ABSTRACT
Vertebrate photoreceptors are highly specialized retinal neurons that have cilium-derived membrane organelles called outer segments, which function as platforms for phototransduction. Male germ cell-associated kinase (MAK) is a cilium-associated serine/threonine kinase, and its genetic mutation causes photoreceptor degeneration in mice and retinitis pigmentosa in humans. However, the role of MAK in photoreceptors is not fully understood. Here, we report that zebrafish mak mutants show rapid photoreceptor degeneration during embryonic development. In mak mutants, both cone and rod photoreceptors completely lacked outer segments and underwent apoptosis. Interestingly, zebrafish mak mutants failed to generate axonemes during photoreceptor ciliogenesis, whereas basal bodies were specified. These data suggest that Mak contributes to axoneme development in zebrafish, in contrast to mouse Mak mutants, which have elongated photoreceptor axonemes. Furthermore, the kinase activity of Mak was found to be critical in ciliary axoneme development and photoreceptor survival. Thus, Mak is required for ciliogenesis and outer segment formation in zebrafish photoreceptors to ensure intracellular protein transport and photoreceptor survival.
Keywords: Zebrafish, Photoreceptor degeneration, MAK, Ciliopathy, Ciliogenesis
Summary: Male germ cell-associated kinase (Mak) is a cilium-associated serine/threonine kinase that promotes axoneme development during ciliogenesis in zebrafish photoreceptors to ensure intracellular protein transport and photoreceptor survival.
INTRODUCTION
The vertebrate photoreceptor is highly compartmentalized to form specialized structures related to phototransduction (Fain et al., 2010). The outer segment (OS) is a specialized cilium of the photoreceptor, in which multiple photoreceptive membrane discs are regularly stacked to accommodate phototransduction molecules such as rhodopsin and opsins (Bachmann-Gagescu and Neuhauss, 2019; Chen et al., 2021). The inner segment (IS) is a mitochondria-enriched region between the OS and the nucleus. Finally, photoreceptors have a specialized synaptic structure in the most basal region that mediates transmission of electric signals to horizontal cells and bipolar cells (Mercer and Thoreson, 2011).
The photoreceptor OS is a highly specialized primary cilium that consists of stacked membrane discs around a microtubule-based backbone called the axoneme (Bachmann-Gagescu and Neuhauss, 2019; Chen et al., 2021). The axoneme is anchored to the basal body in the IS of photoreceptors and extends apically through the connecting cilium. The connecting cilium bridges the IS and the OS, through which phototransduction molecules are transported from the endoplasmic reticulum to the Golgi and then into the OS. Importantly, the connecting cilium is equivalent to the ciliary transition zone in other cell types and functions as a gating system (Park and Leroux, 2022), through which OS-resident proteins are transported to the OS by the intraflagellar transport (IFT) complex (Klena and Pigino, 2022) and BBSome, composed of eight Bardet–Biedl syndrome (BBS) proteins (Tian et al., 2023).
The process of ciliary development is called ciliogenesis, which consists primarily of three steps: centriole (basal body) docking to the apical plasma membrane, establishment of the transition zone and ciliary axoneme extension (Breslow and Holland, 2019; Chen et al., 2021). The first step of ciliogenesis is removal of CP110 from a distal appendage of the mother centriole, which enables ARL13B and components of the IFT-A and IFT-B complexes to assemble at the apical surface of a distal appendage in a RAB8-dependent manner (Breslow and Holland, 2019; Ge et al., 2022). The second step is formation of the transition zone by transport of MKS and NPHP module components (Park and Leroux, 2022). The third step is extension of the axoneme through IFT-mediated ciliary transport, which transports and adds α/β-tubulin dimers to the plus end of the microtubules making up the axoneme (Brouhard and Rice, 2018; Ge et al., 2022; Nakayama and Katoh, 2018). Defects in ciliogenesis cause ciliopathy, which is associated with various abnormalities during organogenesis, including retinal dystrophies, cystic kidney diseases, skeletal dysplasia and polydactyly (Mill et al., 2023). Although many factors are reportedly involved in photoreceptor ciliogenesis and ciliopathy (Cideciyan et al., 2007; den Hollander et al., 2006; Gao et al., 2002; Gorden et al., 2008; Kakakhel et al., 2020; Rao et al., 2015; Zhu et al., 2021), the underlying mechanisms are not fully understood.
Male germ cell-associated kinase (MAK) belongs to the MAK/ICK/MOK serine/threonine kinase family (Chen et al., 2013). MAK was identified by cross-hybridization with tyrosine kinase v-ros in rat testicular cells, so it was considered to be a spermatogenesis regulator (Matsushime et al., 1990). MAK functions as a coactivator of androgen receptors to promote androgen receptor-mediated signaling, which is associated with prostate tumorigenesis (Ma et al., 2006; Wang and Kung, 2012; Xia et al., 2002). Furthermore, Mak transcripts are expressed in the photoreceptor layer in mouse retina (Blackshaw et al., 2004) and MAK protein is localized in cilia of photoreceptors (Omori et al., 2010). In Mak knockout mice, photoreceptors show abnormally elongated axonemes and malformation of membrane discs in the OS, leading to photoreceptor degeneration (Omori et al., 2010). In humans, MAK variants cause retinitis pigmentosa (RP), in which patients progressively lose their vision (Ozgul et al., 2011; Stone et al., 2011). However, human patients carrying MAK variants do not show elongation of the photoreceptor layer (Stone et al., 2011), although fibroblasts derived from patients with RP carrying MAK variants have elongated cilia (Tucker et al., 2022). Thus, it is important to understand how MAK mutations affect ciliary regulation in photoreceptors among vertebrate species.
In this study, we found that photoreceptors of zebrafish mak mutants degenerate during embryonic development. In mak mutants, both rods and cones underwent apoptosis, although their degeneration processes differed. Interestingly, mak mutants failed to form axonemes in photoreceptor cilia, whereas basal bodies were specified, in contrast to mouse Mak knockout photoreceptors, which have elongated ciliary axonemes. Furthermore, both cones and rods completely lacked OSs in mak mutants, leading to ectopic distribution of opsins. Thus, Mak is essential in the formation of axonemes and OSs in photoreceptors. Finally, Mak activity was found to be critical for axoneme formation and photoreceptor survival. Thus, Mak is essential for ciliogenesis, OS formation, and photoreceptor survival.
RESULTS
Zebrafish pday mutants carry a mutation in the mak gene, leading to photoreceptor degeneration
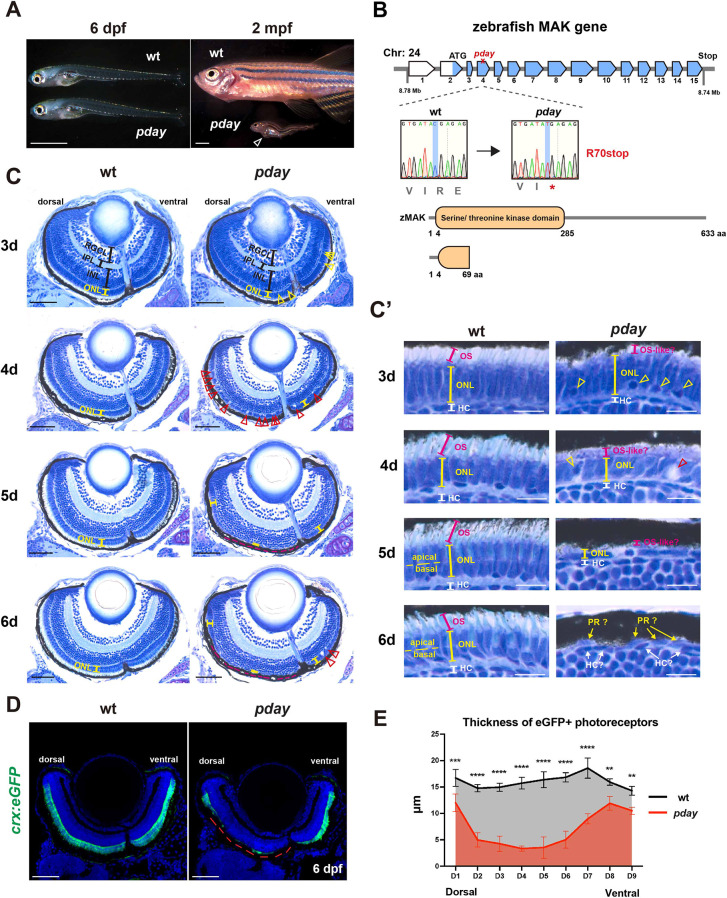
Zebrafish payday (pday) mutants display no visual response during the embryonic stage (Muto et al., 2005). We selected pday homozygous mutant embryos using an optokinetic response (OKR) assay and examined their phenotypes. At 6 days post fertilization (dpf), pday mutants showed no apparent morphological defect (Fig. 1A), but did not survive beyond 10 dpf, suggesting a lethal mutation. However, under intensive feeding, less than 1% of pday mutants survived until 2 months post fertilization (mpf), displaying scoliosis, cardiac edema and smaller body size (Fig. 1A).
Fig. 1.
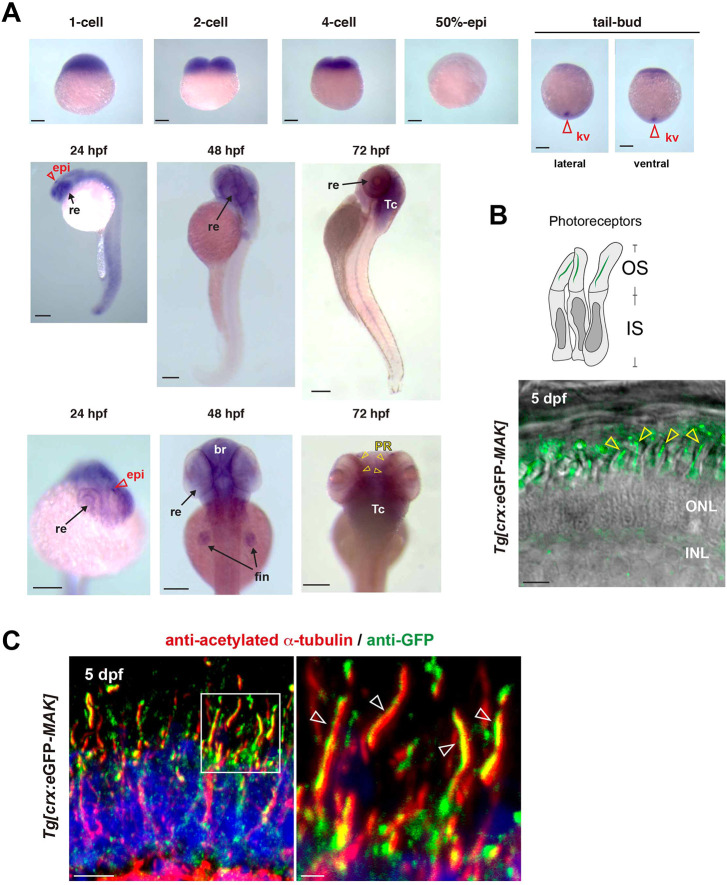
The zebrafish mak mutant pday shows photoreceptor degeneration. (A) pday mutants at 6 days post fertilization (dpf) and 2 months post fertilization (mpf). At 6 dpf, pday mutants showed no morphological differences from wild-type (wt) siblings. At 2 mpf, pday mutants had smaller bodies with less-developed fins, scoliosis and cardiac edema (open arrowhead). (B) The zebrafish mak gene consists of 15 exons and encodes 633 amino acids (aa) with a serine/threonine kinase domain. In pday mutants, a nonsense mutation (R70stop) results in a truncated protein lacking most of the kinase domain. (C) Wild-type and pday mutant retinas. The outer nuclear layer (ONL) in pday mutants showed dense nuclear granules (yellow arrowheads), bubble-like structures (red arrowheads) and reduced ONL thickness (yellow lines). In pday mutants, the dorso-central ONL progressively decreased in thickness after 4 dpf, became extremely flat at 5 dpf and disappeared at 6 dpf (red dotted lines). INL, inner nuclear layer; IPL, inner plexiform layer; RGCL, retinal ganglion cell layer. (C′) Higher magnification of the central ONL. The outer segment (OS) was drastically reduced in pday mutants (red lines). Dense nuclear granules (yellow arrowheads), bubble-like structures (red arrowheads) and the progressive reduction of ONL thickness (yellow lines) were observed in pday mutants. HC, horizontal cells; PR, photoreceptors. (D) Wild-type and pday mutant retinas carrying the transgenic line Tg[crx:eGFP]. Nuclei were counterstained with Hoechst 33342 (blue). crx:eGFP (green) expression was more severely decreased in the dorsal retina (red dotted line) than in the ventral retina of pday mutants. (E) Thickness of eGFP-positive photoreceptors along the dorso-ventral axis of the retina. The thickness was more severely decreased in the dorsal retina than in the ventral retina of pday mutants. Color bars and lines indicate mean±s.d. Statistical difference was evaluated with two-way ANOVA and Sidak's multiple comparison tests; n=3 for each point. **P<0.01; ***P<0.001; ****P<0.0001. Sample sizes are shown in Table S3. Scale bars: 1 mm (A); 50 µm (C); 10 µm (C′); 40 µm (D).
