Abstract
The amounts of Reactive oxygen species (ROS) become higher by strenuous exercises which consume larger amounts of oxygen in active muscles. Since these ROS directly injured muscles, the high ROS concentration involves muscle fatigue. Thus, an immediate ROS scavenging system in the muscle is desired. Since Monascus pigment (MP) involves physiologically active substances which scavenge ROS, it may be a clue to save the muscle injury. However, there are no reports examining MP effects on oxidative stress in skeletal muscle. In this study, we investigated the effect and mechanism of MP on skeletal muscle cells damaged by oxidative stress. The ability to directly eliminate ROS was evaluated by mixing MP solutions with •OH and O2•−, a type of ROS. The effect of peroxidation in C2C12 cells was evaluated by cell viability assay and Western blotting. MP scavenges •OH and O2•−. MP treatment increases the survival rate under oxidative stress. At that time, the expression of catalase was increased: the enzyme change H2O2 into H2O to rescue the cells under oxidative stress. We conclude that monascus pigment suppressed myotube damage under oxidative stress by both non-enzymatic ROS scavenging and up-regulation of catalase expression.
Keywords: monascus pigment, reactive oxygen species, antioxidation, catalase, C2C12 cell
Introduction
Exercise on a daily basis decreases the risk of chronic diseases such as cardiovascular disease, diabetes, and cancer and decreases mortality,(1) while athletes exercise to enhance their physical performance rather than to improve their health. Hard training is essential for the athlete because they have to demonstrate the best performance in the match. In these strenuous exercise, the production of reactive oxygen species (ROS) increase in active muscles. Previous studies have reported that skeletal muscle contraction is a major source of ROS.(2,3) Although the oxidation of biomolecules is important for the activation of signal transduction, excess ROS induces negative effects such as DNA damage, lipid peroxidation, and mitochondrial impairment to inhibit such activations.(4–7) The human body is equipped with antioxidant defense systems. However, the ROS scavenger cannot catch up with the excess ROS.(8) Extrinsic ROS and accumulation of intrinsic ROS from mitochondria are burdening cells as oxidative stress and leading to ATP shortage. Moreover, ROS can suppress the immune system by reducing natural killer cells and ensure a decrease in exercise performance and condition.(9)
ROS are involved in the electron transport chain. superoxide anion radical (O2•−) should change into hydroxyl radical (•OH) via hydrogen peroxide (H2O2). Above all, hydroxyl radical is highly reactive to oxidize lipids, proteins, and nucleic acids, causing damage to the body. To inhibit ROS-derived damage in the body, there are antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) which scavenge ROS. SOD converts superoxide anion radical to hydrogen peroxide. Catalase and GPx detoxify hydrogen peroxide into the water. These enzymes are upregulated in the body in response to ROS. However, aging, disordered eating habits, and strenuous exercise lead to oxidative stress states that exceed these endogenous defense mechanisms of the body. Previous studies have reported that the expression of antioxidant enzymes increased when antioxidants were added to skeletal muscle cells.(10)
On the other hand, ROS can be scavenged by treatments with exogenous antioxidants. Ascorbic acid can directly scavenge ROS by a redox reaction.(11,12) Previous studies have reported that oxidative stress during exercise is inhibited by ascorbic acid.(13) Other antioxidants such as lycopene and β-carotene can scavenge ROS and numerous studies have reported that these pigment antioxidants upregulate the expression of antioxidant enzymes.(14–17)
Monascus pigment (MP) is a red pigment obtained from Monascus purpureus (M. purpureus). Fermented red yeast rice with this strain has been used as a food coloring agent for a long time in Japan and China and has been sold as a supplement all over the world in recent years. MP contains many physiologically active substances. Monacolin k has a lowing cholesterol level and γ-aminobutyric acid (GABA) has an antistress action.(18,19) Furthermore, Dimerumic acid and rubropunctatin have an antioxidant effect.(20,21) Dimerumic acid reduce lipid peroxides and was stronger than ascorbic acid.(22) MTCC-410, a type of M. purpureus, reduced carbonyl proteins and was stronger than rubropunctatin isolated from that strain.(21) Therefore, MP containing several antioxidants is likely to be useful as a powerful antioxidant. However, there are no studies on MP and oxidative stress, and the detailed mechanism remains unclear.
