Abstract
Clinical trials were vital tools to prove the effectiveness and safety of medications. To maximize generalizability, the study sample should represent the sample population and the target population. However, the clinical trial design tends to favor the evaluation of drug safety and procedure (i.e., internal validity) without clear knowledge of its penalty on trial generalizability (i.e., external validity). Alzheimer’s Disease (AD) trials are known to have generalizability issues. Thus, in this study, we explore the effect of eligibility criteria on the AD severity patients and the severe adverse event (SAE) among the eligible patients.
Keywords: clinical trial, eligibility criteria, machine learning, SHAP value
I. Introduction
Clinical trials, especially randomized clinical trials, were vital tools to prove the effectiveness and safety of medications. [1] In clinical trials, three major populations are involved: target population, study population, and study sample. The target population refers to the patients who benefited from the trailed medication. The study population is the patients that fulfill the eligibility criteria (EC). The study sample is the patients enrolled in the study. To maximum generalizability, the study sample should represent the study population and the target population. However, the clinical trial design tends to adopt eligibility criteria in favor of the evaluation of drug safety and procedure (i.e., internal validity). [2–4] Alzheimer’s Disease (AD) trials are known to have the issue of the mismatched study population and target population. [5–7] In this study, we seek to identify ECs that would lead to the mismatch in AD severity and the likelihood of experiencing severe adverse events (SAEs). In this process, we built an EC bank, extracted a cohort of AD patients with AD severity information, and explored the effect of ECs on AD severity and SAE with machine learning (ML) methods and SHapley Additive exPlanation (SHAP) values. The outline of our system is shown in Fig. 2.
Fig. 2.
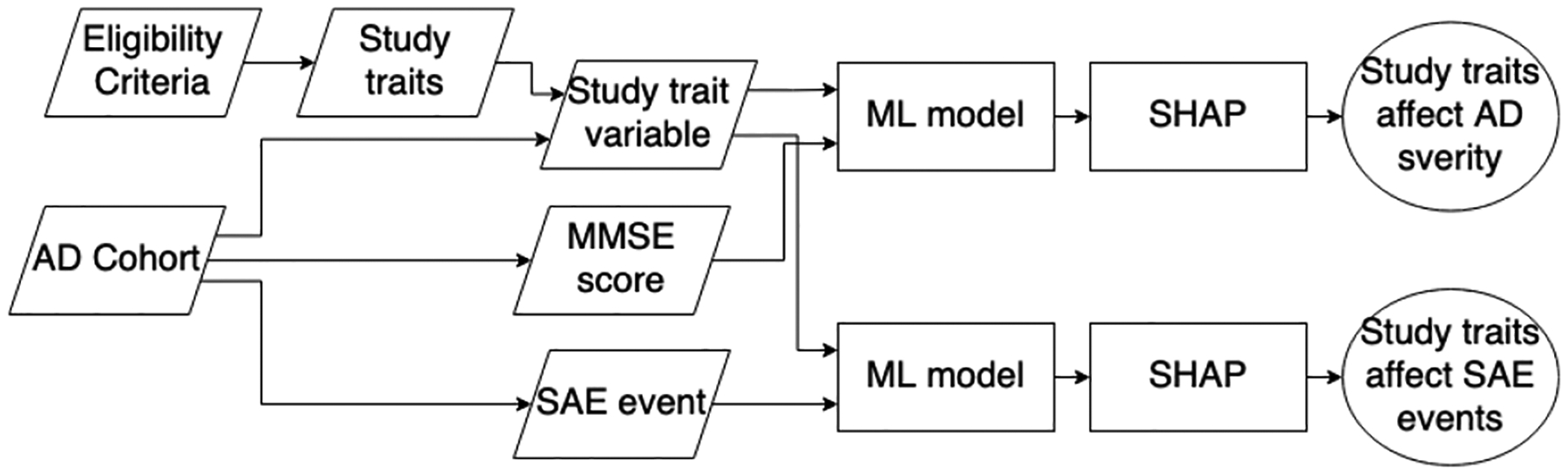
System outline
II. Method
We extracted ECs from 13 phase III and IV AD trials on the safety and effectiveness of four FDA-approved AD medications. ECs were decomposed into study traits that each defines a single demographic (e.g., age, race-ethnicity), diagnoses (e.g., history of diabetes), lab test results (e.g., fasting glucose), or other measurement used to define the study population.
From the University of Florida Integrated Data Repository (IDR), we extracted a cohort of AD patients from 2012 to 2021. We measured AD severity with the Mini-Mental Statement Examination (MMSE) score (0–30, cognitive impairment < 25) [8] extracted from the clinical narratives with an NLP tool [9].
The SAEs reported by the drug label of the corresponding medication were extracted within the SAE observation window between the first prescription of the AD medication and the 30 days after the last prescription (Fig. 3).