Next, we mapped the pday mutant locus with simple sequence length polymorphism (SSLP) markers (Knapik et al., 1998; Shimoda et al., 1999). The pday mutation was mapped between the two SSLP markers z23011 (1/186 meiosis) and z13695 (3/186 meiosis), so the mutant gene is restricted to a genomic region between 8.517 and 9.123 Mb on chromosome 24. In this genomic region, ten genes including mak were annotated in the zebrafish genomic database (GRCz10, Ensemble release 80). As Mak mutations cause photoreceptor degeneration in mice (Omori et al., 2010) and humans (Ozgul et al., 2011; Stone et al., 2011), and because the other nine genes were not reported to be involved in retinal development, degeneration and functions, we focused on the mak gene. First, we designed a new polymorphic marker, called Mak-N1, located around 20 bp upstream of the first exon of the mak gene. The recombination rate of Mak-N1 for pday mutation was 0 of 186 meiosis, suggesting that mak is a strong candidate. Next, we cloned mak cDNA prepared from wild-type embryos and found that mak cDNA comprises 15 exons (Fig. 1B) and that there are two isoforms of mak mRNA. One has and the other lacks exon 14 due to alternative splicing. Then, we focused on the longest isoform, which has exon 14 and is annotated as the full-length isoform in the zebrafish genomic database (mak-201, GRCz11, Ensemble release 110). We compared amino acid sequences of this longest zebrafish Mak isoform with those of human and mouse MAK. Amino acid identity between zebrafish Mak and human/mouse MAK was more than 50% for the full-length protein and more than 86% for the N-terminal kinase domain (Fig. S1). We cloned mak cDNAs from pday mutant mRNA and genomic fragments covering coding exons from the pday mutant genome. Sequencing revealed that a nonsense mutation occurs in exon 4 of the mak gene in pday mutants, which causes a premature termination codon at 70R (Fig. 1B). These data suggest that the pday mutant gene encodes Mak.
Next, we examined retinal phenotypes of wild-type and pday mutant embryos from 3 to 6 dpf (Fig. 1C,C′). In wild-type retinas at 3 dpf, photoreceptors differentiate to form the outer nuclear layer (ONL). The OS is formed in the apical ONL (Fig. 1C′). In pday mutants, the ONL was formed; however, densely labeled nuclei were observed in the basal region of the ventro-central ONL (Fig. 1C,C′) and the OS was markedly reduced (Fig. 1C′). In wild-type photoreceptors at 4 dpf, the OS was elongated; however, in pday mutants, the ONL was decreased in thickness (Fig. 1C,C′) and no OS-like structure was observed (Fig. 1C′). In addition, there were pyknotic nuclei and bubble-like structures (Fig. 1C,C′). In wild-type embryos at 5 dpf, the ONL was divided into two subnuclear layers (Fig. 1C′), the apical and basal regions of which contain nuclei of blue/red/green cones and rods/ultraviolet (UV) cones, respectively (D'Orazi et al., 2020; Oel et al., 2020). However, in pday mutants, the thickness of the dorso-central ONL was drastically decreased (Fig. 1C) and it contained flattened nuclei (Fig. 1C,C′). In wild-type embryos at 6 dpf, photoreceptors showed more mature shapes in the ONL. However, in pday mutants, most photoreceptor nuclei disappeared in the dorso-central retina (Fig. 1C) and there were very flat nuclei in the outermost region of the retina, which were difficult to define as the ONL or horizontal cells (Fig. 1C′). Interestingly, in pday mutants, the ONL thickness was less affected in the retinal region more ventral to the optic nerve head, even at 6 dpf (Fig. 1C). Thus, sensitivity of photoreceptor degeneration differs between the dorso-central and the ventral retina. In addition, the inner nuclear layer (INL), the inner plexiform layer (IPL) and the retinal ganglion cell layer (RGCL) appeared to be intact in pday mutants from 3 to 6 dpf (Fig. 1C), suggesting photoreceptor-specific degeneration in pday mutants.
To confirm photoreceptor degeneration in mutant retinas at 6 dpf, we introduced a transgenic line Tg[crx:eGFP] that expresses eGFP in photoreceptor precursors and mature rod and cone photoreceptors under control of the cone-rod homeobox (crx) promoter (Suzuki et al., 2013) (Fig. 1D). In wild-type retinas, eGFP signals were detected in the ONL at 6 dpf. However, in pday mutant retinas, eGFP signals disappeared in the dorso-central retina (Fig. 1D). We measured the thickness of eGFP-positive areas along the dorso-ventral axis (Fig. S2A) and found that photoreceptor degeneration was more severe in the dorso-central retina than in the ventral retina (Fig. 1E). Thus, photoreceptors undergo degeneration in pday mutants.
Both rods and cones degenerate in pday mutants
In human patients with MAK-associated RP, rods degenerate more prominently (Ozgul et al., 2011; Stone et al., 2011). To evaluate rod and cone degeneration separately, we generated double transgenic lines, Tg[rho:NLS-eGFP; gnat2:NLS-tdTomato], to visualize rods and cones with eGFP and tdTomato fluorescence, respectively, and introduced them into pday mutants (Fig. 2; Fig. S3A). In wild-type retinas, eGFP expression in rods was strong and dense in the ventral ONL but relatively weak and sparse in the dorso-central ONL at 3 dpf (Fig. S3A). In contrast, eGFP expression was very faint in pday mutant retinas under the same conditions. However, when laser intensity was increased, eGFP expression was detected (Fig. S3A), suggesting that rods are maintained in 3 dpf pday mutant retinas, and perhaps that the rhodopsin promoter does not effectively drive eGFP mRNA or that translation of eGFP mRNA to eGFP protein is affected in pday mutants. Therefore, in later experiments using Tg[rho:NLS-eGFP], we applied higher laser intensity to pday mutant scanning and compared rod phenotypes between pday mutants and wild-type siblings.
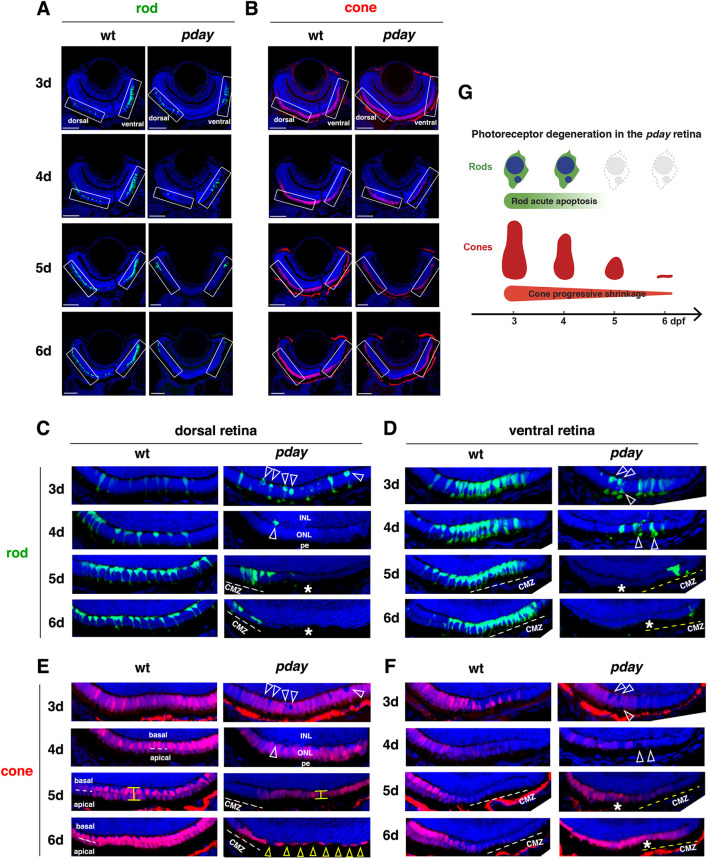
Fig. 2.
Rods undergo apoptotic-like degeneration in pday mutants, whereas cones progressively shrink. (A,B) Images of wild-type and pday mutant retinas carrying the transgenes Tg[rho:NLS-eGFP] and Tg[gnat2:NLS-tdTomato], which visualize rods (green, A) and cones (red, B), respectively. Nuclei were counterstained with Hoechst 33342 (blue). Rectangles indicate the dorsal and ventral ONLs, shown in C-F. (C) The dorsal ONL of wild-type and pday mutant retinas with Tg[rho:NLS-eGFP] (green). In pday mutants, rods had condensed, round nuclei (white arrowheads) at 3 dpf, and rods were drastically reduced in number at 4 dpf and disappeared at 5 and 6 dpf (asterisks), except in the peripheral region near the dorsal ciliary marginal zone (CMZ). pe, retinal pigment epithelium. (D) The ventral ONL of wild-type and pday mutant retinas with Tg[rho:NLS-eGFP] (green). In pday mutants, rods had condensed, round nuclei (white arrowheads) at 3 and 4 dpf white dotted lines. In pday mutants, rods disappeared at 5 and 6 dpf (asterisks), except in the peripheral region near the ventral CMZ. (E) The dorsal ONL of wild-type and pday mutant retinas with Tg[gnat2:NLS-tdTomato] (red). In wild type, cones formed a monolayer at 3 dpf and two subnuclear layers after 4 dpf (white dotted lines). In pday mutants, tdTomato-negative nuclei were observed, the positions of which were identical to those of condensed rod nuclei (white arrowheads). From 4 to 5 dpf, cones shrunk progressively and became flattened (yellow line). At 6 dpf, cones were extremely flattened and disappeared, leaving gaps (yellow arrowheads), except in the peripheral region near the dorsal CMZ. (F) The ventral ONL of wild-type and pday mutant retinas with Tg[gnat2:NLS-tdTomato] (red). In wild type, cones differentiated at 3 dpf, but progressively disappeared toward the periphery of the ventral ONL, where rods densely differentiated. In pday mutants, tdTomato-negative round nuclei appeared at the place where pyknotic-like rod nuclei were observed (white arrowheads, D) at 3 dpf, and ONL thickness was progressively decreased after 4 dpf. Interestingly, after 5 dpf, the cone differentiating area was expanded toward the periphery of the ventral ONL (asterisks), where rods disappeared. (G) Cone and rod degeneration process in pday mutants. Rods undergo acute degeneration with apoptotic-like pyknotic nuclei during 3-4 dpf, whereas cones undergo progressive shrinkage of cell volume during 4-6 dpf and disappear by 6 dpf. Sample sizes are shown in Table S3. Scale bars: 40 µm (A,B).
As photoreceptor degeneration is more severe in the dorso-central retina than in the ventral retina at 6 dpf (Fig. 1D,E), we visualized the morphology of rods and cones using Tg[rho:NLS-eGFP; gnat2:NLS-tdTomato] from 3 to 6 dpf (Fig. 2A,B) and focused on the dorsal and ventral ONL regions (Fig. 2C-F). First, we examined rods (Fig. 2A). In wild-type retinas at 3 dpf, rods were sparse in the dorsal ONL (Fig. 2C), but densely produced in the ventral ONL (Fig. 2D). In pday mutants, many rods showed very round nuclei in both the dorsal and ventral ONL (Fig. 2C,D). Interestingly, eGFP were predominantly observed in the nuclei of wild-type rods because eGFP was tagged with a nuclear localization sequence (NLS); however, eGFP signals were often excluded from the round nuclei of pday mutant rods, suggesting apoptotic-like pyknotic nuclei of pday mutant rods. In wild-type retinas after 4 dpf, rods progressively increased in number and aligned along the dorsal ONL. Their nuclei were positioned in the most basal region of the ONL and rods extended processes to form the OS in the apical ONL (Fig. 2C). In contrast, rods were more densely differentiated in the ventral ONL (Fig. 2D). However, in pday mutants at 4 dpf, rods almost disappeared in both the dorsal and ventral ONL. Only a few rods remained, but these displayed pyknotic nuclei or were excluded from the ONL (Fig. 2C,D). In pday mutants at 5 and 6 dpf, rods disappeared in the ONL (Fig. 2C,D), except in the peripheral retina adjacent to the ciliary marginal zone (CMZ), where new photoreceptors continued to be produced by retinal stem cells. Thus, rods are eliminated soon after they are specified from the CMZ. Evaluation of the numbers of rod nuclei along the dorso-ventral axis of the retina confirmed that rods were progressively diminished in the central ONL of pday mutant retinas and were completely eliminated, except in the CMZ at 6 dpf (Fig. S3B).
Next, we examined cones (Fig. 2B). In wild-type retinas at 3 dpf, cones differentiated uniformly in the dorsal ONL (Fig. 2E); however, cone density became progressively sparse toward the CMZ in the ventral ONL (Fig. 2F), a pattern complementary to that of rods (Fig. 2D). In pday mutant retinas, cones normally differentiated to form a spatial pattern similar to that of wild-type retinas (Fig. 2E,F). However, there were tdTomato-negative rounded nuclei in both the dorsal and ventral ONL (Fig. 2E,F), the positions of which were identical to pyknotic-like rod nuclei (Fig. 2C,D). Thus, rods lost their normal localization in the basal ONL in pday mutants at 3 dpf. After 4 dpf, in wild-type retinas, cones formed two subnuclear layers in the dorso-central ONL (Fig. 2E). However, cone density was complementary to rod density and became progressively sparse toward the CMZ in the ventral ONL (Fig. 2F). In pday mutants at 4 dpf, segregation of two cone subnuclear layers was incomplete in the dorso-central ONL (Fig. 2E). Furthermore, cones were sparse and shortened in the ventral ONL (Fig. 2F). In pday mutants at 5 dpf, cones were shortened in the dorsal ONL (Fig. 2E). Interestingly, cone density increased toward the CMZ in the ventral ONL of pday mutants (Fig. 2F), where rods failed to be maintained (Fig. 2D). In pday mutants at 6 dpf, cones were extremely flattened and there were gaps between them in the dorsal ONL (Fig. 2E), indicating that cones had been eliminated. Furthermore, cones were similarly flattened in the ventro-central ONL (Fig. 2F), whereas cones were uniformly produced and less flattened toward the CMZ of the ventral ONL (Fig. 2F). Therefore, rods undergo acute apoptosis-like cell death in pday mutants from 3 to 4 dpf, whereas cones undergo progressive shrinkage after 4 dpf and eventually disappear by 6 dpf in pday mutants (Fig. 2G), although cone degeneration is less severe in the peripheral region of the ventral ONL.
