journal: Cardiovascular Imaging . 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- [87].Tokodi M, Oláh A, Fábián A, Lakatos BK, Hizoh I, Ruppert M, et al. Novel insights into the athlete’s heart: is myocardial work the new champion of systolic function? European Heart Journal. Cardiovascular Imaging . 2022;23:188–197. doi: 10.1093/ehjci/jeab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Biffi A, Gallo G, Fernando F, Sirico F, Signorello MG, De Martino L, et al. Relationship Between Cardiorespiratory Fitness, Baseline Blood Pressure and Hypertensive Response to Exercise in the Ferrari Corporate Population. High Blood Pressure & Cardiovascular Prevention . 2022;29:81–88. doi: 10.1007/s40292-021-00491-5. [DOI] [PubMed] [Google Scholar]
- [89].Vecchiato M, Neunhaeuserer D, Fabris M, Aghi A, Quinto G, Battista F, et al. The impact of the COVID-19 pandemic on functional capacity in a population of young athletes: should we expect long-time consequences. The Journal of Sports Medicine and Physical Fitness . 2023 doi: 10.23736/S0022-4707.22.14468-3. online ahead of print. [DOI] [PubMed] [Google Scholar]
- [90].Parizher G, Emery MS. Exercise Stress Testing in Athletes. Clinics in Sports Medicine . 2022;41:441–454. doi: 10.1016/j.csm.2022.02.006. [DOI] [PubMed] [Google Scholar]
- [91].Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) Journal of the American College of Cardiology . 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- [92].Löllgen H, Leyk D. Exercise Testing in Sports Medicine. Deutsches Arzteblatt International . 2018;115:409–416. doi: 10.3238/arztebl.2018.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Desai MY, Crugnale S, Mondeau J, Helin K, Mannting F. Slow upsloping ST-segment depression during exercise: does it really signify a positive stress test. American Heart Journal . 2002;143:482–487. doi: 10.1067/mhj.2002.120771. [DOI] [PubMed] [Google Scholar]
- [94].Caselli S, Serdoz A, Mango F, Lemme E, Vaquer Seguì A, Milan A, et al. High blood pressure response to exercise predicts future development of hypertension in young athletes. European Heart Journal . 2019;40:62–68. doi: 10.1093/eurheartj/ehy810. [DOI] [PubMed] [Google Scholar]
- [95].Caselli S, Vaquer Segui A, Quattrini F, Di Gacinto B, Milan A, Assorgi R, et al. Upper normal values of blood pressure response to exercise in Olympic athletes. American Heart Journal . 2016;177:120–128. doi: 10.1016/j.ahj.2016.04.020. [DOI] [PubMed] [Google Scholar]
- [96].D’Ascenzi F, Zorzi A, Alvino F, Bonifazi M, Corrado D, Mondillo S. The prevalence and clinical significance of premature ventricular beats in the athlete. Scandinavian Journal of Medicine & Science in Sports . 2017;27:140–151. doi: 10.1111/sms.12679. [DOI] [PubMed] [Google Scholar]
- [97].Corrado D, Drezner JA, D’Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. British Journal of Sports Medicine . 2020;54:1142–1148. doi: 10.1136/bjsports-2018-100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Boraita A, Heras ME, Valenzuela PL, Diaz-Gonzalez L, Morales-Acuna F, Alcocer-Ayuga M, et al. Holter-determined arrhythmias in young elite athletes with suspected risk: Insights from a 20-year experience. Frontiers in Cardiovascular Medicine . 2022;9:896148. doi: 10.3389/fcvm.2022.896148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gajda R, Biernacka EK, Drygas W. Are heart rate monitors valuable tools for diagnosing arrhythmias in endurance athletes. Scandinavian Journal of Medicine & Science in Sports . 2018;28:496–516. doi: 10.1111/sms.12917. [DOI] [PubMed] [Google Scholar]
- [100].Sciarra L, Cavarretta E, Siciliani S, Sette A, Scarà A, Grieco D, et al. Managing athletes with palpitations of unknown origin with an external loop recorder: a cohort study. The Journal of Sports Medicine and Physical Fitness . 2022;62:554–559. doi: 10.23736/S0022-4707.21.12831-2. [DOI] [PubMed] [Google Scholar]