Fig. 3.
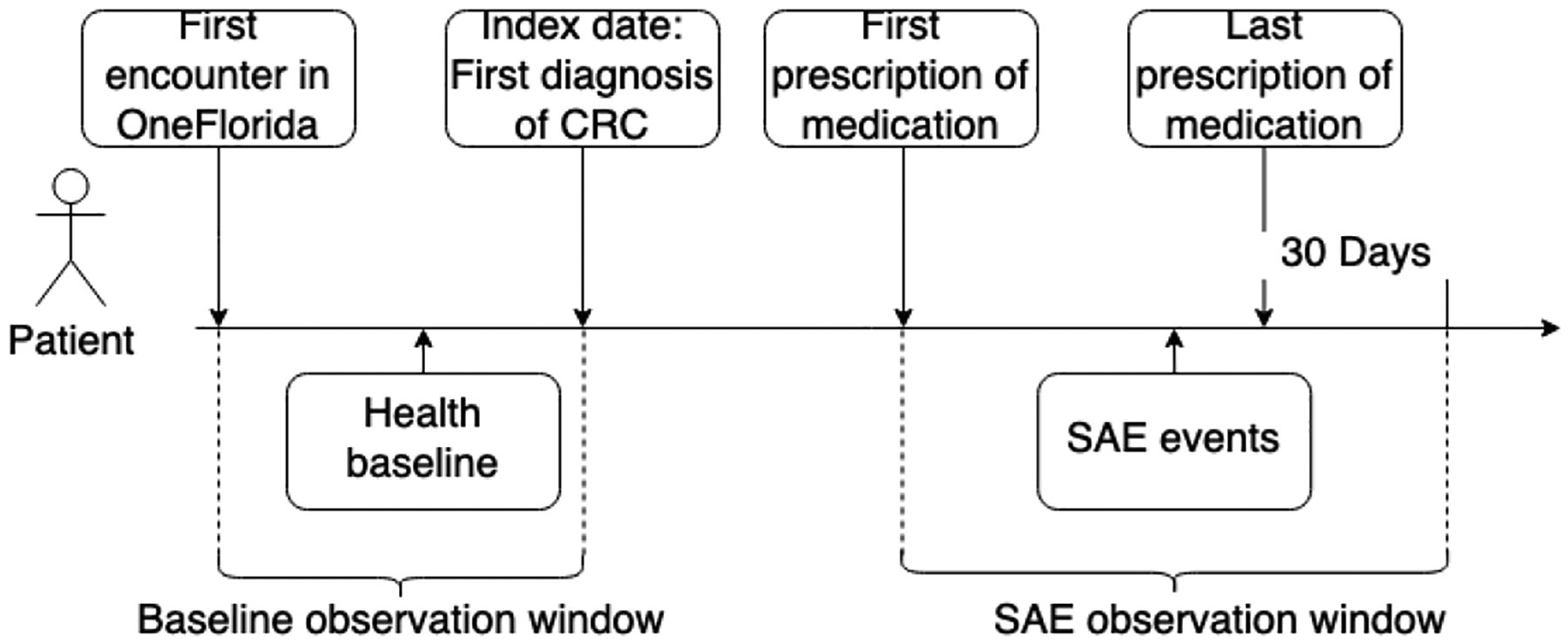
SAE observation window.
For each study trait, we extracted each patient’s eligibility based on their EHR and record them as study trait variables. The corresponding study trait variable will be coded 0 if the patient did not match the measurement described by the corresponding study trait and 1 otherwise.
In our analysis, we first modeled the association between study traits & AD severity, and study traits & number of SAE events with three ML methods: extreme gradient boost regressor (XGBoost) [10], Adaboost regressor (Adaboost) [11], and support vector machine regressor (SVM) [12] stratified by age, sex, race, and ethnicity. We performed the analysis on a trial basis with the patients on the trialed medication in our AD cohort. Then, we calculated the SHAP value with the best-performed model. SHAP value is an artificial intelligence (AI) explanation tool that measured the contribution of each variable to the change in the outcomes. [13] With the SHAP value, we could quantify the change that study traits imposed on AD severity (measured by MMSE score) and the number of SAEs. The study traits with major effects on AD severity and the number of SAEs were identified with the mean absolute SHAP value.
III. Results
In total, we extracted 1,024 AD patients with MMSE scores. The average age of our AD cohort was 80.63 years with the majority being female patients (64.7%). Regarding race and ethnicity, most of the patients were white (76.7%) and non-Hispanic (94.7%) (TABLE I).
TABLE I.
