Abstract
Sustained hepatitis C virus (HCV) RNA clearance is achieved in 8 to 12% of patients with chronic HCV infection treated with alpha interferon (IFN-α) at the approved dose of 3 MU three times a week for 6 months and in about 25% of those receiving this treatment for 12 months. We used single-strand conformation polymorphism analysis combined with cloning and sequencing strategies to characterize the genetic evolution of HCV second envelope gene hypervariable region 1 (HVR1) quasispecies during and after IFN therapy in patients who failed to clear HCV RNA. Sustained HCV RNA clearance was achieved in 6% of patients. Profound changes in HVR1 quasispecies major variants were estimated to occur in 70% of the patients during and after therapy. These changes were evolutionary and were characterized by shifts in the virus population, related to selection and subsequent diversification of minor pretreatment variants. The quasispecies changes appeared to be induced by changes in the host environment likely resulting from the IFN-induced enhancement and post-IFN attenuation of neutralizing and possibly cytotoxic responses against HVR1. The remaining patients had no apparent changes in HVR1 quasispecies major variants, suggesting selection of major pretreatment variants, but some changes were observed in other genomic regions. We conclude that IFN-α administration and withdrawal profoundly alters the nature of circulating HCV quasispecies, owing to profound changes in virus-host interactions, in patients in whom sustained HCV RNA clearance fails to occur. These changes are associated with profound alterations of the natural outcome of HCV-related liver disease, raising the hypothesis of a causal relationship.
Hepatitis C virus (HCV) is a small, enveloped, positive-stranded RNA virus belonging to the Flaviviridae family (9). Acute infection is usually asymptomatic, and persistent infection occurs in more than 80% of cases (1, 12). Chronic hepatitis C is usually paucisymptomatic, but about 20% of patients have cirrhosis as detected by liver biopsy (1, 12, 55). Cirrhosis may lead to life-threatening complications due to portal hypertension or hepatocellular failure. HCV-related end-stage liver cirrhosis has become the main indication for orthotopic liver transplantation in industrialized countries (1). Cirrhosis also predisposes patients to hepatocellular carcinoma, with an estimated yearly incidence of 4 to 5% and a high mortality rate.
The high prevalence of HCV infection in the general population (0.5 to 2% in industrialized countries), the absence of documented spontaneous recovery from chronic infection, and the potentially serious complications of chronic hepatitis C call for an effective treatment. Until recently the only approved treatment for chronic hepatitis C has been alpha interferon (IFN-α), a cytokine with both antiviral and immunomodulatory properties (reviewed in references 2, 44, 51, and 62), administered at a dose of 3 MU three times a week for 6 to 12 months. At this dose, a sustained virological response, defined by normalization of serum alanine aminotransferase (ALT) levels and sustained HCV RNA clearance from serum, i.e., PCR negativity 6 months after treatment withdrawal, is obtained in 8 to 12% of cases after 6 months and in about 25% of cases after 12 months of treatment (37). The interferon-ribavirin combination has recently been shown to improve the results of chronic hepatitis C treatment (10, 41, 53), but the rate of sustained virological responses after 1 year of therapy is still only about 40% in naive patients (41, 53).
HCV circulates in the human host as a pool of genetically distinct but closely related variants referred to collectively as a quasispecies (40, 68). The quasispecies nature of HCV probably confers a significant survival advantage, since the simultaneous presence of multiple variant genomes and the high rate at which new variants are generated mean that mutants better suited to new environmental conditions are rapidly selected (13, 14). It has recently been shown that a small quasispecies repertoire size (i.e., a small number of variants within a quasispecies) at the beginning of therapy is necessary to achieve sustained HCV RNA clearance at the dose of IFN-α presently used (48, 49, 63). Indeed, when the quasispecies repertoire is large at treatment outset, there is a high probability that a few minor variants will gain a survival advantage in the IFN-altered host environment.
We recently observed that HCV genotype 1b resistance to IFN-α therapy is associated with profound changes in the composition of HCV nonstructural (NS) 5A gene central region quasispecies (48). These changes are evolutionary and could result both from high viral replication kinetics when HCV escapes control and from IFN-related positive selection pressures at two amino acid positions (positions 2217 and 2218 of the HCV-1b polyprotein), although the underlying mechanisms are unknown (48). HCV hypervariable region 1 (HVR1) is an 81-nucleotide sequence located at the 5′ end of the E2 envelope gene. The corresponding region of the protein is highly tolerant of amino acid substitutions and, as a target of anti-HCV neutralizing antibodies, is subjected to strong positive selection pressure (18, 27, 67). Qualitative HVR1 quasispecies changes have recently been reported for patients receiving a standard course of IFN therapy who failed to clear HCV RNA (16, 17, 42, 52, 59, 70).
In this study we used single-strand conformation polymorphism (SSCP) analysis, combined with cloning and sequencing strategies, to characterize the evolution of HVR1 quasispecies during and after IFN therapy in patients who did not have sustained virological responses to treatment. The genetic events were compared to clinical, virological, and histological outcomes.
MATERIALS AND METHODS
Patients and samples.
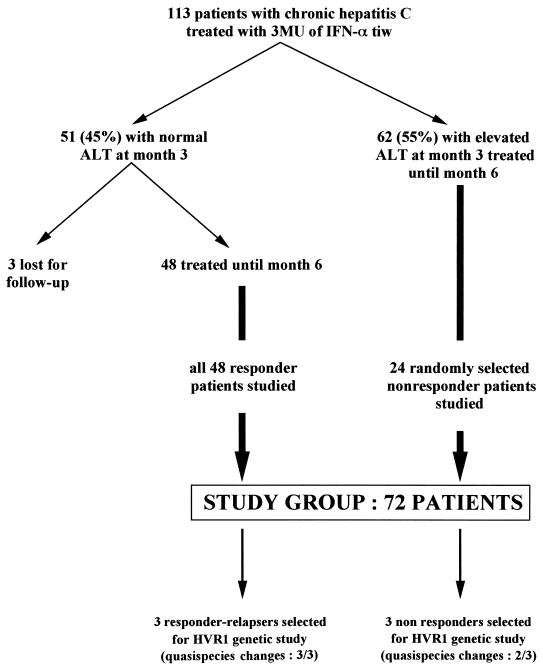
One hundred thirteen consecutive patients with chronic hepatitis C (76 men and 37 women; mean age, 46.2 ± 13.9 years; range, 18 to 74 years) eligible for IFN therapy were included in a clinical trial of IFN-α therapy. The inclusion and exclusion criteria have been described elsewhere (50). Twenty patients (18%) were infected by a genotype 1a strain, 50 (44%) by a genotype 1b strain, 12 (11%) by a genotype 2a strain, 22 (19%) by a genotype 3a strain, and 9 (8%) by a genotype 4a strain. All 113 patients were treated with 3 MU of IFN-α2a (Roferon-A; Roche Laboratories, Basel, Switzerland) subcutaneously three times a week for 6 months and were followed up until month 12, i.e., 6 months after IFN withdrawal.
At month 3, an early biochemical response characterized by normal serum ALT activity was observed in 51 patients (45%). Three of these patients were lost to follow-up, and the remaining 48 were included in this study, whatever their subsequent biochemical or virological responses. Six of these patients, who had biochemical and virological responses at the end of treatment but relapsed after IFN withdrawal, were re-treated at month 9, i.e., 3 months after IFN withdrawal, with the same dose (3 MU three times a week) of the same IFN molecule (IFN-α2a) for the same period (6 months) and were followed up for another 6 months, i.e., until month 21. The remaining 62 patients (55%) had elevated ALT activity at month 3 and were considered biochemical nonresponders. Twenty-four of these patients were randomly selected for the present study. The study group thus comprised a total of 72 patients (48 biochemical responders and 24 biochemical nonresponders) (Fig. 1).
