Abstract
Introduction
There is good evidence that specific autoimmune rheumatic diseases (RDs), for example, rheumatoid arthritis and systemic lupus erythematosus (SLE), are associated with a state of hypercoagulability and an increased risk of venous thromboembolism (VTE). However, limited information regarding this association is available for other autoimmune or autoinflammatory RDs. We sought to address this issue by conducting a systematic review and meta‐analysis of the association between the d‐dimer, an established marker of hypercoagulability and VTE, and RDs and the possible clinical and demographic factors mediating this association.
Methods
We searched the electronic databases PubMed, Web of Science, and Scopus from inception to January 31, 2024. The risk of bias and the certainty of evidence were assessed using the Joanna Briggs Institute Critical Appraisal Checklist and GRADE, respectively.
Results
In 31 studies selected for analysis (2724 RD patients and 3437 healthy controls), RD patients had overall significantly higher d‐dimer concentrations when compared to controls (standard mean difference = 0.93, 95% CI 0.76−1.10, p < .001; I 2 = 86.1%, p < .001; moderate certainty of evidence). The results were stable in a sensitivity analysis. Significant associations were observed between the effect size of the between‐group differences in d‐dimer concentration and age, specific RD and RD category, RD duration, fibrinogen, plasminogen activator inhibitor, C‐reactive protein, and erythrocyte sedimentation rate.
Conclusions
Overall, patients with RDs have significantly higher d‐dimer concentrations when compared with healthy controls, indicating a state of hypercoagulability. The alterations in d‐dimer concentrations are mediated by age, specific RD and RD category, RD duration, and markers of anticoagulation and inflammation. Further research is warranted to investigate d‐dimer concentrations across the spectrum of RDs and their utility in predicting and managing VTE in these patients (PROSPERO registration number: CRD42024517712).
Keywords: autoimmunity, d‐dimer, disease activity, hypercoagulability, inflammation, rheumatic diseases, venous thromboembolism
Our systematic review and meta‐analysis have shown that, overall, patients with rheumatic diseases (RDs) have significantly higher d‐dimer concentrations when compared with healthy controls, indicating a state of hypercoagulability. The alterations in d‐dimer concentrations are mediated by age, specific RD and RD category, RD duration, and markers of anticoagulation and inflammation.
1. INTRODUCTION
Rheumatic diseases (RDs) encompass a wide range of chronic conditions with a predominantly autoimmune (e.g., rheumatoid arthritis, RA), a mixed‐autoimmune‐autoinflammatory (e.g., ankylosing spondylitis), or an autoinflammatory component (e.g., familial Mediterranean fever). 1 , 2 , 3 Regardless of this broad categorization, individual RDs are generally characterized by disabling symptoms, significant complications, and overall poor quality of life despite the availability of safe and effective pharmacological and nonpharmacological treatment strategies. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 One important factor contributing to the health burden of RDs on patients and healthcare systems is represented by a state of hypercoagulability with a predisposition to venous thromboembolism (VTE). 17 , 18 This issue has been well studied in specific RDs, for example, RA (increased risk of VTE by a factor of 2−2.5 vs. general population), 19 , 20 , 21 systemic lupus erythematosus (SLE, increased risk of VTE by a factor of 4.38 vs. general population), 22 , 23 systemic sclerosis (SSc) (increased risk of VTE by a factor of 2.5 vs. general population), 24 ANCA‐associated vasculitis (AAV) (increased risk of VTE by a factor of 3.26 vs. general population), 25 , 26 , 27 , 28 , 29 , 30 , 31 CA and gout (increased risk of VTE by a factor of 1.33 vs. general population), 32 osteoarthritis (OA) (increased risk of VTE by a factor of 1.38 vs. general population), 33 and Behcet disease (BD) (increased risk of VTE by a factor of 2.80 vs. general population). 34 , 35 , 36 , 37
It is commonly accepted that the proinflammatory and pro‐oxidant state in patients with RA and SLE favors the upregulation of procoagulant pathways and the downregulation of anticoagulant and fibrinolytic pathways. 18 , 38 , 39 , 40 In support of this theory, several epidemiological and experimental studies have reported a higher tendency to coagulation and VTE in RA and SLE patients with active disease versus those in remission. 41 , 42 , 43 However, the mechanisms underpinning the complex interplay between inflammation, oxidative stress, coagulation, and thrombosis have been less studied in other RDs, particularly those with a mixed‐autoimmune‐autoinflammatory or autoinflammatory component.
The d‐dimer is one of the main degradation products of fibrin. It is generated following the cleavage of crosslinked fibrin by plasmin and consists of two D domains from adjacent fibrin monomers crosslinked by activated factor XIII. 44 , 45 d‐dimer concentrations are routinely measured when suspecting a state of hypercoagulability and as part of the clinical assessment to determine the probability of VTE. 46 , 47 , 48 In the context of RDs, although the clinical significance of the d‐dimer has been primarily investigated in patients with RA, 49 , 50 , 51 an increasing number of studies has assessed the pathophysiological role of this coagulation biomarker in other autoimmune and autoinflammatory conditions.
Therefore, we critically appraised the available evidence regarding the association between the d‐dimer and RDs by conducting a systematic review and meta‐analysis of studies assessing d‐dimer concentrations in patients with different RDs and healthy controls. Furthermore, we conducted a series of meta‐regression and subgroup analyses to identify possible clinical and demographic factors mediating the association between the d‐dimer and RDs.
2. MATERIALS AND METHODS
2.1. Search strategy and study selection
We systematically searched electronic databases (PubMed, Web of Science, and Scopus) from inception to January 31, 2024, for relevant articles using the following terms: “d‐dimer” AND “rheumatic diseases” OR “rheumatoid arthritis” OR “psoriatic arthritis” OR “reactive arthritis” OR “ankylosing spondylitis” OR “systemic lupus erythematosus” OR “systemic sclerosis” OR “scleroderma” OR “Sjogren's syndrome” OR “connective tissue diseases” OR “vasculitis” OR “Behçet's disease” OR “idiopathic inflammatory myositis” OR “polymyositis” OR “dermatomyositis” OR “gout” OR “pseudogout” OR “systemic vasculitis” OR “ANCA‐associated vasculitis” OR “Takayasu arteritis” OR “polyarteritis nodosa” OR “osteoarthritis” OR “fibromyalgia” OR “granulomatous polyangiitis” OR “Henoch‐Schonlein purpura” OR “Wegener's granulomatosis” OR “familial Mediterranean fever.” Two investigators independently screened abstracts and full‐text articles according to the following inclusion criteria: (i) assessment of d‐dimer, (ii) comparison between RD patients and healthy controls in case‐control studies, (iii) use of English language, and (iv) availability of the full‐text of the article. The references of each article were hand‐searched for additional articles.
The two investigators independently extracted the following variables for analysis: publication year, first author details, the country where the study was conducted, type of RD, d‐dimer concentrations, number of participants, age, male‐to‐female ratio, mean RD duration, fibrinogen, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), tissue plasminogen activator (t‐PA), plasminogen activator inhibitor (PAI‐1), and use of glucocorticoids and disease‐modifying anti‐rheumatic drugs (DMARDs).
We assessed the risk of bias using the Joanna Briggs Institute Critical Appraisal Checklist for analytical studies, which considers the following domains: clear definition of inclusion criteria, detailed description of participants and setting, reliable measurement of the exposure, use of standard criteria to assess the condition, identification, and management of confounding factors, reliable measurement of the outcome, and appropriate use of statistical analysis. 52 The risk was considered high, intermediate, or low for studies that addressed <50%, ≥50% and <75%, and ≥75% of checklist items. The certainty of evidence was assessed using the GRADE system. 53 We complied with the PRISMA 2020 statement (Table S1), 54 and registered our protocol in an international repository (PROSPERO registration number: CRD42024517712).