Both rods and cones undergo apoptosis in pday mutants
The condensed rod nuclei in pday mutants at 3 dpf (Fig. 2C,D) are reminiscent of those seen in apoptosis, in which chromatin condensation is a hallmark (Oberhammer et al., 1994). We performed terminal deoxynucleotidyl transferase-mediated dUTP-nick-end labeling (TUNEL) to pday mutant retinas at 3, 4 and 5 dpf (Fig. 3A,A′; Fig. S4A). In wild-type retinas, the number of TUNEL-positive cells was continuously very low in the ONL (Fig. 3A; Fig. S4A). In pday mutant retinas at 3 dpf, TUNEL signals were detected in the central and ventral ONL (Fig. 3A′), which is consistent with our observation that rod pyknotic nuclei were detected at 3 dpf (Fig. 2C,D), suggesting that these early TUNEL-positive cells are likely to be rods. In pday mutant retinas at 4 and 5 dpf, TUNEL signals increased in the dorsal and central ONL (Fig. 3A′). Interestingly, TUNEL-positive cells were positioned in a very thin central ONL of 5 dpf pday mutant retinas (Fig. 3A′), suggesting that these late TUNEL-positive cells are likely to be cones. TUNEL-positive cells were more numerous in pday mutant ONL than in wild-type ONL at 3 dpf, but the difference was not significant. However, the number of TUNEL-positive cells was the highest in pday mutant ONL at 4 dpf and was still significantly higher in pday mutant ONL at 5 dpf, compared with that in wild-type ONL (Fig. S4A). The number of TUNEL signals in other retinal layers, i.e. the INL and RGCL, was not significantly different between pday mutants and wild-type siblings at 3, 4 or 5 dpf (Fig. S4B,C), suggesting that apoptosis is specific to photoreceptors.
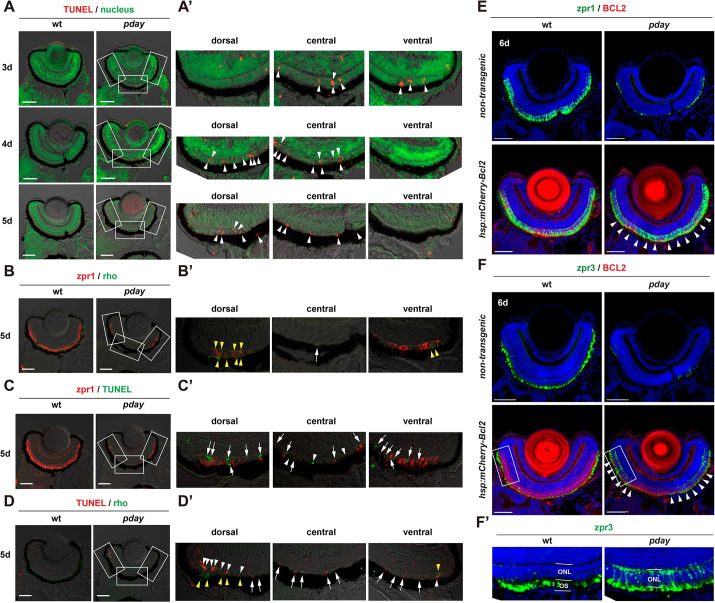
Fig. 3.
Rods and cones undergo apoptosis in pday mutants. (A) TUNEL (red) of wild-type and pday mutant retinas. Nuclei were counterstained with Sytox Green (green). (A′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, which are indicated by white rectangles in A. White arrowheads indicate TUNEL signals (red). (B) Double labeling of 5-dpf wild-type and pday mutant retinas with zpr1 (red) and anti-rhodopsin (green) antibodies. (B′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, which are indicated by white rectangles in B. Both rhodopsin (yellow arrowheads) and zpr1 signals were observed in the peripheral region of the dorsal and ventral ONL. In addition, zpr1-positive flattened cells were located in the central ONL (white arrow). (C) TUNEL (green) of 5-dpf wild-type and pday mutant retinas combined with zpr1 antibody labeling (red). (C′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, indicated by white rectangles in C. Many TUNEL signals were associated with zpr1 signals (white arrows); however, a few were not associated with zpr1 (white arrowheads). (D) TUNEL (red) of 5-dpf wild-type and pday mutant retinas combined with anti-rhodopsin antibody labeling (green). (D′) Higher magnification of the dorsal, central and ventral ONL of pday mutant retinas, indicated by white rectangles in D. Rhodopsin signals were detected in the peripheral region of dorsal and ventral ONL (yellow arrowheads). TUNEL signals associated with rhodopsin signals were observed only in the peripheral region of the dorsal and ventral ONL (white arrowheads). TUNEL signals were not associated with rhodopsin signals in the central ONL and non-peripheral regions of the dorsal and ventral ONL (white arrows). (E,F) Wild-type and pday mutant retinas with and without the transgene Tg[hsp: mCherry-Bcl2], labelled with zpr1 (E) and zpr3 (F) antibodies. Nuclei were counterstained with Hoechst 33342 (blue). Both zpr1 and zpr3 signals in the ONL were recovered in pday mutant retinas with Tg[hsp: mCherry-Bcl2] (white arrowheads). (F′) Higher magnification of the dorsal ONL indicated by white rectangles in F. Sample sizes are shown in Table S3. Scale bars: 50 µm (A-D); 40 µm (E,F).
Next, we examined whether cones undergo apoptosis in pday mutants. First, we carried out double labeling of 5-dpf, wild-type and pday mutant retinas with anti-zebrafish rhodopsin (Vihtelic et al., 1999) and zpr1 (Larison and Bremiller, 1990) antibodies, which label rod OSs and double-cone-type photoreceptors (red/green cones), respectively (Fig. 3B). Consistent with data for Tg[rho:NLS-eGFP; gnat2:NLS-tdTomato] (Fig. 2), rods were only detected in the peripheral region of the dorsal and ventral ONL of pday mutants (Fig. 3B′). In contrast, cones were observed in the peripheral region of the dorsal and ventral ONL and as very flattened cells in the central ONL (Fig. 3B′). Second, we carried out TUNEL of 5-dpf, wild-type and pday mutant retinas combined with zpr1 antibody labeling (Fig. 3C). Many TUNEL-positive cells were observed in the dorsal, central and ventral ONL of pday mutants (Fig. 3C′). A significant fraction of these TUNEL-positive cells was associated with zpr1 signals (Fig. 3C′). Third, we carried out TUNEL in 5-dpf, wild-type and pday mutant retinas combined with anti-rhodopsin antibody labeling (Fig. 3D). Many TUNEL-positive cells were observed in the dorsal, central and ventral ONL of pday mutants (Fig. 3D′). However, rhodopsin signals were detected only in the CMZ of the dorsal and ventral ONL (Fig. 3D′), but not in the central ONL. Thus, many TUNEL signals were not associated with rhodopsin signals in the central ONL (Fig. 3D′). As flattened photoreceptors in the central ONL of 5-dpf pday mutants are cones (Fig. 2), cones undergo apoptosis at or before 5 dpf in pday mutants.
To confirm that both rods and cones undergo apoptosis in pday mutants, we combined the transgenic line Tg[hsp:mCherry-Bcl2], which expresses an anti-apoptotic protein Bcl2 (encoded by bcl2a) (Nishiwaki and Masai, 2020), with pday mutants and evaluated cell survival by labeling with zpr1 (Larison and Bremiller, 1990) and zpr3 (Gao et al., 2022 preprint) antibodies, which visualize double cone photoreceptors and rhodopsin/green opsin, respectively. Combined with quantitative analyses (Fig. S2A,B), we found that Bcl2 overexpression significantly rescued survival of both cones and rods in pday mutants at 6 dpf (Fig. 3E,F; Fig. S4D,E) even along the dorso-ventral axis (Fig. S4F), suggesting that both cones and rods undergo apoptosis in pday mutants.
Although rods and cones survived in pday mutants that overexpressed Bcl2, zpr3 signals failed to be localized in the OS and spread in the plasma membrane throughout the cell body, including the basal synaptic region (Fig. 3F′), suggesting that green opsin and rhodopsin are mislocalized in Bcl2-overexpressing pday mutant photoreceptors. Furthermore, photoreceptor nuclear shape differed between Bcl2-overexpressing pday mutants and wild-type siblings (Fig. S4G). Next, we examined OKR in embryos produced by crosses of pday heterozygous fish. On average, 25% of embryos were homozygous for the pday mutation and did not show OKR. Bcl2 overexpression did not restore OKR of pday mutants (Fig. S4H). Thus, Mak activity is primarily required for visual functions of photoreceptors, disruption of which may subsequently cause Bax-mediated apoptosis (Tait and Green, 2010).
mak is responsible for pday mutant phenotypes
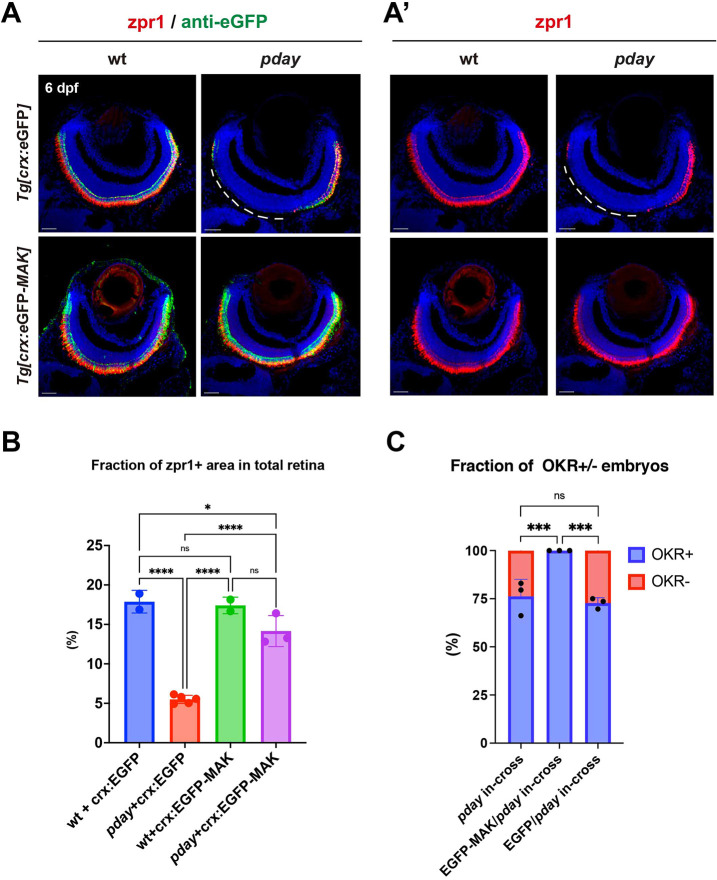
To validate whether the mak gene is responsible for pday mutant phenotypes, we generated a zebrafish transgenic line Tg[crx:eGFP-MAK], which expresses the N-terminal eGFP-tagged, full-length isoform of Mak under control of the crx promotor. A transgenic line Tg[crx:eGFP] was used as a control. Labeling with the zpr1 antibody revealed that Tg[crx:eGFP-MAK] rescued cone photoreceptor degeneration in pday mutants at 6 dpf, whereas Tg[crx:eGFP] did not (Fig. 4A,A′,B). Furthermore, we examined OKR in embryos produced by crosses of pday heterozygous fish. On average, 25% of embryos were homozygous for the pday mutation and did not show OKR. However, Tg[crx:eGFP-MAK] restored OKR of pday mutants at 6 dpf, whereas Tg[crx:eGFP] did not (Fig. 4C). Thus, mak is responsible for pday mutant phenotypes, i.e. photoreceptor degeneration, and visual response defects.
Fig. 4.
Overexpression of Mak restores photoreceptor survival and visual function of pday mutants. (A,Aʹ) Labeling of wild-type and pday mutant retinas with zpr1 (red, A,Aʹ) and anti-eGFP (green, A) antibodies carrying the transgene Tg[crx:eGFP] or Tg[crx:eGFP-MAK]. Nuclei were counterstained with Hoechst 33342 (blue). zpr1 signals were markedly decreased in the dorso-central region of pday mutant retinas carrying Tg[crx:eGFP] (white dotted lines), but maintained in pday mutant retinas carrying Tg[crx:eGFP-MAK]. Scale bars: 20 µm. (B) The fraction of zpr1-positive area in the total retinal area in wild-type and pday mutants with Tg[crx:eGFP] or Tg[crx:eGFP-MAK]. The fraction was markedly reduced in pday mutants with Tg[crx:eGFP] (red bar), but recovered in pday mutants with Tg[crx:eGFP-MAK] (purple bar), equivalent to that in wild-type with Tg[crx:eGFP] (blue bar) or Tg[crx:eGFP-MAK] (green bar). Bars and lines indicate mean±s.d. Statistical significance was evaluated with ordinary one-way ANOVA and Tukey's multiple comparison tests. ns, not significant; *P<0.0332; ****P<0.0002. (C) The fraction of optokinetic response (OKR)+ (blue) and OKR− (red) embryos relative to total progeny produced by crosses of pday+/− parent fish, pday+/− parent fish carrying Tg[crx:eGFP] and pday+/− parent fish carrying Tg[crx:eGFP-MAK]. 57 of 244 (23.3%) embryos produced by three independent crosses of pday+/− parent fish were negative for OKR. However, 100% of total 257 embryos produced by three independent crosses of pday+/− parent fish carrying Tg[crx:eGFP-MAK] were positive for OKR. 68 of 266 (25.5%) embryos produced by three independent crosses of pday+/− parent fish carrying Tg[crx:eGFP] were negative for OKR. Bars and lines indicate mean±s.d. Statistical significance was evaluated with two-way ANOVA and Tukey's multiple comparison tests. ns, not significant; ***P<0.0002.