FIG. 1.
Selection of the study group. tiw, three times a week.
Serum ALT activity, HCV RNA load, and HVR1 quasispecies distribution were studied in every patient, both before treatment and monthly from the beginning of therapy to the end of follow-up. Liver biopsy was performed in every case before therapy, and the findings were compared to those of the posttreatment liver biopsy performed at the end of follow-up, when both were available. The qualitative evolution of HVR1 quasispecies was assessed in all the patients by means of a sensitive standardized SSCP technique (49). Detailed genetic characterization of HVR1 quasispecies evolution was performed for five randomly selected patients with HVR1 quasispecies changes on SSCP analysis and for a patient with no apparent HVR1 quasispecies changes (Fig. 1).
A control group was formed from seven untreated HCV-infected patients, and two to five samples per patient were tested over 6 months for three patients, 8 months for two patients, 14 months for one patient, and 16 months for one patient. These patients had no contraindication to IFN therapy and were subsequently treated. The spontaneous evolution of HVR1 quasispecies over time was studied by means of SSCP for these seven patients, and detailed genetic characterization of HVR1 quasispecies evolution was performed for one randomly selected control patient.
PCR detection of HCV RNA.
To assess the virological response to treatment, the sera of patients with sustained biochemical responses to IFN (i.e., ALT normalization) at month 12 were tested for HCV RNA by means of the Amplicor HCV assay (Roche Molecular Systems, Pleasanton, Calif.) according to the manufacturer’s instructions.
HCV RNA quantification.
The noncompetitive quantitative PCR-based Amplicor HCV Monitor assay (Roche Molecular Systems) was used for HCV RNA quantification according to the manufacturer’s instructions. The stated cutoff is 1,000 genome copies/ml (3 log10 genome copies/ml).
HVR1 quasispecies analysis by SSCP.
HVR1 quasispecies analysis by SSCP and its validation have been described recently (49). Briefly, RNA was extracted from 50 μl of serum with RNAzol (RNA-B; Bioprobe Systems, Montreuil-sous-Bois, France) and chloroform and was reverse transcribed at 42°C for 90 min by using 7 pmol of the downstream primer (5′GGTGTGGAGGGAGTCATTGCAGTT3′; nucleotide positions 1611 to 1634) in the presence of 8 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.). PCR was performed by using 5 pmol of each biotinylated downstream primer and upstream primer (5′GCTTGGGATATGATGATGAACTGGTC3′; nucleotide positions 1284 to 1309) with 2.5 U of Taq DNA polymerase (Pharmacia Biotech, Uppsala, Sweden). After denaturation for 5 min at 94°C, PCR comprised 45 cycles (94°C, 1 min; 68°C, 1 min; 72°C, 1 min). Amplified products were analyzed by electrophoresis through a 3% NuSieve agarose gel (FMC, Rockland, Maine) and staining with ethidium bromide.
Amplified products were extracted from the agarose gel and purified with the Sephaglas BandPrep kit (Pharmacia Biotech) according to the manufacturer’s instructions. Purified PCR products were eluted in 20 μl of distilled water. SSCP analysis was performed by using the PCR Fragment Analysis kit (Pharmacia Biotech). An average of 50 ng of DNA amplified from each serum sample was diluted in 4.5 μl of sterile distilled water and 4.5 μl of a solution of 10 mM NaOH and 2 mM EDTA; bromophenol blue was then added. The samples were denatured for 10 min at 100°C and immediately chilled on ice. Eight microliters of the denatured samples was then loaded into the wells of a discontinuous polyacrylamide gel (CleanGel; Pharmacia Biotech) which had been rehydrated to a thickness of 0.5 mm with a buffer specially designed for DNA separation (pH 7.3). Horizontal electrophoresis was run in a Multiphor II apparatus (Pharmacia Biotech) at 9°C and 100 V for 20 min (penetration in the gel) and then 600 V for 60 min (migration and stacking).
The gel was then submitted to a rapid and sensitive silver-staining procedure by using the Silver Staining kit DNA (Pharmacia Biotech); this procedure can detect 0.5 to 2 ng of DNA. After electrophoresis the gel was fixed for 30 min at room temperature in 10% acetic acid, then washed and incubated for 30 min in 200 ml of a solution of 0.1% AgNO3 (wt/vol) and 0.1% formaldehyde. The gel was rinsed, placed in 200 ml of a solution of 2.5% Na2CO3, 0.1% formaldehyde, and 0.002% sodium thiosulfate, and slowly agitated until staining became visible. The reaction was stopped by incubation for 20 min in 10% acetic acid, and staining was preserved by a 20-min incubation at room temperature in a solution of 5% glycerol and 10% acetic acid.
Cloning, clonal frequency analysis, and sequencing.
PCR products were purified with the Sephaglas BandPrep kit (Pharmacia Biotech) according to the manufacturer’s protocol. Purified products were quantified by ethidium bromide staining, with DNA standards as controls; 50 ng was directly ligated into 50 ng of the pTAg vector (LigATor cloning kit; R&D Systems, Abingdon, United Kingdom). Transformation of recombinant plasmid DNA into Escherichia coli competent cells was performed according to the manufacturer’s protocol, and transformants were grown on ampicillin-tetracycline plates. Cloned DNA was reamplified by using the HVR1-specific PCR procedure described above for SSCP analysis.
After cloning and PCR amplification of 20 clones per time point, clonal frequency analysis was performed as previously described (49) by means of the SSCP technique described above. The two strands of one to three clones per SSCP pattern were then sequenced with the AutoLoad Solid Phase Sequencing kit on an ALF Express automated DNA sequencer (both from Pharmacia Biotech). The sequencing primers were the upstream and downstream PCR primers.
Genetic characterization of HVR1 quasispecies.
We determined the entropy (S) of HVR1 quasispecies, which is defined in terms of the probabilities of the different sequences or clusters of sequences appearing at a given time and measures the quasispecies repertoire size (48). This measure is calculated as S = −Σi [pi ln(pi)], where pi is the frequency of each sequence in the viral quasispecies. Normalized entropy (Sn) was calculated as S/ln20, where 20 is the total number of sequences analyzed per time point. Sn theoretically varies from 0 (no diversity) to 1 (maximum diversity).
Nucleotide sequences were aligned with the Clustal W program, version 1.5 (60). Distances between pairs of sequences were calculated by using the DNADIST module in the PHYLIP package, version 3.572 (distributed by J. Felsenstein, Department of Genetics, University of Washington, Seattle). Calculation was based on a Kimura two-parameter distance matrix with a transition-to-transversion ratio of 1.3. The mean within-sample genetic distances ± the standard errors of the means (SEM) were calculated at each time point. The numbers of synonymous and nonsynonymous substitutions per synonymous and nonsynonymous site, respectively, were calculated at each time point for each patient with the Jukes-Cantor correction for multiple substitutions, by using the MEGA program (25, 33). In addition, the mean between-sample genetic distances ± the SEM were calculated on the basis of distances between pairs of pretreatment (month-0) and posttreatment (month-12) sequences, as were the numbers of synonymous and nonsynonymous substitutions per synonymous and nonsynonymous site. Statistical comparisons were made by t test.
The PHYLIP program, version 3.572 (21), was used to construct phylogenetic trees by means of the neighbor-joining method (56) with a sequence matrix determined by the two-parameter method of Kimura. Bootstrap support was determined by 1,000 resamplings of the sequences (20). Patient trees were constructed with both nucleotide and amino acid sequences. Phylogenetic analyses of all viral sequences generated in this study showed distinct clusters of viral sequences corresponding to each patient (data not shown), indicating the absence of PCR cross-contamination.
RESULTS
Biochemical, virological, and histological outcomes.