2.2. Statistical analysis
We generated forest plots of standardized mean differences (SMDs) and 95% confidence intervals (CIs) to assess differences in d‐dimer concentrations between RD patients and healthy controls (a p < .05 was considered statistically significant). A positive pooled SMD value indicated higher d‐dimer concentrations in RD patients compared to controls. By contrast, a negative pooled SMD value indicated lower d‐dimer concentrations in RD patients compared to controls. If necessary, means and standard deviations were extrapolated using accepted methods. 55 The Q statistic was used to assess the heterogeneity of the SMD across studies (a p < .01 was considered statistically significant). A random‐effect model based on the inverse‐variance method was used if high heterogeneity was present. 56 , 57 Sensitivity analysis and assessment of publication bias were performed according to standard procedures. 58 , 59 , 60 , 61 We conducted meta‐regression and subgroup analyses to investigate associations between the effect size and the following parameters: year of publication, the geographical area where the study was conducted, RD type and RD category, sample size, age, male‐to‐female ratio, mean RD duration, fibrinogen, CRP, ESR, t‐PA, PAI‐1, and use of glucocorticoids and DMARDs. Statistical analyses were performed using Stata 14 (Stata Corp.).
3. RESULTS
Our search criteria identified 2302 articles, of which 2247 were excluded because of irrelevance or duplication. After a full‐text assessment of the remaining 55 articles, 10 were excluded because they did not report critical information, seven because they recruited non‐adult participants, six because they had a different study design, and one because it presented data that duplicated those of another study. Therefore, 31 studies were selected for analysis 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 (Figure 1 and Table 1). The risk of bias was ranked as low in 20 studies 70 , 71 , 74 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 and moderate in the remaining 11 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 72 , 73 , 75 (Table 2). The cross‐sectional nature of the studies selected downgraded the initial level of certainty to low.
Figure 1.
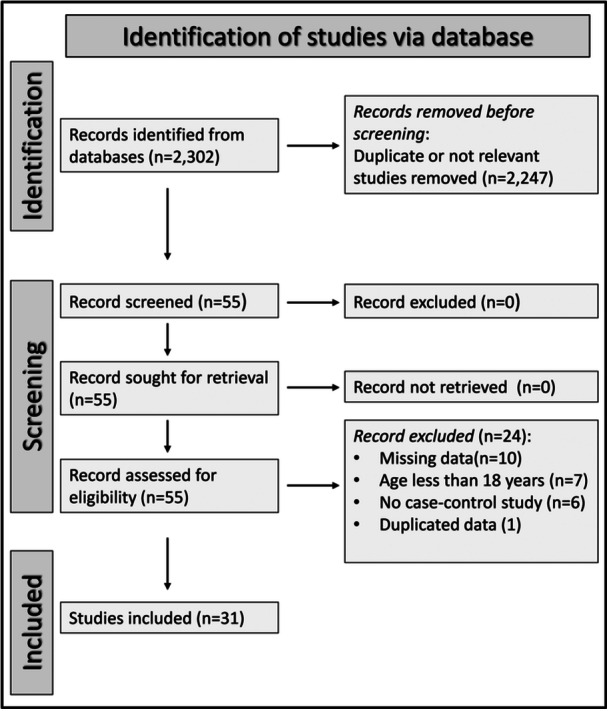
PRISMA 2020 flow diagram.
Table 1.
Characteristics of the studies investigating d‐dimer concentrations in rheumatic diseases.
| Study | Healthy controls | Patients with rheumatic diseases | Disease type | MDD (years) | Design | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (years) | M/F | d‐dimer (mean ± SD) | n | Age (years) | M/F | d‐dimer (mean ± SD) | ||||
| Orem et al. 1995, Turkey 62 | 30 | 32 | 15/15 | 486 ± 106 | 33 | 29 | 19/14 | 463 ± 97 | BD | NR | P |
| Akazawa et al. 1996, Japan 63 | 20 | 44 | 1/19 | 55.6 ± 35.5 | 30 | 45 | 1/29 | 83.6 ± 41.6 | TA | 13 | P |
| Ames et al. 1997, Portugal 64 | 22 | 37 | 2/20 | 59.7 ± 21.1 | 26 | 41 | 3/23 | 112 ± 38.8 | SSc | 8.4 | P |
| Cheras et al. 1997, Australia 65 | 52 | 68 | 31/21 | 0.096 ± 0.06 | 44 | 69 | 26/18 | 0.152 ± 0.114 | OA | NR | P |
| Ichikawa et al. 1998, Japan 66 | 20 | 57 | 0/20 | 63 ± 64.1 | 60 | 56 | 7/53 | 351.2 ± 296.3 | RA | 11.5 | P |
| Ichikawa et al. 1998, Japan 66 | 20 | 57 | 0/20 | 63 ± 64.1 | 21 | 38 | 3/18 | 86.9 ± 85.2 | SLE | 11.7 | P |
| Kamper et al. 2000, Greece 67 | 33 | 53 | 10/23 | 36 ± 33 | 45 | 60 | 12/33 | 442 ± 215 | RA | 4.9 | P |
| McEntegart et al. 2001, UK 68 | 641 | NR | NR | 59 ± 27 | 76 | NR | 13/63 | 80 ± 110 | RA | 12.5 | P |
| Wållberg‐Jonsson et al. 2002, Sweden 69 | 39 | Matched | Matched | 0.138 ± 0.058 | 39 | 52 | 9/30 | 1.088 ± 0.85 | RA | NR | P |
| So et al. 2003, Germany 70 | 21 | 45 | 9/12 | 297 ± 376 | 29 | 67 | 11/18 | 812 ± 690 | OA | NR | P |
| So et al. 2003, Germany 70 | 21 | 45 | 9/12 | 297 ± 376 | 64 | 55 | 15/49 | 2238 ± 1501 | RA | NR | P |
| So et al. 2003, Germany 70 | 21 | 45 | 9/12 | 297 ± 376 | 22 | 38 | 14/8 | 1820 ± 2967 | SpA | NR | P |
| So et al. 2003, Germany 70 | 21 | 45 | 9/12 | 297 ± 376 | 26 | 72 | 14/12 | 2093 ± 1751 | CA | NR | P |
| Bunescu et al. 2004, Sweden 71 | 23 | 55 | 5/18 | 0.233 ± 0.079 | 20 | 56 | 5/15 | 2.53 ± 2.37 | RA | NR | P |
| Afeltra et al. 2005, Italy 72 | 50 | Matched | Matched | 0.32 ± 0.14 | 57 | 40 | 8/49 | 0.58 ± 0.66 | SLE | 9.9 | R |
| Ingegnoli et al. 2008, Italy 73 | 40 | Matched | Matched | 239 ± 88 | 20 | 55 | 5/15 | 2054 ± 2059 | RA | 6.1 | P |
| Marie et al. 2008, France 74 | 69 | Matched | Matched | 284 ± 126 | 69 | 58 | 9/60 | 672 ± 324 | SSc | NR | P |
| Suzuki et al. 2009, Japan 75 | 43 | 28 | 18/25 | 0.6 ± 0.2 | 60 | 45 | 3/57 | 1.3 ± 0.7 | SLE | 15.5 | P |
| Fernández‐Bello et al. 2013, Spain 76 | 33 | 43 | 12/21 | 270 ± 86 | 23 | 49 | 5/18 | 293 ± 65 | BD | 15 | P |
| Mejía et al. 2014, Spain 77 | 56 | 35 | 30/26 | 0.2 ± 0.1 | 56 | 34 | 30/26 | 0.2 ± 0.1 | BD | NR | P |
| Salmela et al. 2015, Finland 78 | 20 | 58 | 14/6 | 0.33 ± 0.24 | 21 | 60 | 16/5 | 3.57 ± 4.37 | AAV | NR | P |
| Ma et al. 2018, China 79 | 100 | 35 | 50/50 | 395 ± 188 | 138 | 65 | 45/93 | 1685 ± 985 | RA | NR | P |
| Chen et al. 2020, China 80 | 101 | 37 | 5/96 | 0.253 ± 0.075 | 334 | 40 | 9/325 | 0.437 ± 0.432 | Gout | NR | R |
| Cicarini et al. 2020, Brazil 81 | 30 | NR | 0/30 | 409 ± 242 | 60 | 40 | 0/60 | 1354 ± 1270 | SLE | 8.5 | P |
| Oh et al. 2020, Korea 82 | 1104 | 38 | 172/932 | 527 ± 573 | 276 | 37 | 35/241 | 1468 ± 1383 | SLE | 8.4 | R |
| Tan et al. 2020, China 83 | 43 | 67 | 22/21 | 1.68 ± 0.98 | 40 | 67 | 23/17 | 3.88 ± 3.69 | AAV | NR | P |
| Tan et al. 2020, China 83 | 43 | 67 | 22/21 | 1.68 ± 0.98 | 34 | 62 | 14/20 | 1.91 ± 2.07 | SLE | NR | P |
| Wu et al. 2020, China 84 | 10 | 56 | 5/5 | 0.234 ± 0.093 | 40 | 58 | 19/21 | 1.5 ± 1.53 | AAV | NR | P |
| Huang et al. 2021, China 86 | 98 | 37 | 20/78 | 0.35 ± 0.07 | 193 | 39 | 10/183 | 1.68 ± 2.07 | SLE | NR | R |