In this study, we created the state of skeletal muscle during exercise by generating oxidative stress using hydrogen peroxide, which is a kind of ROS.(23–25) The purpose of this study was to clarify the mechanism by which MP reduces oxidative stress in skeletal muscle cells.
Materials and Methods
Materials
Monascus pigment, hypoxanthine, and hydrogen peroxide were obtained from Wako Pure Chem., Ind., Ltd., (Osaka, Japan). Xanthine oxidase (Roche, Basel, Switzerland), 2-[5,5-dimethyl-2-oxo-2λ5-(1,3,2)dioxaphosphinan-2-yl]-2-methyl-3,4-dihydro-2H-pyrrole1-oxide (CYPMPO; Radical Research Inc., Tokyo, Japan), Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) were purchased and used without further purification or modification.
Electron spin resonance spectroscopy
The antioxidant effect of monascus pigment was measured by electron spin resonance (ESR). The scavenging activities of monascus pigment in solution were estimated using CYPMPO. Hydroxyl radicals were generated by 3 s. UV (405 ± 20 nm) irradiating for the mixture of 10 mM H2O2 and 10 mM CYPMPO. Superoxide radicals were generated from a xanthine/xanthine oxidase (X/XO) system. This reaction mixture contained phosphate buffered saline (PBS) with 20 mM hypoxanthine, 20 units/ml xanthine oxidase, 10 mM CYPMPO and MP. Each reaction mixture was immediately mixed with a given concentration of MP and placed in a Pasteur pipette. The ESR spectra were recorded using a JEOL-TE X-band spectrometer (JEOL, Tokyo, Japan). ESR spectra of MP solution were obtained under the following conditions: 20 mW incident microwave power, 9.2 GHz frequency, 0.2 mT modulation width, 7.5 mT sweep width, 0.1 s time contrast, and 335.5 mT center field.
Cell culture and cell viability assay
C2C12 skeletal muscle cells were purchased from the RIKEN Cell Bank (Ibaraki, Japan). C2C12 myoblasts are cells that differentiate into myotube cells by a differentiation medium,(26) and differentiation induction was performed with reference to previous research.(27,28) The cells were cultured in a growth medium High-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 1% penicillin/streptomycin solution in a humidified incubator at 37°C with 5% CO2. When C2C12 myoblasts were cultured to 60–80% confluence, the supernatant was aspirated and added DMEM containing 2% horse serum for 0 to 7 days. After incubation, the C2C12 skeletal muscle cells differentiated into myotubes. Cells from Day 0 to Day 7 of differentiation were used for Western blotting analysis.
Cell viability was measured using the Cell Counting Kit-8 according to the manufacturer’s protocol. C2C12 myotubes were grown in 12-well plates. To determine the cytotoxicity of monascus pigment, cells were cultured in the medium containing 0, 25, 50, 100, 200, and 300 μg/ml of monascus pigment respectively, and incubated for 24 h. After incubation, cells were rinsed with PBS twice, then incubated with 10% Cell Counting Kit-8. The absorbance at 450 nm was measured by a microplate reader (Varioskan LUX; Thermo Fisher Scientific, Waltham, MA).
The groups were divided into four groups: control group (CON), monascus pigment group (MP), hydrogen peroxide group (H2O2), and monascus pigment and hydrogen peroxide group (MP + H2O2). MP group and MP + H2O2 group were replaced with a culture medium containing monascus pigment at the concentration determined by the cell viability assay. The other groups were replaced with a fresh culture medium, incubated for 24 h. Thereafter, the H2O2 group and MP + H2O2 group were replaced with a culture medium containing 7 mM H2O2, incubated for 24 h. Samples used for RT-PCR and Western blotting analysis were incubated for 12 h. After incubation, cells were rinsed in the same procedure and cell viability was measured.