Seven patients (6% of the initial group of 113 patients) had sustained virological responses to IFN-α at the end of follow-up (normal ALT activity and HCV RNA PCR negativity). In all seven patients, HCV RNA was undetectable at month 1 and remained negative throughout follow-up. The liver histology, as assessed by Knodell’s score (29), improved significantly in every case (P < 0.03).
Various patterns of biochemical response (ALT) and virological response (HCV RNA) were observed in the patients who did not clear HCV RNA during or after therapy. Seventeen (35%) of the 48 early biochemical responders (patients with normal ALT levels at month 3) had a virological response with a relapse after treatment, 14 (29%) had a virological response with a breakthrough during treatment, and 10 (21%) were virological nonresponders. Among the 24 randomly selected biochemical nonresponders (patients with elevated ALT levels at month 3), wide fluctuations in serum ALT activity were observed in 19 patients during and after therapy, whereas ALT activity remained stable before and after therapy in the remaining 5 patients.
A liver biopsy was performed both before treatment and at the end of follow-up for 53 patients. In each case the Knodell score, which assesses both histological activity and fibrosis (29), was calculated after double pathological examination. Table 1 shows the time courses of the different components of the Knodell score, including indexes of activity (periportal necrosis, intralobular necrosis, and portal inflammation) and fibrosis. Only changes of at least 2 points between the two biopsies were considered significant. Overall, the changes in the scores were not related to the virological responses, although patients whose histological scores worsened tended to be virological nonresponders. Intralobular necrosis was the most frequently aggravated parameter. Fibrosis worsened in three patients during the 1-year follow-up period (Table 1).
TABLE 1.
Changes in the histological scores that make up Knodell’s score (29) between the pretreatment liver biopsy and the end-of-follow-up liver biopsy (month 12) for the 53 patients with both biopsies available who failed to clear HCV RNA
| Histological lesion | No. (%) of early biochemical responders (32 patients) with:
|
No. (%) of nonresponders (21 patients) with:
|
||||
|---|---|---|---|---|---|---|
| Improvement of ≥2 points | No change (±1 point) | Aggravation of ≥2 points | Improvement of ≥2 points | No change (±1 point) | Aggravation of ≥2 points | |
| Periportal necrosis | 9 (28) | 21 (66) | 2 (6) | 4 (19) | 16 (76) | 1 (5) |
| Intralobular necrosis | 5 (16) | 22 (69) | 5 (16) | 5 (24) | 13 (62) | 3 (14) |
| Portal inflammation | 5 (16) | 26 (81) | 1 (3) | 1 (5) | 20 (95) | 0 (0) |
| Fibrosis | 4 (12) | 26 (81) | 2 (6) | 1 (5) | 19 (90) | 1 (5) |
Evolution of HVR1 quasispecies major variants.
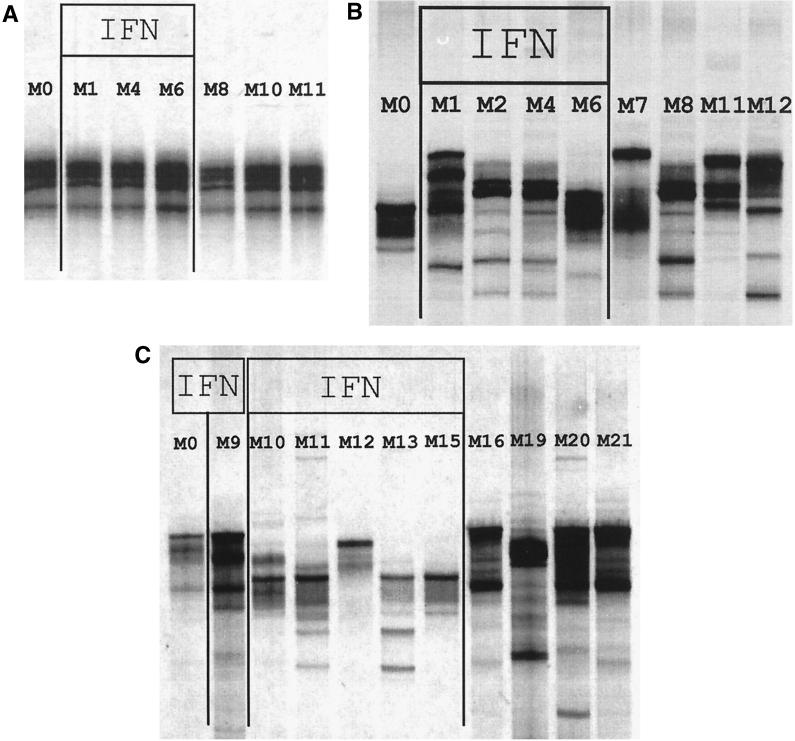
For the 72 treated patients studied, HVR1 was amplified by PCR before treatment and every month until the end of follow-up, i.e., until month 12 (month 21 for the 6 re-treated patients). For the seven untreated control patients, HVR1 was successfully amplified by PCR in all available samples. Serial HCV RNA-positive samples from the same patient were all tested in the same SSCP run. Figure 2A shows results for a patient with no HVR1 quasispecies major variant changes during follow-up: the SSCP patterns were identical at various times before, during, and after IFN treatment. Figure 2B and C show results for two patients with HVR1 quasispecies major variant changes during treatment and until the end of follow-up. The SSCP technique we used is sensitive enough to discriminate among variants bearing single nucleotide substitutions and to detect HVR1 quasispecies major variants, i.e., variants representing 10% or more of the viral quasispecies (49). Minor variant changes could not be ruled out for the patients showing no apparent major variant changes by means of SSCP.
FIG. 2.
SSCP analysis of 351-bp HCV E1/E2 PCR products containing HVR1 in specimens obtained before, during, and after treatment from three patients receiving 3 × 106 U of IFN-α2a subcutaneously three times a week for 6 months. (A) Nonresponder followed up until month 12 (M12). This is an example of a patient without HVR1 quasispecies major variant changes during follow-up: the SSCP patterns were identical at the various times before (M0), during (M1 to M6), and after (M8 to M12) IFN treatment. (B) Nonresponder followed up until month 12 (M12). This is an example of a patient with HVR1 quasispecies major variant changes: HVR1 quasispecies changed from one time point to the next during treatment (M1 to M6) and after IFN withdrawal (M7 to M12) until the end of follow-up. (C) Virological responder-relapser re-treated with the same dose of IFN for 6 months (M9 to M15) and followed up for a further 6 months. This is an example of a patient with HVR1 quasispecies major variant changes: HVR1 quasispecies changed after the first course of IFN (M9 versus M0) and from one time point to the next during re-treatment (M10 to M15) and after IFN withdrawal until the end of follow-up (M16 to M21).
No HVR1 quasispecies major variant changes were observed in the seven untreated control patients. With regard to the 72 treated patients (including the 48 early biochemical responders and the 24 randomly selected nonresponders), (i) PCR failed to amplify HVR1 for 2 patients, (ii) the 7 sustained virological responders cleared HCV RNA at month 1 and remained HCV RNA negative thereafter, (iii) 12 patients had no apparent HVR1 quasispecies major variant changes (for an example, see Fig. 2A), and (iv) profound HVR1 quasispecies major variant changes were observed in the remaining 51 patients. In these patients, the HVR1 quasispecies changed from one time point to the next during treatment but also after IFN withdrawal until the end of follow-up (examples are shown in Fig. 2B and C). New HVR1 quasispecies changes were observed during the second course of IFN-α therapy, as well as after IFN withdrawal (an example is shown in Fig. 2C), in all the re-treated patients but one, who had a sustained virological response to the second course of treatment. The fact that follow-up was limited to 6 months after treatment prevented us from determining the date at which a new quasispecies equilibrium was reached.
