Abstract
Critical care uses syndromic definitions to describe patient groups for clinical practice and research. There is growing recognition that a “precision medicine” approach is required and that integrated biologic and physiologic data identify reproducible subpopulations that may respond differently to treatment. This article reviews the current state of the field and considers how to successfully transition to a precision medicine approach. To impact clinical care, identification of subpopulations must do more than differentiate prognosis. It must differentiate response to treatment, ideally by defining subgroups with distinct functional or pathobiological mechanisms (endotypes). There are now multiple examples of reproducible subpopulations of sepsis, acute respiratory distress syndrome, and acute kidney or brain injury described using clinical, physiological, and/or biological data. Many of these subpopulations have demonstrated the potential to define differential treatment response, largely in retrospective studies, and that the same treatment-responsive subpopulations may cross multiple clinical syndromes (treatable traits). To bring about a change in clinical practice, a precision medicine approach must be evaluated in prospective clinical studies requiring novel adaptive trial designs. Several such studies are underway, but there are multiple challenges to be tackled. Such subpopulations must be readily identifiable and be applicable to all critically ill populations around the world. Subdividing clinical syndromes into subpopulations will require large patient numbers. Global collaboration of investigators, clinicians, industry, and patients over many years will therefore be required to transition to a precision medicine approach and ultimately realize treatment advances seen in other medical fields.
Keywords: sepsis, respiratory distress syndrome, acute kidney injury, brain injury, precision medicine
Contents
- Summary of Existing Evidence for Subphenotypes in Critical Care
- Biologic Subphenotypes of Sepsis, ARDS, and AKI
- Physiology of ARDS and TBI to Guide Precision Medicine
- Limitations of Current Subphenotyping Evidence
Developing Areas of Research with Promise for Advancing Subphenotyping and Precision Medicine in Critical Care
Potential Pitfalls to Consider in the Transition to a Precision Phenotype-based Approach
Consensus Priorities: How to Move Forward
A Call for Global Collaboration
For several decades, critical care has organized both clinical practice and research around syndromes: sepsis, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and traumatic brain injury (TBI), among others. Yet, these syndromes, although recognizable to clinicians, are inherently heterogeneous and capture numerous pathophysiologic processes present to varying degrees within individual patients. Not only do the syndromes involve heterogeneous biologic processes, but similar or highly overlapping biologic processes can manifest as multiple related critical care syndromes (1, 2). This heterogeneity has likely contributed to the failure of multiple pharmacologic and supportive care interventions that appeared promising in preclinical models but made no difference, or in some cases may have caused harm, in critically ill patients (2). Integrated biologic and physiologic data increasingly demonstrate reproducible subpopulations within a given critical care syndrome. In some cases, these “subphenotypes” (Box 1) respond differently to interventions tested in randomized controlled trials (RCTs) (3), indicating that targeting them in future therapeutic studies may increase our chances of finding beneficial effects. Such a “precision medicine” approach has had a transformative impact in oncology and other fields (4) but has yet to significantly impact critical care medicine.
Box 1: Phenotyping Terminology Used in This Review (Adapted From PMIDs 32526190, 37326646, and 36070787)
-
1.
Phenotype: a clinically observable set of clinical features; syndromes such as acute respiratory distress syndrome (ARDS) and sepsis could be considered as phenotypes.
-
2.
Subgroup: any subset of patients within a phenotype, which may be defined using any cutoff in any clinical variable or measure (e.g., PaO2/FiO2-based severity classification of ARDS).
-
3.
Subphenotype: a distinct subgroup of a phenotype based on a shared set or pattern of observable or measurable properties, which can be reliably discriminated from other subphenotypes.
-
4.
Endotype: a subphenotype with distinct functional or pathobiological mechanisms that may or may not be associated with a specific treatment response.
-
5.
Treatable trait: a set of clinical characteristics and/or biomarkers, indicative of an underlying pathogenetic mechanism that responds to a specific intervention and may be present across multiple clinical syndromic diagnoses (e.g., hyperinflammation in ARDS and sepsis).
To review the current state of the field and consider how to successfully transition from a syndrome-based approach in critical care to a precision subphenotype-based approach, a roundtable conference was held in Brussels, March 18–20, 2023. Twenty-five clinician scientists with expertise in critical care subphenotyping, physiology, epidemiology, clinical trials, and global health and from geographically and demographically diverse backgrounds were invited. The program was structured to present the evidence for subphenotypes, consider alternative approaches to precision medicine, and discuss challenges and pitfalls to delivering this paradigm shift around the world. To mentor and nurture the next generation of critical care clinician scientists, eight early career researchers from groups that may be underrepresented in academic critical care medicine were supported to attend as part of a “Rising Stars” program. These researchers were nominated by roundtable participants and selected on the basis of their interest in the field to provide a broader diversity of perspectives; they were encouraged to propose alternative approaches, challenge existing opinion, and help ensure that solutions would be widely applicable. This roundtable conference formed the basis for this review.
Summary of Existing Evidence for Subphenotypes in Critical Care
Biologic Subphenotypes of Sepsis, ARDS, and AKI
Sepsis epitomizes the concept of a heterogeneous syndrome; any pathogen can infect any site in the body and initiate a myriad of dysregulated host immune processes that result in one or more acutely dysfunctional organs (5). Sepsis subphenotyping based on observed clinical severity or selection of specific organ dysfunction(s) has generally not yielded successful treatments, despite strong grounding in physiologic rationale. For example, the SCARLET (Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin) trial did not report a survival benefit from randomized recombinant human soluble thrombomodulin for patients with sepsis-associated coagulopathy (6). However, an exploratory study using observational registry data to classify patients into clusters defined by clinical and laboratory data identified likely treatment heterogeneity, even within sepsis-associated coagulopathy (7). This and other examples (8) illustrate how using clinical severity of illness criteria to subclassify patients can achieve prognostic enrichment (i.e., enrolling patients at higher risk of experiencing adverse outcomes) but has generally failed to enrich for treatment response.
Further work used machine learning on more than 25 routinely measured clinical parameters in electronic health data and found four distinct sepsis subphenotypes on emergency department arrival, with markedly different clinical trajectories, outcomes, and biologic signatures (9). These four types were reproduced in more than 100,000 patients in six studies (10) and suggested a differential response to fluid resuscitation using in silico models. With embedding of clinical subphenotypes in electronic health records, a randomized trial including prospective analyses of these subtypes is ongoing (11).
An alternative approach to enriching on the basis of clinical severity is to add molecular testing. Using whole-blood mRNA or transcriptional data, several groups have identified transcriptomic clusters within patients with sepsis that are associated with differential survival (12–14). Perhaps more important, these transcriptional signatures may predict a differential response to treatments. For example, in a reanalysis of the VANISH (Vasopressin vs Norepinephrine as Initial Therapy in Septic Shock) trial testing corticosteroid use in septic shock, patients who displayed the “sepsis response signature 2” (SRS2) low-mortality subphenotype had significantly higher mortality when randomized to corticosteroids than to placebo (15). These findings could potentially explain the inconsistent treatment effects seen across different corticosteroid trials (16), although the retrospective nature of this analysis in a subset of the whole trial limits the conclusions that can be drawn. Subclassification based on peripheral blood leukocyte or monocyte populations has also been pursued both in all-cause sepsis (17) and in SARS-CoV-2–associated sepsis (18). Low expression of monocyte HLA-DR isotype, a ligand for the T cell receptor, has been proposed as a biomarker for sepsis-induced immunoparalysis that might identify participants for immune-enhancing therapy in clinical trials (19). In practice, however, it has been challenging to define a threshold of monocyte HLA-DR that reliably identifies an immunoparalyzed state (20). Nonetheless, there remains great interest in combining cellular, proteomic. and genomic expression to conduct precision RCTs based on molecularly defined sepsis subphenotypes (21).