| Roldan et al. 2021, USA 87 | 26 | 32 | 4/22 | 0.25 ± 0.21 | 70 | 36 | 6/64 | 0.42 ± 0.3 | SLE | 8 | P |
| Xue et al. 2021, China 88 | 102 | 54 | 26/76 | 907 ± 546 | 105 | 55 | 27/78 | 2963 ± 1893 | RA | NR | R |
| Zheng et al. 2021, China 89 | 60 | 39 | 6/54 | 0.14 ± 0.08 | 105 | 42 | 10/95 | 0.87 ± 0.94 | SLE | NR | R |
| Feng et al. 2022, China 85 | 70 | 34 | 59/11 | 0.19 ± 0.16 | 65 | 26 | 61/4 | 0.36 ± 0.45 | SpA | NR | P |
| Gögebakan et al. 2022, Turkey 90 | 204 | 34 | 152/52 | 913 ± 541 | 210 | 35 | 156/54 | 2875 ± 1885 | AS | 9.4 | R |
| Qiang et al. 2022, China 91 | 50 | NR | NR | 0.5 ± 0.15 | 112 | 55 | 24/88 | 1.29 ± 1.12 | RA | NR | P |
| Wang et al. 2022, China 92 | 101 | 54 | 2/99 | 0.37 ± 0.31 | 101 | 54 | 2/99 | 1.07 ± 2.3 | pSS | NR | P |
Note: d‐dimer serum concentrations are expressed as ng/mL, µg/mL, mg/dL, or g/mL.
Abbreviations: AAV, ANCA‐associated vasculitis; AS, ankylosing spondylitis; BD, Behcet disease; CA, crystal arthritis; MDD, mean disease duration; NR, not reported; OA, osteoarthritis; P, prospective; pSS, primary Sjögren's Syndrome; R, retrospective; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SpA, spondyloarthritis; SSc, systemic sclerosis; TA, Takayasu arteritis.
Table 2.
Assessment of the risk of bias using the Joanna Briggs Institute critical appraisal checklist.
| Study | Were the inclusion criteria clearly defined? | Were the subjects and the setting described in detail? | Was the exposure measured in a reliable way? | Were standard criteria used to assess the condition? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Were the outcomes measured in a reliable way? | Was appropriate statistical analysis used? | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Orem et al. 62 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Akazawa et al. 63 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Ames et al. 64 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Cheras et al. 65 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Ichikawa et al. 66 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Kamper et al. 67 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| McEntegart et al. 68 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Wållberg‐Jonsson et al. 69 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| So et al. 70 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Bunescu et al. 71 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Afeltra et al. 72 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Ingegnoli et al. 73 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Marie et al. 74 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Suzuki et al. 75 | No | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Fernández‐Bello et al. 76 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Mejía et al. 77 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Salmela et al. 78 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ma et al. 79 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Chen et al. 80 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Cicarini et al. 81 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Oh et al. 82 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tan et al. 83 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Wu et al. 84 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Huang et al. 86 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Roldan et al. 87 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Xue et al. 88 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Zheng et al. 89 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Feng et al. 85 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Gögebakan et al. 90 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Qiang et al. 91 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Wang et al. 92 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
The 31 selected studies, including a total of 36 group comparators, assessed the d‐dimer in 2724 RD patients (mean age 46 years, 75% females) and 3437 healthy controls (mean age 42 years, 72% females). The continents where the studies were conducted included Asia (n = 16), 62 , 63 , 66 , 75 , 79 , 80 , 82 , 83 , 84 , 85 , 86 , 88 , 89 , 90 , 91 , 92 Europe (n = 12), 64 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 76 , 77 , 78 America (n = 2), 81 , 87 and Oceania (n = 1). 65 RA was investigated in 10 study groups, 66 , 67 , 68 , 69 , 70 , 71 , 73 , 79 , 88 , 91 SLE in nine, 66 , 72 , 75 , 81 , 82 , 83 , 86 , 87 , 89 BD in three, 62 , 76 , 77 AAV in three, 78 , 83 , 84 spondyloarthritis (SpA) and AS in three, 70 , 85 , 90 SSc in two, 64 , 74 OA in two, 65 , 70 gout in one, 80 crystal arthritis in one, 70 Takayasu arteritis (TA) in one, 63 and primary Sjögren's Syndrome (pSS) in one. 92 Twenty‐four studies were prospective 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 81 , 83 , 84 , 85 , 87 , 91 , 92 and the remaining seven retrospective. 72 , 80 , 82 , 86 , 88 , 89 , 90 Mean disease duration, reported in 14 study groups, ranged between 4.9 and 15 years. 63 , 64 , 66 , 67 , 68 , 72 , 73 , 75 , 76 , 81 , 82 , 87 , 90 Only three studies reported the use of duplex ultrasonography and/or venography 72 , 77 , 82 and six reported the percentage of subjects with deep vein thrombosis or VTE. 71 , 72 , 74 , 77 , 78 , 82 However, this information was not further analyzed in these studies.
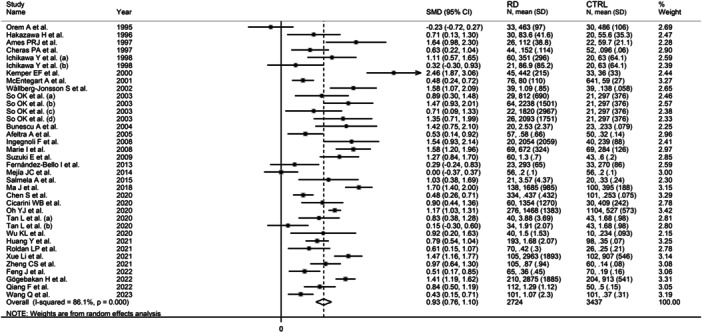
The forest plot showed that, overall, RD patients had significantly higher d‐dimer concentrations when compared to controls (SMD = 0.93, 95% CI 0.76−1.10, p < .001; I 2 = 86.1%, p < .001; Figure 2), with stable corresponding pooled SMD in the sensitivity analysis (range 0.89−0.96, Figure 3).
Figure 2.
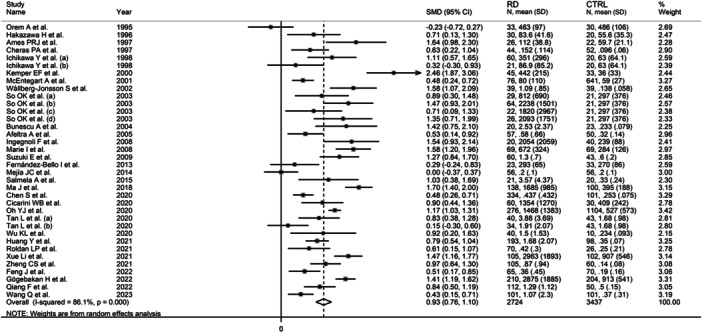
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls.
Figure 3.