Eleven of the 12 patients without HVR1 quasispecies changes were biochemical and virological nonresponders (the remaining patient had biochemical and virological responses, with a breakthrough during therapy). Overall, no HVR1 quasispecies changes were observed in 11 of the 24 randomly selected nonresponders (46%). Given that 55% of the initial population of 113 patients were biochemical nonresponders to IFN, it can be extrapolated from our results that about 25% of patients receiving standard IFN-α therapy do not have HVR1 quasispecies major variant changes during or after treatment. As 6% of our patients were sustained virological responders, an estimated 70% of patients receiving standard IFN-α therapy have profound HVR1 quasispecies major variant changes during and after IFN therapy.
Genetic evolution of HVR1 in the patients with no apparent HVR1 changes and in untreated controls.
The apparent lack of HVR1 major variant changes in SSCP experiments was confirmed by the analysis of 20 clones per time point in specimens from one treated nonresponder with no HVR1 changes on SSCP and one untreated control patient. In both cases, phylogenetic analyses showed substantial intermingling of viral sequences isolated at different times (data not shown), a finding in keeping with the lack of significant genetic evolution during the follow-up period. In both cases the dominant HVR1 sequences continued to represent 75 to 100% of the quasispecies sequences.
Genetic characterization of HVR1 quasispecies changes.
Five of the 51 patients with HVR1 quasispecies changes during follow-up were selected for the genetic characterization of HVR1 quasispecies evolution. They comprised two nonresponders (patients who had elevated ALT and detectable viremia throughout follow-up [patients 1 and 2]), one responder-relapser (a patient with normal ALT and undetectable viremia during therapy, who relapsed after IFN withdrawal [patient 3]), one responder-relapser re-treated at the same dose of IFN-α2a from month 9 to month 15 and followed up until month 21, who had a sustained virological response to the second course of IFN (patient 4), and one responder-relapser who did not respond to the second course of IFN (patient 5). Twenty HVR1 clones per HCV RNA-positive time point were generated, and a total of 480 HVR1 clones from these patients were analyzed for clonal frequency by means of SSCP and were sequenced. Amino acid sequences were deduced from nucleotide sequences.
Table 2 shows the number of within-sample synonymous mutations per synonymous site and the number of within-sample nonsynonymous mutations per nonsynonymous site, calculated for each of the five patients at each time. In four of these five patients (patients 1 to 4), the proportion of nonsynonymous mutations was significantly higher than the proportion of synonymous mutations at month 0 and at most subsequent times, suggesting that HVR1 quasispecies heterogeneity was principally the result of positive selection pressures, a finding in keeping with the view that HVR1 is a target for anti-HCV responses (6, 18, 27, 67). In the remaining patient (patient 5), the numbers of synonymous and nonsynonymous mutations at the various times were high but not significantly different, suggesting both positive selection pressures and a high rate of accumulation of random mutations, likely owing to rapid replication kinetics.
TABLE 2.
Proportions of synonymous and nonsynonymous mutations at different times in five patients with HVR1 quasispecies major variant changesa
| Patient and time | Avg no. of nonsynonymous mutations/nonsynonymous site (SEM) | P | Avg no. of synonymous mutations/synonymous site (SEM) |
|---|---|---|---|
| Patient 1 | |||
| Mo 0 | 0.1450 (0.0114) | <0.0001 | 0.0446 (0.0058) |
| Mo 3 | 0.1148 (0.0230) | 0.009 | 0.0143 (0.0090) |
| Mo 12 | 0.1054 (0.0270) | <0.003 | 0.0000 (0.0000) |
| Patient 2 | |||
| Mo 0 | 0.0961 (0.0152) | <0.0001 | 0.0000 (0.0000) |
| Mo 3 | 0.0299 (0.0034) | <0.02 | 0.0596 (0.0087) |
| Mo 6 | 0.0627 (0.0095) | <0.02 | 0.0321 (0.0078) |
| Mo 12 | 0.0368 (0.0067) | NSb | 0.0303 (0.0074) |
| Patient 3 | |||
| Mo 0 | 0.1043 (0.0141) | <0.003 | 0.0531 (0.0074) |
| Mo 9 | 0.1821 (0.0412) | <0.02 | 0.0564 (0.0108) |
| Mo 12 | 0.1662 (0.0748) | NS | 0.0461 (0.0231) |
| Patient 4 | |||
| Mo 0 | 0.3072 (0.0279) | <0.05 | 0.0985 (0.0084) |
| Mo 9 | 0.0111 (0.0056) | NS | 0.0336 (0.0168) |
| Patient 5 | |||
| Mo 0 | 0.1170 (0.0201) | NS | 0.1459 (0.0351) |
| Mo 7 | 0.2166 (0.0261) | NS | 0.1958 (0.0264) |
| Mo 9 | 0.1998 (0.0208) | NS | 0.2232 (0.0259) |
| Mo 15 | 0.0000 (0.0000) | NS | 0.0000 (0.0000) |
| Mo 21 | 0.2551 (0.0150) | NS | 0.2625 (0.0209) |
During treatment, there was a tendency towards a decrease in HVR1 entropy (an estimate of the size of the HVR1 quasispecies sequence repertoire) at both the nucleotide and amino acid sequence levels, followed by an increase after the end of therapy. However, these changes did not reach statistical significance. Similarly, there was a tendency towards a decrease in average HVR1 within-sample genetic distances during treatment, followed by an increase after the end of therapy, but again it failed to reach statistical significance (data not shown).
As shown in Table 3, the average between-sample genetic distances (at the end of follow-up versus month 0) were significantly higher than the average pretreatment within-sample genetic distances for four patients (patients 1, 2, 3, and 5), indicating that evolutionary genetic changes occurred in HVR1 sequences during the study period. The data for the remaining patient (patient 4), who had very low pre- and posttreatment entropy, i.e., very few different sequences at both time points, could not be reliably interpreted. We then studied the relative accumulation rates of nonsynonymous and synonymous substitutions for the five patients throughout the study period by pairwise comparison of the quasispecies sequences isolated before treatment and at the end of follow-up (in the case of patient 4, who cleared HCV RNA after month 9, the last HCV RNA-positive sample was used), respectively (Table 3). The proportion of nonsynonymous mutations per nonsynonymous site was significantly higher than the proportion of synonymous mutations per synonymous site for four patients (patients 1 to 4), suggesting that HVR1 quasispecies evolution was principally due to positive selection for change rather than random genetic drift. In patient 5, high rates of accumulation of random mutations and positive selection appeared to contribute equally to HVR1 quasispecies evolution.
TABLE 3.
Evolution of HVR1 quasispecies in five patients with changes during follow-upa
| Patient no. | Avg (SEM) genetic distance
|
Between-sample mutational change
|
||||
|---|---|---|---|---|---|---|
| Within sample | P | Between samples | Avg no. of nonsynonymous mutations/nonsynonymous site (SEM) | P | Avg no. of synonymous mutations/synonymous site (SEM) | |
| 1 | 0.1139 (0.0091) | <0.04 | 0.1326 (0.0083) | 0.1677 (0.0094) | <0.0001 | 0.0514 (0.0093) |
| 2 | 0.0677 (0.0106) | <0.03 | 0.1004 (0.0061) | 0.1367 (0.0089) | <0.0001 | 0.0154 (0.0034) |
| 3 | 0.0926 (0.0111) | <0.0001 | 0.1872 (0.0142) | 0.2251 (0.0169) | <0.0001 | 0.0828 (0.0096) |
| 4 | 0.2478 (0.0171) | NS | 0.1814 (0.0433) | 0.2117 (0.0536) | <0.05 | 0.1027 (0.0193) |
| 5 | 0.1258 (0.0239) | <0.0001 | 0.2512 (0.0116) | 0.2377 (0.0104) | NS | 0.2651 (0.0184) |
The average within-sample genetic distances (month 0) were compared with the average between-sample genetic distances (end of follow-up versus month 0), as were the respective proportions of between-sample nonsynonymous and synonymous mutations. NS, not significant.