Subphenotyping algorithms have also identified reproducible subgroups among patients with ARDS. Applying latent class analysis (LCA) to multiple trial populations from the NIH/NHLBI ARDS Network and observational cohorts, two subphenotypes of patients with ARDS with differential mortality have consistently been identified using clinical and plasma inflammatory biomarker data (22–26). Importantly, for several randomized interventions, including positive end-expiratory pressure, fluid therapy, simvastatin, and activated protein C, the treatment effect was divergent in the two subphenotypes. To operationalize the LCA subphenotyping strategy in a clinical setting, parsimonious three- or four-variable classifiers (including plasma biomarkers) have been identified that can reliably classify patients into one or the other subphenotype (27). A clinical study using one of these parsimonious classifiers has now completed recruitment (ClinicalTrials.gov identifier NCT04009330). Similar clusters termed “reactive” and “uninflamed” have been identified in other ARDS cohorts using plasma inflammatory, coagulation, and endothelial plasma proteins as the sole data inputs. Importantly, these plasma protein-derived ARDS subphenotypes express different whole-blood transcriptional signatures (28) and different plasma metabolite profiles (29), supporting the concept that the subphenotypes are biologically distinct endotypes. Furthermore, these subphenotypes are also present in critically ill patients with various organ dysfunctions, including mechanically ventilated patients without ARDS (30) and patients with sepsis (2), and have been associated with heterogeneity of treatment effect in sepsis (31). These findings suggest shared biologic dysregulation that transcends our current syndromic classifications (1).
Like sepsis and ARDS, AKI is recognized as a multifactorial syndrome with significant heterogeneity (32). In addition to different nephrotoxic triggers and multiple pathophysiologic mechanisms, the risk of developing AKI and the subsequent clinical trajectories are impacted by host susceptibility factors, illness severity, and concomitant therapies (33). New biomarkers have provided opportunities for earlier detection and identification of AKI subphenotypes characterized by different biological mechanisms, treatment responses, and prognosis (34). Using LCA of clinical and inflammatory/endothelial plasma biomarker data in patients with sepsis-associated AKI, two AKI subphenotypes were identified with different short-term renal recovery and 90-day mortality rates (35). Applying a parsimonious subphenotyping strategy to participants in VASST (Vasopressin and Septic Shock Trial), differences in treatment effect were seen across the two AKI subphenotypes, with patients categorized to AKI subphenotype 1 suggesting a potential survival benefit with receipt of low-dose vasopressin (36).
Physiology of ARDS and TBI to Guide Precision Medicine
Although the concept of precision medicine is often used to refer to subphenotyping patients via molecular measurements, physiologic measurements remain valuable for developing a precision medicine approach as well. In cases in which physiology is tied to the underlying mechanisms of intervention and outcome, physiologic measurements can be used to predict the benefit or harm of an intervention in individual patients (i.e., heterogeneity of treatment effect [HTE]). In such cases, physiological measures serve to specify treatable traits to define subgroups that may benefit from specific interventions. For example, patients with ARDS with reduced respiratory system compliance appear to sustain greater survival benefit from low-Vt ventilation compared with patients with high respiratory system compliance (37). Patients with a high ventilatory ratio, which estimates ventilatory efficiency and CO2 production, have a higher probability of survival benefit from extracorporeal CO2 removal for acute hypoxemic respiratory failure (38). The association between neuromuscular blockade and mortality may depend on baseline diaphragm thickness (39). These findings suggest that physiologic features may characterize relevant heterogeneity of treatment effects in various critical illness syndromes. They could also be used to select patients for therapeutic interventions, especially because they can be obtained easily in real time at the bedside using existing equipment or devices.
In TBI, physiological monitoring (classically, intracranial pressure and cerebral perfusion pressure monitoring) has commonly been used in neurocritical care (40) to titrate therapy intensity, with more hazardous therapies (e.g., decompressive craniectomy) showing benefit only in patients with refractory intracranial hypertension (41) but harm in early or less severe disease (42). More recently, the combined use of intracranial pressure, brain tissue Po2 monitoring, and brain microdialysis has allowed more specific titration of therapies (43) to nuanced physiological endotypes (classical ischemia, diffusion hypoxia, mitochondrial dysfunction, substrate deficiency, disturbed autoregulation). These endotypes have been identified using advanced monitoring and signal processing and imaging techniques, and they provide a basis for rational targeting of therapy (43–46). These associations are now being tested in formal prospective trials (47–49). The physiologic insights obtained from studying TBI are also being extended to other contexts, such as subarachnoid hemorrhage (50) and hypoxic ischemic encephalopathy after cardiac arrest (51). Preliminary results suggest that approximately 25% of the variance in TBI outcome is heritable (52); outcome may be influenced by the intensity and nature of the inflammatory host response. However, the ability to find distinct endotypes derived from such genetic insights remains a challenge.
Limitations of Current Subphenotyping Evidence
Although numerous studies have identified between two and four subphenotypes within sepsis and ARDS, it is likely that multiple different subphenotypes are present beyond the first stratification. In ARDS, this heterogeneity is most evident in the lung compartment, where lack of overlap is reported between the inflammatory state of alveolar fluid and plasma (53, 54). Respiratory outcomes may be more closely associated with the alveolar inflammatory state (53). In pediatric influenza-related ARDS, endotracheal aspirate and serum inflammatory cytokine associations differed markedly and were also differentially associated with clinical outcomes (55).
Subphenotypes observed in adults are not universally observed in children and vice versa. Age is a critical factor influencing immunity. From birth through adolescence, the innate and adaptive immune systems undergo marked developmental changes that influence disease susceptibility and outcomes (56). In addition, rare primary inherited immune deficiencies usually present in early childhood markedly influence susceptibility to severe infection (57). Transcriptional profiling in sepsis has shown that pediatric and adult subphenotypes do not perfectly overlap (58). Developmental differences in the immune system may explain why application of pediatric sepsis endotypes A and B to an adult cohort did not identify consistent differences in mortality between groups, in contrast to findings in children (58). On the other hand, some subphenotypes of ARDS observed in adult populations (e.g., LCA-derived subphenotypes) do appear to have similar correlates in children. Endocrine, immune, and other differences between adults and children may in part explain differences across biomarker studies (59–61). Of note, a secondary analysis of the HALF-PINT (Heart and Lung Failure – Pediatric Insulin Titration) and IIT-SBPP (Intensive Insulin Treatment – Severely Burned Pediatric Patients) trials suggested that critically ill patients with a hyperinflammatory molecular subphenotype had a mortality benefit from tight glycemic control, unlike those with a hypoinflammatory subphenotype (62). Unique, discernible AKI subphenotypes in children differ from those in adults (53).