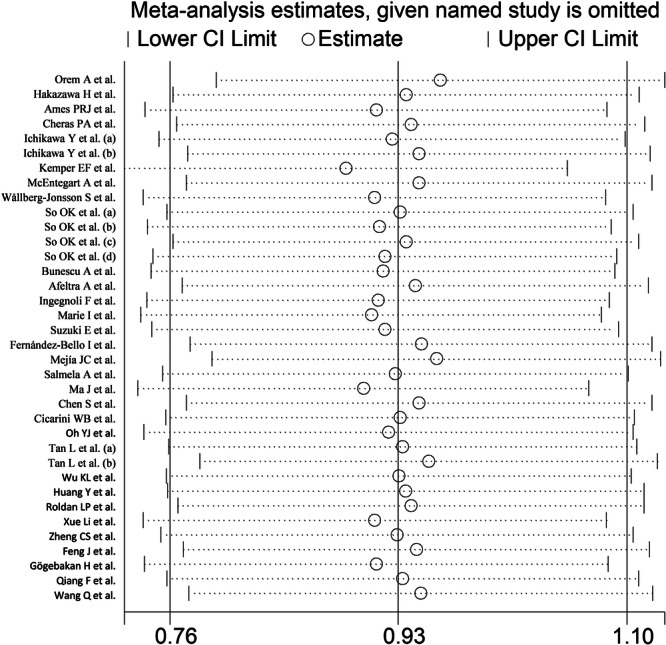
Sensitivity analysis of the association between the d‐dimer and rheumatic diseases.
Neither Begg's test (p = .32), Egger's test (p = .79), nor the “trim‐and‐fill” method showed significant publication bias (Figure 4).
Figure 4.
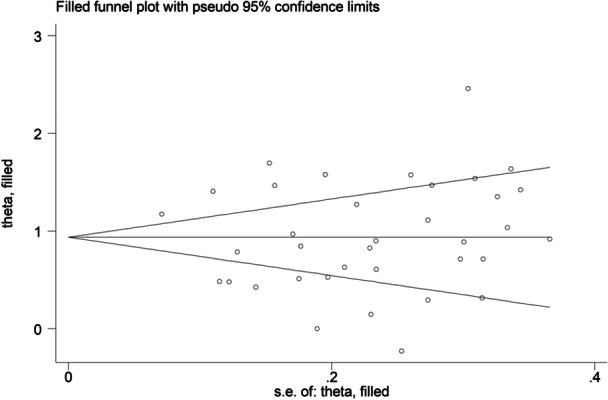
Funnel plot of studies investigating the association between d‐dimer concentrations and rheumatic diseases after “trimming‐and‐filling.”
The effect size was not associated with the male‐to‐female ratio, sample size, publication year, or use of glucocorticoids and DMARDs. By contrast, significant associations were observed with age, mean RD duration, fibrinogen, CRP, and ESR (Figures 5 and 6; Table 3).
Figure 5.
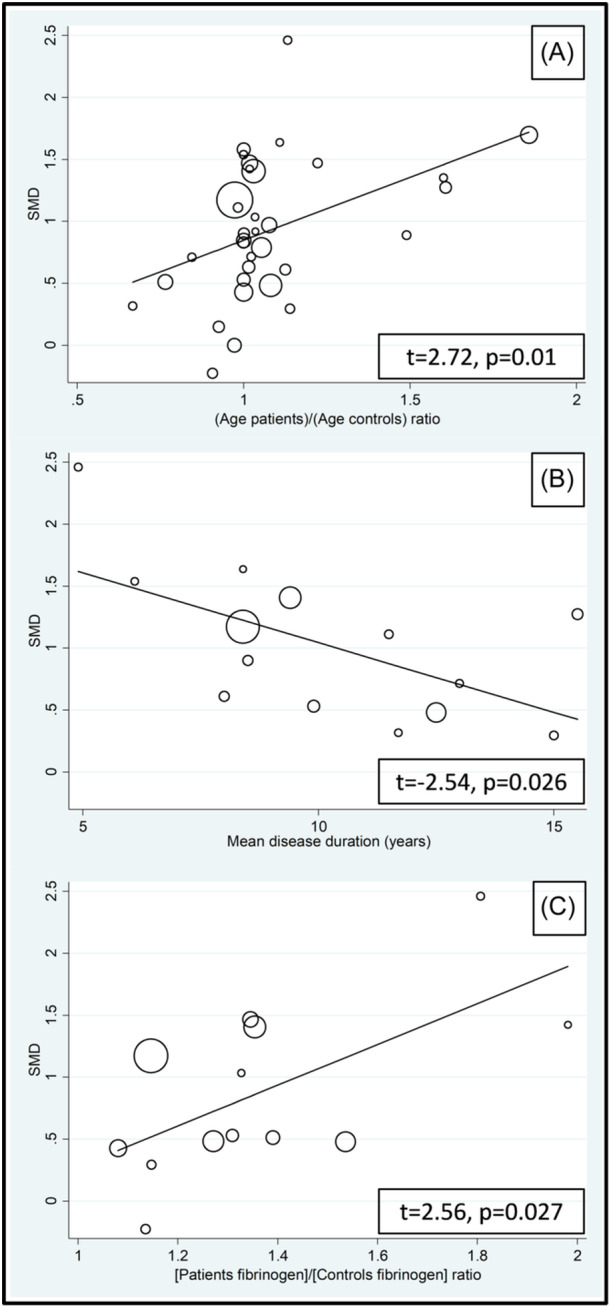
Bubble plot of the univariate meta‐regression analysis between the effect size and age (A), mean disease duration (B), and fibrinogen (C).
Figure 6.
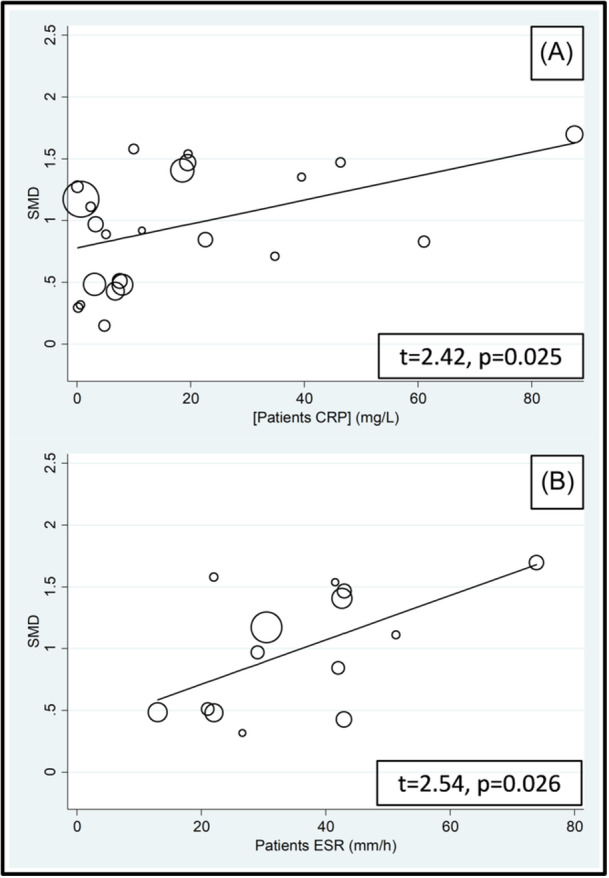
Bubble plot of the univariate meta‐regression analysis between the effect size and C‐reactive protein (CRP) (A) and erythrocyte sedimentation rate (ESR) (B).
Table 3.
Meta‐regression analysis to evaluate the association between study and patient characteristics and the SMD.
| t‐value | p Value | |
|---|---|---|
| Age | 2.72 | .01 |
| Male‐to‐female ratio | 0.69 | .50 |
| Sample size | 0.14 | .89 |
| Publication year | −0.53 | .60 |
| Use of glucocorticoids | −1.83 | .10 |
| Fibrinogen | 2.56 | .027 |
| CRP | 2.42 | .025 |
| ESR | 2.54 | .026 |
| Use of DMARDS | 1.34 | .22 |
| Mean RD duration | −2.54 | .026 |
Abbreviations: CRP, C‐reactive protein; DMARD, disease‐modifying anti‐rheumatic drugs; ESR, erythrocyte sedimentation rate; SMD, standard mean difference.