The demographic of the AD cohort with mmse
| AD patient with MMSE (n = 1,026) | |
|---|---|
| Age (SDa) | 80.63 (9.40) |
| Sex | |
| female (%) | 664 (64.7%) |
| male (%) | 362 (35.3%) |
| Race | |
| White (%) | 787 (76.7%) |
| Black (%) | 191 (18.6%) |
| Other (%) | 39 (3.8%) |
| Unknown (%) | 9 (0.9%) |
| Ethnicity | |
| Hispanic (%) | 42 (4.1%) |
| Non-Hispanic (%) | 972 (94.7%) |
| Unknown (%) | 12 (1.2%) |
SD: standard deviation.
We excluded the trials that contains too few patients and analyzed the eligibility criteria from 4 trials: NCT00097916, NCT00235716, NCT00305903, and NCT00478205. The SHAP values were calculated based on the XGBoost models as it outperformed the rest in all 4 trials on mean absolute error.
From NCT00097916, we identified 5 study traits (shown in Fig, 4, i.e., mental disorder, disorder of endocrine system, kidney disease, Donepezil prescription, and liver disease) affecting the AD severity of the patients, and 7 study traits (shown in Fig, 5, i.e., disorder of coronary artery, peripheral nerve system, drug dependence, cardiac dysrhythmias, renal failure, transient ischemic attack, diabetes, and asthma) related to the number of SAEs (absolute SHAP value > 0.1).
Fig. 4.
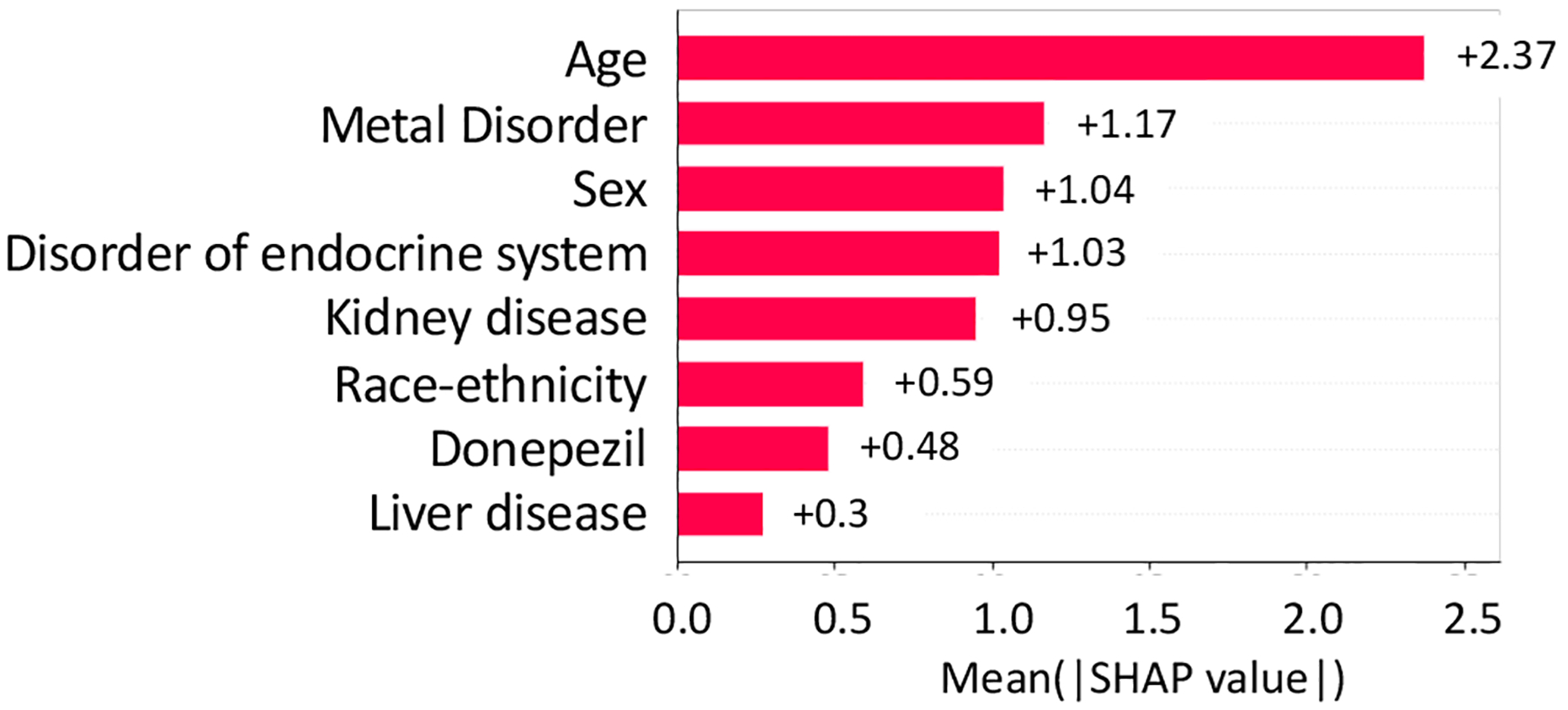
Absolute SHAP value of variables on AD severity in NCT00097916
Fig. 5.