Developing Areas of Research with Promise for Advancing Subphenotyping and Precision Medicine in Critical Care
It is encouraging that many of the subphenotyping studies, as detailed above, have identified distinct subgroups with implications for prognostic and, in some cases, predictive enrichment. Additional studies are needed to determine how the numerous published subphenotyping schema overlap and to provide additional external validation. To date, most published subphenotyping work is limited to cohort studies and retrospective analyses of previously collected samples from clinical trials. Prospective studies of these subphenotypes will be needed to move the field toward clinical implementation. Several current studies are testing the feasibility of real-time subphenotyping that could influence decision making in clinical settings (21, 63–66).
Clinical trials using novel designs that enable the evaluation of targeted therapies are also in development, adapting strategies successfully deployed in other fields, such as oncology (67, 68). The goal of these trial designs is often predictive enrichment (69), selecting patients with specific biological or physiological features who are likely responders to a mechanism-targeted therapeutic intervention (69, 70). Examples of such predictive enrichment factors include lung compliance or lung morphology and response to ventilation strategies (ClinicalTrials.gov identifiers NCT05440851 and NCT05492344) or the inflammatory response for immunomodulating therapies (66). Features that putatively indicate treatment responsiveness may also be termed “treatable traits” (71–73) (Box 1). Treatable traits are sets of characteristics and biomarkers indicative of an underlying pathogenetic mechanism that may respond to a specific intervention and may be present across clinical syndromic diagnoses. Such traits may be observable characteristics (e.g., clinical, biological features, physiological characteristics, functional patterns, imaging studies), molecular pathways, or trajectories of the above. Table 1 illustrates examples of biomarker panels or traits associated with heterogeneity of treatment effect that may be candidates for use in future studies (16–19, 23–33).
Table 1.
Promising Biomarker-Intervention Pairs with Potential to Be Used as Treatable Traits in Critical Care
| Syndrome Originally Tested | Biomarker Panel or Trait | Subtype Derivation | Treatment | Randomized Exposure | Outcome (Reference) |
|---|---|---|---|---|---|
| Sepsis | PT, PTT, platelets, FDP, D-dimers, bilirubin, AST, ALT (HBD and DIC as MAS features) | A priori mechanism-based hypothesis of subtype difference | Anakinra | Yes; subgroup/secondary analysis | Decreased 28-d mortality (34.6% vs. 64.7%; P = 0.0006) (73) |
| Ferritin and HLA-DR (MALS) | A priori mechanism-based hypothesis | Anakinra | Yes | Survival with SOFA decrease at Day 7 (42.9% vs. 10%; P = 0.042) (20) | |
| SRS2 transcriptomic endotype (DYRK2, CCNB1IP1, TDRD9, ZAP70, ARL14EP, MDC1, ADGRE3) | Agnostic data-derived subtypes | Hydrocortisone | Yes; subgroup/secondary analysis | Increased 28-d mortality; OR, 4.6; 95% CI, 1.5–14.4; P = 0.006 (15) | |
| SENECA delta-type (29 clinical variables within 6 h of arrival) | Agnostic data derived | Aggressive fluid resuscitation | Yes; subgroup/secondary analysis | Simulations indicated possible differential response by subtype in ProCESS, ACCESS trials (9) | |
| Septic shock | “Hyperinflammatory” | Agnostic data derived | Activated protein C | Yes; subgroup/secondary analysis | Differential response by phenotype (31) |
| ARDS | “Hyperinflammatory” | Agnostic data derived | Liberal vs. conservative fluid management | Yes; subgroup/secondary analysis | Differential response to fluids by phenotype (23) |
| “Hyperinflammatory” | Agnostic data derived | Simvastatin | Yes; subgroup/secondary analysis | Improved 28-d survival (24) | |
| “Hyperinflammatory” | Agnostic data derived | High vs. low PEEP | Yes in one study; subgroup/secondary analysis (22)/no in a second study (85) | Differential response to PEEP by phenotype | |
| Low respiratory system compliance, high alveolar dead space fraction | A priori mechanism-based hypothesis | ECCO2R | No | Larger predicted decrease in driving pressure, lower predicted mortality (102) | |
| Focal or nonfocal pattern of lung damage | A priori mechanism-based hypothesis | Personalized (Vt 8 ml/kg, low PEEP for focal, Vt 6 ml/kg, high titrated PEEP for nonfocal ARDS) vs. usual care | Yes; prespecified per-protocol analysis | Decreased 90-d mortality in the personalized group (HR, 0.58; 95% CI, 0.37–0.93; P = 0.024) among correctly classified patients (114) | |
| “Hyperinflammatory” COVID-19 ARDS | Agnostic data derived | Corticosteroids | No | Differential response to corticosteroids by phenotype (115) | |
| High plasma IL-18 | A priori defined on basis of previous studies | Simvastatin | Yes; subgroup/secondary analysis | Decreased 28-d mortality (24.0% vs. 36.8%; P = 0.01) | |
| Acute kidney injury | AKI SP-1 (Ang-1, bicarbonate, platelets, IL-6, IL-8, sTNFR1, Ang-2, RAGE) | Agnostic data derived | Vasopressin (vs. noradrenaline) | Yes; subgroup/secondary analysis | Decreased 90-d mortality (27% vs. 46%; P = 0.02) (36) |
| Sepsis-induced coagulopathy | Cluster A (hyperfibrinolysis, D-dimers, FDP) | Agnostic data derived | rhTM | No | Decreased 28-d mortality (adjusted risk difference, −17.8% (95% CI, −28.7% to −6.9%) (7) |
| Traumatic brain injury | Endotype 2 (multiomic, including clinical, cytokine, endotheliopathy biomarker, lipidome, metabolome, and proteome data) | Agnostic data derived | Prehospital plasma | Yes; subgroup/secondary analysis | Decreased 30-d mortality (HR, 0.31; 0.12–0.81; P = 0.0015) (116) |
Definition of abbreviations: AKI = acute kidney injury; ALT = alanine transaminase; AST = aspartate transaminase; ARDS = acute respiratory distress syndrome; ARR = absolute risk reduction; ALT = alanine aminotransferase; AST = aspartate aminotransferase; Ang-1 or -2 = angiopoetin-1 or -2; CCL3 = C-C motif chemokine ligand 3; CI = confidence interval; DIC = disseminated intravascular coagulation; ECCO2R = extracorporeal CO2 removal; FDP = fibrin degradation product; GZMB = granzyme B; HBD = hepatobiliary dysfunction; HR = hazard ratio; HSPA1B = heat shock protein family A member 1B; MA(L)S = macrophage activation(-like) syndrome; MMP-8 = matrix metalloproteinase 8; OR = odds ratio; PAI-1 = plasminogen activator inhibitor-1; PEEP = positive end-expiratory pressure; PT = prothrombin time; PTT = partial thromboplastin time; RAGE = receptor for advanced glycation end products; rhTM = recombinant human thrombomodulin; RR = risk ratio; sTNFR1 = soluble tumor necrosis factor receptor 1; vWF = von Willebrand factor.