Phylogenetic analysis of HVR1 quasispecies sequences in 5 patients with HVR1 quasispecies major variant changes.
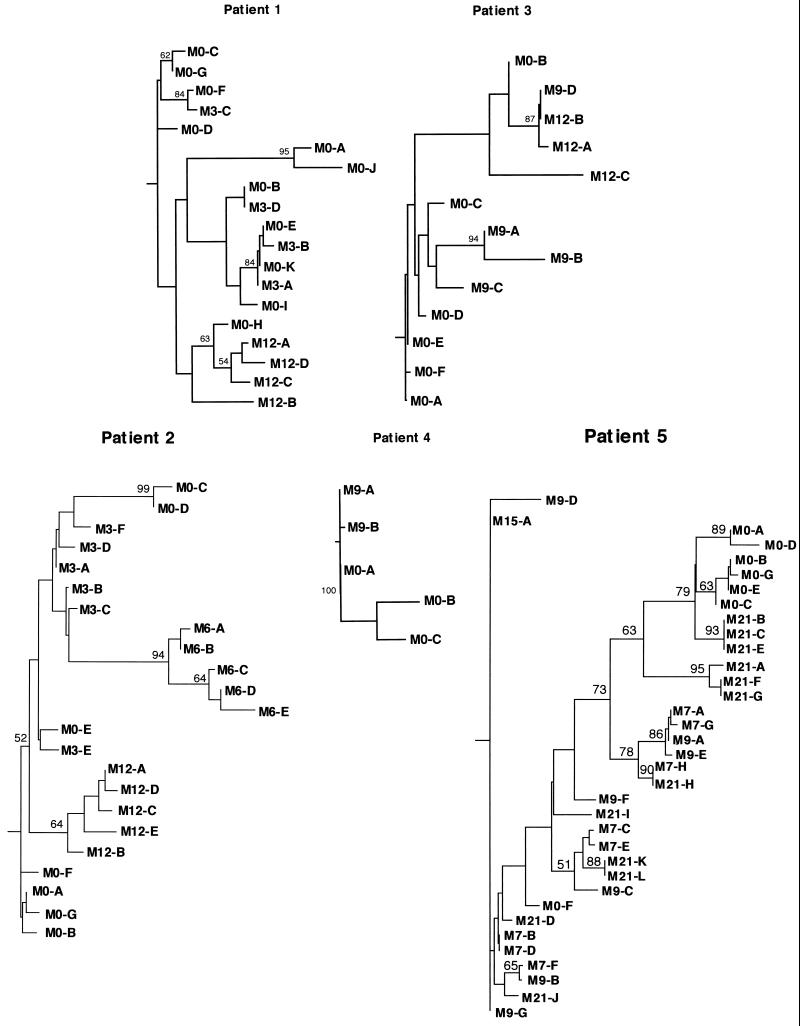
Phylogenetic analysis was used to characterize the evolution and diversification of HVR1 sequences over time in the same five patients. Phylogenetic trees were plotted with both nucleotide and amino acid sequences, and bootstrap support was determined by 1,000 resamplings of the sequences. Figure 3 shows the phylogenetic trees plotted with the HVR1 amino acid sequences from patients 1 to 5.
FIG. 3.
Phylogenetic trees of HVR1 in the five patients studied before IFN treatment and at various times during and after therapy. The phylogenetic reconstructions are neighbor-joining trees with bootstrap proportions of more than 500 of 1,000 bootstrap replicates shown (in percentages) at appropriate branch points. Letters stand for amino acid sequences, and numbers following “M” stand for the months when the sequences were isolated (e.g., M3-B, amino acid sequence B isolated at month 3).
For patients 1, 2, 3, and 4, phylogenetic analyses showed distinctive clustering of the sequences isolated at various sampling times (Fig. 3). This suggested that a true evolutionary process occurred, characterized by sequential shifts in the population of viruses, likely driven by positive selection, as suggested by the significantly higher rate of accumulation of nonsynonymous relative to synonymous mutations over time (Table 3). The sequences present during and after therapy were genetically closer to certain pretreatment sequences than to any other. For instance, for patient 1, the sequences isolated at month 12 were genetically closer to sequence M0-H, a sequence that represented 5.0% (95% confidence interval [CI]; 0 to 15.0%) of the viral quasispecies before treatment, than to any other pretreatment sequence. For patient 3, two distinct groups of viral sequences appeared to have been selected from two distinct groups of pretreatment variants at month 9 (i.e., 3 months after IFN withdrawal): sequences M9-A, M9-B, and M9-C on the one hand (representing 90.0% [95% CI, 67.0 to 100.0%] of quasispecies sequences) and sequence M9-D on the other hand (representing 10% [95% CI, 0 to 23.0%] of quasispecies sequences). All the sequences isolated 3 months later, i.e., at month 12, appeared to be derived from the second, minor group of pretreatment sequences. Altogether, this suggested that the sequences present during and after therapy were the result of selection and subsequent genetic diversification of minor pretreatment variants or groups of variants over time.
Patient 4 had very low quasispecies entropy before each of the two courses of treatment. The variants isolated at month 9 (i.e., 6 months after the end of the first course of treatment, and the date when the second course was started) were likely derived from pretreatment variants selected by IFN therapy. However, these variants appeared to be unfit during the second course of IFN, which led to sustained HCV RNA clearance. This suggests that IFN-α does not select intrinsically IFN-resistant HCV variants but rather selects variants with better fitness in the host environment at a given time point during therapy.
Phylogenetic analyses of patient 5 showed both clustering according to sampling time and substantial intermingling of viral sequences isolated at different time points. This pattern was in keeping with the accumulation of synonymous mutations relative to nonsynonymous mutations, which were not significantly different (Table 3). These results suggest that HVR1 quasispecies changes were less driven by positive selection and that the persistence of high viral replication kinetics probably played an important role in this patient.
DISCUSSION
To characterize the effect of IFN-α administration and its subsequent withdrawal on HCV quasispecies, we chose to study HVR1 for the following reasons: (i) the high variability of this region, likely due to its high (although not absolute) tolerance of amino acid substitutions and to the fact that it is subjected to strong selection pressures (67, 68), maximizes HCV quasispecies variant detection, and (ii) HVR1 is one of the main targets of anti-HCV neutralizing antibodies (18, 27, 67) and, possibly, of cytotoxic responses (64). IFN-α exerts its antiviral action partly by enhancing HCV-specific immune responses. Indeed, IFN-α induces a number of immunological changes, including increased expression of class I major histocompatibility complex antigens, activation of cytotoxic T cells, natural killer cells, and macrophages, and complex interactions with the cytokine cascade (reviewed in references 2, 44, 51, and 62). We therefore used HVR1 as a model for variable genomic regions targeted by IFN-inducible antiviral effectors, such as regions encoding neutralizing or cytotoxic epitopes.
Qualitative HVR1 quasispecies changes over time have previously been reported in untreated immunocompetent patients and experimentally infected chimpanzees with persistent HCV infections (26, 28, 35, 38, 65, 66) and in patients with chronic hepatitis C receiving IFN-α therapy (17, 42, 52, 59, 70). The former changes result from random genetic drift (26, 28, 35, 38, 65, 66), whereas the latter had not been genetically characterized so far. In the present study IFN-α-induced qualitative changes in HVR1 quasispecies major variants were estimated (by means of SSCP analysis) to occur in about 70% of the patients receiving standard IFN-α therapy who failed to clear HCV RNA. Sequence analysis of a large number of HVR1 clones showed that these changes were evolutionary and followed a classical Darwinian process. They were characterized by shifts in the population of viruses, related to continuous production of new variants and subsequent selection and diversification of the variants best fitted to the host environment at a given time. Our observations suggest that the quasispecies equilibrium is abruptly and irreversibly disrupted by IFN administration, which is known to profoundly alter the host environment by inducing numerous enzymatic pathways and interacting with the immune system. It is of particular interest, however, that IFN-α withdrawal also abruptly disrupted the quasispecies equilibrium, probably by abruptly removing IFN-induced pressures, thereby inducing drastic changes in the host environment.