RNA isolation and quantitative real-time RT-PCR
The mRNA expression was measured using real-time RT-PCR. To extract total RNA, C2C12 myotubes were collected on ice in the Sepasol-RNAISuper G kit (Nakalai Tesque, Kyoto, Japan) and then separated into organic and aqueous phases with chloroform. Samples were allowed to sit at room temperature for 3 min and then centrifuged (4°C, 12,000 × g, 15 min). The separated aqueous phase in the tube was dispensed to the new tube and 2-isopropanol was added. The mixture was mixed by inversion and centrifuged (4°C, 12,000 × g, 15 min) after incubating for 10 min at room temperature. The supernatant was removed and 70% ethanol was added for suspension. Centrifugation (4°C, 12,000 × g, 5 min) was performed to remove the supernatant completely. Finally, RNase-free Water (9012; Takara Bio Inc., Shiga, Japan) was added and the sample was incubated at 65°C for 5 min. After RNA concentration was measured by spectrophotometry (Nanodrop ND1000; Thermo Fisher Scientific), cDNA synthesis by PrimeScript RT master mix (Takara Bio Inc.) was performed as per the manufacturer’s instructions. To quantify gene expression levels, we performed PCR using a KAPA SYBR FAST qPCR kit (Kapa Biosystems, Wilmington, MA) on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific), according to the manufacturer’s instructions. The cycling program included an initial denaturation at 95°C for 20 s, followed by 40 cycles of denaturation at 95°C for 3 s, and annealing and elongation at 60°C for 3 s. A melting curve analysis confirmed that the PCR product did not contain nonspecific by products. The mRNA expression of Tbp was measured as the housekeeping gene. The mRNA content was determined using the standard curve method. The target gene’s cycle threshold (Ct) value was standardized to the Ct value of the housekeeping gene (ΔΔCt method). The primer sequences used in this study are shown in Table 1.
Table 1.
Primer sequences used for mRNA analysis
| Name | Forward (5'→3') | Reverse (5'→3') |
|---|---|---|
| Tbp | CAGCCAAGATTCACGGTAGAT | CCAATGACTCCTATGACCCCTA |
| Catalase | CAAGTTTTTGATGCCCTGGT | CCTTCAAGTTGGTTAATGCAGA |
| Gpx1 | CAGGTCGGACGTACTTGAGG | GTTTCCCGTGCAATCAGTTC |
| Sod2 | GTAGTAAGCGTGCTCCCACAC | TGCTCTAATCAGGACCCATTG |
Western blotting
Whole cell lysates were prepared by rinsing cells three times with PBS, adding NuPAGE LDS Sample buffer (Life Technologies, Carlsbad, CA) on ice, then heating at 95°C for 5 min. For SDS-polyacrylamide gel electrophoresis, the cell lysates were added into wells of NuPAGE 4 to 12% Bis-Tris Gel (Thermo Fisher Scientific). The gel was electrophoresed at 100 V for 60 min and proteins were transferred onto a PVDF membrane (Millipore Corp.) by electrophoresis at 0.2 A for 60 min. The membrane was blocked at room temperature for 60 min with PVDF blocking reagent for Can Get Signal (TOYOBO Co., Ltd., Osaka, Japan). Membranes were washed three times for 10 min each with PBS-T. Anti-rabbit β-Actin (4967; Cell Signaling Technology), Myogenin (ab1248000; Abcam), SOD2 (13141; Cell Signaling Technology), Catalase (14097; Cell Signaling Technology), GPx1 (ab22604; Abcam) antibodies (1:1,000) were added to Can Get Signal® Immunoreaction Enhancer Solution 1. The membrane was exposed to this solution overnight at 4°C. Thereafter, membranes were washed. The secondary HRP-linked anti-rabbit IgG antibody (7074; Cell Signaling Technology) (1:3,000) was added to the Can Get Signal Immunoreaction Enhancer Solution 2 (TOYOBO Co., Ltd.) and exposed to the membrane at room temperature for 60 min. The membranes were then washed three times in PBS-T for 10 min. The proteins were visualized with Western blot chemiluminescence reagent (Lumina Forte Western HRP Substrate; Millipore Corp.) and detected using a FUSION FX7.EDGE (Vilber Lourmat, Eberhardzell, Germany). Ponceau staining was used to verify consistent loading. Ponceau has been shown to be an inner control that can substitute for housekeeping genes,(29) and there is an example of using ponceau as a control in a previous study on mouse skeletal muscle.(30,31)
Statistical analysis
Data are shown as mean ± SE. For all measurements, a two-way analysis of variance or t test was conducted. In the case of significant F values, comparisons were made using Tukey’s post hoc test. GraphPad Prism 7 software (GraphPad, Inc., San Diego, CA) was used for all statistical calculations. P value of less than 0.05 was statistically significant.