Mak is localized in the connecting cilium and promotes axoneme development during ciliogenesis
We examined mak mRNA expression during zebrafish embryonic development by whole-mount in situ hybridization (Fig. 5A). mak mRNA was ubiquitously expressed from the one- to four-cell stages, indicating maternal mRNA expression. mak mRNA expression disappeared at the 50% epiboly stage, but was detected in Kupffer's vesicle, a ciliated embryonic organ that is important for left–right asymmetry patterning (Essner et al., 2005), at the tail-bud stage. At 24 h post fertilization (hpf), mak mRNA was expressed in the central nervous system, and especially strongly expressed in the epiphysis and retina. At 48 hpf, mak mRNA was prominently expressed in the brain, the retina and the pectoral fin bud. At 72 hpf, mak mRNA was highly expressed in the brain, including the optic tectum and the photoreceptor layer of the retina. We also confirmed that mak mRNA expression was markedly decreased in pday mutant embryos at 72 hpf, probably due to nonsense-mediated mRNA decay (Fig. S5A). Thus, mak mRNA is expressed in various cell types in zebrafish embryos, including Kupffer's vesicle and retinal photoreceptors, in which ciliation supports left-right asymmetry patterning and IFT-mediated ciliary transport, respectively (Essner et al., 2005; Liu et al., 2004; Sukumaran and Perkins, 2009; Zhai et al., 2014). In addition, zebrafish mutants with cilium defects tend to develop scoliosis (Gray et al., 2021; Latour et al., 2020; Wang et al., 2022), which was observed in pday mutants at 2 mpf (Fig. 1A). Thus, Mak regulates cilium-associated mechanisms in photoreceptors in zebrafish.
Fig. 5.
Mak is localized in axonemes of photoreceptor cilia. (A) Whole-mount in situ hybridization of zebrafish embryos with mak RNA probe. mak mRNA expression was observed in Kupffer's vesicles (red open arrowheads) at the tail-bud stage, in the retina (arrows) and epiphysis (red open arrowheads) at 24 hpf, and in the retina, brain and fin (arrows) at 48 hpf. Retinal expression was restricted to the photoreceptor layer (yellow arrowheads) at 72 hpf. kv, Kupffer's vesicle; re, retina; epi, epiphysis; br, brain; fin, pectoral fin; Tc, optic tectum; PR, photoreceptor layer. (B) Confocal live image of Tg[crx: eGFP-MAK] transgenic retina at 5 dpf. eGFP-Mak (green) was localized in the OS of photoreceptors (yellow arrowheads). (C) Labeling of 5-dpf Tg[crx: eGFP-MAK] transgenic retina with anti-acetylated α-tubulin (red) and anti-GFP (green) antibodies. Nuclei were counterstained with Hoechst 33342 (blue). The right panel shows a higher magnification of the square shown in the left panel. eGFP-Mak was localized in the axoneme (white open arrowheads, right panel). Sample sizes are shown in Table S3. Scale bars: 200 µm (A); 5 µm (B, left panel in C); 1 µm (right panel in C).
Next, we examined Mak protein localization in zebrafish photoreceptors by tracking the eGFP signal in Tg[crx:eGFP-MAK] retinas. eGFP-Mak was localized in a stem-like structure inside the OS of live photoreceptors (Fig. 5B). Next, we examined double labeling of Tg[crx:eGFP-MAK] retinas with anti-GFP antibody and anti-acetylated α-tubulin antibody. The latter mainly labels the ciliary axoneme. eGFP-Mak was localized to the ciliary axoneme as visualized by the anti-acetylated α-tubulin antibody (Fig. 5C; Fig. S5B). Although overexpression of eGFP-Mak could lead to its localization to places where the endogenous protein does not localize, it is likely that Mak is a ciliary protein in photoreceptors.
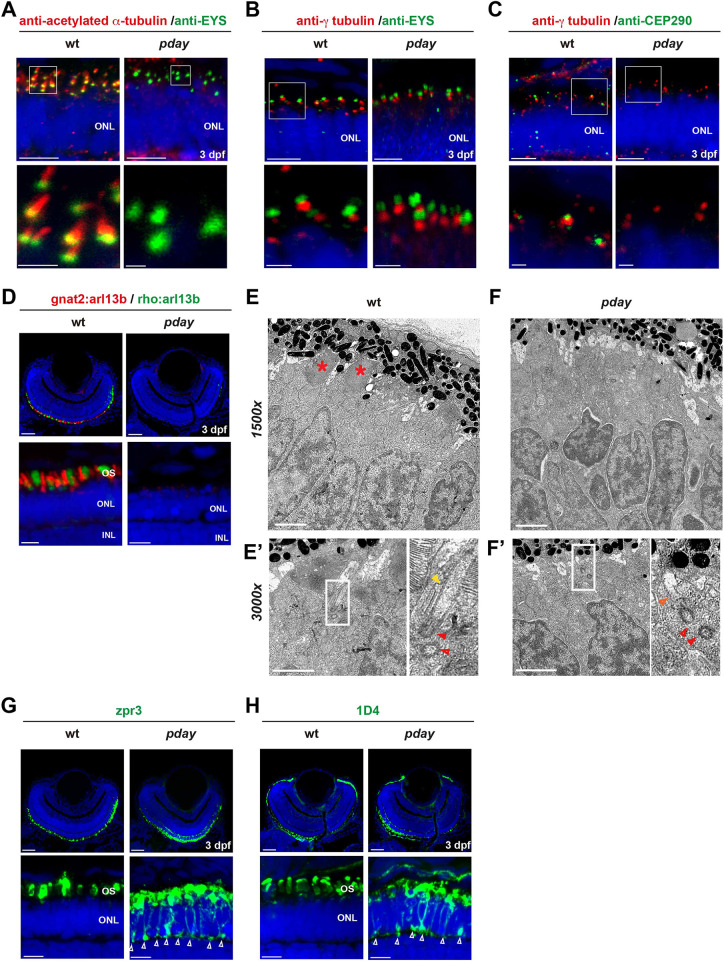
In zebrafish, photoreceptor precursors undergo one round of symmetric cell division to produce two daughter cones after 60 hpf, in which apically localized centrosomes start ciliogenesis (Zolessi et al., 2021). Thus, we examined the ciliary structure in pday mutant photoreceptors at 3 dpf. We used anti-γ-tubulin and anti-acetylated α-tubulin antibodies, which visualize the basal body and the axoneme of the cilium, respectively (Chen et al., 2021). Eyes shut (Eys) is a secreted extracellular matrix protein localized near the transition zone of photoreceptors and is required for ciliary pocket formation in zebrafish (Yu et al., 2016). Cep290 (also known as NPHP6) is a large multidomain coiled-coil protein localized at the proximal part of the transition zone (Goncalves and Pelletier, 2017; Park and Leroux, 2022). Genetic variants of CEP290 are associated with Leber's congenital amaurosis in humans (den Hollander et al., 2006) and photoreceptor degeneration in zebrafish (Cardenas-Rodriguez et al., 2021). Thus, we used anti-Eys and anti-Cep290 antibodies, which visualize transition zone-related areas. Labeling with anti-acetylated α-tubulin and anti-Eys antibodies revealed that Eys signals were normally detected, but acetylated α-tubulin signals were absent in pday mutant photoreceptors (Fig. 6A). Labeling with anti-γ-tubulin and anti-Eys antibodies revealed that both γ-tubulin and Eys signals were normally associated in pday mutant photoreceptors (Fig. 6B). Next, labeling with anti-γ-tubulin and anti-Cep290 antibodies revealed that γ-tubulin signals were normal in pday mutant photoreceptors; however, Cep290 signals were drastically reduced (Fig. 6C). These data indicate that the ciliary axoneme is absent and the transition zone is also defective in pday mutants, whereas the basal body appears to be normal, suggesting that the ciliary axoneme and the transition zone are the primary target of Mak. In contrast, normal Eys signals in pday mutant photoreceptors suggest that Mak is not involved in Eys-mediated ciliary pocket formation.
Fig. 6.
The axoneme is absent in pday mutant photoreceptors. (A-C) Wild-type and pday mutant retinas with anti-acetylated α-tubulin (red) and anti-Eys (green) antibodies (A); anti-γ-tubulin (red) and anti-Eys (green) antibodies (B); and anti-γ-tubulin (red) and anti-Cep290 (green) antibodies (C). (D) Wild-type and pday mutant retinas carrying the transgenes Tg[gnat2: arl13b-tdTomato] (red) and Tg[rho: ail13b-eGFP] (green). (E-Fʹ) Electron microscopy analysis of wild-type (E) and pday mutant (F) photoreceptors at 3 dpf. The bottom-left panels show a higher magnification (3000×) of the apical region of photoreceptors in wild-type siblings (E′) and pday mutants (F′). The bottom-right panels are enlarged images of the rectangular region shown in the bottom left panels. Red asterisks indicate the OS. Red and yellow arrowheads indicate the basal body and ciliary axoneme, respectively. The orange arrowhead indicates the short ciliary extension from the basal body observed in pday mutants. (G,H) Labeling of wild-type and pday mutant retinas with zpr3 (G) and 1D4 (H) antibodies. The open arrowheads indicate ectopic distribution of zpr3 and 1D4 signals in the plasma membrane including the synaptic area in pday mutants. In A-D,G,H, nuclei were counterstained with Hoechst 33342 (blue). In A-C, except for the mutant image in B, the bottom panels show a higher magnification of the ONL shown in the top panels (white boxes). Sample sizes are shown in Table S3. Scale bars: 5 µm (top panels of A-C; bottom panels of D,G,H); 1 µm (bottom panels of A-C; E-F′); 20 µm (top panels of D,G,H).
The OS is a specialized cilium of photoreceptors (Bachmann-Gagescu and Neuhauss, 2019; Chen et al., 2021). Arl13b is a Joubert syndrome protein, which is localized in the primary cilium and mutated in zebrafish scorpion (sco) mutants (Duldulao et al., 2009). In addition, eGFP-tagged Arl13b visualizes cilia in zebrafish (Bachmann-Gagescu et al., 2011; Borovina et al., 2010). We established a zebrafish double transgenic line, Tg[gnat2:arl13b-tdTomato; rho:arl13b-eGFP], and found that fluorescent protein-tagged Arl13b overexpressed under the gnat2 and rho promoters was localized more broadly within the OSs of cones and rods in wild-type retinas (Fig. 6D). However, expression of both Arl13b-tdTomato and Arl13b-eGFP was absent in 3-dpf pday mutant photoreceptors. These data suggest that OS formation is compromised in both rod and cone photoreceptors in pday mutants.
Next, we used electron microscopy (EM) to examine the ultrastructure of photoreceptors at 3 dpf (Fig. 6E,F). Wild-type photoreceptors at 3 dpf showed columnar shapes and their OSs had begun to form (Fig. 6E). In contrast, OSs were not observed in pday mutant photoreceptors (Fig. 6F). Higher-magnification EM images detected a basal body (a pair of centrioles) near the apical domain underneath the OS, as well as a long extension from the basal body, which corresponds to the transition zone and the axoneme, in wild-type photoreceptors (Fig. 6E′). However, a basal body and only a short extension from the basal body, which might correspond to part of the transition zone, were observed in pday mutant photoreceptors (Fig. 6F′). These data suggest that the OS and the ciliary axoneme are absent in pday mutant photoreceptors, whereas the basal body is normally formed. Thus, Mak regulates photoreceptor ciliogenesis by promoting axonemal formation.
Phototransduction molecules fail to be transported to the OS in pday mutant photoreceptors
Phototransduction molecules are normally transported to the OS through the cilium (Athanasiou et al., 2018; Gulati and Palczewski, 2023; Karan et al., 2008; Wang and Deretic, 2014). However, mislocalization of opsins leads to photoreceptor degeneration, although detailed mechanisms are not fully understood (Alfinito and Townes-Anderson, 2002; Lopes et al., 2010; Rohrer et al., 2005). Therefore, we examined distributions of opsins and rhodopsin in pday mutant photoreceptors using zpr3 (Gao et al., 2022 preprint) and 1D4 (Yin et al., 2012) antibodies, which label green opsin/rhodopsin and red opsin in zebrafish, respectively. In 3-dpf wild-type photoreceptors, rhodopsin and red/green opsins were normally localized in the OS (Fig. 6G,H). However, in pday mutants, these opsins were mislocalized to the IS, plasma membrane and the basal synaptic region (Fig. 6G,H). Thus, transport of phototransduction molecules to the OS is compromised in mak mutants.