Interestingly, this phenomenon associated with the lack of HCV clearance during IFN therapy appears to be similar to one of the main mechanisms involved in viral persistence (6). Indeed, during acute HCV infection, the neutralizing response against HVR1 allows those variants bearing HVR1 peptide sequences with low affinity for the antibodies induced by the major HVR1 variants to escape neutralization and, subsequently, to emerge as a different quasispecies (27, 30, 31, 34, 45, 57, 69), whereas cytotoxic responses directed against HVR1 (and probably other epitopes) appear to select variants capable of escaping cytotoxic T-cell activity (64). In addition, low quasispecies genetic complexity (i.e., a small quasispecies sequence repertoire) appears to be necessary for both clearance of hepatitis C viremia during acute infection (54) and sustained viral clearance after IFN-α therapy (48, 49, 63).
About 25% of the patients in this study had no apparent changes in HVR1 quasispecies major variants either during or after treatment. Pretreatment major variants rather than minor variants were thus selected by therapy in these patients. It remains to be determined whether these patients are unable to synthesize high-affinity neutralizing antibodies and/or to mount efficient cytotoxic responses against their HVR1 major variants or, conversely, whether their major variants escape recognition by efficient neutralizing and cytotoxic responses. In this respect, certain HVR1 sequences could be resistant to antibody binding and neutralization (31). It has been suggested that, in some patients, almost all HCV particles may be bound to β-lipoproteins and thus may be immune to precipitation by anti-immunoglobulin G antibodies (61). It is conceivable that viral envelope proteins are protected from recognition by host immune responses in such cases.
It is unclear whether the HCV strains that did not undergo HVR1 quasispecies changes during and after therapy were intrinsically resistant to IFN-α. If this were the case, efficient induction of anti-HVR1 neutralizing responses would be necessary to achieve a sustained virological response to IFN-α therapy. This is supported by the fact that most of the patients without HVR1 quasispecies changes in this study were nonresponders. However, significant changes in ALT levels and HCV RNA loads were observed in these patients, suggesting that IFN-α had an effect on the virus and the disease course. We and others have observed quasispecies changes in genomic regions other than HVR1 in patients who received the standard dose of IFN-α and who had no apparent HVR1 quasispecies changes (reference 52 and our unpublished data), suggesting that genomic regions other than HVR1 may be sensitive to the action of IFN in such patients. Altogether, these data suggest that an efficient neutralizing response is important but that the combination of several efficient IFN-induced antiviral actions is necessary to achieve sustained viral clearance in patients receiving the standard dose of IFN-α.
The key genomic region(s) and IFN-induced effect(s) are unknown. Quasispecies changes have been reported in the NS5A gene central region in patients receiving IFN-α (16, 48, 52), and we recently identified a 2-amino-acid stretch within this region (amino acid positions 2217 and 2218 of the HCV polyprotein), the sequence of which could be related to the response to IFN therapy and the evolution of which during treatment appears to be driven by positive selection pressures (48). The possibility that the forces driving selection in this region are related to the recently reported interactions of NS5A protein with IFN-induced pathways such as the double-stranded RNA-dependent protein kinase PKR (22, 23) or, further upstream, the Jak-Stat pathway (24, 46) is currently under investigation. Similar phenomena could also occur in other genomic regions, such as the core, E1, NS2, and NS3, which are known to change during therapy but to a lesser extent than HVR1 and the NS5A gene central region (16), as a result of the interaction with other IFN-induced selection pressures. Changes in different regions may even be linked, as has already been suggested for other viruses (11).
IFN-induced quasispecies changes were associated with profound changes in the biochemical and virological course of HCV-related liver disease, probably as a result of qualitative modifications of the virus-host interaction. ALT and HCV RNA fluctuations were observed in most patients in whom viremia remained detectable during therapy, possibly owing to successive shifts in the virus population. Relapses and breakthroughs were almost always associated with peaks of HCV replication and serum ALT activity, followed by rapid decreases and subsequent fluctuations (data not shown). This pattern was identical to that usually observed during acute HCV infection progressing to chronicity (3, 58) and during liver graft reinfection after transplantation for HCV-related end-stage cirrhosis (7, 15). This strongly suggests that relapses and breakthroughs could be related to acute reinfection of the liver by a new, selected quasispecies that subsequently gives rise to a chronic infection. The role of hepatic or extrahepatic sites of replication that could act as IFN sanctuaries and sources of HCV reinfection with qualitatively different variants is under investigation.
Various histological outcomes were observed in our patients after therapy. Our results are consistent with the notion that qualitative HCV quasispecies changes could be responsible for most of the observed (beneficial or deleterious) changes in liver histology. Indeed, (i) the incidence of histological improvement is higher in patients with chronic hepatitis C receiving IFN-α than in those given no treatment or a placebo (5), whereas the annual rate of spontaneous chronic hepatitis C remission was recently estimated at 0.2% (4), and (ii) a 20% incidence of liver histology aggravation was recently reported in a series of 1,071 patients treated with IFN-α (19), whereas the annual risk of spontaneous hepatitis C aggravation was recently estimated at only 4.1% (4). Different HCV quasispecies may be more or less pathogenic. However, no direct cytopathic effect of HCV has yet been observed. More likely, different viral antigens presented at the surfaces of infected hepatocytes may trigger qualitatively and quantitatively different immune responses in the liver as a result of shifts in cytotoxic epitope immunodominance (43). Local cytokine secretion, which plays a major role in the onset of liver lesions in chronic hepatitis C (reviewed in references 32 and 47), could also be qualitatively and quantitatively altered. For most of our patients the aggravation of the Knodell score was due to a worsening of intralobular necrosis, a finding compatible with immune-mediated mechanisms. It must be stressed, however, that liver biopsy was performed very early (6 months) after the end of IFN therapy, when the HCV quasispecies had not yet reached a new equilibrium. Subsequent qualitative quasispecies changes and related disease changes were therefore likely.
In conclusion, IFN-α therapy induces profound changes in the nature of circulating HCV quasispecies in patients in whom sustained HCV RNA clearance is not achieved. These changes are characterized by shifts in the virus population, apparently owing to profound changes in the nature of the virus-host interaction following IFN administration and withdrawal. HCV quasispecies changes are associated with significant changes in the natural course of HCV disease, in which they could play a role. These results support the use of chronic hepatitis C treatments yielding sustained HCV RNA clearance, the only factor consistently associated with long-term improvement in HCV-related liver disease (8, 36, 39).
ACKNOWLEDGMENTS
This work was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique 1996, contract AOM96-136).
We thank Anne Bastie, Jean-Michel Métreau, Ariane Mallat, Jean-Philippe Mavier, Catherine Douvin, and Christophe Duvoux for providing patient samples; Jeanne Tran Van Nhieu and Elie-Serge Zafrani for reading liver biopsies; and Jocelyne Rémiré and Françoise Darthuy for excellent technical assistance. We are grateful to Gérard Babany and Marie-France Saint-Marc-Girardin (Roche Products, Neuilly-sur-Seine, France) and to Karen Gutekunst (Roche Molecular Systems) for their constant help. Finally, we are indebted to Avidan U. Neumann for helpful discussions and critical review of the manuscript.