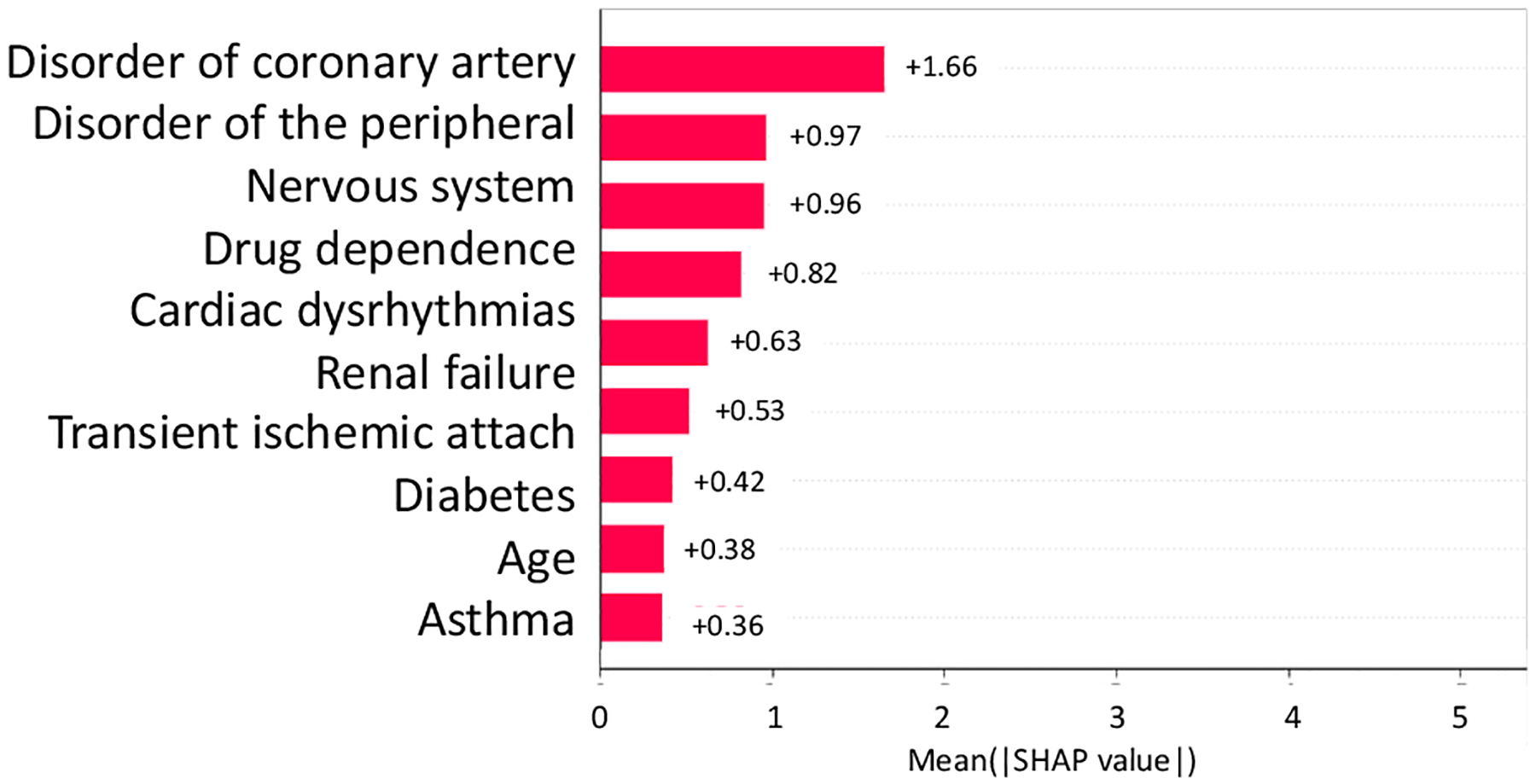
Absolute SHAP value of variables on SAE in NCT00097916
From NCT00235716, we identified 3 study traits (shown in Fig, 6 and Fig. 7, i.e., delirium, vitamin B12 deficiency, and Donepezil prescription) affecting both the AD severity and the number of SAEs (absolute SHAP value > 0.1).
Fig. 6.