- [101].Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC: Heart Failure . 2016;4:607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- [102].De Rocco Ponce M, Vecchiato M, Neunhaeuserer D, Battista F, Caretta N, Savalla F, et al. Association Between Penile Color Doppler Ultrasonography and Cardiorespiratory Fitness in Patients With Vascular Erectile Dysfunction. Sexual Medicine . 2021;9:100347. doi: 10.1016/j.esxm.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary Exercise Testing: What Is its Value. Journal of the American College of Cardiology . 2017;70:1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- [104].Neunhaeuserer D, Battista F, Mazzucato B, Vecchiato M, Meneguzzo G, Quinto G, et al. Exercise Capacity and Cardiorespiratory Fitness in Children with Congenital Heart Diseases: A Proposal for an Adapted NYHA Classification. International Journal of Environmental Research and Public Health . 2022;19:5907. doi: 10.3390/ijerph19105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Vecchiato M, Ermolao A, Zanardo E, Battista F, Ruvoletto G, Palermi S, et al. Overshoot of the Respiratory Exchange Ratio during Recovery from Maximal Exercise Testing in Young Patients with Congenital Heart Disease. Children . 2023;10:521. doi: 10.3390/children10030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pieles GE, Gowing L, Ryding D, Perry D, McNally SR, Stuart AG, et al. Characterisation of LV myocardial exercise function by 2-D strain deformation imaging in elite adolescent footballers. European Journal of Applied Physiology . 2021;121:239–250. doi: 10.1007/s00421-020-04510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mazaheri R, Schmied C, Niederseer D, Guazzi M. Cardiopulmonary Exercise Test Parameters in Athletic Population: A Review. Journal of Clinical Medicine . 2021;10:5073. doi: 10.3390/jcm10215073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kaminsky LA, Arena R, Myers J, Peterman JE, Bonikowske AR, Harber MP, et al. Updated Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Mayo Clinic Proceedings . 2022;97:285–293. doi: 10.1016/j.mayocp.2021.08.020. [DOI] [PubMed] [Google Scholar]
- [109].Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. American Journal of Physiology: Heart and Circulatory Physiology . 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- [110].Petek BJ, Gustus SK, Wasfy MM. Cardiopulmonary Exercise Testing in Athletes: Expect the Unexpected. Current Treatment Options in Cardiovascular Medicine . 2021;23:49. doi: 10.1007/s11936-021-00928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation . 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. European Heart Journal . 2018;39:1144–1161. doi: 10.1093/eurheartj/ehw180. [DOI] [PubMed] [Google Scholar]
- [113].Petek BJ, Tso JV, Churchill TW, Guseh JS, Loomer G, DiCarli M, et al. Normative cardiopulmonary exercise data for endurance athletes: the Cardiopulmonary Health and Endurance Exercise Registry (CHEER) European Journal of Preventive Cardiology . 2022;29:536–544. doi: 10.1093/eurjpc/zwab150. [DOI] [PubMed] [Google Scholar]
- [114].Sharma S, Elliott PM, Whyte G, Mahon N, Virdee MS, Mist B, et al. Utility of metabolic exercise testing in distinguishing hypertrophic cardiomyopathy from physiologic left ventricular hypertrophy in athletes. Journal of the American College of Cardiology . 2000;36:864–870. doi: 10.1016/s0735-1097(00)00816-0. [DOI] [PubMed] [Google Scholar]
- [115].D’Ascenzi F, Cavigli L, Pagliaro A, Focardi M, Valente S, Cameli M, et al. Clinician approach to cardiopulmonary exercise testing for exercise prescription in patients at risk of and with cardiovascular disease. British Journal of Sports Medicine . 2022 doi: 10.1136/bjsports-2021-105261. online ahead of print. [DOI] [PubMed] [Google Scholar]
- [116].Lamberti V, Palermi S, Franceschin A, Scapol G, Lamberti V, Lamberti C, et al. The Effectiveness of Adapted Personalized Motor Activity (AMPA) to Improve Health in Individuals with Mental Disorders and Physical Comorbidities: A Randomized Controlled Trial. Sports . 2022;10:30. doi: 10.3390/sports10030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Compagno S, Palermi S, Pescatore V, Brugin E, Sarto M, Marin R, et al. Physical and psychological reconditioning in long COVID syndrome: Results of an out-of-hospital exercise and psychological - based rehabilitation program. International Journal of Cardiology. Heart & Vasculature . 2022;41:101080. doi: 10.1016/j.ijcha.2022.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bayonas-Ruiz A, Muñoz-Franco FM, Ferrer V, Pérez-Caballero C, Sabater-Molina M, Tomé-Esteban MT, et al. Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine . 2021;10:2312. doi: 10.3390/jcm10112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cavigli L, Olivotto I, Fattirolli F, Mochi N, Favilli S, Mondillo S, et al. Prescribing, dosing and titrating exercise in patients with hypertrophic cardiomyopathy for prevention of comorbidities: Ready for prime time. European Journal of Preventive Cardiology . 2021;28:1093–1099. doi: 10.1177/2047487320928654. [DOI] [PubMed] [Google Scholar]
