Abstract
We investigated the reduction in regional brain volume and cerebral blood flow (CBF) with aging and explored potential sex differences in healthy brains. Three-dimensional (3D) T1-weighted magnetic resonance imaging (MRI), time-of-flight magnetic resonance angiography, and four-dimensional (4D) flow MRI were performed on 129 healthy volunteers aged 22–92 years. The brains of healthy volunteers were segmented into 21 subregions using 3D T1-weighted MRI and CBFs in 16 major intracranial arteries were measured using 4D flow MRI. The cortical gray matter volume decreased linearly with aging, whereas the cerebral white matter volume increased until the 40s and then decreased, and the subcortical gray matter volume changed little with aging. The cortical gray matter volume was significantly associated with the total CBF of the major intracranial arteries distal to the circle of Willis; however, the cerebral white matter and subcortical gray matter volumes were not. Generally, women have higher total CBF than men, particularly in their 40s and younger, despite the smaller intracranial volume and smaller diameters of intracranial arteries than men. This may contribute to the higher incidence of subarachnoid hemorrhage due to cerebral aneurysms and migraine in women.
Keywords: Sex difference, aging, regional brain volume, cerebral blood flow, circle of Willis
Why is cerebral blood flow elevated in young women?
Brain volume and cerebral blood flow (CBF) gradually decrease with normal aging. We aimed to simulate the natural aging process of the human brain by constructing the intracranial environment of human cerebral blood circulation and cerebrospinal fluid movement and to elucidate pathological conditions related to the brain environment, such as stroke, dementia, and neurodegenerative diseases. As part of this project, we published two papers in 2023.1,2 The first paper is a study on age-related volume changes in the brain subregions and cerebrospinal fluid volumes of healthy volunteers aged 20s to 90s using an automatic brain region segmentation application. 1 From the three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient-echo sequence, the brain was automatically segmented into 21 subregions using artificial intelligence; however, they were reintegrated into the cortical gray matter, subcortical gray matter, and cerebral white matter. For example, the frontal, temporal, parietal, and occipital cortices were combined as the cortical gray matter, and the hippocampus, basal ganglia, and brainstem were combined as the subcortical gray matter. The first important common knowledge is that women have a significantly smaller intracranial cavity than men, which does not change after adulthood. Therefore, we used the volume ratio; that of the cortical gray matter linearly decreased with aging; however, that of the cerebral white matter increased in those in their 40s, and that of the subcortical gray matter was less affected by aging. No sex differences in age-related changes in the volume ratios of the three subregions were observed.
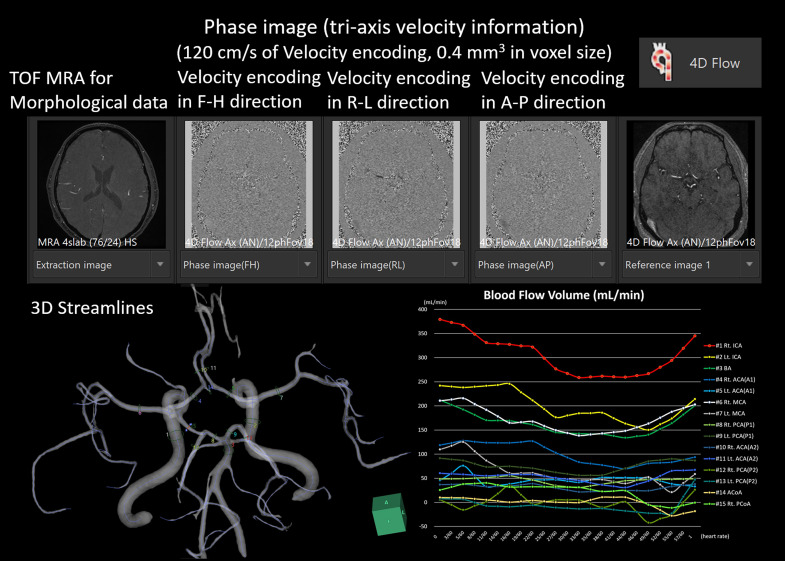
The second paper focused on age and sex differences in the relationship between segmented brain volume and CBF of intracranial arteries measured using four-dimensional (4D) flow magnetic resonance imaging (MRI). 2 From the 4D flow MRI, time-resolved velocity data were obtained with 120 cm/s velocity encoding in three dimensions while synchronizing the peripheral pulse rate (Figure 1).
Figure 1.
Four-dimensional (4D) flow analyses were conducted using the 4D flow application in an independent three-dimensional (3D) volume analyzer workstation (SYNAPSE 3D; FUJIFILM Corporation, Tokyo, Japan).