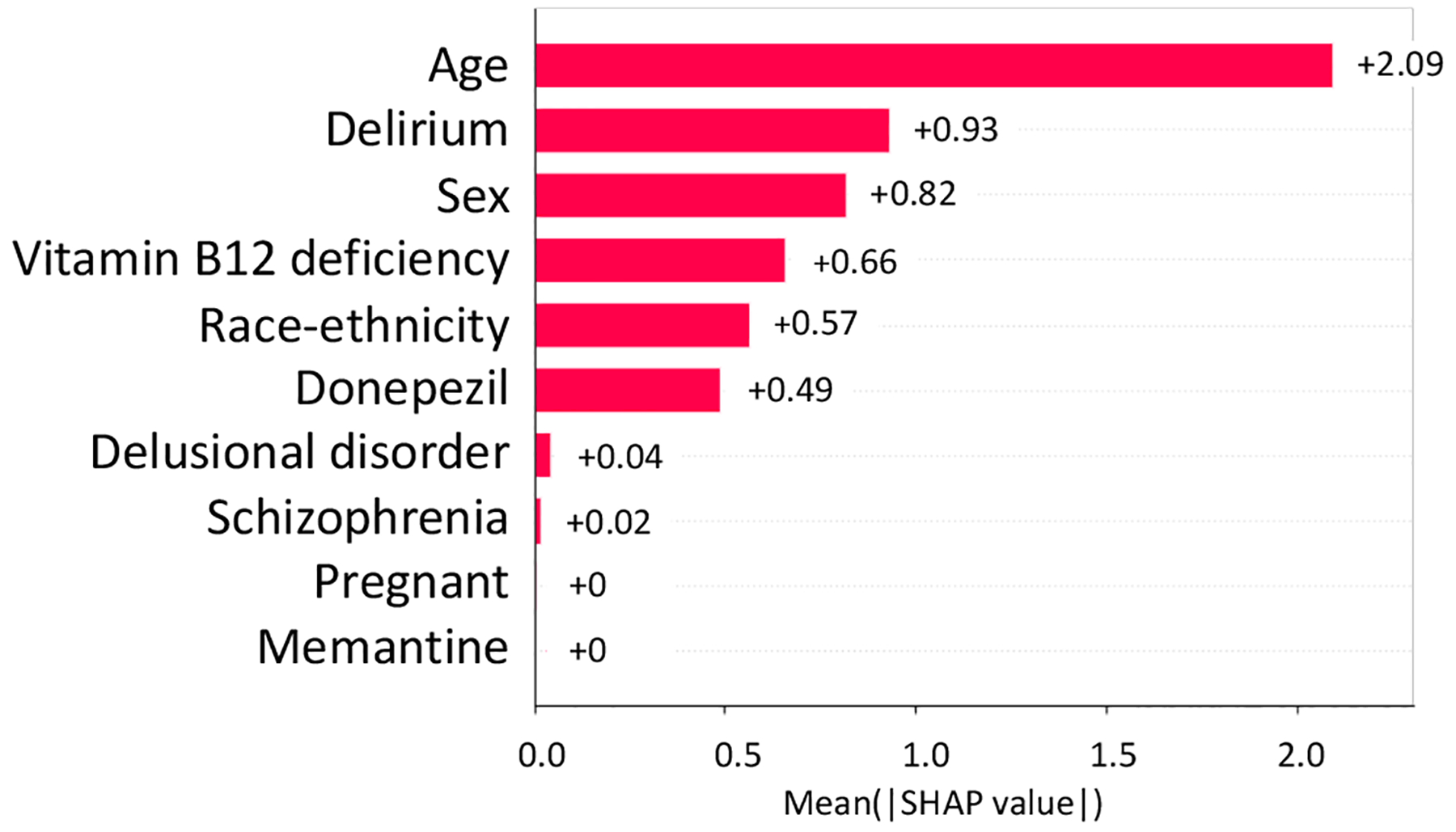
Absolute SHAP value of variables on AD severity in NCT00235716
Fig. 7.

Absolute SHAP value of variables on SAE in NCT00235716
From NCT00305903, we identified 5 study traits (shown in Fig, 8 and Fig. 9, i.e., kidney disease, disorder of endocrine system, myocardial infarction, disorder of respiratory system, and disorder of digest system) affecting both the AD severity and the number of SAEs (absolute SHAP value > 0.1).
Fig. 8.