Results
MP included the ROS scavenging activity
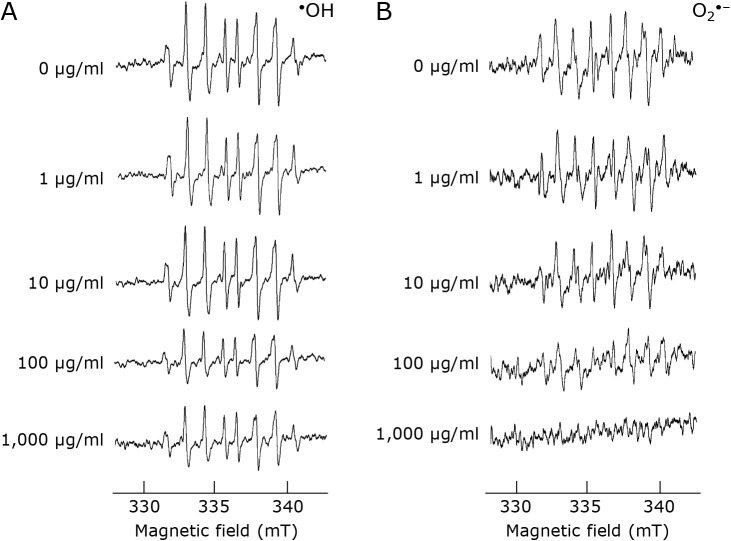
Figure 1 shows the results of ESR measurements for hydroxyl radicals and superoxide anion radicals. The peak of hydroxyl radicals with MP was decreased in a dose-dependent manner (Fig. 1A). The peak of superoxide anion radicals also decreased in MP dose-dependent manner (Fig. 1B).
Fig. 1.
ESR spectra of (A) hydroxyl radicals or (B) superoxide anion radicals. CYPMPO was used as the spin-trapping regent. 0 μg/ml has only PBS added as a negative control.
Differentiation from myoblasts to myotubes
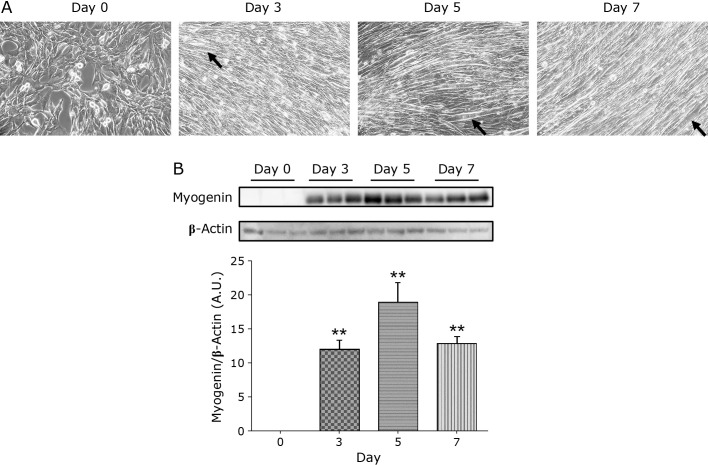
C2C12 myoblasts differentiated into mature multinucleated myotubes on Day 3, 5, and 7 (Fig. 2A, black arrows). Myogenin acts as a transcriptional activator that promotes the transcription of muscle-specific target genes and plays a role in muscle differentiation. Myogenin protein expression was significantly increased at all culture days compared to Day 0 (Fig. 2B).
Fig. 2.
Morphological change from C2C12 myoblasts to myotubes. (A) The phase contrast images from Day 0 to Day 7. The cells were counted under ×200 magnification. Arrow shows an example of a myoblast transformed into a myotube. (B) The protein expression level of myogenin, a factor regulating myogenesis, was measured using a Western blotting assay. Data are shown as mean ± SE (n = 6). **p<0.01.