Novel trial designs that incorporate the subphenotype/endotype/treatable trait paradigm may include stratification, umbrella designs, basket trials, and/or adaptive platform trials. Depending on the strength of evidence for a particular trait, targeted therapies can be tested in all participants or only those with a specific trait. Testing in all participants is necessary when there is weak evidence for a differential treatment effect and possible benefit in both groups. In contrast, selective testing may be preferred if, for example, there is potential harm in patients without the trait and unlikely benefit in both groups (69). Umbrella trials evaluate several mechanism-based, targeted therapies in patients with one condition; for example, a sepsis umbrella trial could test therapies targeting coagulopathy in one subset of patients and endothelial dysfunction in another (68). However, the same biological traits can exist across different syndromes, such as sepsis, ARDS, trauma, pancreatitis, and patients at risk for organ dysfunction (30, 74). This overlap suggests that critically ill patients with different clinical syndromes may respond similarly to the same targeted interventions (1). Basket trials can evaluate therapies targeting specific traits across multiple related diseases (e.g., IL-6 inhibition in ARDS and sepsis) (68). Both umbrella and basket trials can be used to test multiple different interventions within a platform design (68, 75, 76). Adaptive platform trials have particular value for precision medicine, allowing evaluation of multiple biomarkers and targeted therapies simultaneously or sequentially while adjusting sample size, randomization strategy, or treatment dose during the trial on the basis of emerging findings (67, 77, 78).
Although preclinical studies will continue to be important for identifying potential treatable traits for prospective validation, biological samples collected during clinical trials, as demonstrated above, have led to the discovery of many subphenotypes. Retrospective analyses leverage the power of randomization, identifying traits that appear to respond differently to therapy. Such “backward translation” can be enormously powerful in understanding HTE for many interventions and in identifying interventions and study populations for future trials (79, 80). Novel machine learning methods such as causal forests may also help uncover previously unrecognized HTE, including in large RCTs with neutral outcomes (81, 82). The Predictive Approaches to Treatment effect Heterogeneity statement provides useful guidance on alternative approaches to identifying HTE, with the goal of providing patient-centered estimates of outcome risks within clinical trials (82). Novel methodologies, including artificial intelligence (AI) methods such as unsupervised learning, are increasingly being used to identify potential enrichment strategies. Recent examples include trajectory-based subphenotypes of sepsis (83), COVID-19–associated ARDS subphentoypes (84), AI-based algorithms to identify previously defined subphenotypes of ARDS (22, 85, 86), and reinforcement learning to identify “optimal” treatment policies in individual patients (87). The challenge remains to implement these methods during trial conduct to optimize the trial design adaptively, thereby maximizing the opportunity to demonstrate benefit in the treatment-responsive subset.
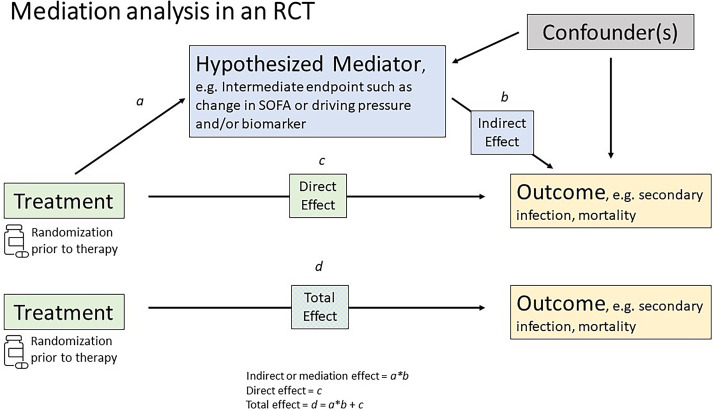
Identification of predictive traits and mechanisms could also provide novel, mechanistically based surrogate endpoints for clinical trials in sepsis and ARDS. Causal inference methods, such as mediation analysis, have been proposed for validating surrogate endpoints (Figure 1). These allow separation of the total treatment effect of an intervention on a (final) endpoint into indirect and direct effects through a mediator (the surrogate) (88). A secondary analysis of an RCT in patients with severe COVID-19 randomized to imatinib versus placebo identified changes in biomarkers of innate immune response and endothelial function that might serve as surrogate endpoints (89). However, such candidate surrogate endpoints will require validation before use in prospective clinical trials. Mediation analyses of trajectory data can also help define the temporal kinetics of a particular phenotype. SRS subphenotypes change over time, with 46% of subjects switching their SRS subphenotype between Days 1, 3, and 7 (90). It remains to be seen if these trajectories correspond to disease progression or are modified by treatment. Resolution of a dysfunctional phenotype, if consistently linked to longer-term outcomes, may be a more feasible outcome to study in early-stage clinical trials than mortality (91).
Figure 1.
Mediation analysis in a randomized controlled trial. In a randomized controlled trial, mediation analysis may be used to validate a surrogate endpoint by separating the total treatment effect of an intervention on an outcome into direct and indirect effects through a mediator. The direct effect (c) is the effect of the treatment on the outcome, independent of the effect of any mediating variable. The indirect, or mediation, effect (b) quantifies whether and how much of the effect of the treatment on the outcome goes through, or is mediated by, the surrogate endpoint. The total effect (d) is the effect of the treatment on the outcome while ignoring, or not adjusting for, the mediator variable. Path a and total effect (a × b + c = d) are assumed to be free of confounding because of randomization. However, path b may contain confounders because both the mediator and outcome are outcomes of randomization (108, 109), which may be important in explaining the mediating pathway (110). Adapted from Reference 111. This figure provides an illustrative and simplified mediation framework. More complex model construction is likely necessary to disentangle complex longitudinal relationships over time (112, 113). SOFA = Sequential Organ Failure Assessment.
Potential Pitfalls to Consider in the Transition to a Precision Phenotype-based Approach
As precision subphenotyping is being incorporated into clinical trials and practice, there are several potential pitfalls. First, more data are needed to prove that identified subphenotypes will have utility in clinical practice. Heterogeneous populations of critically ill patients may be better described or characterized by multiple subgroups or subphenotypes, and these subgroups may be associated with differential outcomes; however, these subphenotypes may not necessarily be useful when making treatment decisions. Useful subphenotypes must be prospectively identifiable, be readily measurable with acceptable operating characteristics, provide actionable information to tailor care, predict treatment responsiveness, and thereby improve patient-important outcomes. Subphenotypes must be consistently reproducible across numerous populations to avoid overinterpreting signals identified in any one dataset. Furthermore, additional studies will be useful to determine how the numerous proposed subphenotyping approaches overlap with or are orthogonal to each other and to provide additional external validation. A recent evaluation demonstrated mixed concordance between different subphenotypes and that combining multiple subphenotypes may be required (92). The majority of currently defined subphenotypes are assigned on the basis of probabilistic models or scores (93) and then assigned to categories. It will be important to optimally define these cutoff thresholds, which may vary between different interventions to optimize the benefit/risk ratio; for instance, a high risk intervention may require a specific threshold to minimize risk.
Second, subphenotypes derived in high-resource settings may not extrapolate to lower-resource settings where patient characteristics, epidemiology of critical illness, and healthcare systems differ markedly. Rather, phenotypes will need to be validated, if not rederived, in settings with differing patient case mix (94). Studies on subphenotyping of critical illness have generally used data from intensive care cohorts from North America or Europe; however, low- and middle-income countries experience the bulk of the global burden of sepsis (95) and other critical illnesses, often with higher mortality rates. Research and therapeutic advances must have global relevance and be responsive to regions of the world with the greatest need.
Third, as alluded to above, subphenotypes that have clinical utility must be readily measurable to allow global evaluation and incorporation into routine practice. Subphenotypes derived from complex and multimodal data may be readily approximated with more pragmatic approaches. For example, subphenotypes of pediatric septic shock can be identified with a limited set of biomarkers (96). Similarly, subphenotypes of ARDS derived using plasma biomarkers can be accurately identified using routine clinical data (86). Physiologic subphenotypes are typically already identifiable using clinical data. Pragmatic and parsimonious approaches to phenotype identification will be important for facilitating implementation.