There were nonsignificant differences (p = .76) in the pooled SMD between studies conducted in Asia (SMD = 0.84, 95% CI 0.63−1.06, p < .001; I 2 = 88.0%, p < .001), Europe (SMD = 1.11, 95% CI 0.76−1.46, p < .001; I 2 = 86.8%, p < .001), and America (SMD = 0.76, 95% CI 0.43−1.08, p < .001; I 2 = 0.0%, p = .378, Figure 7), with a virtually absent heterogeneity in the American subgroup.
Figure 7.
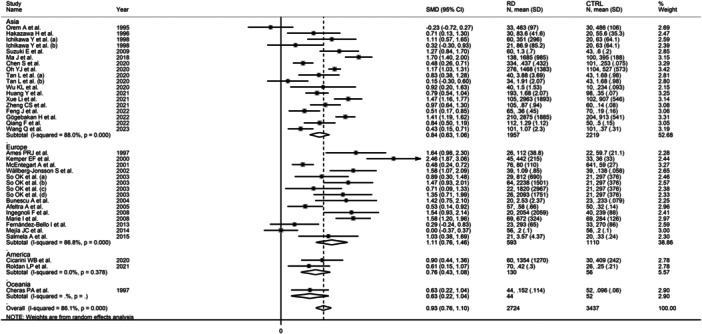
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to country where the study was conducted.
By contrast, there was a significant difference (p < .001) in the pooled SMD among different RDs, which progressively decreased in SSc (SMD = 1.59, 95% CI 1.26−1.92, p < .001; I 2 = 0.0%, p = .88), RA (SMD = 1.38, 95% CI 1.01−1.76, p < .001; I 2 = 87.9%, p < .001), SpA (SMD = 0.90, 95% CI 0.23−1.56, p = .008; I 2 = 90.3%, p < .001), AAV (SMD = 0.90, 95% CI 0.57−1.23, p < .001; I 2 = 0.0%, p = .878), CA and gout (SMD = 0.86, 95% CI 0.02−1.71, p = .045; I 2 = 84.1%, p = .012), SLE (SMD = 0.78, 95% CI 0.54−1.02, p < .001; I 2 = 78.1%, p < .001), OA (SMD = 0.71, 95% CI 0.38−1.05, p < .001; I 2 = 0.0%, p = .482), and BD (SMD = 0.01, 95% CI −0.25 to 0.27, p = .96; I 2 = 0.0%, p = .375, Figure 8) with a virtually absent heterogeneity in the SSc, AAV, OA, and BD subgroups (the SMD in the BD subgroup was not statistically significant).
Figure 8.
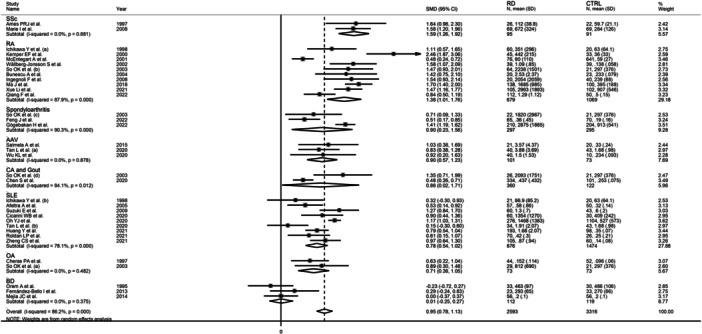
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to specific types of rheumatic disease.
Furthermore, the pooled SMD was significant in studies conducted in patients with autoimmune (SMD = 1.07, 95% CI 0.88−1.26, p < .001; I 2 = 84.0%, p < .001) and autoinflammatory diseases (SMD = 0.72, 95% CI 0.45−1.00, p < .001; I 2 = 45.5%, p = .119), but not mixed autoimmune‐autoinflammatory diseases (SMD = 0.46, 95% CI−0.13 to 1.05, p = .13; I 2 = 93.0%, p < .001, Figure 9), with lower between‐study variance in the autoinflammatory disease subgroup.
Figure 9.
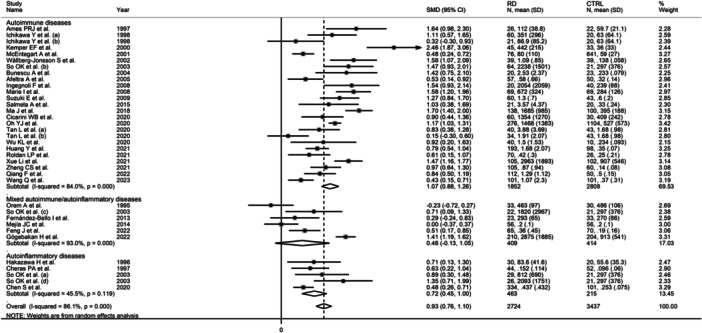
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to the category of rheumatic diseases.
There were no significant differences in the pooled SMD between prospective (SMD = 0.92, 95% CI 0.70−1.14, p < .001; I 2 = 84.9%, p < .001) and retrospective studies (SMD = 0.98, 95% CI 0.70−1.26, p < .001; I 2 = 89.3%, p < .001; Figure 10).
Figure 10.
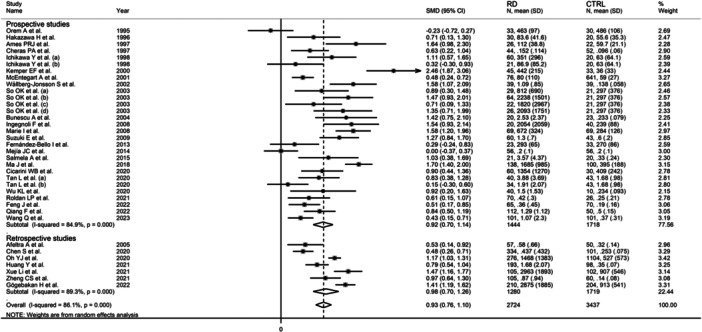
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to study design.
Eight studies also reported serum PAI‐1 concentrations. 62 , 64 , 65 , 67 , 68 , 69 , 74 , 76 Following the creation of two subgroups based on the median value of the (PAI‐1 RD patients)/(PAI‐1 controls) ratio, 1.8, there was a significant difference (p = .002) in the pooled SMD between studies with ratio <1.8 (SMD = 0.33, 95% CI 0.00−1.66, p = .048; I 2 = 62.0%, p = .048) and ratio ≥1.8 (SMD = 1.79, 95% CI 1.39−2.18, p < .001; I 2 = 55.9%, p = .079, Figure 11).
Figure 11.
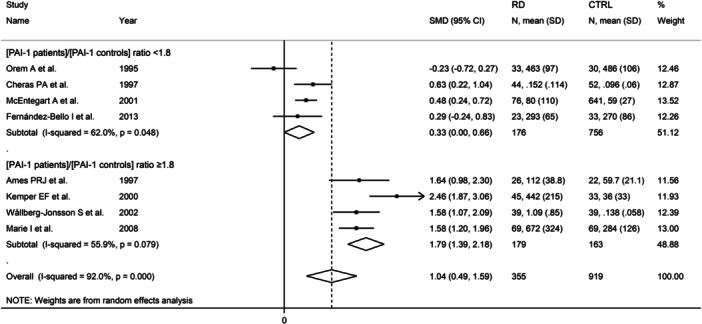
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to the median value of the (PAI‐1 patients)/(PAI‐1 controls) ratio. AI‐1, plasminogen activator inhibitor.
Finally, seven studies also reported serum t‐PA concentrations. 62 , 64 , 65 , 67 , 68 , 69 , 76 After creating two subgroups based on the median value of the (t‐PA patients)/(t‐PA controls) ratio, 1.4, the pooled SMD was significant when the ratio was ≥1.4 (SMD = 1.58, 95% CI 0.29−2.68, p = .015; I 2 = 95.6%, p < .001) but not when it was <1.4 (SMD = 0.56, 95% CI −0.12 to 1.24, p = .10; I 2 = 85.4%, p < .001, Figure 12).
Figure 12.