MP can suppress cytotoxicity by H2O2
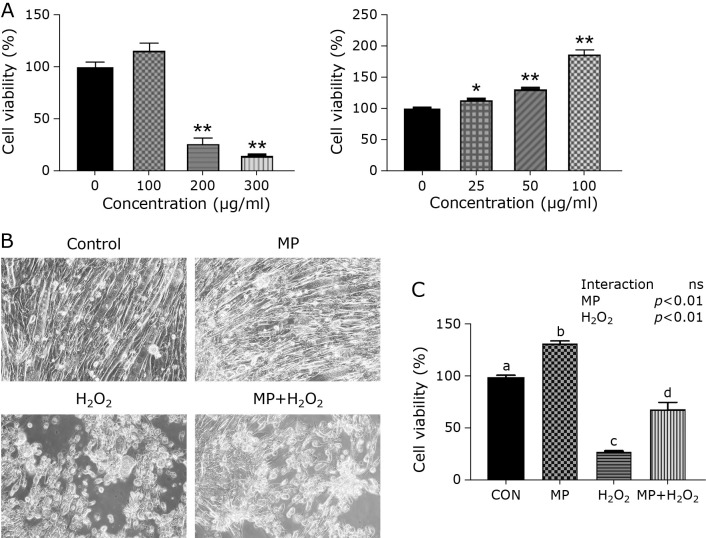
The cytotoxicity of MP in C2C12 myotubes was evaluated by the cell viability assay. Cell viability was significantly decreased at 200 and 300 μg/ml compared with 0 μg/ml (Fig. 3A left). Cell viability gradually increased with the addition of MP at concentrations up to 100 μg/ml (Fig. 3A right). From these results, the following experiment was demonstrated with the 100 μg/ml MP.
Fig. 3.
Cell viability and cell morphology under the microscopy. (A) Cytotoxicity of MP in C2C12 myotubes. Data are shown as mean ± SE (n = 3). *p<0.05, **p<0.01. (B) The phase contrast images in each group of cells. The cells were counted under ×200 magnification. (C) The cytotoxicity effect of H2O2. Compared to CON, the cell viability in H2O2 was significantly decreased. The addition of MP could suppress H2O2-induced cytotoxicity. Data are shown as mean ± SE (n = 4). Different letters are significantly different (p<0.05).
Figure 3B shows the phase contrast image of C2C12 cells in each group. The cell morphology was deformed and the number of myotube cells was decreased in the H2O2 and MP + H2O2 group compared with the CON and MP group. Figure 3C shows the cell viability assay. Cell viability in the H2O2 group was significantly decreased compared with the CON + H2O2 group. As a result of this, MP can suppress cytotoxicity by H2O2.
Antioxidant enzymes gene and protein expression
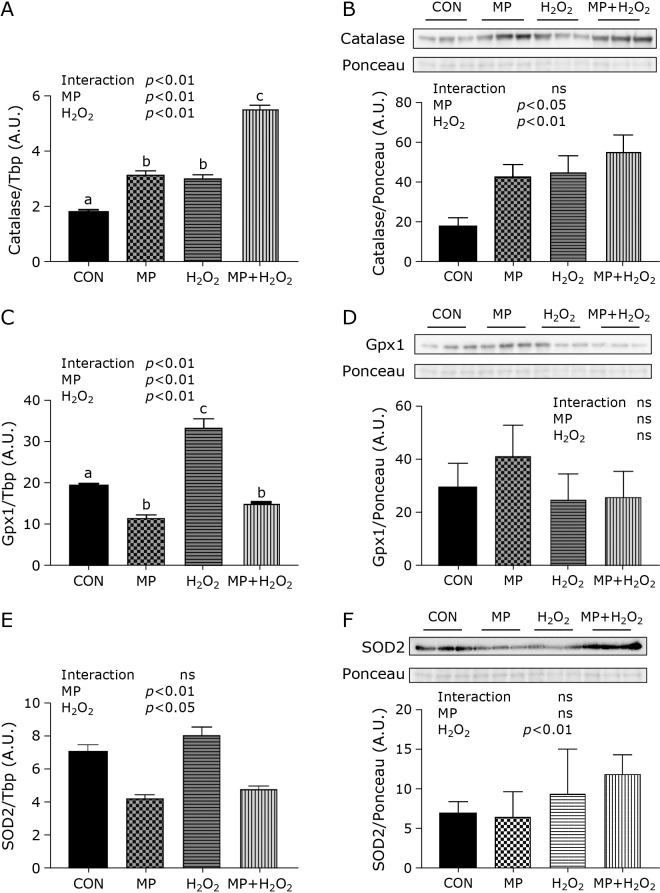
Antioxidant enzymes gene and protein expression levels in C2C12 myotubes were measured. Both MP and H2O2 treatment showed the synergistic effect on catalase gene expression. In the MP or H2O2 group, the gene expression showed a significant increase compared to the CON group. (Fig. 4A). Moreover, this expression in the MP + H2O2 group was highest in four groups and significantly increased compared with the MP group or H2O2 group. The catalase protein expression was also increased in the MP, H2O2, and MP + H2O2 group compared with the CON group (Fig. 4B). Both MP and H2O2 treatments also showed synergistic effects on Gpx1 gene expression. In the H2O2 group, the gene expression showed a significant increase compared to the CON group and significant decrease compared to MP and MP + H2O2 group (Fig. 4C). Compared with the CON group, GPx1 protein expression in the MP group increased, however, there was no significant difference (Fig. 4D). The SOD2 gene expression increased in the H2O2 group, while it decreased in the MP and MP + H2O2 group (Fig. 4E). SOD2 protein expression in each group was no significant difference (Fig. 4F).