REFERENCES
- 1.Alter H. Natural history and clinical aspects of hepatitis C virus infection. In: Schinazi R F, Sommadossi J P, Thomas H C, editors. Therapies for viral hepatitis. London, United Kingdom: International Medical Press; 1998. pp. 43–50. [Google Scholar]
- 2.Baron S, Tyring S K, Fleischmann W R, Coppenhaver D H, Niesel D W, Klimpel G R, Stanton G J, Hughes T K. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 3.Barrera J M, Bruguera M, Ercilla M G, Gil C, Celis R, Gil M P, del Valle Onorato M, Rodes J, Ordinas A. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology. 1995;21:639–644. [PubMed] [Google Scholar]
- 4.Bennett W G, Inoue Y, Beck J R, Wong J B, Pauker S G, Davis G L. Estimates of the cost-effectiveness of a single course of interferon-α2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Carithers R L, Jr, Emerson S S. Therapy of hepatitis C: meta-analysis of interferon alfa-2b trials. Hepatology. 1997;26(Suppl. 1):83S–88S. doi: 10.1002/hep.510260715. [DOI] [PubMed] [Google Scholar]
- 6.Chang K M. The mechanisms of chronicity in hepatitis C virus infection. Gastroenterology. 1998;115:1015–1017. doi: 10.1016/s0016-5085(98)70277-x. [DOI] [PubMed] [Google Scholar]
- 7.Chazouillères O, Kim M, Combs C, Ferrell L, Bacchetti P, Roberts J, Ascher N L, Neuwald P, Wilber J, Urdea M, Quan S, Sanchez-Pescador R, Wright T L. Quantitation of hepatitis C virus RNA in liver transplant recipients. Gastroenterology. 1994;106:994–999. doi: 10.1016/0016-5085(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 8.Chemello L, Cavalletto L, Casarin C, Bonetti P, Bernardinello E, Pontisso P, Donada C, Belussi F, Martinelli S, Alberti A the TriVeneto Viral Hepatitis Group. Persistent hepatitis C viremia predicts late relapse after sustained response to interferon-α in chronic hepatitis C. Ann Intern Med. 1996;124:1058–1060. doi: 10.7326/0003-4819-124-12-199606150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 10.Davis G L, Esteban-Mur R, Rustgi V, Hoefs J, Gordon S C, Trépo C, Shiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 11.Delwart E L, Pan H, Neumann A, Markowitz M. Rapid, transient changes at the env locus of plasma human immunodeficiency virus type 1 populations during the emergence of protease inhibitor resistance. J Virol. 1998;72:2416–2421. doi: 10.1128/jvi.72.3.2416-2421.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhumeaux D, Doffoël M, Galmiche J P. A French consensus conference on hepatitis C: screening and treatment. J Hepatol. 1997;27:941–944. doi: 10.1016/s0168-8278(97)80337-6. [DOI] [PubMed] [Google Scholar]
- 13.Domingo E. Biological significance of viral quasispecies. Viral Hepatitis Rev. 1996;2:247–261. [Google Scholar]
- 14.Duarte E A, Novella I S, Weaver S C, Domingo E, Wain-Hobson S, Clarke D K, Moya A, Elena S F, de la Torre J C, Holland J J. RNA virus quasispecies: significance for viral disease and epidemiology. Infect Agents Dis. 1994;3:201–214. [PubMed] [Google Scholar]
- 15.Duvoux C, Pawlotsky J M, Cherqui D, Tran van Nhieu J, Métreau J M, Fagniez P L, Duval J, Zafrani E S, Dhumeaux D. Serial quantitative determination of hepatitis C virus RNA levels after liver transplantation. A useful test for diagnosis of hepatitis C virus reinfection. Transplantation. 1995;60:457–461. doi: 10.1097/00007890-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Izumi N, Marumo F, Sato C. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. J Clin Investig. 1995;96:224–230. doi: 10.1172/JCI118025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enomoto N, Kurosaki M, Tanaka Y, Marumo F, Sato S. Fluctuation of hepatitis C virus quasispecies in persistent infection and interferon treatment revealed by single-strand conformation polymorphism analysis. J Gen Virol. 1994;75:1361–1369. doi: 10.1099/0022-1317-75-6-1361. [DOI] [PubMed] [Google Scholar]
- 18.Farci P, Alter H J, Wong D C, Miller R H, Govindarajan S, Engle R, Shapiro M, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell G C, Bacon B R, Goldin R D the Clinical Advisory Group for the Hepatitis C Comparative Study. Lymphoblastoid interferon alfa-n1 improves the long-term response to a 6-month course of treatment in chronic hepatitis C compared with recombinant interferon alfa-2b: results of an international randomized controlled trial. Hepatology. 1998;27:1121–1127. doi: 10.1002/hep.510270429. [DOI] [PubMed] [Google Scholar]
- 20.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein J. PHYLIP (phylogeny inference package), version 3.57. Distributed by the author. Seattle: Department of Genetics, University of Washington; 1995. [Google Scholar]
- 22.Gale M, Jr, Blakely C M, Kwieciszewski B, Tan S L, Dossett M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale M J, Jr, Korth M J, Tang N M, Tan S L, Hopkins D A, Dever T E, Polyak S J, Gretch D R, Katze M G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 24.Heim M K, Moradpour D, Blum H E. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-Stat pathway. Hepatology. 1998;28:321A. doi: 10.1128/jvi.73.10.8469-8475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jukes T H, Cantor T R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 26.Kao J H, Chen P J, Lai M Y, Wang T H, Chen D S. Quasispecies of hepatitis C virus and genetic drift of the hypervariable region in chronic type C hepatitis. J Infect Dis. 1994;172:261–264. doi: 10.1093/infdis/172.1.261. [DOI] [PubMed] [Google Scholar]
- 27.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knodell R G, Ishak K G, Black W C, Chen T S, Craig R, Kaplowitz N, Kiernan T W, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 30.Kojima M, Osuga T, Tsuda F, Tanaka T, Okamoto H. Influence of antibodies to the hypervariable region of E2/NS1 glycoprotein on the selective replication of hepatitis C virus in chimpanzees. Virology. 1994;204:665–672. doi: 10.1006/viro.1994.1582. [DOI] [PubMed] [Google Scholar]
- 31.Korenaga M, Hino K, Okazaki M, Okuda M, Okita K. Differences in hypervariable region 1 quasispecies between immune complexed and non-immune complexed hepatitis C virus particles. Biochem Biophys Res Commun. 1997;240:677–682. doi: 10.1006/bbrc.1997.7693. [DOI] [PubMed] [Google Scholar]
- 32.Koziel M J. The role of the immune responses in the pathogenesis of hepatitis C virus infection. J Viral Hepatitis. 1997;4(Suppl. 2):31–41. doi: 10.1111/j.1365-2893.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetic analysis for microcomputers. Comput Appl Biosci. 1993;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 34.Kumar U, Monjardino J, Thomas H C. Hypervariable region of hepatitis C virus envelope glycoprotein (E2/NS1) in an agammaglobulinemic patient. Gastroenterology. 1994;106:1072–1075. doi: 10.1016/0016-5085(94)90770-6. [DOI] [PubMed] [Google Scholar]
- 35.Kurosaki M, Enomoto N, Marumo F, Sato C. Rapid sequence variation of the hypervariable region of hepatitis C virus during the course of chronic infection. Hepatology. 1993;18:1293–1299. [PubMed] [Google Scholar]
- 36.Lau D T Y, Kleiner D E, Ghany M G, Park Y, Schmid P, Hoofnagle J H. Ten-year follow-up after interferon-α therapy for chronic hepatitis C. Hepatology. 1998;28:1121–1127. doi: 10.1002/hep.510280430. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay K L. Therapy of hepatitis C: overview. Hepatology. 1997;26(Suppl. 1):71S–77S. doi: 10.1002/hep.510260713. [DOI] [PubMed] [Google Scholar]