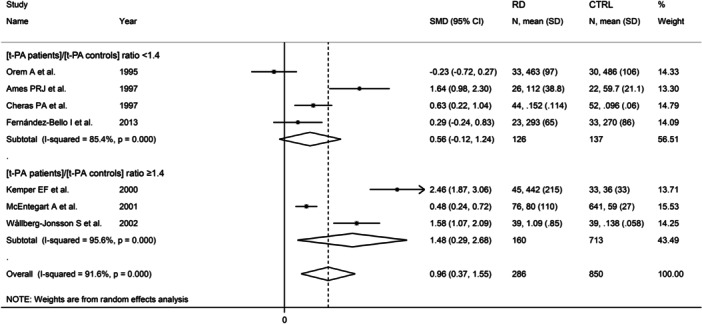
Forest plot of studies investigating d‐dimer concentrations in patients with rheumatic diseases and healthy controls according to the median value of the (t‐PA patients)/(t‐PA controls) ratio. t‐PA, tissue plasminogen activator.
The overall level of certainty was upgraded to moderate after considering the low‐moderate risk of bias in all studies (no change), the high but partially explainable heterogeneity (no change), the lack of indirectness (no change), the large effect size (SMD = 0.93, upgrade one level), 93 and the absence of publication bias (no change).
4. DISCUSSION
This systematic review and meta‐analysis showed that, overall, patients with RDs have significantly higher d‐dimer concentrations when compared to healthy controls. However, such elevations differ according to individual RDs and broad RD categories. Specifically, the between‐group differences in d‐dimer progressively decreased in studies of patients with SSc, RA, SpA, AAV, CA, and gout, SLE, OA, and BD. Furthermore, the alterations in d‐dimer concentrations were significant versus controls in patients with autoimmune and autoinflammatory RDs but not in patients with not mixed autoimmune‐autoinflammatory RDs. In meta‐regression and subgroup analyses, significant associations were observed between the effect size of the between‐group differences in d‐dimer concentrations and age, mean RD duration, fibrinogen, CRP, ESR, type of RD, RD subgroup, PAI‐1, and t‐PA. By contrast, no associations were observed with sex, sample size, publication year, use of glucocorticoids and DMARDs, study geographical location, or study design.
The elevations in d‐dimer concentrations in RD patient groups that, unlike RA and SLE, have been relatively less studied in terms of hypercoagulability and risk of VTE, for example, SpA, is likely to foster additional research in this field. Furthermore, despite the known association between BD and DVT in epidemiological and clinical studies, 34 , 35 , 36 , 37 the effect size of the between‐group differences in d‐dimer concentrations in this patient group was small and nonsignificant compared to other RDs. However, it is important to emphasize that calculating the increased risk of VTE given a particular SMD value is not possible as the SMD is used when studies use different units of measurement for a given variable, in this case d‐dimer concentrations. 94 Additional research is warranted to investigate whether the relationship between d‐dimer concentrations, hypercoagulability, and risk of VTE is consistent across different RDs, particularly in patients with active disease and/or not receiving optimal pharmacological treatment, or whether other markers of coagulation play a more prominent pathophysiological role in the prevention and management of VTE in specific RDs.
The reported positive associations between the effect size of the between‐group differences in d‐dimer concentrations and age are in line with the known increase in d‐dimer concentrations with advancing age, 95 , 96 which has led to the development of age‐adjusted d‐dimer cutoffs to increase the diagnostic performance for VTE. 97 , 98 Notably, epidemiological studies investigating the association between RA and VTE have reported an increasing risk with advancing age. 41 , 99 However, opposite trends have been observed with SLE. 23 The positive associations observed between the SMD of d‐dimer concentrations and other markers of thrombosis and coagulation (fibrinogen and PAI‐1) further support a state of hypercoagulability and increased risk of thrombosis in various RDs, whereas the positive associations with established inflammatory biomarkers (CRP and ESR) are in line with the results of studies reporting an increased risk of VTE in RA and SLE patients with increased disease activity. 41 , 42 , 43 The observed significant and negative association between the SMD of d‐dimer concentrations and RD duration is also in line with the results of epidemiological studies in RA patients reporting that the risk of VTE is higher shortly after diagnosis and tends to be stable or decrease afterward. 99 , 100 , 101 However, other studies have reported an increased risk of VTE with longer RD duration. 102 , 103 Future studies should investigate whether the alterations in d‐dimer concentrations are subject to temporal variations with longer disease duration, whether these trajectories are similar across different RDs, and whether relatively higher d‐dimer concentrations have a causal relationship with incident VTE in these patients.
Another interesting observation in our subgroup analysis was the absence of significant differences in the pooled SMD between studies conducted on different continents. Although this suggests that the reported elevations in d‐dimer in RDs can be generalized to other ethnic groups, studies in non‐RD populations have consistently reported relatively higher d‐dimer concentrations in African American subjects. 104 , 105 , 106
Strengths of our study include the assessment of d‐dimer concentrations in several types of RDs and broad RD groups, the evaluation of possible associations between the effect size of the between‐group differences in d‐dimer concentrations and various study and patient characteristics, particularly age, CRP, ESR, other markers of coagulation and thrombosis, and RD duration, and a comprehensive assessment of the risk of bias and the certainty of evidence. Furthermore, the results of the meta‐analysis were stable in sensitivity analysis, and no publication bias was observed. The main limitations include the relatively limited number of RDs captured in our systematic search (BD, TA, SSc, OA, RA, SLE, SpA, CA, AAV, gout, AS, and pSS) and the fact that no information was available regarding e causal relationship between d‐dimer alterations and occurrence of DVT/PE.
5. CONCLUSIONS
The results of our systematic review and meta‐analysis suggest the presence of significant elevations in d‐dimer concentrations in patients with RDs taken together. However, such alterations depend on specific RD types and categories and are also significantly associated with age, mean RD duration, and other coagulation and inflammatory biomarkers. Additional research is warranted to investigate d‐dimer concentrations in a wider range of RDs and their relationship with disease activity and the occurrence of VTE in this patient group. The results of these studies will determine the true pathophysiological and clinical role of the d‐dimer as a marker of hypercoagulability in patients with RDs and its potential utility in the prevention and management of VTE in these patients.
AUTHOR CONTRIBUTIONS
Angelo Zinellu: Conceptualization; formal analysis; methodology; writing—review and editing. Arduino A. Mangoni: Data curation; methodology; project administration; validation; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethics approval was not required as this was a systematic review of published studies. Patient consent was not required as this was a systematic review of published studies.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Open access publishing facilitated by Flinders University, as part of the Wiley ‐ Flinders University agreement via the Council of Australian University Librarians.
Zinellu A, Mangoni AA. A systematic review and meta‐analysis of the association between the D‐dimer and rheumatic diseases. Immun Inflamm Dis. 2024;12:e1349. 10.1002/iid3.1349
DATA AVAILABILITY STATEMENT
The data supporting the findings of this systematic review and meta‐analysis are available from A. Z. upon reasonable request.