Fig. 4.
Expression levels of antioxidant enzymes. Antioxidant enzymes measured catalase (A, B), GPx1 (C, D), and SOD2 (E, F). Data are shown as mean ± SE (n = 6). Western blotting images represent three representative samples per group. Different letters are significantly different (p<0.05).
Discussion
The aim of this study was to clarify the mechanism by which MP reduces oxidative stress in skeletal muscle cells under oxidative stress states. We demonstrated that MP scavenges hydroxyl radicals in a dose-dependent manner for the first time. MP also scavenged superoxide anion radicals in a dose-dependent manner same as the previous report.(1) Dhale et al.(21) reported that rubropunctatin, which was extracted and isolated from red yeast rice, scavenge superoxide anion radicals. A part of the scavenging ability of superoxide anion radicals in MP can be attributed to rubropunctatin. MP is constituted by compounds. We demonstrated that these compounds detoxified ROS into the water and other substances by redox reactions.(3) Taken together, we concluded that MP has the ability to scavenge the hydroxyl radicals and superoxide anion radicals.
We differentiate the myoblast into the myotube which is a state virtually the same as the muscle fiber for our experiments. Myotubes were observed at all culture days, with a particularly large number of myotubes observed on the 5th day of differentiation. Myogenin, a factor regulating myogenesis, protein expression was also significantly increased at all culture days, confirming that the cells were transformed into myotubes. Many previous studies have reported that myoblast becomes myotube 5 days after differentiation.(28,32,33) Based on these results, we concluded that the myotube derived myoblast can use for the evaluation and decided to conduct experiments using myotube cells cultured for 5 days.
We investigated the antioxidant effect of MP in vitro. MP can increase cell viability and attenuate the cytotoxicity derived from hydrogen peroxide in C2C12 myotubes. Increased cell viability has been reported in vitro studies with the treatment of antioxidants,(34) and it is possible that a similar cytoprotective effect characteristic of antioxidants occurred in this study. To clarify the antioxidant mechanism of MP, we focused on antioxidant enzymes such as catalase, GPx1, and SOD2. Gene and protein expression levels of catalase in C2C12 myotubes were increased by MP. We considered that the catalase reaction, which detoxifies ROS into water and oxygen, was activated in the C2C12 myotubes. Previous studies have indicated the antioxidants pigment such as anthocyanins, lutein, and lycopene increased catalase levels in cells, animals, and humans.(14,15,17) Catalase levels were significantly increased in the MP + H2O2 group compared with the MP and H2O2 groups, we considered that the MP treatment scavenge the ROS by catalase activation. On the other hand, the gene expression level of GPx1, which is an enzyme that detoxifies ROS like catalase, decreased the MP group compared with the CON group. Several antioxidants enhance the GPx expression in vivo,(14,17) the opposite result was obtained in this experiment. Moreover, the MP + H2O2 group also showed a significant decrease compared to the CON group, indicating that the contribution of GPx1 was small even during oxidative stress generation by H2O2 treatment. Therefore, we concluded that not GPx but a catalase-mediated pathway may be preferentially used to scavenge the ROS by MP treatment. The gene expression level of SOD2 was also reduced by MP treatment. Wang et al.(35) reported that the SOD level in the red yeast rice group was decreased. Red yeast rice is an antioxidant and is included in MP. From these results, we considered that MP downregulates the SOD expression at the gene level. Although an increase in the SOD expression has been reported using other antioxidant pigments except for MP,(16,17) the antioxidant effect of MP is not SOD or GPx but catalase.