Fourth, disparities should not be exacerbated. Racial and ethnic minorities are underrepresented in critical care trials and precision medicine trials, as are children and pregnant women (97). Disparities in research participation not only limit access to novel therapies offered in trials but also result in lower-quality evidence and a limited ability to assess racial or ethnic differences in treatment outcomes (98). To ensure international relevance, the use of optimal outcomes that are patient centered is imperative.
Fifth, machine learning or AI models used to identify subphenotypes are sometimes viewed as black boxes because of internal mechanisms that are difficult to understand (99). The lack of transparency and difficulty in explaining such models that determine treatment strategy for patients with critical illness are challenging (100); physicians may have difficulty in informing patients of the rationale for different treatment options and may be uncomfortable making decisions on the basis of these model-derived results (101).
Sixth, precision subphenotyping generally requires reducing the proportion of a population submitted to treatment evaluation in a trial, increasing the total number of patients who need to be screened to enroll in a trial (102). Sample size has traditionally been a key constraint in critical care trials, and incorporating subphenotyping into trials may exacerbate this challenge. Ethical issues concerning equipoise for randomizing patients who have subphenotypes unlikely to benefit from the therapy under study also need to be considered, taking into account issues such as biomarker credentials (69).
Consensus Priorities: How to Move Forward
Despite considerable progress toward understanding heterogeneity in ICU syndromes, several important questions remain (Box 2). First, the terms “phenotypes,” “subtypes,” “subphenotypes,” “subgroups,” and others are often used interchangeably in research and practice. To facilitate effective communication and seamless translation of findings across different platforms, agreement on a standardized approach to terminology is needed; we suggest the approach in Box 1, which is based on previous attempts to standardize that have not been adopted consistently, perhaps in part because true endotypes in critical care have not yet been identified. As more is understood in the field, further changes may be required, but using standardized terms will help ensure that emerging evidence can be better assimilated.
Box 2: Future Research Priorities for Subphenotyping of Critical Illness
-
1.
Optimal and consistent terminology to communicate research findings
-
2.
Greater integration of data sources used for precision phenotyping studies (e.g., clinical, biologic, radiographic, physiologic)
-
3.
Understanding natural history of subphenotypes from onset to resolution
-
4.
Preclinical and translational studies that enhance the understanding of mechanisms underlying the development and evolution of phenotypes
-
5.
Comparative study of phenotypes integrating biospecimens from multiple organ systems and compartments
-
6.
Broader set of indicators of treatment response at short- and long-term time points
Second, multiple efforts in subphenotyping have used methods that compartmentalize into clinical, biological, radiographic, and physiological domains. However, interactions between these domains must be considered. To gain a deeper understanding of proposed subphenotypes, underlying biologic mechanisms, and potential targeted therapies, efforts to harmonize these complementary data domains through multidisciplinary engagement are critically needed.
Third, current proposed subphenotypes are often derived from cross-sectional clinical and biological data with limited knowledge about their temporality. This knowledge gap hampers the understanding of dynamic biological mechanisms through the trajectory of illness and the optimal time windows for the delivery of targeted treatment.
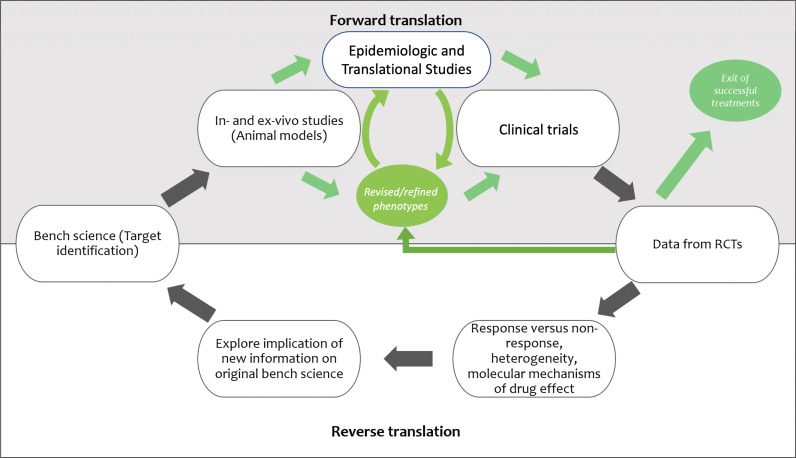
Fourth, further investigation of phenotypes in preclinical models may help to understand the mechanisms underlying observed differences and to inform development of targeted therapies, although exactly how preclinical studies should be designed and conducted to best model specific aspects of sepsis (103), ARDS (104), and related syndromes remains somewhat controversial. Iterative forward and reverse translation will be needed (Figure 2). Epidemiologic and translational studies that delineate pathophysiology in human diseases will play an important part in both refining and revising phenotypes and in establishing how human disease maps to (or differs from) mechanisms and therapy responses seen in animal models (105).
Figure 2.
Proposed paradigm for iterative forward and reverse translation for studies in precision medicine. RCTs = randomized controlled trials.
Fifth, most studies of critical illness subphenotyping use biospecimens from a single compartment: the blood. Greater exploration across organ systems is crucial to enhance understanding of biological mechanisms.
Sixth, although studies in critical care primarily focus on outcomes such as 28- or 90-day mortality, the investigation of more proximate measures of treatment response may be essential for studying subphenotype-targeted therapies in clinical trials to identify differential biological effects. However, any proximate indicators must be predictive of longer-term patient-centered outcomes, which are ultimately needed to change clinical practice.
A Call for Global Collaboration
Moving from the possibility of precision medicine in critical care to its realization will require new models of global research collaboration. The lessons of oncology are instructive. The first international cancer congress was held in Heidelberg in 1906 and now, more than 100 years later, comprises 1,200 member groups from 172 countries. The Union for International Cancer Control has transformed cancer care and research, developing the TNM (tumor, nodes, metastasis) staging system (106) and providing a forum for cancer researchers, health providers, patients, and policy makers to connect as part of a global cancer community.
The challenge in critical care is arguably orders of magnitude greater. Abnormal cell growth in cancer can be directly investigated by interrogating dysregulated tumor tissue. By contrast, the innate inflammatory response that causes organ dysfunction in critical illness not only involves differential expression of thousands of genes (107) but also is critical to survival after infection or injury.
Meeting this challenge will require models of collaboration at a scale unprecedented in critical care that will unfold over a timeline measured in years if not decades. Such a model is emerging among those active in investigator-led acute care research through the formation of national clinical trials groups, international platform trials, and collaborative biologic consortia. Research networks or, more recently, research platforms conduct research outside the confines of a single university, research institute, or commercial sponsor and serve as the primary body conducting research. Collaborative interactions between these networks should address common themes and interests: data sharing, harmonization of research metrics, development of definitions and outcome measures, and communication with stakeholders, including funders, policy makers, and patients and their families. New levels of global collaboration will be required to accrue sufficiently large populations of patients to test phenotype-specific treatment hypotheses in a timely manner. Differences in regulatory and funding mechanisms among different jurisdictions remain a major hurdle to such effective collaboration. Engaging both industry and academia will be important for advancing this vision, although current models of industry-led research support a competitive model of knowledge generation, and the role of individual academic institutions and academic career development is challenged by large-scale collaboration. Finally, the patient perspective must also be considered and incorporated throughout this process, ideally via patient and family/caregiver participation in the design and conduct of subphenotype-driven clinical research and clinical trials.