REFERENCES
- 1. Calle E, Gómez‐Puerta JA. The spectrum of rheumatic diseases. Surgery in rheumatic and musculoskeletal disease. Handbook Systemic Autoimmune Dis. 2018;15:1‐13. [Google Scholar]
- 2. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3(8):e297. 10.1371/journal.pmed.0030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moutsopoulos HM. Autoimmune rheumatic diseases: one or many diseases. J Translational Autoimmunity. 2021;4:100129. 10.1016/j.jtauto.2021.100129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(3):351‐360. 10.1016/j.semarthrit.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 5. Korotaeva T, Dina O, Holdsworth E, et al. Investigating diagnosis, treatment, and burden of disease in patients with ankylosing spondylitis in Central Eastern Europe and the United States: a real‐world study. Clin Rheumatol. 2021;40(12):4915‐4926. 10.1007/s10067-021-05864-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Wu L, Hernández‐Muñoz J, et al. The economic burden of systemic sclerosis—a systematic review. Int J Rheumatic Diseases. 2022;25(2):110‐120. 10.1111/1756-185X.14270 [DOI] [PubMed] [Google Scholar]
- 7. Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990‐2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819‐828. 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 8. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12(10):605‐620. 10.1038/nrrheum.2016.137 [DOI] [PubMed] [Google Scholar]
- 9. Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990‐2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463‐1471. 10.1136/annrheumdis-2019-215920 [DOI] [PubMed] [Google Scholar]
- 10. Ben Mrid R, Bouchmaa N, Ainani H, El Fatimy R, Malka G, Mazini L. Anti‐rheumatoid drugs advancements: new insights into the molecular treatment of rheumatoid arthritis. Biomed Pharmacother. 2022;151:113126. 10.1016/j.biopha.2022.113126 [DOI] [PubMed] [Google Scholar]
- 11. Jung SM, Kim WU. Targeted immunotherapy for autoimmune disease. Immune Netw. 2022;22(1):e9. 10.4110/in.2022.22.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reynolds JA, Putterman C. Progress and unmet needs in understanding fundamental mechanisms of autoimmunity. J Autoimmun. 2023;137:102999. 10.1016/j.jaut.2023.102999 [DOI] [PubMed] [Google Scholar]
- 13. Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63‐80. 10.1016/j.cell.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 14. Havnaer A, Han G. Autoinflammatory disorders: a review and update on pathogenesis and treatment. Am J Clin Dermatol. 2019;20(4):539‐564. 10.1007/s40257-019-00440-y [DOI] [PubMed] [Google Scholar]
- 15. Efthimiou P, Petryna O, Nakasato P, Kontzias A. New insights on multigenic autoinflammatory diseases. Ther Adv Musculoskelet Dis. 2022;14:1759720X221117880. 10.1177/1759720X221117880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawakami A, Endo Y, Koga T, Yoshiura K, Migita K. Autoinflammatory disease: clinical perspectives and therapeutic strategies. Inflamm Regen. 2022;42(1):37. 10.1186/s41232-022-00217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Misra DP, Ahmed S, Goyal M, Sharma A, Agarwal V. Venous thromboembolism in the inflammatory rheumatic diseases. Rheumatic Dis Clin North Am. 2023;49(1):97‐127. 10.1016/j.rdc.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 18. Menichelli D, Cormaci VM, Marucci S, et al. Risk of venous thromboembolism in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2023;22(11):103447. 10.1016/j.autrev.2023.103447 [DOI] [PubMed] [Google Scholar]
- 19. Ketfi C, Boutigny A, Mohamedi N, et al. Risk of venous thromboembolism in rheumatoid arthritis. Joint Bone Spine. 2021;88(3):105122. 10.1016/j.jbspin.2020.105122 [DOI] [PubMed] [Google Scholar]
- 20. Wang F, Liu J, Fang Y, et al. Hypercoagulability in rheumatoid arthritis: a bibliometric analysis and retrospective data mining study. ACS Omega. 2023;8(50):48522‐48534. 10.1021/acsomega.3c08460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omair MA, Alkhelb SA, Ezzat SE, Boudal AM, Bedaiwi MK, Almaghlouth I. Venous thromboembolism in rheumatoid arthritis: the added effect of disease activity to traditional risk factors. Open Access Rheumatol: Res Rev. 2022;14:231‐242. 10.2147/OARRR.S284757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung WS, Lin CL, Chang SN, Lu CC, Kao CH. Systemic lupus erythematosus increases the risks of deep vein thrombosis and pulmonary embolism: a nationwide cohort study. J Thromb Haemostasis. 2014;12(4):452‐458. 10.1111/jth.12518 [DOI] [PubMed] [Google Scholar]
- 23. Bello N, Meyers KJ, Workman J, Marcano Belisario J, Cervera R. Systematic literature review and meta‐analysis of venous thromboembolism events in systemic lupus erythematosus. Rheumatol Ther. 2023;10(1):7‐34. 10.1007/s40744-022-00513-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ungprasert P, Srivali N, Kittanamongkolchai W. Systemic sclerosis and risk of venous thromboembolism: a systematic review and meta‐analysis. Modern Rheumatol. 2015;25(6):893‐897. 10.3109/14397595.2015.1038456 [DOI] [PubMed] [Google Scholar]
- 25. Liapi M, Jayne D, Merkel PA, Segelmark M, Mohammad AJ. Venous thromboembolism in ANCA‐associated vasculitis: a population‐based cohort study. Rheumatology. 2021;60(10):4616‐4623. 10.1093/rheumatology/keab057 [DOI] [PubMed] [Google Scholar]
- 26. Isaacs B, Gapud EJ, Antiochos B, Seo P, Geetha D. Venous thrombotic events in ANCA‐associated vasculitis: incidence and risk factors. Kidney360. 2020;1(4):258‐262. 10.34067/KID.0000572019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansrivijit P, Trongtorsak A, Gadhiya KP, et al. Incidence and risk factors of venous thromboembolism in ANCA‐associated vasculitis: a meta‐analysis and metaregression. Clin Rheumatol. 2021;40(7):2843‐2853. 10.1007/s10067-021-05589-8 [DOI] [PubMed] [Google Scholar]
- 28. Stassen PM, Derks RPH, Kallenberg CGM, Stegeman CA. Venous thromboembolism in ANCA‐associated vasculitis: incidence and risk factors. Rheumatology. 2007;47(4):530‐534. 10.1093/rheumatology/ken035 [DOI] [PubMed] [Google Scholar]
- 29. Berti A, Matteson EL, Crowson CS, Specks U, Cornec D. Risk of cardiovascular disease and venous thromboembolism among patients with incident ANCA‐associated vasculitis: a 20‐year population‐based cohort study. Mayo Clin Proc. 2018;93(5):597‐606. 10.1016/j.mayocp.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang A, Antonelou M, Wong NL, et al. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody‐associated vasculitis. J Rheumatol. 2019;46(3):285‐293. 10.3899/jrheum.170896 [DOI] [PubMed] [Google Scholar]
- 31. Kronbichler A, Leierer J, Leierer G, et al. Clinical associations with venous thromboembolism in anti‐neutrophil cytoplasm antibody‐associated vasculitides. Rheumatology. 2017;56(5):kew465. 10.1093/rheumatology/kew465 [DOI] [PubMed] [Google Scholar]
- 32. Guo Y, Zhou F, Xu H. Gout and risk of venous thromboembolism: a systematic review and meta‐analysis of cohort studies. Int J Rheumatic Dis. 2023;26(2):344‐353. 10.1111/1756-185X.14524 [DOI] [PubMed] [Google Scholar]
- 33. Zeng C, Bennell K, Yang Z, et al. Risk of venous thromboembolism in knee, hip and hand osteoarthritis: a general population‐based cohort study. Ann Rheum Dis. 2020;79(12):1616‐1624. 10.1136/annrheumdis-2020-217782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee NH, Bae M, Jin M, Chung SW, Lee CW, Jeon CH. Characterization of venous involvement in Vasculo‐Behçet disease. Korean J Thorac Cardiovasc Surg. 2020;53(6):381‐386. 10.5090/kjtcs.20.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alibaz‐Oner F, Aldag B, Aldag M, et al. Post‐thrombotic syndrome and venous disease‐specific quality of life in patients with vascular Behçet's disease. J Vascular Surg: Venous Lymphatic Disorders. 2016;4(3):301‐306. 10.1016/j.jvsv.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 36. Toledo‐Samaniego N, Oblitas CM, Peñaloza‐Martínez E, et al. Arterial and venous involvement in Behçet's syndrome: a narrative review. J Thromb Thrombolysis. 2022;54(1):162‐171. 10.1007/s11239-022-02637-1 [DOI] [PubMed] [Google Scholar]