Although we used hydrogen peroxides as a ROS, the gene expression of SOD2, which response to superoxide anion radicals, tended to increase by hydrogen peroxides exposure. We hypothesized that the increased SOD2 gene expression was induced by the superoxide anion radical production via the mitochondria. Defect of the electron transport chain (ETC) was induced the ROS generation.(36) In this study, we considered that hydrogen peroxides injure the mitochondrial ETC, causing the production of superoxide anion radicals, which in turn increased the gene expression of SOD2. However, there was no significant difference in the protein expression levels of the antioxidant enzymes GPx1 and SOD2. We considered that hydrogen peroxide regulates these enzymes expression at the gene level, while it cannot regulate these enzymes expression at the protein level. Otherwise, the expression GPx1 and SOD2 expression in protein level may regulate not only hydrogen peroxides but also other components synergistically. Since it was confirmed that there was a change in the expression of each antioxidant enzyme in this experiment, future studies will clarify whether each antioxidant enzyme is expressed in collaboration with each other.
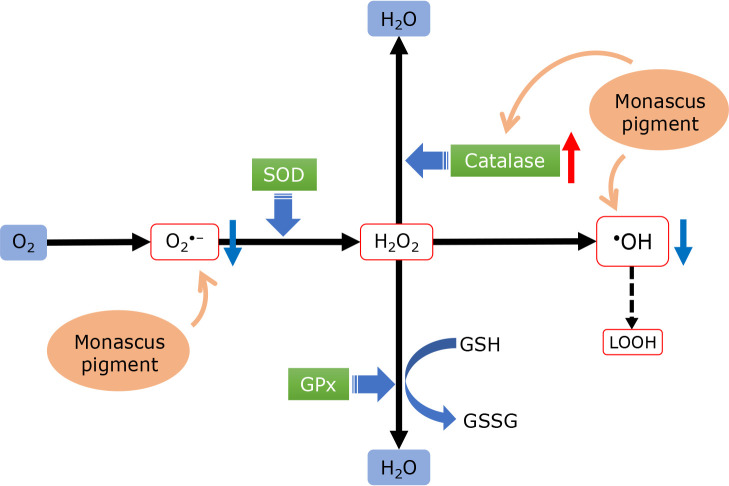
From the results in this study, we considered that the MP treatment in cells under oxidative stress causes the reaction shown in Fig. 5. 1) Superoxide anion radicals and hydroxyl radicals react directly with MP and are removed. 2) Hydrogen peroxides are removed in a catalase reaction upregulated by MP. 3) While GPx and SOD gene expression are downregulated.
Fig. 5.
Based on the results of this study, the mechanism by which MP acts on the ROS scavenging mechanism in the cell is outlined. MP scavenges superoxide anion radicals and hydroxyl radicals by a direct reaction. Moreover, the expression of the antioxidant enzyme catalase is increased, while the expression of GPx and SOD is suppressed.
In conclusion, Monascus pigment suppressed the damage to myotube cells under oxidative stress. There are two possible mechanisms as following: the non-enzymatic ROS scavenging ability of MP and the ROS scavenging ability of skeletal muscle by increasing the expression of catalase, an antioxidant enzyme. We consider that these capacities remove major ROS such as superoxide anion radical, hydrogen peroxide, and hydroxyl radical, and suppress myotube cell damage.