The kinase activity of Mak is essential for photoreceptor survival and ciliary axoneme formation
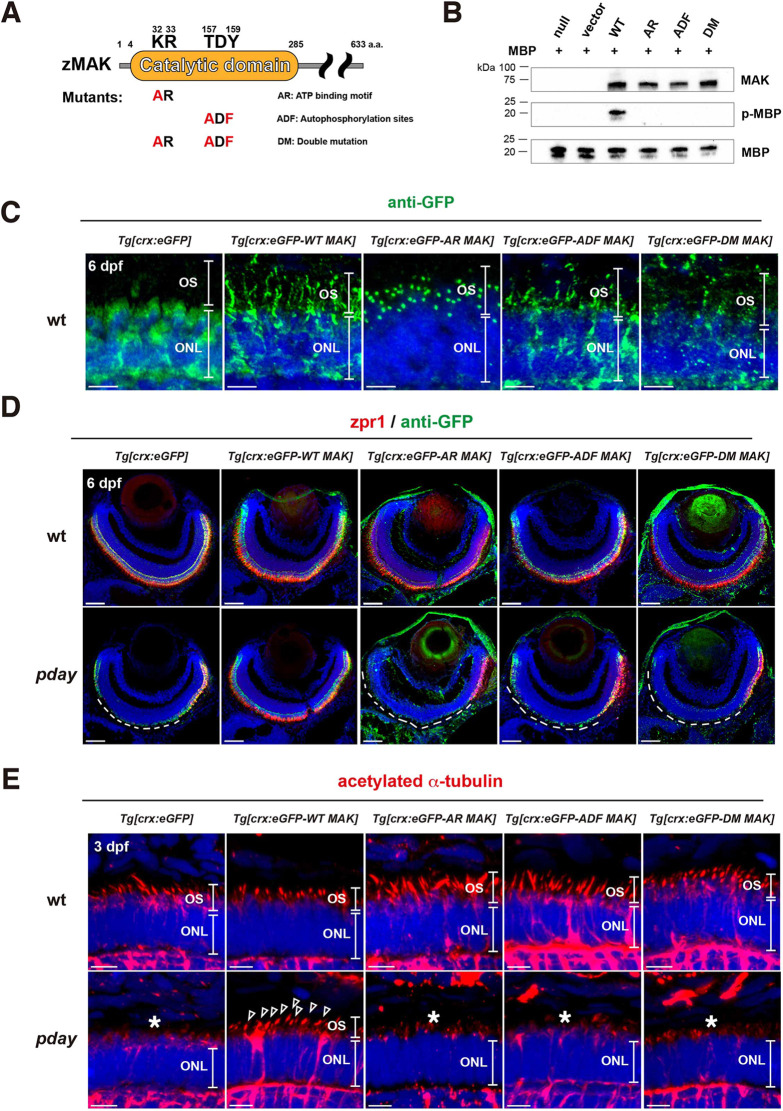
To understand how Mak regulates photoreceptor survival and ciliary axoneme formation, we focused on kinase-associated motifs (Wang and Kung, 2012) of Mak and conducted site-directed mutagenesis to silence kinase activity (Fig. 7A). Similar to human and mouse MAK, the kinase domain is located at the N-terminal region of zebrafish Mak (Fig. S1). In this kinase domain, KR (32-33 amino acids) and TDY (157-159 amino acids) are required for kinase activity as an ATP-binding motif and an autophosphorylation site, respectively (Wang and Kung, 2012). We designed two kinase-dead mutants of zebrafish Mak, namely AR Mak and ADF Mak, by replacing KR and TDY in wild-type zebrafish Mak with the residues AR and ADF, respectively (Fig. 7A). We also prepared double-mutant (DM) Mak, which carries both AR and ADF mutant motifs. In vitro kinase assay using Mak proteins purified from an Escherichia coli expression system revealed that all three kinase-dead Mak mutants, AR Mak, ADF Mak and DM Mak, failed to phosphorylate a Mak mock substrate, myelin basic protein (MBP), indicating that Mak kinase activity is silenced in all three mutants (Fig. 7B).
Fig. 7.
Mak kinase activity is required for Mak protein localization in the ciliary axoneme, axoneme formation and photoreceptor maintenance. (A) A scheme of generation of three types of Mak kinase-dead mutants: AR Mak, ADF Mak and DM Mak. Zebrafish Mak has a catalytic domain, which contains an ATP-binding motif, KR, and an autophosphorylation site, TDY. AR Mak and ADF Mak were generated by conversion of KR and TDY into AR and ADF, respectively. DM Mak has both the AR and ADF mutations. (B) In vitro kinase assay of Mak using a substrate, MBP. Only wild-type Mak phosphorylated MBP. The number of blots is shown in Table S3. (C) Labeling of 6-dpf wild-type ONL carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with anti-GFP antibody (green). (D) Labeling of 6-dpf wild-type and pday mutant retinas carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with zpr1 (red) and anti-GFP (green) antibodies. zpr1-positive photoreceptors were degenerated in dorso-central retinas of pday mutants carrying Tg[crx:eGFP] (white dotted line). However, photoreceptor degeneration was rescued only in pday mutants carrying Tg[crx:eGFP-WT MAK], but not in pday mutants carrying kinase-dead mutant transgenes (white dotted line). Images of wild-type and pday mutant retinas carrying the transgenes Tg[crx:eGFP] and Tg[crx:eGFP-WT MAK] are serial sections of the same retinas shown in Fig. 4A. (E) Labeling of 3-dpf wild-type and pday mutant ONL carrying the transgene Tg[crx:eGFP], Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] or Tg[crx:eGFP-DM MAK] with anti-acetylated α-tubulin antibody (red). Axoneme formation defects were rescued only in pday mutants carrying Tg[crx:eGFP-WT MAK] (arrowheads), but not in pday mutants carrying kinase-dead mutant transgenes (asterisks). In C-E, nuclei were counterstained with Hoechst 33342 (blue). Sample sizes are shown in Table S3. Scale bars: 5 µm (C,E); 40 µm (D).
Next, we generated zebrafish transgenic lines, Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] and Tg[crx:eGFP-DM MAK], by introducing kinase-dead mutant motifs into the wild-type Mak-expressing construct, Tg[crx:eGFP-WT MAK]. First, we examined the subcellular localization of these three kinase-dead mutant Mak proteins in zebrafish photoreceptors by labeling these transgenic wild-type retinas with anti-GFP antibody (Fig. 7C). eGFP-wild-type (WT) Mak was localized in a stem-like pattern similar to the axoneme of the photoreceptor cilium. However, eGFP-AR Mak was localized in a spot-like region near the proximal region of the photoreceptor cilium, which appears to correspond to either the basal body or the transition zone. Thus, ATP-binding activity is important for proper localization of Mak in the axoneme of the photoreceptor cilium. eGFP-ADF Mak was likely to be localized in the axoneme of the photoreceptor cilium, but its expression level was low, indicating that eGFP-ADF Mak may be unstable. eGFP-DM Mak showed low expression level as well, which made it difficult to define its subcellular localization. Thus, Mak kinase activity influences its localization in the axoneme of the photoreceptor cilium.
Next, using the zpr1 antibody, we examined whether Mak kinase activity is required for survival of double-cone photoreceptors. We combined the transgenic lines Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] and Tg[crx:eGFP-DM MAK] with the pday mutants. In contrast to Tg[crx:eGFP-WT MAK], all three kinase-dead mutant mak transgenes failed to rescue survival of double-cone photoreceptors at 6 dpf (Fig. 7D), suggesting that Mak kinase activity is important for photoreceptor survival. Furthermore, we examined ciliary axonemes in pday mutants by labeling pday mutants combined with Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] and Tg[crx:eGFP-DM MAK] with anti-acetylated α-tubulin antibody. In contrast to Tg[crx:eGFP-WT MAK], all three kinase-dead mutant mak transgenes failed to rescue axoneme formation at 6 dpf (Fig. 7E). Thus, Mak kinase activity is critical for ciliary axoneme development and photoreceptor survival (Fig. 8).
Fig. 8.
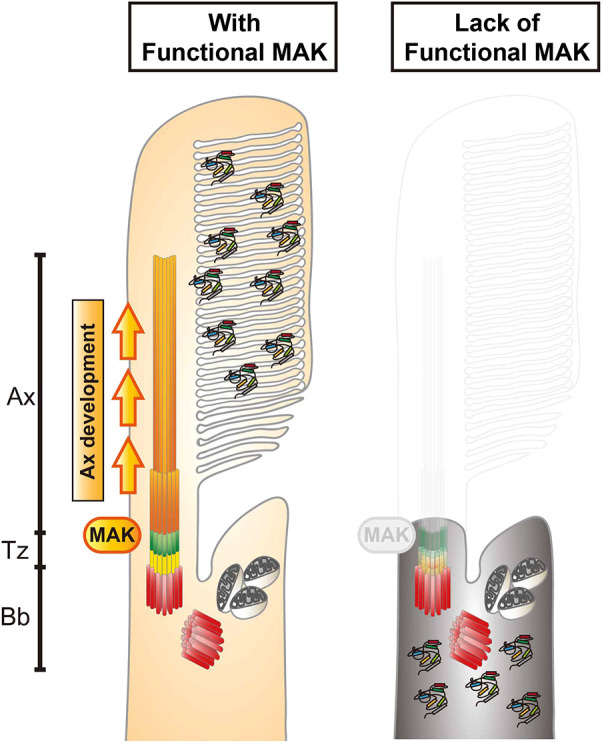
Mak is essential for ciliary axoneme formation in zebrafish photoreceptor ciliogenesis. Phenotypes of zebrafish mak mutant photoreceptors. In the absence of Mak, the axoneme (orange) does not form and the transition zone (green) is affected, resulting in failure to form the OS and to transport phototransduction molecules into the OS. Ax, axoneme; Bb, basal body; Tz, transition zone.
DISCUSSION
In humans and other animal models including zebrafish, many genetic mutations showing defects in photoreceptor ciliogenesis have been identified (Chen et al., 2021). In zebrafish mutants of transition zone components, selective transport of phototransduction molecules into the OS is compromised, resulting in ectopic distribution of these OS-resident proteins outside the OS (Wang et al., 2022). However, the axoneme itself is formed in almost all of these transition zone-defective mutants. In zebrafish mutants of BBSome components, which cooperate with IFT to regulate intraciliary transport (Tian et al., 2023), OS non-resident proteins are mislocalized into the OS, but the axoneme and the OS are formed (Masek et al., 2022). Furthermore, in zebrafish arl13b mutants with compromised cargo release in the OS, the axoneme and the OS are reduced in size but still formed (Song et al., 2016). Thus, most ciliogenesis-defective mutants show formation of the axoneme structure in photoreceptors. However, there are a few exceptions. In zebrafish mutants of the IFT-B component Ift88 (also named oval), Traf3ip1 (also named elipsa) and the kinesin II family protein Kif3a, the axoneme and the OS are totally absent in photoreceptors (Krock and Perkins, 2008; Omori et al., 2008; Sukumaran and Perkins, 2009; Zhu et al., 2021). Interestingly, in ift88 and traf3ip1 mutants, a single spot-like γ-tubulin or centrin signal is detected in the apical region of photoreceptors, similar to that seen in zebrafish mak mutants, suggesting that basal bodies are formed in both mutants (Omori et al., 2008; Sukumaran and Perkins, 2009; Tsujikawa and Malicki, 2004). Thus, it is likely that Mak cooperates with anterograde IFT complexes including Ift88 to promote axoneme formation during ciliogenesis.
Overexpression of wild-type Mak recovers the formation of axonemes, leading to photoreceptor survival, in mak mutants. However, overexpression of kinase-mutant forms of Mak fail to rescue defects in axoneme and cell survival, so phosphorylation events on Mak substrates are necessary for axoneme formation. Interestingly, eGFP-tagged AR Mak is localized in a single spot area near the apical region of photoreceptors, which appears to correspond to the basal body or transition zone, whereas eGFP-tagged wild-type Mak is located along the axoneme. One possibility is that target proteins of Mak-mediated phosphorylation exist near the basal body or transition zone and move with Mak along the axoneme after phosphorylation by Mak. Another possibility is that Mak interacts with adaptor proteins that are localized in the basal body or transition zone to support Mak substrate phosphorylation for ciliogenesis. At present, Mak phosphorylation substrates and adaptor proteins that facilitate Mak substrate phosphorylation are unknown in zebrafish; however, as the IFT complex is assembled and transported through the transition zone in the first step of ciliogenesis (Breslow and Holland, 2019), IFT complex components may be promising candidates to promote Mak substrate phosphorylation.
MAK proteins share a highly conserved amino acid sequence between zebrafish and mice. However, in contrast to zebrafish mak mutants, the axoneme is elongated in mouse Mak mutants (Omori et al., 2010). Why does mak gene knockout induce such opposite phenotypes in zebrafish and mice? From a phylogenetic point of view, MAK belongs to the MAK/ICK/MOK serine/threonine kinase family, all members of which share a highly conserved kinase domain (Chen et al., 2013; Miyata and Nishida, 1999). CILK1 (previously known as ICK) is a member of this kinase family and is now classified as a paralog of Mak. Indeed, human CILK1 shows a highly similar amino acid sequence in the kinase domain to that of zebrafish Mak (81.5% amino acid identity). Interestingly, in Cilk1 mouse mutants, the ciliary axonemes of fibroblasts are shortened compared with those of wild-type controls (Chaya et al., 2014). Furthermore, mouse CILK1 interacts with IFT-B components, including IFT88 (Nakamura et al., 2020). As a CILK1 homologous gene has not been identified in the zebrafish genomic database, zebrafish Mak and mouse CILK1 may share a common regulatory pathway for axoneme formation during ciliogenesis. It will be important to investigate whether zebrafish Mak cooperates with IFT-B to regulate axoneme formation during ciliogenesis.
Another interesting finding is that there is a difference in the degeneration of rods and cones in mak mutants. In mak mutants, rods undergo acute apoptosis from 3 to 4 dpf, whereas cones undergo progressive shrinkage from 4 to 6 dpf. Interestingly, early pyknotic nuclei and later progressive shrinkage of the photoreceptor layer was also observed in zebrafish ift88 and traf3ip1 mutants, both of which show defects in axoneme formation (Bahadori et al., 2003; Doerre and Malicki, 2002; Sukumaran and Perkins, 2009; Wang et al., 2022). Recently, it was reported that photoreceptor degeneration in ciliogenesis-defective zebrafish tulp1a and tulp1b mutants is associated with pathological features of ferroptosis (Jia et al., 2022), which is a new form of cell death linked to accumulation of lipid peroxides (Yu et al., 2017). Although both rod and cone degeneration in mak mutants depend on apoptosis, it is interesting to examine why cones show progressive shrinkage in the absence of axoneme formation. Recent metabolic transcriptome analysis revealed that the cellular metabolism of rods and cones depends on aerobic glycolysis and oxidative phosphorylation, respectively, but that cones shift toward glycolysis in the early pathological stage of RP, suggesting an intrinsic difference of metabolic regulation between rods and cones (Lee et al., 2023). It is interesting to examine how metabolic states are regulated differently in rods and cones in mak mutants.