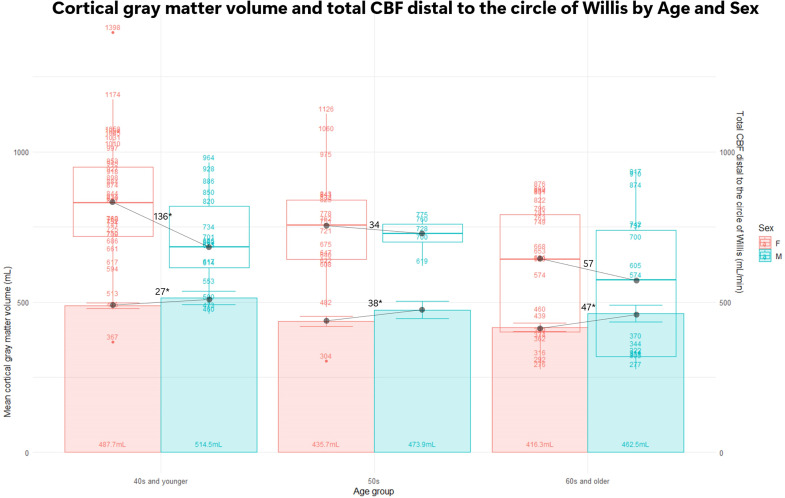
On the proximal and distal sides of the circle of Willis, 3D-flow velocities of the bilateral internal carotid arteries (ICAs), anterior, middle, and posterior cerebral arteries (ACA, MCA, and PCA), and basilar artery (BA) were measured in a cross-section perpendicular to the centerlines of the arteries. The strongest association was observed between cortical gray matter volume and total CBF in the ACA, MCA, and PCA distal to the circle of Willis (r = 0.425), and a moderate association was observed between cortical gray matter volume and bilateral ICAs and BA proximal to the circle of Willis (r = 0.326). The cerebral white matter volume had a weaker association with total CBF than the cortical gray matter volume, and subcortical gray matter was not significantly associated with any total CBF. Furthermore, healthy women had significantly higher mean total CBF than healthy men. As shown in Figure 2, sex differences in total CBF were more prominent in younger age groups, although there was greater variability. The cortical gray matter volume in the 40s and younger was significantly larger in men (mean ± standard deviation: 515 ± 51 mL) than in women (488 ± 31 mL) by an average of 27 mL (p = 0.036), whereas the total CBF distal to the circle of Willis was significantly larger in women (831 ± 204 mL/min) than in men (695 ± 154 mL/min) by an average of 136 mL/min (0.011). However, the sex difference in the cortical gray matter volume increased to 38 mL (0.029) in the 50s and 47 mL (0.005) in the 60s and older, whereas the sex difference in total CBF distal to the circle of Willis was 34 mL/min (0.59) in the 50s and 57 mL/min (0.44) in the 60s and older; no significant differences were observed in either group.
Figure 2.
Bars show cortical gray matter volume (mL) with the left axis, and mean values are noted within the bars. Box plots show total cerebral blood flow (CBF) distal to the circle of Willis (mL/min) with the right axis, and the values of the total CBF are plotted. Females (F) are shown in pink and males (M) are shown in sky blue. * Indicates statistical difference (p < 0.05).
A significantly higher CBF in healthy young women than in age-matched men was first reported in 1984 in a study using 133Xe gas-inhalation positron emission tomography. 3 Regional CBFs at the capillary level, as measured by nuclear medicine studies, are fundamentally different from the flow in the artery, as measured using 4D flow MRI.
The mean diameters of the ICA and MCA were reported to be significantly larger in men than in women. 4 However, accurately measuring the diameter of arteries is difficult using the time-of-flight magnetic resonance angiography used in our study because the flow velocity affects the strength of the signal. Furthermore, normal cardiac output has been reported to be significantly higher in women than in men. 5 Approximately 10% of cardiac output demands CBF, although the reason for the sex difference in the total CBF and cardiac output has not been clarified. Based on our findings and previous reports, CBF may be more influenced by peripheral vascular resistance in the brain than by the diameters of the major intracranial arteries that comprise the circle of Willis. Regarding why CBF is higher in young women than in men, based on the lack of sex differences in normal brain activity, we hypothesized that the smaller volume of cerebral gray matter may require higher CBF in younger women than in men. Another hypothesis is that women have lower hematocrit levels than men. Lower hematocrit levels increase cerebral oxygen consumption, increase the partial pressure of carbon dioxide, dilate peripheral vessels, and increase cerebral circulatory blood flow. However, there is no evidence that healthy women are more prone to future ischemic stroke or cerebral infarction than men. The sex differences in brain volume and CBF may have implications for understanding the risks of several cerebrovascular diseases, such as cerebral aneurysms, 6 subarachnoid hemorrhage,7,8 and migraine.9,10 Compared with men, women are known to have twice the frequency of cerebral aneurysms and subarachnoid hemorrhage caused by their rupture and three times the frequency of migraine; however, the pathogenesis has not been clarified. The family history of cerebral aneurysms is autosomal dominant,11,12 and many genes associated with cerebral aneurysms have been reported.13–15 However, genetic factors have not been reported as a reason for the higher frequency in women. Similarly, why migraine is more common in women has not been fully proven, although it has been reported to be associated with sex hormones, genetic polymorphisms or mutations, stress, and neuronal activity. 10 It is easy to speculate that if the blood flow volume is higher in the same arterial diameter, the pressure and shear stress on the arterial walls will naturally be greater, making them more susceptible to artery-associated diseases.