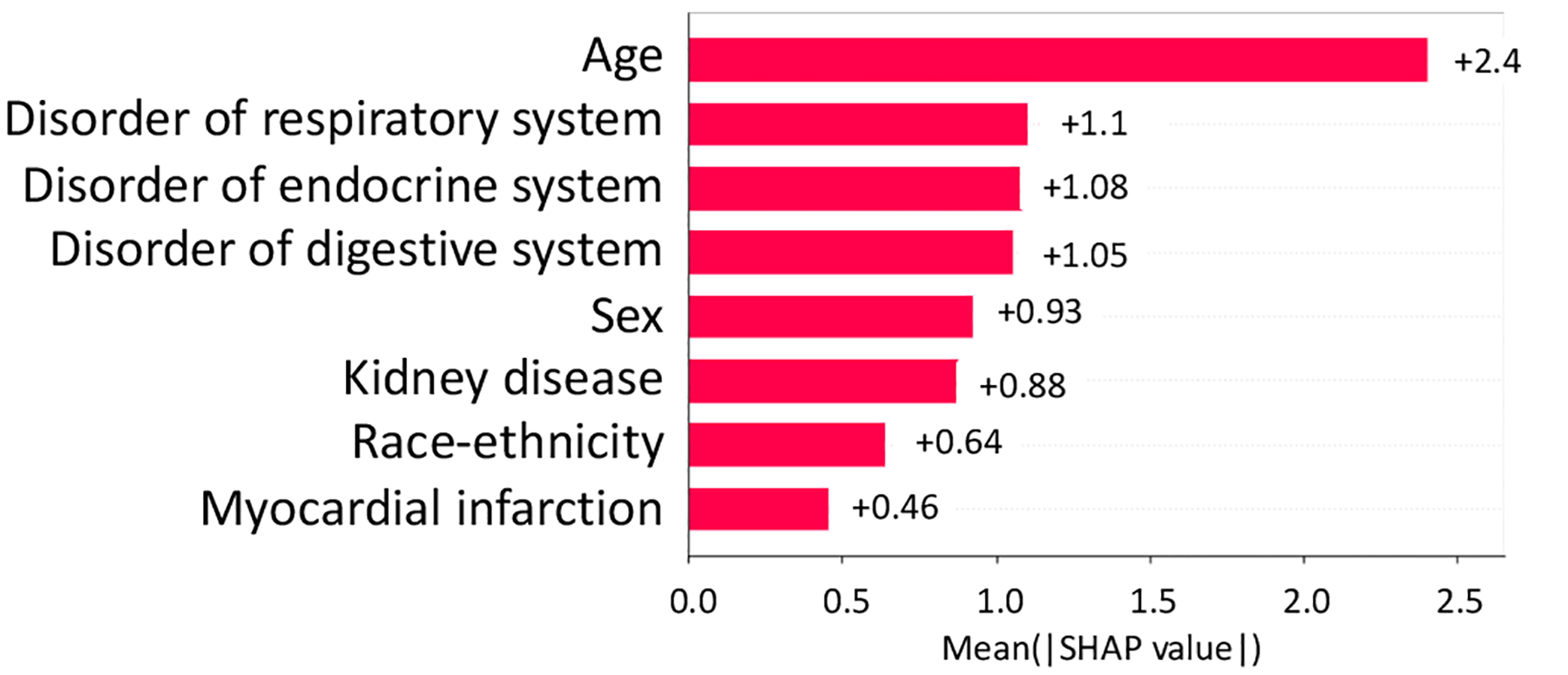
Absolute SHAP value of variables on AD severity in NCT00305903
Fig. 9.
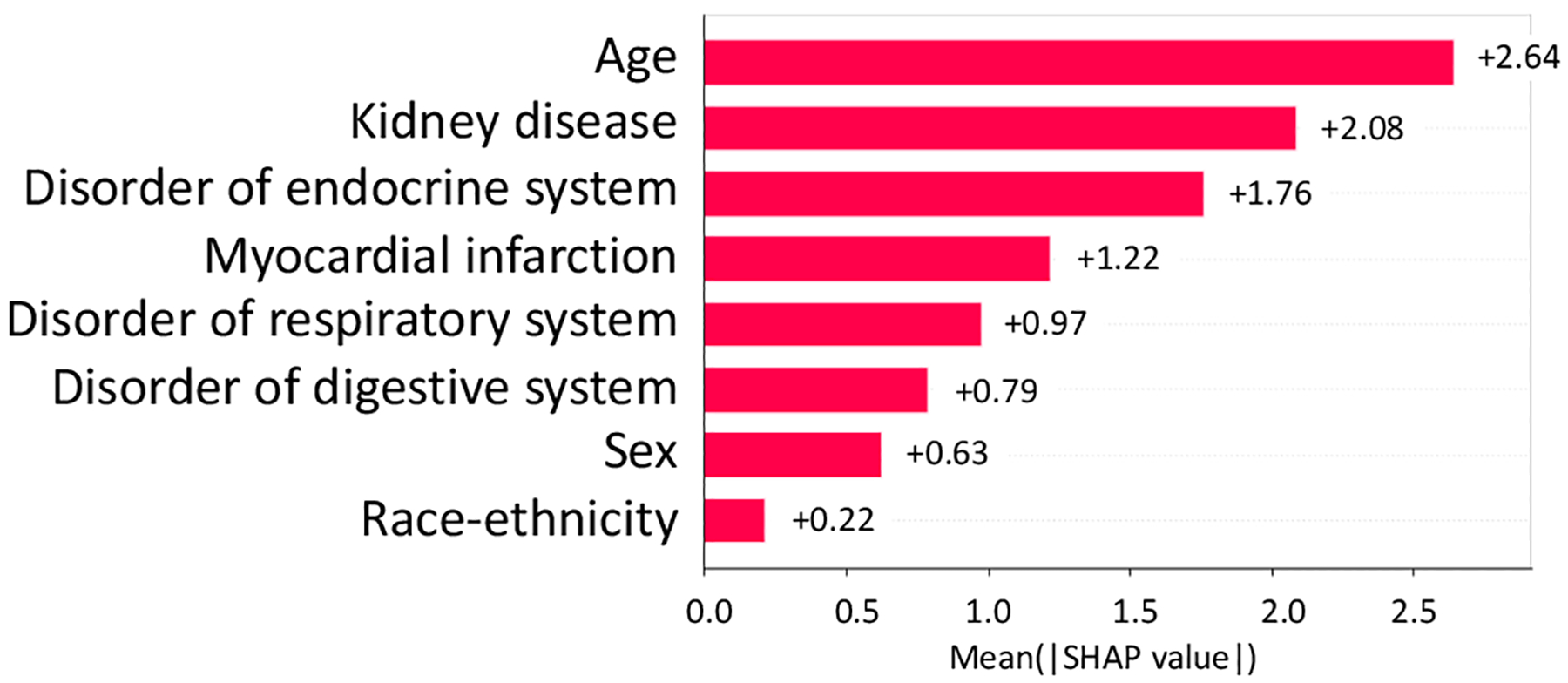
Absolute SHAP value of variables on SAE in NCT00305903
From NCT00478205, we identified 8 study traits (shown in Fig. 10, i.e., kidney disease, Benzodiazepine prescription, Memantine prescription, disorder of endocrine system, disorder of respiratory system, sleep disorder, urinary incontinence) affecting the number of SAEs (absolute SHAP value > 0.1).
Fig. 10.

Absolute SHAP value of variables on AD severity in NCT00478205
IV. Conclusion
In this study, we identified 14 unique study traits that affected the patients’ AD severity and 20 unique study traits that affected the number of SAEs. This analysis served as the first step in the development of a future system to guide AD clinical trial design.
Fig. 1.
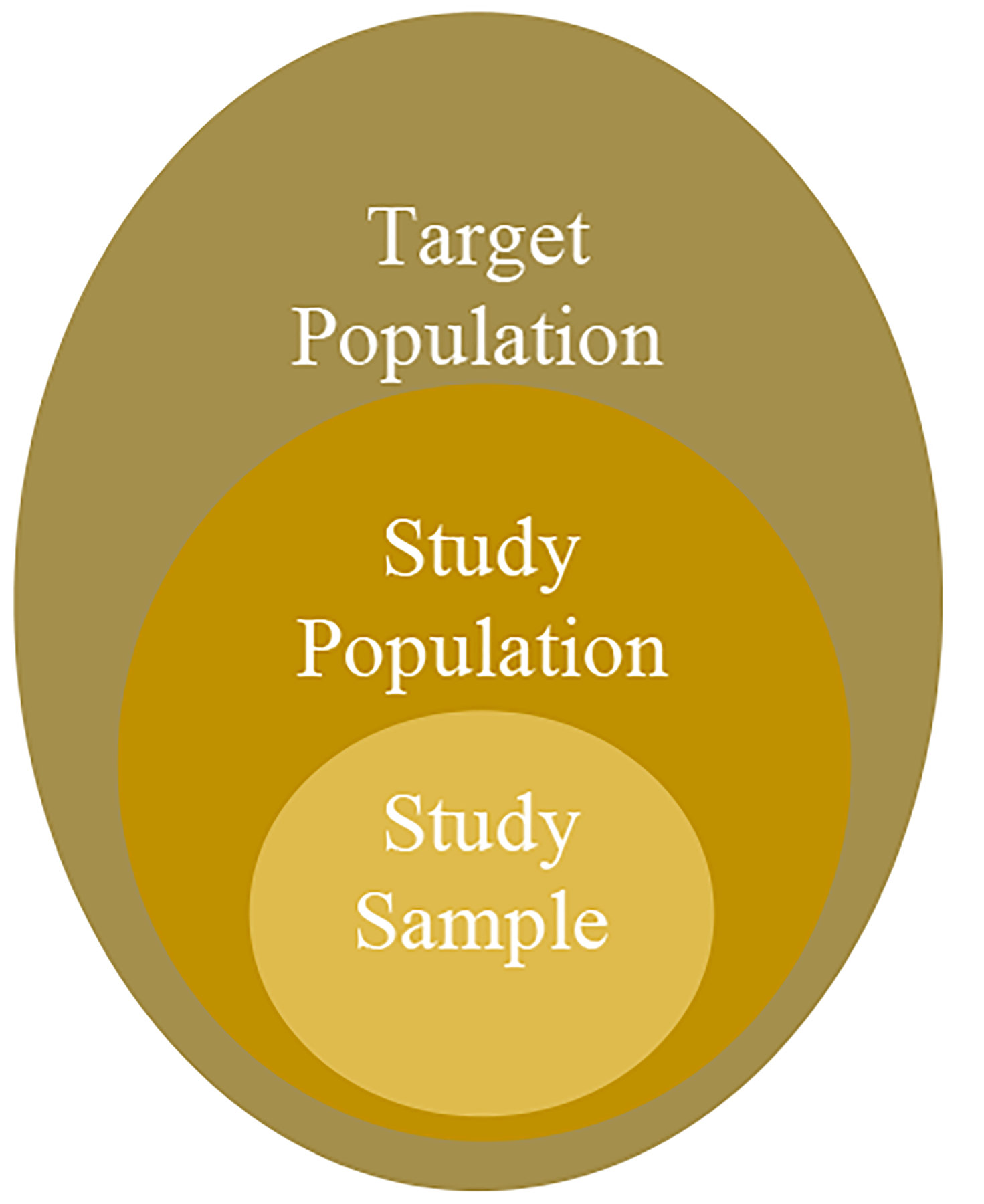
Populations in the clinical trial.
Contributor Information
Aokun Chen, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
Qian Li, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
Elizabeth Shenkman, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
Yonghui Wu, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
Yi Guo, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
Jiang Bian, Department of Health Outcome and Biomedical Informatics, College of Medicine University of Florida Gainesville, FL,USA.