Footnotes
Author Contributions: A.C.G. and C.S.C. conceived the initial draft. All authors made substantial contributions to further drafting and critical review. All authors approved the final version.
Originally Published in Press as DOI: 10.1164/rccm.202311-2086SO on April 30, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, et al. Redefining critical illness. Nat Med . 2022;28:1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]
- 2.Sinha P, Meyer NJ, Calfee CS. Biological phenotyping in sepsis and acute respiratory distress syndrome. Annu Rev Med. 2023;74:457–471. doi: 10.1146/annurev-med-043021-014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alipanah N, Calfee CS. Phenotyping in acute respiratory distress syndrome: state of the art and clinical implications. Curr Opin Crit Care . 2022;28:1–8. doi: 10.1097/MCC.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med . 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA . 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, et al. SCARLET Trial Group Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA . 2019;321:1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kudo D, Goto T, Uchimido R, Hayakawa M, Yamakawa K, Abe T, et al. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: an analysis of three multicentre observational studies. Crit Care . 2021;25:114. doi: 10.1186/s13054-021-03541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. PROWESS-SHOCK Study Group Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med . 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 9. Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA . 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brant EB, Kennedy JN, King AJ, Gerstley LD, Mishra P, Schlessinger D, et al. Developing a shared sepsis data infrastructure: a systematic review and concept map to FHIR. NPJ Digit Med . 2022;5:44. doi: 10.1038/s41746-022-00580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good Clinical Practice Network. 2023. https://ichgcp.net/clinical-trials-registry/NCT05491941
- 12. Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med . 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med . 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL, et al. MARS consortium Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med . 2017;5:816–826. doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 15. Antcliffe DB, Burnham KL, Al-Beidh F, Santhakumaran S, Brett SJ, Hinds CJ, et al. Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am J Respir Crit Care Med . 2019;199:980–986. doi: 10.1164/rccm.201807-1419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antcliffe DB, Gordon AC. Why understanding sepsis endotypes is important for steroid trials in septic shock. Crit Care Med . 2019;47:1782–1784. doi: 10.1097/CCM.0000000000003833. [DOI] [PubMed] [Google Scholar]
- 17. Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med . 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 18. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. UPenn COVID Processing Unit Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science . 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med . 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 20. Leventogiannis K, Kyriazopoulou E, Antonakos N, Kotsaki A, Tsangaris I, Markopoulou D, et al. Toward personalized immunotherapy in sepsis: the PROVIDE randomized clinical trial. Cell Rep Med . 2022;3:100817. doi: 10.1016/j.xcrm.2022.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotsaki A, Pickkers P, Bauer M, Calandra T, Lupse M, Wiersinga WJ, et al. ImmunoSep (Personalised Immunotherapy in Sepsis) international double-blind, double-dummy, placebo-controlled randomised clinical trial: study protocol. BMJ Open . 2022;12:e067251. doi: 10.1136/bmjopen-2022-067251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. ARDS Network Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med . 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Irish Critical Care Trials Group Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med . 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, NHLBI ARDS Network Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med . 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinha P, Delucchi KL, Chen Y, Zhuo H, Abbott J, Wang C, et al. Latent class analysis-derived subphenotypes are generalisable to observational cohorts of acute respiratory distress syndrome: a prospective study. Thorax . 2022;77:13–21. doi: 10.1136/thoraxjnl-2021-217158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinha P, Delucchi KL, McAuley DF, O’Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med . 2020;8:247–257. doi: 10.1016/S2213-2600(19)30369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, et al. MARS consortium Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax . 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alipanah-Lechner N, Neyton L, Mick E, Willmore A, Leligdowicz A, Contrepois K, et al. Plasma metabolic profiling implicates dysregulated lipid metabolism and glycolytic shift in hyperinflammatory ARDS. Am J Physiol Lung Cell Mol Physiol . 2023;324:L297–L306. doi: 10.1152/ajplung.00278.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heijnen NFL, Hagens LA, Smit MR, Cremer OL, Ong DSY, van der Poll T, et al. Biological subphenotypes of acute respiratory distress syndrome show prognostic enrichment in mechanically ventilated patients without acute respiratory distress syndrome. Am J Respir Crit Care Med . 2021;203:1503–1511. doi: 10.1164/rccm.202006-2522OC. [DOI] [PubMed] [Google Scholar]
- 31. Sinha P, Kerchberger VE, Willmore A, Chambers J, Zhuo H, Abbott J, et al. Identifying molecular phenotypes in sepsis: an analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir Med . 2023;11:965–974. doi: 10.1016/S2213-2600(23)00237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostermann M, Basu RK, Mehta RL. Acute kidney injury. Intensive Care Med . 2023;49:219–222. doi: 10.1007/s00134-022-06946-0. [DOI] [PubMed] [Google Scholar]
- 33. Stanski NL, Rodrigues CE, Strader M, Murray PT, Endre ZH, Bagshaw SM. Precision management of acute kidney injury in the intensive care unit: current state of the art. Intensive Care Med . 2023;49:1049–1061. doi: 10.1007/s00134-023-07171-z. [DOI] [PubMed] [Google Scholar]
- 34. Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative Consensus Conference: a consensus statement. JAMA Netw Open . 2020;3:e2019209. doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 35. Wiersema R, Jukarainen S, Vaara ST, Poukkanen M, Lakkisto P, Wong H, et al. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit Care . 2020;24:150. doi: 10.1186/s13054-020-02866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhatraju PK, Zelnick LR, Herting J, Katz R, Mikacenic C, Kosamo S, et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med . 2019;199:863–872. doi: 10.1164/rccm.201807-1346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, et al. Effect of lowering Vt on mortality in acute respiratory distress syndrome varies with respiratory system elastance. Am J Respir Crit Care Med . 2021;203:1378–1385. doi: 10.1164/rccm.202009-3536OC. [DOI] [PubMed] [Google Scholar]
- 38. Dianti J, McNamee JJ, Slutsky AS, Fan E, Ferguson ND, McAuley DF, et al. Determinants of effect of extracorporeal CO2 removal in hypoxemic respiratory failure. NEJM Evid . 2023;2:a2200295. doi: 10.1056/EVIDoa2200295. [DOI] [PubMed] [Google Scholar]
- 39. Dianti J, Angriman F, Ferreyro BL, Sklar MC, Brochard L, Ferguson ND, et al. Association of mortality with neuromuscular blockade differs according to baseline diaphragm thickness. Am J Respir Crit Care Med . 2020;202:1717–1720. doi: 10.1164/rccm.202004-1157LE. [DOI] [PubMed] [Google Scholar]
- 40. Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intensive Care Med . 2019;45:1783–1794. doi: 10.1007/s00134-019-05805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hutchinson PJ, Adams H, Mohan M, Devi BI, Uff C, Hasan S, et al. British Neurosurgical Trainee Research Collaborative, NIHR Global Health Research Group on Acquired Brain and Spine Injury, and RESCUE-ASDH Trial Collaborators; RESCUE-ASDH Trial Collaborators Decompressive craniectomy versus craniotomy for acute subdural hematoma. N Engl J Med . 2023;388:2219–2229. doi: 10.1056/NEJMoa2214172. [DOI] [PubMed] [Google Scholar]