Lastly, mak mutants show scoliosis and smaller body size in later development. A comprehensive analysis of zebrafish transition zone mutants revealed phenotypic variations between these ciliary-defective mutants (Wang et al., 2022). Among them, cep290 mutants showed severe scoliosis in the adult stage, whereas tmem216 mutants showed smaller body size. Indeed, Cep290 is markedly decreased in mak mutants, so it is possible that scoliosis and smaller body size in mak mutants are caused by reduced activity of Cep290 and Tmem216, respectively. Further study on the ciliary regulatory network in mak mutants will reveal the role of Mak in ciliopathy mechanisms.
MATERIALS AND METHODS
Zebrafish maintenance and ethics statement
Zebrafish were maintained at 28.5°C, on a 14 h light/10 h dark cycle, following standard procedures (Westerfield, 2000). Collected embryos were cultured in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2 and 0.33 mM MgSO4) containing 0.003% 1-phenyl-2-thiourea (PTU) to prevent pigmentation and 0.0002% methylene blue to prevent fungal growth. All experiments were performed on zebrafish embryos between the one-cell stage and 6 dpf prior to sexual differentiation. Therefore, sexes of embryos could not be determined. All zebrafish experiments were performed under the Okinawa Institute of Science and Technology (OIST) Animal Care and Use Program based on the Guide for the Care and Use of Laboratory Animals by the National Research Council of the Nation Academies and approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The OIST Institutional Animal Care and Use Committee approved all experimental protocols (protocols: ACUP-2023-016, ACUP −2023-017, ACUP −2023-018, ACUP −2023-019 and ACUP −2023-020).
Zebrafish strains
Okinawa wild-type (oki) was used as the wild-type strain to maintain all mutant and transgenic strains. The zebrafish mutant, pdays351, was originally isolated by the Herwig Baier laboratory (Muto et al., 2005). Mapping of the pdays351 mutation was carried out in the genetic background of WIK. Zebrafish transgenic lines, Tg[gnat2:NLS-tdTomato]oki070 and Tg[rho:NLS-eGFP]oki071, were used to visualize cone and rod nuclei, respectively. Tg[hsp:mCherry-Bcl2]oki029 (Nishiwaki and Masai, 2020) was used to express mCherry-tagged zebrafish Bcl2 under control of the zebrafish heat shock 70 (hsp70) promoter. Tg[crx:eGFP]oki072 was used to express eGFP, Tg[crx:eGFP-WT MAK]oki073 was used to express eGFP-tagged wild-type Mak, and Tg[crx:eGFP-AR MAK]oki074, Tg[crx:eGFP-ADF MAK]oki075 and Tg[crx:eGFP-DM MAK]oki076 were used to express kinase-deficient mutant Mak. Tg[gnat2:arl13b-tdTomato]oki077 and Tg[rho:arl13b-eGFP]oki078 were used to visualize cone and rod OSs, respectively.
Mapping and cloning of the pday mutant gene
pday+/− fish maintained in the oki wild-type background were outcrossed with the WIK wild-type strain to generate F1 pday+/− fish with an oki and WIK trans-heterozygous wild-type background. These F1 pday+/− male and female fish were crossed to produce F2 embryos. F2 pday–/– embryos were selected by measuring OKR at 5 dpf and stored in methanol at −80°C. Genomic DNA was extracted from individual F2 pday–/– embryos. First, two DNA pools of 20 wild-type and 20 homozygous mutant embryos, respectively, were used for PCR-mediated amplification of SSLP markers (Knapik et al., 1998; Shimoda et al., 1999) to examine which chromosome hosts the pday mutation. Furthermore, 93 F2 pday−/− embryos (186 meiosis) were used to restrict the cytogenetic position of the pday mutation using SSLP markers mapped on the linked chromosome. We restricted the pday mutation within the genomic region flanked between two SSLP markers, z23011 and z13695, which were annotated at positions 8.517 and 9.123 Mb on chromosome 24 (zebrafish genomic database, GRCz10, Ensemble release 80). This region contained tengenes (tfap2a, tmem14ca, mak, gcm2, elovl2, BX546453, gnal, mppe1, CR318624 and dlgap1b). As, among these, mak is the only gene for which mutations cause inherited photoreceptor degeneration diseases, such as RP in humans (Ozgul et al., 2011; Stone et al., 2011), we designed a new SSLP marker, namely Mak-N1, which was located around 20 bp upstream of the first exon of the mak gene, and confirmed no recombination of Mak-N1 in all 93 F2 pday−/− embryos (186 meiosis). Sequence information on forward and reverse primers of z23011, z13695 and Mak-N1 is provided in Table S1.
To confirm that the pday gene encodes Mak, full-length mak cDNA was amplified by PCR using mRNA prepared from wild-type and pdays351 homozygous embryos at 6 dpf. However, only a partial cDNA fragment that corresponds to the C-terminal coding region, which starts from the middle of exon 7 and ends at exon 15, was amplified by PCR from pday homozygous mutant mRNA. We sequenced the full-length cDNA of wild-type and the C-terminal partial cDNA of pday homozygous mutants and found no amino acid change in the Mak-coding region from exons 8 to 15 between wild-type and pday−/− cDNA. Next, we amplified six DNA fragments from the pday−/− genome, each of which covers six N-terminal coding exons (exons 2-7), respectively. Sequencing of these six DNA fragments revealed a nonsense mutation in exon 4 of the pday−/− genome. To confirm that the pday mutant gene encodes Mak, we combined pday mutants with the transgenic line Tg[crx:eGFP-WT MAK]oki073 and showed that expression of eGFP-tagged Mak in photoreceptor precursors rescues photoreceptor degeneration in pday mutants.
Generation of DNA expression constructs and their transgenic lines
Tol2[crx: membrane targeted YFP (MYFP)] was used as a template to make the expression construct Tol2[crx: eGFP-MAK]. Tol2[crx: MYFP] was constructed using the Tol2kit multiple gateway-based construction system (Kwan et al., 2007), in which the Tol2 vector backbone contains the cmcl2:CFP transgenesis marker (Suzuki et al., 2013). mak cDNA corresponding to a full-length isoform annotated on the zebrafish genomic database (mak-201, GRCz11, Ensemble release 110) was amplified by PCR using an mRNA pool of 3-dpf wild-type embryos and was subcloned into pCR TOPO vector (Invitrogen). After the eGFP cDNA fragment was tagged in-frame to the mak full-length cDNA at the N-terminus, the MYFP region of Tol2[crx: MYFP] was replaced with the DNA fragment encoding eGFP-tagged mak cDNA at the BamHI and ClaI sites to make the expression construct pTol2[crx:eGFP-WT MAK]. The pTol2[crx:eGFP-WT MAK] was used as a template in site-directed mutagenesis to make the three kinase-dead MAK constructs Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK] and Tg[crx:eGFP-DM MAK].
The EF1α promoter of the Tol2 transposon vector pT2AL200R150G (Urasaki et al., 2006) was replaced with the zebrafish gnat2 promoter (Iribarne et al., 2019) at the XhoI and BamHI sites to make Tol2[gnat2:eGFP]. Zebrafish arl13b cDNA was amplified by PCR using an mRNA pool prepared from 4-dpf wild-type embryos and subcloned into the pCR TOPO vector (Invitrogen). After the tdTomato cDNA fragment was tagged in-frame to the arl13b cDNA at the C-terminus, the DNA fragment of tdTomato-tagged arl13b cDNA was further subcloned into the modified pT2AL200R150G carrying the gnat2 promoter at the BamHI and ClaI sites to make the expression construct pTol2[gnat2:arl13b-tdTomato]. Similarly, the EF1α promoter of the Tol2 transposon vector pT2AL200R150G was replaced with the zebrafish rhodopsin promoter (1.1 kb upstream of the transcription unit) at the XhoI and BamHI site. A DNA fragment of C-terminal eGFP-tagged arl13b cDNA was prepared and subcloned into the modified pT2AL200R150G carrying the rhodopsin promoter at the BamHI and ClaI sites to make the expression construct pTol2[rho:arl13b-eGFP].
The NLS was fused to cDNA encoding tdTomato or eGFP at the N-terminus. DNA fragments of NLS-tdTomato and NLS-eGFP were subcloned into the modified pT2AL200R150G carrying the zebrafish gnat2 promoter and the zebrafish rhodopsin promoter, respectively, at the BamHI and ClaI sites to make the expression constructs pTol2[gnat2:NLS-tdTomato] and pTol2[rho:NLS-eGFP].
Next, these Tol2 expression construct plasmids and Tol2 transposase mRNA were co-microinjected into one-cell-stage zebrafish fertilized eggs for generation of the transgenic lines Tg[crx:eGFP-WT MAK], Tg[crx:eGFP-AR MAK], Tg[crx:eGFP-ADF MAK], Tg[crx:eGFP-DM MAK], Tg[gnat2:arl13b-tdTomato], Tg[rho:arl13b-eGFP], Tg[gnat2:NLS-tdTomato] and Tg[rho:NLS-eGFP]. Injected F0 fish expressing fluorescent protein (eGFP or tdTomato) in the ONL were bred until adulthood and used to identify founder fish, which produce fluorescent protein-expressing F1 progeny. These transgenic F1 embryos were raised and used to produce F2 generation for establishment of the transgenic strains.
Histology
For plastic sections, zebrafish embryos were anesthetized in 0.02% 3-aminobenzoic acid ethyl ester (tricaine) and fixed in PBS (150 mM NaCl, 10 mM PO43−, pH 7.4) (Invitrogen) with 4% paraformaldehyde (PFA) at 4°C overnight. Samples were dehydrated with an ethanol gradient before being embedded in JB4 resin (Polysciences). Samples were sectioned at 5 µm with a rotary microtome (HM335E, MICROM International) and stained with 0.1% toluidine blue for further analysis.
For TUNEL, samples were covered with the staining mixture at 37°C for 1 h following instructions of the In Situ Cell Death Detection Kit (Roche, 11684795910). After the TUNEL reaction, nuclei were stained with 60 mM PO4 buffer (PB) (42.6 mM NaH2PO4, 17.4 mM Na2HPO4, pH 7.3) containing Hoechst 33342 (Fujifilm, 346-07951) at 1:1000, followed by washing with PB containing 0.1% Triton X-100 (0.1% PBTx) three times for 5 min each. Samples were mounted with Fluoromount (Diagnostic BioSystems, K024) for further analysis.
For immunohistochemistry, 3- to 6-dpf embryos were fixed with 4% PFA at room temperature for 2 h and transferred to 30% sucrose in PB overnight before being embedded and frozen in OCT compound (Sakura Finetek Japan). However, we used non-PFA-fixed samples for anti-GFP labeling shown in Fig. 6C, because the eGFP-Mak signals were stably detected only in non-fixed tissues. Cryosections were prepared at 7 µm with a cryostat (Cryostar NX70, Thermo Fisher Scientific), and at 20 µm for imaging cilia components. Cryosections were air dried for at least 2 h, then rehydrated in 0.5% PBTx three times for 5 min each. Rehydrated sections were immersed in blocking solution (10% goat serum in 0.5% PBTx) for 1 h at room temperature. Then, primary antibody in blocking solution at an appropriate dilution (see Table S2) was applied, and sections were washed with 0.5% PBTx three times for 10 min each. Next, primary antibody-labeled sections were treated with fluorophore-conjugated secondary antibody at 1:500 and Hoechst 33342 at 1:1000 (DOJINDO Laboratories, CAS23491-52-3) in blocking solution for 1 h at room temperature, or with 50 nM Sytox Green (Molecular Probes) in 0.1% PBTx three times for 10 min after secondary antibody incubation, and then washed with 0.1% PBTx three times for 10 min. Sections were finally mounted with Fluoromount. Confocal images were scanned with a confocal laser scanning microscope [LSM780 (Carl Zeiss) or Fluoview FV3000 (Olympus, Evident)]. Information on primary and secondary antibodies is provided in Table S2.
For EM analysis, 3-dpf embryos were fixed in PB with fixative (2.5% glutaraldehyde, 1% paraformaldehyde, 3% sucrose and 30 mM HEPES) on ice for 2 h, followed by washing with PB with 3% sucrose and 30 mM HEPES three times for 5 min. Embryos were postfixed in 60 mM PB with 1% OsO4 for 1 h on ice, followed by washing with Milli-Q water three times. Samples were dehydrated with a gradient series of ethanol that was replaced with acetone. After treatment with a gradient series of propylene oxide, epoxy resin EPON812 (Nisshin-EM, 3402) was applied to the samples before they were embedded in resin and solidified for 2 days at 60°C. Ultra-thin sections at 50 nm were prepared with a Leica UC7 ultra-microtome and processed with Reynolds' stain (Reynolds, 1963). Images were observed and captured using a JOEL JEM-1230R transmission electron microscope.
Bcl2 overexpression in pday mutant retinas
The transgenic line Tg[hsp:mCherry-Bcl2] (Nishiwaki and Masai, 2020) was combined with pday mutants. Heat shock treatment was carried out by incubation of embryos at 39°C for 1 h every 12 h after 1 dpf until 6 dpf.