- [120].Castelletti S, Gray B, Basso C, Behr ER, Crotti L, Elliott PM, et al. Indications and utility of cardiac genetic testing in athletes. European Journal of Preventive Cardiology . 2022;29:1582–1591. doi: 10.1093/eurjpc/zwac080. [DOI] [PubMed] [Google Scholar]
- [121].Pelliccia A, Caselli S, Sharma S, Basso C, Bax JJ, Corrado D, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. European Heart Journal . 2018;39:1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- [122].D’Andrea A, Mele D, Palermi S, Rizzo M, Campana M, Di Giannuario G, et al. Grey zones in cardiovascular adaptations to physical exercise: how to navigate in the echocardiographic evaluation of the athlete’s heart. Giornale Italiano di Cardiologia (2006) . 2020;21:457–468. doi: 10.1714/3359.33330. (In Italian) [DOI] [PubMed] [Google Scholar]
- [123].Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete’s heart: an expert consensus of the European Association of Cardiovascular Imaging. European Heart Journal: Cardiovascular Imaging . 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- [124].Szabo L, Brunetti G, Cipriani A, Juhasz V, Graziano F, Hirschberg K, et al. Certainties and Uncertainties of Cardiac Magnetic Resonance Imaging in Athletes. Journal of Cardiovascular Development and Disease . 2022;9:361. doi: 10.3390/jcdd9100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].D’Ascenzi F, Anselmi F, Piu P, Fiorentini C, Carbone SF, Volterrani L, et al. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC: Cardiovascular Imaging . 2019;12:1755–1765. doi: 10.1016/j.jcmg.2018.09.021. [DOI] [PubMed] [Google Scholar]
- [126].Scharf M, Brem MH, Wilhelm M, Schoepf UJ, Uder M, Lell MM. Atrial and ventricular functional and structural adaptations of the heart in elite triathletes assessed with cardiac MR imaging. Radiology . 2010;257:71–79. doi: 10.1148/radiol.10092377. [DOI] [PubMed] [Google Scholar]
- [127].Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European Heart Journal . 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- [128].Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. Journal of the American College of Cardiology . 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- [129].Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation . 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- [130].Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. European Heart Journal . 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Francone M, Aquaro GD, Barison A, Castelletti S, de Cobelli F, de Lazzari M, et al. Appropriate use criteria for cardiovascular MRI: SIC - SIRM position paper Part 2 (myocarditis, pericardial disease, cardiomyopathies and valvular heart disease) Journal of Cardiovascular Medicine . 2021;22:515–529. doi: 10.2459/JCM.0000000000001170. [DOI] [PubMed] [Google Scholar]
- [132].Castelletti S, Menacho K, Davies RH, Maestrini V, Treibel TA, Rosmini S, et al. Hypertrophic cardiomyopathy: insights from extracellular volume mapping. European Journal of Preventive Cardiology . 2022;28:e39–e41. doi: 10.1093/eurjpc/zwaa083. [DOI] [PubMed] [Google Scholar]
- [133].Treibel TA, Kozor R, Menacho K, Castelletti S, Bulluck H, Rosmini S, et al. Left Ventricular Hypertrophy Revisited: Cell and Matrix Expansion Have Disease-Specific Relationships. Circulation . 2017;136:2519–2521. doi: 10.1161/CIRCULATIONAHA.117.029895. [DOI] [PubMed] [Google Scholar]
- [134].Czimbalmos C, Csecs I, Toth A, Kiss O, Suhai FI, Sydo N, et al. The demanding grey zone: Sport indices by cardiac magnetic resonance imaging differentiate hypertrophic cardiomyopathy from athlete’s heart. PLoS ONE . 2019;14:e0211624. doi: 10.1371/journal.pone.0211624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Luijkx T, Cramer MJ, Prakken NHJ, Buckens CF, Mosterd A, Rienks R, et al. Sport category is an important determinant of cardiac adaptation: an MRI study. British Journal of Sports Medicine . 2012;46:1119–1124. doi: 10.1136/bjsports-2011-090520. [DOI] [PubMed] [Google Scholar]