- 42. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med . 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 43. Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intensive Care Med . 2020;46:919–929. doi: 10.1007/s00134-019-05900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Launey Y, Fryer TD, Hong YT, Steiner LA, Nortje J, Veenith TV, et al. Spatial and temporal pattern of ischemia and abnormal vascular function following traumatic brain injury. JAMA Neurol . 2020;77:339–349. doi: 10.1001/jamaneurol.2019.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veenith TV, Carter EL, Geeraerts T, Grossac J, Newcombe VF, Outtrim J, et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury. JAMA Neurol . 2016;73:542–550. doi: 10.1001/jamaneurol.2016.0091. [DOI] [PubMed] [Google Scholar]
- 46. Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab . 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med . 2017;45:1907–1914. doi: 10.1097/CCM.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beqiri E, Smielewski P, Robba C, Czosnyka M, Cabeleira MT, Tas J, et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open . 2019;9:e030727. doi: 10.1136/bmjopen-2019-030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open . 2022;12:e060188. doi: 10.1136/bmjopen-2021-060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veldeman M, Albanna W, Weiss M, Park S, Hoellig A, Clusmann H, et al. Invasive multimodal neuromonitoring in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke . 2021;52:3624–3632. doi: 10.1161/STROKEAHA.121.034633. [DOI] [PubMed] [Google Scholar]
- 51. Sekhon MS, Ainslie PN, Menon DK, Thiara SS, Cardim D, Gupta AK, et al. Brain hypoxia secondary to diffusion limitation in hypoxic ischemic brain injury postcardiac arrest. Crit Care Med . 2020;48:378–384. doi: 10.1097/CCM.0000000000004138. [DOI] [PubMed] [Google Scholar]
- 52. Kals M, Kunzmann K, Parodi L, Radmanesh F, Wilson L, Izzy S, et al. Genetic Associations In Neurotrauma (GAIN) Consortium (with contribution from the CENTER-TBI, TRACK-TBI, CABI, MGB, and TBIcare studies) A genome-wide association study of outcome from traumatic brain injury. EBioMedicine . 2022;77:103933. doi: 10.1016/j.ebiom.2022.103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sathe NA, Morrell ED, Bhatraju PK, Fessler MB, Stapleton RD, Wurfel MM, et al. Alveolar biomarker profiles in subphenotypes of the acute respiratory distress syndrome. Crit Care Med . 2023;51:e13–e18. doi: 10.1097/CCM.0000000000005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heijnen NFL, Hagens LA, Smit MR, Schultz MJ, van der Poll T, Schnabel RM, et al. BASIC consortium Biological subphenotypes of acute respiratory distress syndrome may not reflect differences in alveolar inflammation. Physiol Rep . 2021;9:e14693. doi: 10.14814/phy2.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fiore-Gartland A, Panoskaltsis-Mortari A, Agan AA, Mistry AJ, Thomas PG, Matthay MA, et al. PALISI PICFlu Investigators Cytokine profiles of severe influenza virus-related complications in children. Front Immunol . 2017;8:1423. doi: 10.3389/fimmu.2017.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol . 2022;23:177–185. doi: 10.1038/s41590-021-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Randolph AG, McCulloh RJ. Pediatric sepsis: important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence . 2014;5:179–189. doi: 10.4161/viru.27045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong HR, Sweeney TE, Hart KW, Khatri P, Lindsell CJ. Pediatric sepsis endotypes among adults with sepsis. Crit Care Med . 2017;45:e1289–e1291. doi: 10.1097/CCM.0000000000002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dahmer MK, Yang G, Zhang M, Quasney MW, Sapru A, Weeks HM, et al. RESTORE and BALI study investigators; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med . 2022;10:289–297. doi: 10.1016/S2213-2600(21)00382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Flori HR, Zhang M, Xie J, Yang G, Sapru A, Calfee CS, et al. Subphenotypes assigned to pediatric acute respiratory failure patients show differing outcomes. Am J Respir Crit Care Med . 2023;208:331–333. doi: 10.1164/rccm.202301-0070LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yehya N, Zinter MS, Thompson JM, Lim MJ, Hanudel MR, Alkhouli MF, et al. Identification of molecular subphenotypes in two cohorts of paediatric ARDS. Thorax . 2024;79:128–134. doi: 10.1136/thorax-2023-220130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zinter MS, Markovic D, Asaro LA, Nadkarni VM, McQuillen PS, Sinha P, et al. CAF-PINT Investigators of the PALISI Network Tight glycemic control, inflammation, and the ICU: evidence for heterogeneous treatment effects in two randomized controlled trials. Am J Respir Crit Care Med . 2023;207:945–949. doi: 10.1164/rccm.202210-1988LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med . 2020;8:1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.EIT Health. 2023. https://eithealth.eu/product-service/impacct/
- 65.Collaborative Pediatric Critical Care Research Network. 2023. https://www.cpccrn.org/precise-study/
- 66.European Respiratory Society. 2023. https://www.ersnet.org/science-and-research/clinical-research-collaboration-application-programme/panther-precision-medicine-adaptive-network-platform-trial-in-hypoxemic-acute-respiratory-failure/
- 67. Beitler JR, Thompson BT, Baron RM, Bastarache JA, Denlinger LC, Esserman L, et al. Advancing precision medicine for acute respiratory distress syndrome. Lancet Respir Med . 2022;10:107–120. doi: 10.1016/S2213-2600(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med . 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 69. Freidlin B, Korn EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol . 2014;11:81–90. doi: 10.1038/nrclinonc.2013.218. [DOI] [PubMed] [Google Scholar]
- 70. Wolf DM, Yau C, Wulfkuhle J, Brown-Swigart L, Gallagher RI, Lee PRE, et al. I-SPY2 Investigators Redefining breast cancer subtypes to guide treatment prioritization and maximize response: Predictive biomarkers across 10 cancer therapies. Cancer Cell . 2022;40:609–623.e6. doi: 10.1016/j.ccell.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J . 2021;59:2102730. doi: 10.1183/13993003.02730-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Panelists of the St Gallen Consensus Conference Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol . 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med . 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med . 2019;47:1724–1734. doi: 10.1097/CCM.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park JJH, Detry MA, Murthy S, Guyatt G, Mills EJ. How to use and interpret the results of a platform trial: users’ guide to the medical literature. JAMA . 2022;327:67–74. doi: 10.1001/jama.2021.22507. [DOI] [PubMed] [Google Scholar]