References
- [1].Spieth PM, Kubasch AS, Penzlin AI, Illigens BM-W, Barlinn K, and Siepmann T, “Randomized controlled trials - a matter of design,” Neuropsychiatr. Dis. Treat, vol. 12, pp. 1341–1349, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karim S, Xu Y, Kong S, Abdel-Rahman O, Quan ML, and Cheung WY, “Generalisability of common oncology clinical trial eligibility criteria in the real world,” Clin. Oncol. (R Coll. Radiol.), vol. 31, no. 9, pp. e160–e166, Sep. 2019. [DOI] [PubMed] [Google Scholar]
- [3].He J, Morales DR, and Guthrie B, “Exclusion rates in randomized controlled trials of treatments for physical conditions: a systematic review,” Trials, vol. 21, no. 1, p. 228, Feb. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Q, Guo Y, He Z, Zhang H, George TJ Jr, and Bian J, “Using real-world data to rationalize clinical trials eligibility criteria design: A case study of Alzheimer’s disease trials,” AMIA Annu. Symp. Proc, vol. 2020, pp. 717–726, 2020. [PMC free article] [PubMed] [Google Scholar]
- [5].Raman R et al. , “Disparities by Race and Ethnicity Among Adults Recruited for a Preclinical Alzheimer Disease Trial,” JAMA Netw Open, vol. 4, no. 7, p. e2114364, Jul. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shin J and Doraiswamy PM, “Underrepresentation of African-Americans in Alzheimer’s trials: A call for affirmative action,” Front. Aging Neurosci, vol. 8, p. 123, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fredriksen-Goldsen K et al. , “Design and development of the first randomized controlled trial of an intervention (IDEA) for sexual and gender minority older adults living with dementia and care partners,” Contemp. Clin. Trials, vol. 128, no. 107143, p. 107143, Mar. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Folstein MF, Robins LN, and Helzer JE, “The Mini-Mental State Examination,” Arch. Gen. Psychiatry, vol. 40, no. 7, p. 812, Jul. 1983. [DOI] [PubMed] [Google Scholar]
- [9].Chen Z et al. , “Assess the documentation of cognitive tests and biomarkers in electronic health records via natural language processing for Alzheimer’s disease and related dementias,” Int. J. Med. Inform, vol. 170, no. 104973, p. 104973, Feb. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen T and Guestrin C, “XGBoost,” in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco California USA, 2016. [Google Scholar]
- [11].Freund Y and Schapire RE, “A decision-theoretic generalization of on-line learning and an application to boosting,” J. Comput. Syst. Sci, vol. 55, no. 1, pp. 119–139, Aug. 1997. [Google Scholar]
- [12].Awad M and Khanna R, “Support Vector Regression,” in Efficient Learning Machines, Berkeley, CA: Apress, 2015, pp. 67–80. [Google Scholar]
- [13].Lundberg S and Lee S-I, “A unified approach to interpreting model predictions,” arXiv [cs.AI], 22-May-2017. [Google Scholar]