- [136].D’Ascenzi F, Baggiano A, Cavigli L, Mandoli GE, Andreini D, Marallo C, et al. The role of cardiac computed tomography in sports cardiology: back to the future. European Heart Journal: Cardiovascular Imaging . 2022;23:e481–e493. doi: 10.1093/ehjci/jeac069. [DOI] [PubMed] [Google Scholar]
- [137].Christou GA, Deligiannis AP, Kouidi EJ. The role of cardiac computed tomography in pre-participation screening of mature athletes. European Journal of Sport Science . 2022;22:636–649. doi: 10.1080/17461391.2021.1883125. [DOI] [PubMed] [Google Scholar]
- [138].van de Sande DA, Liem IH, Hoogsteen J, Kemps HM. The diagnostic accuracy of myocardial perfusion scintigraphy in athletes with abnormal exercise test results. European Journal of Preventive Cardiology . 2017;24:1000–1007. doi: 10.1177/2047487317693135. [DOI] [PubMed] [Google Scholar]
- [139].Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal . 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- [140].Al Moudi M, Sun Z, Lenzo N. Diagnostic value of SPECT, PET and PET/CT in the diagnosis of coronary artery disease: A systematic review. Biomedical Imaging and Intervention Journal . 2011;7:e9. doi: 10.2349/biij.7.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bartram P, Toft J, Hanel B, Ali S, Gustafsson F, Mortensen J, et al. False-positive defects in technetium-99m sestamibi myocardial single-photon emission tomography in healthy athletes with left ventricular hypertrophy. European Journal of Nuclear Medicine . 1998;25:1308–1312. doi: 10.1007/s002590050300. [DOI] [PubMed] [Google Scholar]
- [142].Kajander S, Joutsiniemi E, Saraste M, Pietilä M, Ukkonen H, Saraste A, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation . 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- [143].Castelletti S, Zorzi A, Ballardini E, Basso C, Biffi A, Brancati F, et al. Molecular genetic testing in athletes: Why and when a position statement from the Italian Society of Sports Cardiology. International Journal of Cardiology . 2022;364:169–177. doi: 10.1016/j.ijcard.2022.05.071. [DOI] [PubMed] [Google Scholar]
- [144].Ahmad F, McNally EM, Ackerman MJ, Baty LC, Day SM, Kullo IJ, et al. Establishment of Specialized Clinical Cardiovascular Genetics Programs: Recognizing the Need and Meeting Standards: A Scientific Statement From the American Heart Association. Circulation: Genomic and Precision Medicine . 2019;12:e000054. doi: 10.1161/HCG.0000000000000054. [DOI] [PubMed] [Google Scholar]
- [145].Monda E, Sarubbi B, Russo MG, Caiazza M, Mazzaccara C, Magrelli J, et al. Unexplained sudden cardiac arrest in children: clinical and genetic characteristics of survivors. European Journal of Preventive Cardiology . 2021;28:1134–1137. doi: 10.1177/2047487320940863. [DOI] [PubMed] [Google Scholar]
- [146].Limongelli G, Nunziato M, D’Argenio V, Esposito MV, Monda E, Mazzaccara C, et al. Yield and clinical significance of genetic screening in elite and amateur athletes. European Journal of Preventive Cardiology . 2021;28:1081–1090. doi: 10.1177/2047487320934265. [DOI] [PubMed] [Google Scholar]
- [147].Sheikh N, Papadakis M, Wilson M, Malhotra A, Adamuz C, Homfray T, et al. Diagnostic Yield of Genetic Testing in Young Athletes With T-Wave Inversion. Circulation . 2018;138:1184–1194. doi: 10.1161/CIRCULATIONAHA.118.034208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ingles J, Semsarian C. The value of cardiac genetic testing. Trends in Cardiovascular Medicine . 2014;24:217–224. doi: 10.1016/j.tcm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- [149].Caruso MR, Garg L, Martinez MW. Cardiac Imaging in the Athlete: Shrinking the “Gray Zone”. Current Treatment Options in Cardiovascular Medicine . 2020;22:5. doi: 10.1007/s11936-020-0802-8. [DOI] [PubMed] [Google Scholar]
- [150].Augustine DX, Howard L. Left Ventricular Hypertrophy in Athletes: Differentiating Physiology From Pathology. Current Treatment Options in Cardiovascular Medicine . 2018;20:96. doi: 10.1007/s11936-018-0691-2. [DOI] [PubMed] [Google Scholar]
- [151].Millar LM, Fanton Z, Finocchiaro G, Sanchez-Fernandez G, Dhutia H, Malhotra A, et al. Differentiation between athlete’s heart and dilated cardiomyopathy in athletic individuals. Heart . 2020;106:1059–1065. doi: 10.1136/heartjnl-2019-316147. [DOI] [PubMed] [Google Scholar]