- 38.Manzin A, Solforosi L, Petrelli E, Macarri G, Tosone G, Piazza M, Clementi M. Evolution of hypervariable region 1 of hepatitis C virus in primary infection. J Virol. 1998;72:6271–6276. doi: 10.1128/jvi.72.7.6271-6276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou J P, Degott C, Erlinger S. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-α therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 40.Martell M, Esteban J I, Quer J, Genesca J, Weiner A, Esteban R, Guardia J, Gomez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 42.Nagasaka A, Hige S, Tsunematsu I, Yoshida J, Sasaki Y, Matsushima T, Asaka M. Changes in hepatitis C virus quasispecies and density populations in patients before and after interferon therapy. J Med Virol. 1996;50:214–220. doi: 10.1002/(SICI)1096-9071(199611)50:3<214::AID-JMV2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Nowak M A, May R M, Phillips R E, Rowland-Jones S, Lalloo D G, McAdam S, Klenerman P, Koppe B, Sigmund K, Bangham C R M, McMichael A J. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature. 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- 44.O’Connel J F. Mechanisms of action of interferon: potential role in hepatitis C. Viral Hepatitis Rev. 1997;3:121–128. [Google Scholar]
- 45.Odeberg J, Yun Z, Sönnerborg A, Bjoro K, Uhlen M, Lundenberg J. Variation of hepatitis C virus hypervariable region 1 in immunocompromised patients. J Infect Dis. 1997;175:938–943. doi: 10.1086/513995. [DOI] [PubMed] [Google Scholar]
- 46.Paterson M, Laxton C D, Sully R, Thomas H C, Ackrill A, Foster G R. The non-structural 5A protein of the hepatitis C virus blocks the antiviral effects triggered by IFN-α. Hepatology. 1998;28:370A. [Google Scholar]
- 47.Pawlotsky J M. Hepatitis C virus infection: virus/host interactions. J Viral Hepatitis. 1998;5(Suppl. 1):3–8. doi: 10.1046/j.1365-2893.1998.0050s1003.x. [DOI] [PubMed] [Google Scholar]
- 48.Pawlotsky J M, Germanidis G, Neumann A U, Pellerin M, Frainais P O, Dhumeaux D. Interferon resistance of hepatitis C virus genotype 1b: relationship to nonstructural 5A gene quasispecies mutations. J Virol. 1998;72:2795–2805. doi: 10.1128/jvi.72.4.2795-2805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawlotsky J M, Pellerin M, Bouvier M, Roudot-Thoraval F, Germanidis G, Bastie A, Darthuy F, Rémiré J, Soussy C J, Dhumeaux D. Genetic complexity of the hypervariable region 1 (HVR1) of hepatitis C virus (HCV): influence on characteristics of HCV infection and responses to interferon alfa therapy in patients with chronic hepatitis C. J Med Virol. 1998;54:256–264. [PubMed] [Google Scholar]
- 50.Pawlotsky J M, Roudot-Thoraval F, Bastie A, Darthuy F, Rémiré J, Métreau J M, Zafrani E S, Duval J, Dhumeaux D. Factors affecting treatment responses to interferon-α in chronic hepatitis C. J Infect Dis. 1996;174:1–7. doi: 10.1093/infdis/174.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Peters M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology. 1996;23:909–916. doi: 10.1053/jhep.1996.v23.ajhep0230909. [DOI] [PubMed] [Google Scholar]
- 52.Polyak S J, McArdle S, Liu S L, Sullivan D G, Chung M, Hofgärtner W T, Carithers R L, McMahon B J, Mullins J I, Corey L, Gretch D R. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J Virol. 1998;72:4288–4296. doi: 10.1128/jvi.72.5.4288-4296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trépo C, Albrecht J. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 54.Ray S C, Wang Y M, Laeyendecker O, Ticehurst J R, Villano S A, Thomas D L. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J Virol. 1999;73:2938–2946. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roudot-Thoraval F, Bastie A, Pawlotsky J M, Dhumeaux D the Study Group for the Prevalence and the Epidemiology of Hepatitis C Virus. Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6664 patients. Hepatology. 1997;26:485–490. doi: 10.1002/hep.510260233. [DOI] [PubMed] [Google Scholar]
- 56.Saitou N, Mei N. The neighbor-joining methods: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Purcell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shindo M, Di Bisceglie A M, Biswas R, Mihalik K, Feinstone S M. Hepatitis C virus replication during acute infection in the chimpanzee. J Infect Dis. 1992;166:424–427. doi: 10.1093/infdis/166.2.424. [DOI] [PubMed] [Google Scholar]
- 59.Shindo M, Hamada K, Koya S, Arai K, Sokawa Y, Okuno T. The clinical significance of changes in genetic heterogeneity of the hypervariable region 1 in chronic hepatitis C with interferon therapy. Hepatology. 1996;24:1018–1023. doi: 10.1053/jhep.1996.v24.pm0008903369. [DOI] [PubMed] [Google Scholar]
- 60.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomssen R, Bonk S, Thiele A. Density heterogeneities of hepatitis C virus in human sera due to the binding of β-lipoproteins and immunoglobulins. Med Microbiol Immunol. 1994;182:329–334. doi: 10.1007/BF00191948. [DOI] [PubMed] [Google Scholar]
- 62.Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- 63.Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Kinoshita M, Hadama T. Quasispecies nature of hepatitis C virus and response to alpha interferon: significance as a predictor of direct response to interferon. J Hepatol. 1997;26:6–13. doi: 10.1016/s0168-8278(97)80002-5. [DOI] [PubMed] [Google Scholar]
- 64.Tsai S L, Chen Y M, Chen M H, Huang C Y, Sheen I S, Yeh C T, Huang J H, Kuo G C, Liaw Y F. Hepatitis C virus variants circumventing cytotoxic T lymphocyte activity as a mechanism of chronicity. Gastroenterology. 1998;115:954–965. doi: 10.1016/s0016-5085(98)70268-9. [DOI] [PubMed] [Google Scholar]
- 65.van Doorn L J, Capriles I, Maertens G, DeLeys R, Murray K, Kos T, Schellekens H, Quint W. Sequence evolution of the hypervariable region in the putative envelope region E2/NS1 of hepatitis C virus is correlated with specific humoral immune responses. J Virol. 1995;69:773–778. doi: 10.1128/jvi.69.2.773-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Doorn L J, Quint W, Tsiquaye K, Voermans J, Paelinck D, Kos T, Maertens G, Schellekens H, Murray K. Longitudinal analysis of hepatitis C virus infection and genetic drift of the hypervariable region. J Infect Dis. 1994;169:1226–1235. doi: 10.1093/infdis/169.6.1226. [DOI] [PubMed] [Google Scholar]
- 67.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A, Brunetto M, Barr P J, Miyamura T, McHutchison J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiner A J, Brauer M J, Rosenblatt J, Richman K H, Tung J, Crawford K, Bonino F, Saracco G, Choo Q L, Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991;180:842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- 69.Wyatt C A, Andrus L, Brotman B, Huang F, Lee D H, Prince A M. Immunity in chimpanzees chronically infected with hepatitis C virus: role of minor quasispecies in reinfection. J Virol. 1998;72:1725–1730. doi: 10.1128/jvi.72.3.1725-1730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeh B I, Han K H, Oh S H, Kim H S, Hong S H, Oh S H, Kim Y S. Nucleotide sequence variation in the hypervariable region of the hepatitis C virus in the sera of chronic hepatitis C patients undergoing controlled interferon-α therapy. J Med Virol. 1996;49:95–102. doi: 10.1002/(SICI)1096-9071(199606)49:2<95::AID-JMV5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]