- 76. Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol . 2020;16:20–31. doi: 10.1038/s41581-019-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, van Bentum-Puijk W, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc . 2020;17:879–891. doi: 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, et al. ACTIV-4B Investigators Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA . 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wong HR. Pediatric sepsis biomarkers for prognostic and predictive enrichment. Pediatr Res . 2022;91:283–288. doi: 10.1038/s41390-021-01620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Odum JD, Standage S, Alder M, Zingarelli B, Devarajan P, Wong HR. Candidate biomarkers for sepsis-associated acute kidney injury mechanistic studies. Shock . 2022;57:687–693. doi: 10.1097/SHK.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goligher EC, Lawler PR, Jensen TP, Talisa V, Berry LR, Lorenzi E, et al. REMAP-CAP, ATTACC, and ACTIV-4a Investigators Heterogeneous treatment effects of therapeutic-dose heparin in patients hospitalized for COVID-19. JAMA . 2023;329:1066–1077. doi: 10.1001/jama.2023.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kent DM, Paulus JK, van Klaveren D, D’Agostino R, Goodman S, Hayward R, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med . 2020;172:35–45. doi: 10.7326/M18-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu Z, Mao C, Su C, Zhang H, Siempos I, Torres LK, et al. Sepsis subphenotyping based on organ dysfunction trajectory. Crit Care . 2022;26:197. doi: 10.1186/s13054-022-04071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bos LDJ, Sjoding M, Sinha P, Bhavani SV, Lyons PG, Bewley AF, et al. PRoVENT-COVID collaborative group Longitudinal respiratory subphenotypes in patients with COVID-19-related acute respiratory distress syndrome: results from three observational cohorts. Lancet Respir Med . 2021;9:1377–1386. doi: 10.1016/S2213-2600(21)00365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Maddali MV, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, et al. LUNG SAFE Investigators and the ESICM Trials Group Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med . 2022;10:367–377. doi: 10.1016/S2213-2600(21)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sinha P, Churpek MM, Calfee CS. Machine learning classifier models can identify acute respiratory distress syndrome phenotypes using readily available clinical data. Am J Respir Crit Care Med . 2020;202:996–1004. doi: 10.1164/rccm.202002-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med . 2018;24:1716–1720. doi: 10.1038/s41591-018-0213-5. [DOI] [PubMed] [Google Scholar]
- 88. Le Coënt Q, Legrand C, Rondeau V. Time-to-event surrogate endpoint validation using mediation analysis and meta-analytic data. Biostatistics . 2023;25:98–116. doi: 10.1093/biostatistics/kxac044. [DOI] [PubMed] [Google Scholar]
- 89. de Brabander J, Duijvelaar E, Schippers JR, Smeele PJ, Peters-Sengers H, Duitman JW, et al. CounterCOVID Study Group Immunomodulation and endothelial barrier protection mediate the association between oral imatinib and mortality in hospitalised COVID-19 patients. Eur Respir J . 2022;60:2200780. doi: 10.1183/13993003.00780-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burnham KL, Davenport EE, Radhakrishnan J, Humburg P, Gordon AC, Hutton P, et al. Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am J Respir Crit Care Med . 2017;196:328–339. doi: 10.1164/rccm.201608-1685OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mayr VD, Dünser MW, Greil V, Jochberger S, Luckner G, Ulmer H, et al. Causes of death and determinants of outcome in critically ill patients. Crit Care . 2006;10:R154. doi: 10.1186/cc5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Amstel RBE, Kennedy JN, Scicluna BP, Bos LDJ, Peters-Sengers H, Butler JM, et al. MARS Consortium Uncovering heterogeneity in sepsis: a comparative analysis of subphenotypes. Intensive Care Med . 2023;49:1360–1369. doi: 10.1007/s00134-023-07239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cano-Gamez E, Burnham KL, Goh C, Allcock A, Malick ZH, Overend L, et al. GAinS Investigators An immune dysfunction score for stratification of patients with acute infection based on whole-blood gene expression. Sci Transl Med . 2022;14:eabq4433. doi: 10.1126/scitranslmed.abq4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cummings MJ, Bakamutumaho B, Tomoiaga AS, Owor N, Jain K, Price A, et al. A transcriptomic classifier model identifies high-risk endotypes in a prospective study of sepsis in Uganda. Crit Care Med . 2024;52:475–482. doi: 10.1097/CCM.0000000000006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet . 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wong HR, Sweeney TE, Lindsell CJ. Simplification of a septic shock endotyping strategy for clinical application. Am J Respir Crit Care Med . 2017;195:263–265. doi: 10.1164/rccm.201607-1535LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open . 2021;4:e2133205. doi: 10.1001/jamanetworkopen.2021.33205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reeves A, Elliott MR, Lewis TT, Karvonen-Gutierrez CA, Herman WH, Harlow SD. Study selection bias and racial or ethnic disparities in estimated age at onset of cardiometabolic disease among midlife women in the US. JAMA Netw Open . 2022;5:e2240665. doi: 10.1001/jamanetworkopen.2022.40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cadario R, Longoni C, Morewedge CK. Understanding, explaining, and utilizing medical artificial intelligence. Nat Hum Behav . 2021;5:1636–1642. doi: 10.1038/s41562-021-01146-0. [DOI] [PubMed] [Google Scholar]
- 100. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med . 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Song X, Yu ASL, Kellum JA, Waitman LR, Matheny ME, Simpson SQ, et al. Cross-site transportability of an explainable artificial intelligence model for acute kidney injury prediction. Nat Commun . 2020;11:5668. doi: 10.1038/s41467-020-19551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goligher EC, Combes A, Brodie D, Ferguson ND, Pesenti AM, Ranieri VM, et al. SUPERNOVA investigators (European Society of Intensive Care Medicine trials group) and for the International ECMO Network (ECMONet) Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med . 2019;45:1219–1230. doi: 10.1007/s00134-019-05708-9. [DOI] [PubMed] [Google Scholar]
- 103. Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Infection . 2018;46:687–691. doi: 10.1007/s15010-018-1183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kulkarni HS, Lee JS, Bastarache JA, Kuebler WM, Downey GP, Albaiceta GM, et al. Update on the features and measurements of experimental acute lung injury in animals: an official American Thoracic Society workshop report. Am J Respir Cell Mol Biol . 2022;66:e1–e14. doi: 10.1165/rcmb.2021-0531ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med . 2010;2:27rv1. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- 106. Gospodarowicz M, Benedet L, Hutter RV, Fleming I, Henson DE, Sobin LH. History and international developments in cancer staging. Cancer Prev Control . 1998;2:262–268. [PubMed] [Google Scholar]
- 107. Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. Inflamm and Host Response to Injury Large Scale Collaborative Research Program A network-based analysis of systemic inflammation in humans. Nature . 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 108. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods . 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Imai K, Jo B, Stuart EA. Using potential outcomes to understand causal mediation analysis: comment on. Multivariate Behav Res . 2011;46:861–873. doi: 10.1080/00273171.2011.606743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Whittle R, Mansell G, Jellema P, van der Windt D. Applying causal mediation methods to clinical trial data: What can we learn about why our interventions (don’t) work? Eur J Pain . 2017;21:614–622. doi: 10.1002/ejp.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mascha EJ, Dalton JE, Kurz A, Saager L. Statistical grand rounds: understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg . 2013;117:980–994. doi: 10.1213/ANE.0b013e3182a44cb9. [DOI] [PubMed] [Google Scholar]
- 112. Nguyen TQ, Schmid I, Stuart EA. Clarifying causal mediation analysis for the applied researcher: defining effects based on what we want to learn. Psychol Methods . 2021;26:255–271. doi: 10.1037/met0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lynch KG, Cary M, Gallop R, Ten Have TR. Causal mediation analyses for randomized trials. Health Serv Outcomes Res Methodol . 2008;8:57–76. doi: 10.1007/s10742-008-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. AZUREA Network Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med . 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 115. Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med . 2021;204:1274–1285. doi: 10.1164/rccm.202105-1302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wu J, Vodovotz Y, Abdelhamid S, Guyette FX, Yaffe MB, Gruen DS, et al. PAMPer study group Multi-omic analysis in injured humans: patterns align with outcomes and treatment responses. Cell Rep Med . 2021;2:100478. doi: 10.1016/j.xcrm.2021.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]