Acknowledgments
The author would like to thank Professor H. Ohmori for his cooperation and advice in carrying out the experiments.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006; 174: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 2001; 280: C621–C627. [DOI] [PubMed] [Google Scholar]
- 3.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 2004; 37: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 4.Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol 1985; 79: 675–686. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AJ, Jude B, Lanner JT. Intramuscular mechanisms of overtraining. Redox Biol 2020; 35: 101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaahtera L, Brosché M, Wrzaczek M, Kangasjärvi J. Specificity in ROS signaling and transcript signatures. Antioxid Redox Signal 2014; 21: 1422–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignolet-Spruyt L, Xu E, Idänheimo N, et al. Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot 2016; 67: 3831–3844. [DOI] [PubMed] [Google Scholar]
- 8.Leopold JA, Loscalzo J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov Today Ther Strateg 2008; 5: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K. Oxygen radical-induced natural killer cell dysfunction: role of myeloperoxidase and regulation by serotonin. J Leukoc Biol 2004; 75: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 10.Nishida H, Ichikawa H, Konishi T. Shengmai-san enhances antioxidant potential in C2C12 myoblasts through the induction of intracellular glutathione peroxidase. J Pharmacol Sci 2007; 105: 342–352. [DOI] [PubMed] [Google Scholar]
- 11.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 2016; 15: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen J, Griffiths PT, Campbell SJ, Utinger B, Kalberer M, Paulson SE. Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants. Sci Rep 2021; 11: 7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans LW, Zhang F, Omaye ST. Vitamin C supplementation reduces exercise-induced oxidative stress and increases peak muscular force. Food Nutr Sci 2017; 8: 812–822. [Google Scholar]
- 14.Sukprasansap M, Chanvorachote P, Tencomnao T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement Med Ther 2020; 20: 46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Madhavan J, Chandrasekharan S, Priya MK, Godavarthi A. Modulatory effect of carotenoid supplement constituting lutein and zeaxanthin (10:1) on anti-oxidant enzymes and macular pigments level in rats. Pharmacogn Mag 2018; 14: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamoshita M, Toda E, Osada H, et al. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep 2016; 6: 30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkar PD, Gupt T, Sahu A. Comparative analysis of lycopene in oxidative stress. J Assoc Physicians India 2012; 60: 17–19. [PubMed] [Google Scholar]
- 18.Abdou AM, Higashiguchi S, Horie K, Kim M, Hatta H, Yokogoshi H. Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 2006; 26: 201–208. [DOI] [PubMed] [Google Scholar]
- 19.Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot (Tokyo) 1979; 32: 852–854. [DOI] [PubMed] [Google Scholar]
- 20.Aniya Y, Ohtani II, Higa T, et al. Dimerumic acid as an antioxidant of the mold, Monascus anka. Free Radic Biol Med 2000; 28: 999–1004. [DOI] [PubMed] [Google Scholar]
- 21.Dhale MA, Javagal M, Puttananjaiah MH. Protective and antioxidative effect of rubropunctatin against oxidative protein damage induced by metal catalyzed reaction. Int J Biol Macromol 2018; 116: 409–416. [DOI] [PubMed] [Google Scholar]
- 22.Taira J, Miyagi C, Aniya Y. Dimerumic acid as an antioxidant from the mold, Monascus anka: the inhibition mechanisms against lipid peroxidation and hemeprotein-mediated oxidation. Biochem Pharmacol 2002; 63: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 23.Wei ZJ, Sun L, Li YL, et al. Low-calorie sweetener D-psicose promotes hydrogen peroxide-mediated apoptosis in C2C12 myogenic cells favoring skeletal muscle cell injury. Mol Med Rep 2021; 24: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 2010; 49: 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci 2009; 84: 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffle D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977; 270: 725–727. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Tokuyama K. ACE activity affects myogenic differentiation via mTOR signaling. Biochem Biophys Res Commun 2007; 363: 597–602. [DOI] [PubMed] [Google Scholar]
- 28.Cai JG, Luo LM, Tang H, Zhou L. Cytotoxicity of malondialdehyde and cytoprotective effects of taurine via oxidative stress and PGC-1α signal pathway in C2C12 cells. Mol Biol (Mosk) 2018; 52: 616–627. (in Russian) [DOI] [PubMed] [Google Scholar]
- 29.Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal Biochem 2013; 440:186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes AV, Waddell DS, Siu R, et al. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J 2012; 26: 2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokinoya K, Shirai T, Ota Y, Takemasa T, Takekoshi K. Denervation-induced muscle atrophy suppression in renalase-deficient mice via increased protein synthesis. Physiol Rep 2020; 8: e14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno Y, Ando K, Ito T, et al. Lactate stimulates a potential for hypertrophy and regeneration of mouse skeletal muscle. Nutrients 2019; 11: 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivakumar AS, Ochirbat C, Cho SH, Yang J, Hwang I. Antiapoptotic effect of a novel synthetic peptide from bovine muscle and MPG peptide on H2O2-induced C2C12 cells. In Vitro Cell Dev Biol Anim 2014; 50: 630–639. [DOI] [PubMed] [Google Scholar]
- 34.Ziemlewska A, Zagórska-Dziok M, Nizioł-Łukaszewska Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci Rep 2021; 11: 3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol 2006; 70: 247–253. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Zhong W, Zhang W, Zhou Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: role of zinc deficiency. Am J Physiol Gastrointest Liver Physiol 2016; 310: G205–G214. [DOI] [PMC free article] [PubMed] [Google Scholar]