In situ hybridization
Whole-mount in situ hybridization was carried out using a published protocol (Thisse and Thisse, 2008). A DNA fragment containing mak full-length cDNA combined with the T7 RNA polymerase promoter, which is located at the 3′ end of the mak full-length cDNA, was amplified by PCR. A digoxigenin (DIG)-labeled antisense mak RNA probe was generated by in vitro transcription from this T7 promoter:mak cDNA fragment as the template using a DIG RNA labeling kit (Roche, 11175025910). Zebrafish embryos from the one-cell stage to 72 hpf were fixed with 4% PFA at 4°C overnight and were stored in 100% methanol at −70°C. Samples were rehydrated from methanol to PBS, and then treated with proteinase K (10 µg/ml) to increase permeability. After refixing with 4% PFA for 20 min, samples were soaked with prehybridization buffer at 65°C for 2 h, then incubated with hybridization buffer containing mak antisense RNA probe at 65°C overnight. Samples were washed with 2× SSC with 0.1% Tween 20 for 5 min, room temperature, and then with 0.2× SSC with 0.1% Tween 20 three times for 30 min each at 65°C. Washed samples were transferred to maleic acid buffer with 0.1% Tween 20 (MABT), and then to MABT with 2% blocking reagent (Roche, 11096176001) at 4°C overnight. Samples were incubated with anti-DIG-AP antibody (Roche, 11093274910) at 1:6000 in MABT for 1 h at room temperature, washed with MABT three times for 15 min, and then transferred to Tris-HCl buffer (pH 9.5) for 15 min. For the alkaline phosphatase (AP) reaction, samples were transferred to BM-Purple (Roche, 11442074001) until a blue signal developed, and were then fixed with 4% PFA overnight at 4°C. These samples were mounted in 70% glycerol-PBS and stored at 4°C for long-term preservation. Images of the embryos were observed with a SteREO Discovery V12 dissection microscope (Carl Zeiss).
Live imaging
For live imaging using confocal laser scanning microscopy, embryos were anesthetized in 0.02% tricaine and mounted on glass slides in appropriate positions and glued with 3% methylcellulose. An upright confocal laser scanning microscope (FV3000, Olympus, Evident) was used for scanning fluorescence signals in the photoreceptor OS of Tg[crx:GFP-MAK].
In vitro kinase assay
The wild-type zebrafish mak cDNA fragment was introduced into pGEX6p3 (GE Health Care Life Science). Kinase-dead mak cDNA fragments were generated from pGEX6p3-zMAK(wt) with primers for site-directed mutagenesis. Plasmids were transformed into E. coli strain BL21 competent cells (TaKaRa Bio, TKR9126). Colonies were inoculated into LB broth for starter cultures incubated at 37°C overnight, followed by amplification cultures containing 1% of the starters. Amplification cultures were cooled on ice once the optical density reached 0.6, then IPTG was added (TaKaRa Bio, TKR9030) to a final concentration of 1 mM and the cultures were incubated at 20°C overnight. The cultures were pelleted, washed with cold PBS and sonicated in PBS containing 1% Triton X-100 (PBSTx) with 1× protease inhibitor (Roche, 04693132001). The debris was then pelleted and supernatant fractions were incubated with Glutathione Sepharose 4B beads (Cyvita, 17-0756-01) for 1.5 h at 4°C. After being washed with ice-cold PBS three times, sepharose beads were incubated with PreScission protease (Cyvita, 27084301) in the solution at 4°C to release Mak from the GST tag. The digested products were pelleted and supernatant fractions were used for in vitro kinase assays.
Each reaction for in vitro kinase assays was composed of 50 mM Tris-HCl (pH 7.0), 10 mM MgCl2, 5 mM ATP (Thermo Fisher Scientific, R0441), 1 mM dithiothreitol (Thermo Fisher Scientific, 20290), 5 µg dephosphorylated myelin basic protein (Merck, 13-110) and purified Mak protein. Reactions were incubated 1 h at 30°C. Samples were mixed with SDS sample dye and incubated for 20 min at 75°C to stop the reactions. Samples were separated by SDS-PAGE and transferred onto PVDF membranes for later analyses. Membranes were blocked with 5% skim milk for 1 h at room temperature, followed by probing with primary antibodies and HRP-conjugated secondary antibodies (see Table S2). ECL substrate (Thermo Fisher Scientific, 34577) was applied to visualize signals using an iBright FL1500 imager (Invitrogen).
Data quantification and statistical analysis
To qualify the thickness of photoreceptor layers, photoreceptor layers in the images were straightened using the straighten tool in ImageJ (Fig. S2A). A line dividing the photoreceptor layer into ten parts was generated with Adobe Illustrator. Photoreceptor layer thickness at each decile was measured with ImageJ by following the conversion ratio of pixels to the actual length of the scale bar. To qualify photoreceptor numbers, photoreceptor layers in the images were straightened with ImageJ, and a reference line marking every one in ten points was drawn with Adobe Illustrator. Cell numbers were manually counted. TUNEL signals in retinas were manually counted. The zpr1-positive area in the total retina was calculated as previously described (Fig. S2B) (Nishiwaki et al., 2013). All statistical analyses were performed with GraphPad Prism 9.2.1. Significance levels, numbers of samples and details of analyses are indicated in each figure and figure legend. Sample sizes (fish numbers) for each figure panel are provided in Table S3.
Supplementary Material
Acknowledgements
We are grateful to Herwig Baier and Akira Muto for providing the pday mutant line. We also thank Tadashi Yamamoto for providing the vector pGEX6p3. We thank previous laboratory members, Brandy Lee Denis and Maria Iribarne for supporting the experiment to clone pday mutant gene; Shohei Suzuki for cloning of the zebrafish rhodopsin promoter; Sachihiro Suzuki for cloning of the zebrafish gnat2 promoter; and Yutaka Kojima, Ayano Harata and Hiroshi Izumi for supporting histological analyses of pday mutant phenotypes. We are grateful to the Imaging Section and the sequencing section of the Research Support Division of Okinawa Institute of Science and Technology for assistance in imaging and sequencing experiments, respectively. We thank Steven D. Aird for critical reading and editing of the manuscript.
Footnotes
Author contributions
Conceptualization: H.-J.C., I.M.; Methodology: H.-J.C., Y.N., I.M.; Software: H.-J.C., I.M.; Validation: H.-J.C., Y.N., W.-C.C., I.M.; Formal analysis: H.-J.C., Y.N., I.M.; Investigation: H.-J.C., Y.N., W.-C.C., I.M.; Resources: H.-J.C., Y.N., I.M.; Data curation: H.-J.C., Y.N., I.M.; Writing - original draft: H.-J.C., I.M.; Writing - review & editing: H.-J.C., I.M.; Visualization: H.-J.C., Y.N., W.-C.C., I.M.; Supervision: I.M.; Project administration: H.-J.C., Y.N., I.M.; Funding acquisition: I.M.
Funding
This work was funded by a grant from the Okinawa Institute of Science and Technology Graduate University to I.M. Open access funding provided by Okinawa Institute of Science and Technology Graduate University. Deposited in PMC for immediate release.
Data availability
All relevant data can be found within the article and its supplementary information.
References
- Alfinito, P. D. and Townes-Anderson, E. (2002). Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc. Natl. Acad. Sci. USA 99, 5655-5660. 10.1073/pnas.072557799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou, D., Aguila, M., Bellingham, J., Li, W., McCulley, C., Reeves, P. J. and Cheetham, M. E. (2018). The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 62, 1-23. 10.1016/j.preteyeres.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann-Gagescu, R. and Neuhauss, S. C. (2019). The photoreceptor cilium and its diseases. Curr. Opin. Genet. Dev. 56, 22-33. 10.1016/j.gde.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Bachmann-Gagescu, R., Phelps, I. G., Stearns, G., Link, B. A., Brockerhoff, S. E., Moens, C. B. and Doherty, D. (2011). The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum. Mol. Genet. 20, 4041-4055. 10.1093/hmg/ddr332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori, R., Huber, M., Rinner, O., Seeliger, M. W., Geiger-Rudolph, S., Geisler, R. and Neuhauss, S. C. (2003). Retinal function and morphology in two zebrafish models of oculo-renal syndromes. Eur. J. Neurosci. 18, 1377-1386. 10.1046/j.1460-9568.2003.02863.x [DOI] [PubMed] [Google Scholar]
- Blackshaw, S., Harpavat, S., Trimarchi, J., Cai, L., Huang, H., Kuo, W. P., Weber, G., Lee, K., Fraioli, R. E., Cho, S. H.et al. (2004). Genomic analysis of mouse retinal development. PLoS Biol. 2, E247. 10.1371/journal.pbio.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina, A., Superina, S., Voskas, D. and Ciruna, B. (2010). Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 12, 407-412. 10.1038/ncb2042 [DOI] [PubMed] [Google Scholar]
- Breslow, D. K. and Holland, A. J. (2019). Mechanism and Regulation of Centriole and Cilium Biogenesis. Annu. Rev. Biochem. 88, 691-724. 10.1146/annurev-biochem-013118-111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard, G. J. and Rice, L. M. (2018). Microtubule dynamics: an interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451-463. 10.1038/s41580-018-0009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Rodriguez, M., Austin-Tse, C., Bergboer, J. G. M., Molinari, E., Sugano, Y., Bachmann-Gagescu, R., Sayer, J. A. and Drummond, I. A. (2021). Genetic compensation for cilia defects in cep290 mutants by upregulation of cilia-associated small GTPases. J. Cell Sci. 134, Jcs258568. 10.1242/jcs.258568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaya, T., Omori, Y., Kuwahara, R. and Furukawa, T. (2014). ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J. 33, 1227-1242. 10.1002/embj.201488175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. Y., Kelley, R. A., Li, T. and Swaroop, A. (2021). Primary cilia biogenesis and associated retinal ciliopathies. Semin. Cell Dev. Biol. 110, 70-88. 10.1016/j.semcdb.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T., Wu, D., Moskaluk, C. A. and Fu, Z. (2013). Distinct expression patterns of ICK/MAK/MOK protein kinases in the intestine implicate functional diversity. PLoS ONE 8, e79359. 10.1371/journal.pone.0079359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan, A. V., Aleman, T. S., Jacobson, S. G., Khanna, H., Sumaroka, A., Aguirre, G. K., Schwartz, S. B., Windsor, E. A., He, S., Chang, B.et al. (2007). Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum. Mutat. 28, 1074-1083. 10.1002/humu.20565 [DOI] [PubMed] [Google Scholar]
- D'Orazi, F. D., Suzuki, S. C., Darling, N., Wong, R. O. and Yoshimatsu, T. (2020). Conditional and biased regeneration of cone photoreceptor types in the zebrafish retina. J. Comp. Neurol. 528, 2816-2830. 10.1002/cne.24933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander, A. I., Koenekoop, R. K., Yzer, S., Lopez, I., Arends, M. L., Voesenek, K. E., Zonneveld, M. N., Strom, T. M., Meitinger, T., Brunner, H. G.et al. (2006). Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 79, 556-561. 10.1086/507318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerre, G. and Malicki, J. (2002). Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech. Dev. 110, 125-138. 10.1016/S0925-4773(01)00571-8 [DOI] [PubMed] [Google Scholar]
- Duldulao, N. A., Lee, S. and Sun, Z. (2009). Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development 136, 4033-4042. 10.1242/dev.036350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner, J. J., Amack, J. D., Nyholm, M. K., Harris, E. B. and Yost, H. J. (2005). Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247-1260. 10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- Fain, G. L., Hardie, R. and Laughlin, S. B. (2010). Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114-R124. 10.1016/j.cub.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J., Cheon, K., Nusinowitz, S., Liu, Q., Bei, D., Atkins, K., Azimi, A., Daiger, S. P., Farber, D. B., Heckenlively, J. R.et al. (2002). Progressive photoreceptor degeneration, outer segment dysplasia, and rhodopsin mislocalization in mice with targeted disruption of the retinitis pigmentosa-1 (Rp1) gene. Proc. Natl. Acad. Sci. USA 99, 5698-5703. 10.1073/pnas.042122399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, P., Qin, Y., Qu, Z., Huang, Y., Liu, X., Li, J., Liu, F. and Liu, M. (2022). The Zpr-3 antibody recognizes the 320-354 region of Rho and labels both rods and green cones in zebrafish. biorXiv 2022.02.21.481375. 10.1101/2022.02.21.481375 [DOI] [Google Scholar]
- Ge, R., Cao, M., Chen, M., Liu, M. and Xie, S. (2022). Cytoskeletal networks in primary cilia: Current knowledge and perspectives. J. Cell. Physiol. 237, 3975-3983. 10.1002/jcp.30865 [DOI] [PubMed] [Google Scholar]
- Goncalves, J. and Pelletier, L. (2017). The ciliary transition zone: finding the pieces and assembling the gate. Mol. Cells 40, 243-253. 10.14348/molcells.2017.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden, N. T., Arts, H. H., Parisi, M. A., Coene, K. L., Letteboer, S. J., van Beersum, S. E., Mans, D. A., Hikida, A., Eckert, M., Knutzen, D.et al. (2008). CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am. J. Hum. Genet. 83, 559-571. 10.1016/j.ajhg.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, R. S., Gonzalez, R., Ackerman, S. D., Minowa, R., Griest, J. F., Bayrak, M. N., Troutwine, B., Canter, S., Monk, K. R., Sepich, D. S.et al. (2021). Postembryonic screen for mutations affecting spine development in zebrafish. Dev. Biol. 471, 18-33. 10.1016/j.ydbio.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati, S. and Palczewski, K. (2023). Structural view of G protein-coupled receptor signaling in the retinal rod outer segment. Trends Biochem. Sci. 48, 172-186. 10.1016/j.tibs.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarne, M., Hyde, D. R. and Masai, I. (2019). TNFalpha induces muller glia to transition from non-proliferative gliosis to a regenerative response in mutant zebrafish presenting chronic photoreceptor degeneration. Front. Cell Dev. Biol. 7, 296. 10.3389/fcell.2019.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, D., Gao, P., Lv, Y., Huang, Y., Reilly, J., Sun, K., Han, Y., Hu, H., Chen, X., Zhang, Z.et al. (2022). Tulp1 deficiency causes early-onset retinal degeneration through affecting ciliogenesis and activating ferroptosis in zebrafish. Cell Death Dis. 13, 962. 10.1038/s41419-022-05372-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakakhel, M., Tebbe, L., Makia, M. S., Conley, S. M., Sherry, D. M., Al-Ubaidi, M. R. and Naash, M. I. (2020). Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proc. Natl. Acad. Sci. USA 117, 20615-20624. 10.1073/pnas.2010751117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan, S., Zhang, H., Li, S., Frederick, J. M. and Baehr, W. (2008). A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 48, 442-452. 10.1016/j.visres.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena, N. and Pigino, G. (2022). Structural biology of cilia and intraflagellar transport. Annu. Rev. Cell Dev. Biol. 38, 103-123. 10.1146/annurev-cellbio-120219-034238 [DOI] [PubMed] [Google Scholar]