The limitations of this study include the cross-sectional design, potential biases in the volunteer sample, and the need for further validation of the CBF measurements.
Conclusions
The mean total CBF was greater in healthy women than in healthy men, particularly in their 40s and younger, despite the smaller intracranial volume and smaller diameters of intracranial arteries than men. This fact sheds light on the intricate relationships among aging, sex differences, brain volume, and CBF, providing insights into potential factors contributing to sex-specific neurological conditions and diseases. Future comprehensive research must explore diseases related to brain subregions and CBF in relation to aging and sex.
Acknowledgements
We thank the radiology staff of the Shiga University of Medical Science, particularly Masahiro Yoshimura, Shinnosuke Hiratsuka, Asuka Nishihara, Kouhei Ohashi, and Mika Adachi. We thank Enago (www.enago.jp) for the English language review.
Footnotes
Author contributions: S.Y. contributed to conceptualization, methodology, data curation, formal analysis, investigation, resources, writing—original draft, visualization, project administration, and funding acquisition. H.K. contributed to data curation and investigation. T.O., S.I., C.I., M.T., K.Y., Y.W., S.W., M.O., and M.M. contributed to supervision. H.I. and K.O. contributed to software development.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by research grants from the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) for 3 years, beginning in 2021 (Grant no. 21K09098); JSPS Grant-in-Aid for Scientific Research (B) for 4 years, beginning in 2022 (Grant no. 22H03020); and JSPS Grant-in-Aid for Scientific Research (A) for 4 years, beginning in 2022 (Grant no. 22H00190); JSPS Grant-in-Aid for Scientific Research (B) for 4 years, beginning in 2024 (Grant no. 24K02557); from the Ministry of Education, Culture, Sports, Science and Technology as “Program for Promoting Researches on the Supercomputer Fugaku” (Development of human digital twins for cerebral circulation using Fugaku, JPMXP1020230118) for 3 years, beginning in 2023; and from the FUJIFILM Corporation for 6 years, beginning in 2019. The funders had no effect or involvement in the writing of this article. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Shigeki Yamada https://orcid.org/0000-0001-7158-5569
References
- 1.Yamada S, Otani T, Ii S, et al. Aging-related volume changes in the brain and cerebrospinal fluid using artificial intelligence-automated segmentation. Eur Radiol 2023; 33: 7099–7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawano H, Yamada S, Watanabe Y, et al. Aging and sex differences in brain volume and cerebral blood flow. Aging Dis 2023. doi: 10.14336/AD.2023.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw TG, Mortel KF, Meyer JS, et al. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 1984; 34: 855–862. [DOI] [PubMed] [Google Scholar]
- 4.Davison MA, Ouyang B, Keppetipola KM, et al. Arterial diameter and the gender disparity in stroke thrombectomy outcomes. J Neurointerv Surg 2018; 10: 949–952. [DOI] [PubMed] [Google Scholar]
- 5.Xing CY, Tarumi T, Liu J, et al. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 2017; 37: 2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordan E, Lanzino G, Rangel-Castilla L, et al. Risk of de novo aneurysm formation in patients with a prior diagnosis of ruptured or unruptured aneurysm: Systematic review and meta-analysis. J Neurosurg 2018; 131: 14–24. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes AM, Stone McGuire L, Amin-Hanjani S. Sex differences in cerebral aneurysms and subarachnoid hemorrhage. Stroke 2022; 53: 624–633. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Koizumi A, Iso H, et al. Risk factors for fatal subarachnoid hemorrhage: The Japan collaborative cohort study. Stroke 2003; 34: 2781–2787. [DOI] [PubMed] [Google Scholar]
- 9.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017; 16: 76–87. [DOI] [PubMed] [Google Scholar]
- 10.Allais G, Chiarle G, Sinigaglia S, et al. Gender-related differences in migraine. Neurol Sci 2020; 41: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada S, Utsunomiya M, Inoue K, et al. Genome-wide scan for Japanese familial intracranial aneurysms: Linkage to several chromosomal regions. Circulation 2004; 110: 3727–3733. [DOI] [PubMed] [Google Scholar]
- 12.Bakker MK, van der Spek RAA, van Rheenen W, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet 2020; 52: 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Mineharu Y, Inoue S, et al. Search on chromosome 17 centromere reveals TNFRSF13B as a susceptibility gene for intracranial aneurysm: A preliminary study. Circulation 2006; 113: 2002–2010. [DOI] [PubMed] [Google Scholar]
- 14.Bakker MK, Ruigrok YM. Genetics of intracranial aneurysms. Stroke 2021; 52: 3004–3012. [DOI] [PubMed] [Google Scholar]
- 15.Mineharu Y, Inoue K, Inoue S, et al. Association analysis of common variants of ELN, NOS2A, APOE and ACE2 to intracranial aneurysm. Stroke 2006; 37: 1189–1194. [DOI] [PubMed] [Google Scholar]