- Knapik, E. W., Goodman, A., Ekker, M., Chevrette, M., Delgado, J., Neuhauss, S., Shimoda, N., Driever, W., Fishman, M. C. and Jacob, H. J. (1998). A microsatellite genetic linkage map for zebrafish (Danio rerio). Nat. Genet. 18, 338-343. 10.1038/ng0498-338 [DOI] [PubMed] [Google Scholar]
- Krock, B. L. and Perkins, B. D. (2008). The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 121, 1907-1915. 10.1242/jcs.029397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, K. M., Fujimoto, E., Grabher, C., Mangum, B. D., Hardy, M. E., Campbell, D. S., Parant, J. M., Yost, H. J., Kanki, J. P. and Chien, C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099. 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- Larison, K. D. and Bremiller, R. (1990). Early onset of phenotype and cell patterning in the embryonic zebrafish retina. Development 109, 567-576. 10.1242/dev.109.3.567 [DOI] [PubMed] [Google Scholar]
- Latour, B. L., Van De Weghe, J. C., Rusterholz, T. D., Letteboer, S. J., Gomez, A., Shaheen, R., Gesemann, M., Karamzade, A., Asadollahi, M., Barroso-Gil, M.et al. (2020). Dysfunction of the ciliary ARMC9/TOGARAM1 protein module causes Joubert syndrome. J. Clin. Invest. 130, 4423-4439. 10.1172/JCI131656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J., Emery, D., Vukmanic, E., Wang, Y., Lu, X., Wang, W., Fortuny, E., James, R., Kaplan, H. J., Liu, Y.et al. (2023). Metabolic transcriptomics dictate responses of cone photoreceptors to retinitis pigmentosa. Cell Rep. 42, 113054. 10.1016/j.celrep.2023.113054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., Zuo, J. and Pierce, E. A. (2004). The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J. Neurosci. 24, 6427-6436. 10.1523/JNEUROSCI.1335-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, V. S., Jimeno, D., Khanobdee, K., Song, X., Chen, B., Nusinowitz, S. and Williams, D. S. (2010). Dysfunction of heterotrimeric kinesin-2 in rod photoreceptor cells and the role of opsin mislocalization in rapid cell death. Mol. Biol. Cell 21, 4076-4088. 10.1091/mbc.e10-08-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, A. H., Xia, L., Desai, S. J., Boucher, D. L., Guan, Y., Shih, H. M., Shi, X. B., deVere White, R. W., Chen, H. W., Tepper, C. G.et al. (2006). Male germ cell-associated kinase, a male-specific kinase regulated by androgen, is a coactivator of androgen receptor in prostate cancer cells. Cancer Res. 66, 8439-8447. 10.1158/0008-5472.CAN-06-1636 [DOI] [PubMed] [Google Scholar]
- Masek, M., Etard, C., Hofmann, C., Hulsmeier, A. J., Zang, J., Takamiya, M., Gesemann, M., Neuhauss, S. C. F., Hornemann, T., Strahle, U.et al. (2022). Loss of the Bardet-Biedl protein Bbs1 alters photoreceptor outer segment protein and lipid composition. Nat. Commun. 13, 1282. 10.1038/s41467-022-28982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime, H., Jinno, A., Takagi, N. and Shibuya, M. (1990). A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol. Cell. Biol. 10, 2261-2268. doi:10.1128/mcb.10.5.2261-2268.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, A. J. and Thoreson, W. B. (2011). The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis. Neurosci. 28, 453-471. 10.1017/S0952523811000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill, P., Christensen, S. T. and Pedersen, L. B. (2023). Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat. Rev. Genet. 24, 421-441. 10.1038/s41576-023-00587-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata, Y. and Nishida, E. (1999). Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem. Biophys. Res. Commun. 266, 291-295. 10.1006/bbrc.1999.1705 [DOI] [PubMed] [Google Scholar]
- Muto, A., Orger, M. B., Wehman, A. M., Smear, M. C., Kay, J. N., Page-McCaw, P. S., Gahtan, E., Xiao, T., Nevin, L. M., Gosse, N. J.et al. (2005). Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 1, e66. 10.1371/journal.pgen.0010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K., Noguchi, T., Takahara, M., Omori, Y., Furukawa, T., Katoh, Y. and Nakayama, K. (2020). Anterograde trafficking of ciliary MAP kinase-like ICK/CILK1 by the intraflagellar transport machinery is required for intraciliary retrograde protein trafficking. J. Biol. Chem. 295, 13363-13376. 10.1074/jbc.RA120.014142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, K. and Katoh, Y. (2018). Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors. J. Biochem. 163, 155-164. 10.1093/jb/mvx087 [DOI] [PubMed] [Google Scholar]
- Nishiwaki, Y. and Masai, I. (2020). . beta-SNAP activity in the outer segment growth period is critical for preventing BNip1-dependent apoptosis in zebrafish photoreceptors. Sci. Rep. 10, 17379. 10.1038/s41598-020-74360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki, Y., Yoshizawa, A., Kojima, Y., Oguri, E., Nakamura, S., Suzuki, S., Yuasa-Kawada, J., Kinoshita-Kawada, M., Mochizuki, T. and Masai, I. (2013). The BH3-only SNARE BNip1 mediates photoreceptor apoptosis in response to vesicular fusion defects. Dev. Cell 25, 374-387. 10.1016/j.devcel.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Oberhammer, F. A., Hochegger, K., Froschl, G., Tiefenbacher, R. and Pavelka, M. (1994). Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J. Cell Biol. 126, 827-837. 10.1083/jcb.126.4.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oel, A. P., Neil, G. J., Dong, E. M., Balay, S. D., Collett, K. and Allison, W. T. (2020). Nrl Is Dispensable for Specification of Rod Photoreceptors in Adult Zebrafish Despite Its Deeply Conserved Requirement Earlier in Ontogeny. iScience 23, 101805. 10.1016/j.isci.2020.101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori, Y., Chaya, T., Katoh, K., Kajimura, N., Sato, S., Muraoka, K., Ueno, S., Koyasu, T., Kondo, M. and Furukawa, T. (2010). Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc. Natl. Acad. Sci. USA 107, 22671-22676. 10.1073/pnas.1009437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori, Y., Zhao, C., Saras, A., Mukhopadhyay, S., Kim, W., Furukawa, T., Sengupta, P., Veraksa, A. and Malicki, J. (2008). Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10, 437-444. 10.1038/ncb1706 [DOI] [PubMed] [Google Scholar]
- Ozgul, R. K., Siemiatkowska, A. M., Yucel, D., Myers, C. A., Collin, R. W., Zonneveld, M. N., Beryozkin, A., Banin, E., Hoyng, C. B., van den Born, L. I.et al. (2011). Exome sequencing and cis-regulatory mapping identify mutations in MAK, a gene encoding a regulator of ciliary length, as a cause of retinitis pigmentosa. Am. J. Hum. Genet. 89, 253-264. 10.1016/j.ajhg.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. and Leroux, M. R. (2022). Composition, organization and mechanisms of the transition zone, a gate for the cilium. EMBO Rep. 23, e55420. 10.15252/embr.202255420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, K. N., Li, L., Anand, M. and Khanna, H. (2015). Ablation of retinal ciliopathy protein RPGR results in altered photoreceptor ciliary composition. Sci. Rep. 5, 11137. 10.1038/srep11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, E. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208-212. 10.1083/jcb.17.1.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, B., Lohr, H. R., Humphries, P., Redmond, T. M., Seeliger, M. W. and Crouch, R. K. (2005). Cone opsin mislocalization in Rpe65-/- mice: a defect that can be corrected by 11-cis retinal. Invest. Ophthalmol. Vis. Sci. 46, 3876-3882. 10.1167/iovs.05-0533 [DOI] [PubMed] [Google Scholar]
- Shimoda, N., Knapik, E. W., Ziniti, J., Sim, C., Yamada, E., Kaplan, S., Jackson, D., de Sauvage, F., Jacob, H. and Fishman, M. C. (1999). Zebrafish genetic map with 2000 microsatellite markers. Genomics 58, 219-232. 10.1006/geno.1999.5824 [DOI] [PubMed] [Google Scholar]
- Song, P., Dudinsky, L., Fogerty, J., Gaivin, R. and Perkins, B. D. (2016). Arl13b interacts with Vangl2 to regulate cilia and photoreceptor outer segment length in zebrafish. Invest. Ophthalmol. Vis. Sci. 57, 4517-4526. 10.1167/iovs.16-19898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, E. M., Luo, X., Héon, E., Lam, B. L., Weleber, R. G., Halder, J. A., Affatigato, L. M., Goldberg, J. B., Sumaroka, A., Schwartz, S. B.et al. (2011). Autosomal recessive retinitis pigmentosa caused by mutations in theMAKGene. Invest. Opthalmol. Vis. Sci. 52, 9665-9673. 10.1167/iovs.11-8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran, S. and Perkins, B. D. (2009). Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 49, 479-489. 10.1016/j.visres.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S. C., Bleckert, A., Williams, P. R., Takechi, M., Kawamura, S. and Wong, R. O. (2013). Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc. Natl. Acad. Sci. USA 110, 15109-15114. 10.1073/pnas.1303551110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait, S. W. and Green, D. R. (2010). Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621-632. 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- Thisse, C. and Thisse, B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59-69. 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- Tian, X., Zhao, H. and Zhou, J. (2023). Organization, functions, and mechanisms of the BBSome in development, ciliopathies, and beyond. Elife 12, e87623. 10.7554/eLife.87623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa, M. and Malicki, J. (2004). Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42, 703-716. 10.1016/S0896-6273(04)00268-5 [DOI] [PubMed] [Google Scholar]
- Tucker, B. A., Burnight, E. R., Cranston, C. M., Ulferts, M. J., Luse, M. A., Westfall, T., Scott, C. A., Marsden, A., Gibson-Corley, K., Wiley, L. A.et al. (2022). Development and biological characterization of a clinical gene transfer vector for the treatment of MAK-associated retinitis pigmentosa. Gene Ther. 29, 259-288. 10.1038/s41434-021-00291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasaki, A., Morvan, G. and Kawakami, K. (2006). Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174, 639-649. 10.1534/genetics.106.060244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic, T. S., Doro, C. J. and Hyde, D. R. (1999). Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis. Neurosci. 16, 571-585. 10.1017/S0952523899163168 [DOI] [PubMed] [Google Scholar]
- Wang, J. and Deretic, D. (2014). Molecular complexes that direct rhodopsin transport to primary cilia. Prog. Retin. Eye Res. 38, 1-19. 10.1016/j.preteyeres.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Thomas, H. R., Thompson, R. G., Waldrep, S. C., Fogerty, J., Song, P., Li, Z., Ma, Y., Santra, P., Hoover, J. D.et al. (2022). Variable phenotypes and penetrance between and within different zebrafish ciliary transition zone mutants. Dis. Model. Mech. 15, dmm049568. 10.1242/dmm.049568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. Y. and Kung, H. J. (2012). Male germ cell-associated kinase is overexpressed in prostate cancer cells and causes mitotic defects via deregulation of APC/CCDH1. Oncogene 31, 2907-2918. 10.1038/onc.2011.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield, M. (2000). The Zebrafish Book. A Guide for the Laboratory use of Zebrafish (Danio rerio), 4th edn Eugene: Univ. of Oregon Press. https://zfin.org/zf_info/zfbook/zfbk.html [Google Scholar]
- Xia, L., Robinson, D., Ma, A. H., Chen, H. C., Wu, F., Qiu, Y. and Kung, H. J. (2002). Identification of human male germ cell-associated kinase, a kinase transcriptionally activated by androgen in prostate cancer cells. J. Biol. Chem. 277, 35422-35433. 10.1074/jbc.M203940200 [DOI] [PubMed] [Google Scholar]
- Yin, J., Brocher, J., Linder, B., Hirmer, A., Sundaramurthi, H., Fischer, U. and Winkler, C. (2012). The 1D4 antibody labels outer segments of long double cone but not rod photoreceptors in zebrafish. Invest. Ophthalmol. Vis. Sci. 53, 4943-4951. 10.1167/iovs.12-9511 [DOI] [PubMed] [Google Scholar]
- Yu, H., Guo, P., Xie, X., Wang, Y. and Chen, G. (2017). Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 21, 648-657. 10.1111/jcmm.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M., Liu, Y., Li, J., Natale, B. N., Cao, S., Wang, D., Amack, J. D. and Hu, H. (2016). Eyes shut homolog is required for maintaining the ciliary pocket and survival of photoreceptors in zebrafish. Biol. Open 5, 1662-1673. 10.1242/bio.021584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, G., Gu, Q., He, J., Lou, Q., Chen, X., Jin, X., Bi, E. and Yin, Z. (2014). Sept6 is required for ciliogenesis in Kupffer's vesicle, the pronephros, and the neural tube during early embryonic development. Mol. Cell. Biol. 34, 1310-1321. 10.1128/MCB.01409-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, P., Xu, J., Wang, Y. and Zhao, C. (2021). Loss of Ift74 Leads to Slow Photoreceptor Degeneration and Ciliogenesis Defects in Zebrafish. Int. J. Mol. Sci. 22, 9329. 10.3390/ijms22179329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi, F. R., Berois, N., Brauer, M. M. and Castillo, E. (2021). Building the embryo of Developmental Biology in Uruguay. Int. J. Dev. Biol. 65, 71-76. 10.1387/ijdb.200141fz [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.