- 37. Houman MH, Ben Ghorbel I, Khiari Ben Salah I, Lamloum M, Ben Ahmed M, Miled M. Deep vein thrombosis in Behcet's disease. Clin Exp Rheumatol. 2001;19(5 suppl 24):S48‐S50. [PubMed] [Google Scholar]
- 38. Keser G. Inflammation‐induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18(11):1478‐1493. 10.2174/138161212799504731 [DOI] [PubMed] [Google Scholar]
- 39. Peshkova AD, Evdokimova TA, Sibgatullin TB, Ataullakhanov FI, Litvinov RI, Weisel JW. Accelerated spatial fibrin growth and impaired contraction of blood clots in patients with rheumatoid arthritis. Int J Mol Sci. 2020;21(24):9434. 10.3390/ijms21249434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamaki H, Khasnis A. Venous thromboembolism in systemic autoimmune diseases: a narrative review with emphasis on primary systemic vasculitides. Vasc Med. 2015;20(4):369‐376. 10.1177/1358863X15573838 [DOI] [PubMed] [Google Scholar]
- 41. Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80(2):169‐175. 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 42. Yoshimura M, Fujieda Y, Sugawara M, et al. Disease activity as a risk factor for venous thromboembolism in rheumatoid arthritis analysed using time‐averaged DAS28CRP: a nested case‐control study. Rheumatol Int. 2022;42(11):1939‐1946. 10.1007/s00296-022-05121-4 [DOI] [PubMed] [Google Scholar]
- 43. Ferreira KS, Cicarini WB, Alves LCV, et al. Correlation between active disease and hypercoagulability state in patients with systemic lupus erythematosus. Clin Chim Acta. 2019;490:107‐112. 10.1016/j.cca.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 44. Olson JD. D‐dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1‐46. 10.1016/bs.acc.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 45. Johnson ED, Schell JC, Rodgers GM. The D‐dimer assay. Am J Hematol. 2019;94(7):833‐839. 10.1002/ajh.25482 [DOI] [PubMed] [Google Scholar]
- 46. Favresse J, Lippi G, Roy PM, et al. D‐dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548‐577. 10.1080/10408363.2018.1529734 [DOI] [PubMed] [Google Scholar]
- 47. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D‐dimer. J Am Coll Cardiol. 2017;70(19):2411‐2420. 10.1016/j.jacc.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 48. Wauthier L, Favresse J, Hardy M, et al. D‐dimer testing: a narrative review. Adv Clin Chem. 2023;114:151‐223. 10.1016/bs.acc.2023.02.006 [DOI] [PubMed] [Google Scholar]
- 49. Bloom BJ, Tucker LB, Miller LC, Schaller JG. Fibrin D‐dimer as a marker of disease activity in systemic onset juvenile rheumatoid arthritis. J Rheumatol. 1998;25(8):1620‐1625. [PubMed] [Google Scholar]
- 50. Mukubo Y, Kawamata M. Higher preoperative D‐dimer value remain high postoperatively in patients with rheumatoid arthritis compared with those with osteoarthrosis. J Anesth. 2006;20(1):51‐53. 10.1007/s00540-005-0368-3 [DOI] [PubMed] [Google Scholar]
- 51. Mori S, Soejima H, Hokamaki J, Tsujita K. Clinical disease activity is a major determinant of plasma D‐dimer elevation in outpatients with rheumatoid arthritis: a hospital‐based cross‐sectional study. Modern Rheumatol. 2024;34(2):313‐321. 10.1093/mr/road018 [DOI] [PubMed] [Google Scholar]
- 52. Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer's Manual. Johanna Briggs Institute; 2017. [Google Scholar]
- 53. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. J Clin Epidemiol. 2011;64(4):401‐406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 54. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 57. Higgins JPT. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tobias A. Assessing the influence of a single study in the meta‐analysis estimate. Stata Technical Bulletin. 1999;47:15‐17. [Google Scholar]
- 59. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 60. Sterne JAC, Egger M. Funnel plots for detecting bias in meta‐analysis. J Clin Epidemiol. 2001;54(10):1046‐1055. 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 61. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 62. Orem A, Deger O, Memis O, Bahadir S, Ovali E, Cimsit G. Lp(a) lipoprotein levels as a predictor of risk for thrombogenic events in patients with Behcet's disease. Ann Rheum Dis. 1995;54(9):726‐729. 10.1136/ard.54.9.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Akazawa H, Ikeda U, Yamamoto K, Kuroda T, Shimada K. Hypercoagulable state in patients with Takayasu's arteritis. Thromb Haemostasis. 1996;75(5):712‐716. [PubMed] [Google Scholar]
- 64. Ames PR, Lupoli S, Alves J, et al. The coagulation/fibrinolysis balance in systemic sclerosis: evidence for a haematological stress syndrome. Rheumatology. 1997;36(10):1045‐1050. 10.1093/rheumatology/36.10.1045 [DOI] [PubMed] [Google Scholar]
- 65. Cheras PA, Whitaker AN, Blackwell EA, Sinton TJ, Chapman MD, Peacock KA. Hypercoagulability and hypofibrinolysis in primary osteoarthritis. Clin Orthop Relat Res. 1997;334:57‐67. [PubMed] [Google Scholar]
- 66. Ichikawa Y, Yamada C, Horiki T, Hoshina Y, Uchiyama M. Serum matrix metalloproteinase‐3 and fibrin degradation product levels correlate with clinical disease activity in rheumatoid arthritis. Clin Exp Rheumatol. 1998;16(5):533‐540. [PubMed] [Google Scholar]
- 67. Kamper EF, Kopeikina LT, Trontzas P, Potamianou A, Tsiroglou E, Stavridis JC. The effect of disease activity related cytokines on the fibrinolytic potential and cICAM‐1 expression in rheumatoid arthritis. J Rheumatol. 2000;27(11):2545‐2550. [PubMed] [Google Scholar]
- 68. McEntegart A. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology. 2001;40(6):640‐644. 10.1093/rheumatology/40.6.640 [DOI] [PubMed] [Google Scholar]
- 69. Wållberg‐Jonsson S, Cvetkovic JT, Sundqvist KG, Lefvert AK, Rantapää‐Dahlqvist S. Activation of the immune system and inflammatory activity in relation to markers of atherothrombotic disease and atherosclerosis in rheumatoid arthritis. J Rheumatol. 2002;29(5):875‐882. [PubMed] [Google Scholar]
- 70. So AK, Varisco PA, Kemkes‐Matthes B, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemostasis. 2003;1(12):2510‐2515. 10.1111/j.1538-7836.2003.00462.x [DOI] [PubMed] [Google Scholar]
- 71. Bunescu A, Seideman P, Lenkei R, Levin K, Egberg N. Enhanced Fcgamma receptor I, alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J Rheumatol. 2004;31(12):2347‐2355. [PubMed] [Google Scholar]
- 72. Afeltra A, Vadacca M, Conti L, et al. Thrombosis in systemic lupus erythematosus: congenital and acquired risk factors. Arthritis Rheumatism. 2005;53(3):452‐459. 10.1002/art.21172 [DOI] [PubMed] [Google Scholar]
- 73. Ingegnoli F, Fantini F, Favalli EG, et al. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor‐α blockade. J Autoimmun. 2008;31(2):175‐179. 10.1016/j.jaut.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 74. Marie I, Borg JY, Hellot MF, Levesque H. Plasma D‐dimer concentration in patients with systemic sclerosis. Br J Dermatol. 2007;158(2):392‐395. 10.1111/j.1365-2133.2007.08313.x [DOI] [PubMed] [Google Scholar]
- 75. Suzuki E, Amengual O, Atsumi T, et al. Increased expression of phospholipid scramblase 1 in monocytes from patients with systemic lupus erythematosus. J Rheumatol. 2010;37(8):1639‐1645. 10.3899/jrheum.091420 [DOI] [PubMed] [Google Scholar]
- 76. Fernández‐Bello I, López‐Longo FJ, Arias‐Salgado EG, Jiménez‐Yuste V, Butta NV. Behçet's disease: new insight into the relationship between procoagulant state, endothelial activation/damage and disease activity. Orphanet J Rare Dis. 2013;8:81. 10.1186/1750-1172-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mejia JC, Espinosa G, Tassies D, Reverter JC, Cervera R. Endogenous thrombin potential in Behcet's disease: relationship with thrombosis and anticoagulant therapy. Clin Exp Rheumatol. 2014;32(4 suppl 84):S67‐S71. [PubMed] [Google Scholar]
- 78. Salmela A, Ekstrand A, Joutsi‐Korhonen L, Räisänen‐Sokolowski A, Lassila R. Activation of endothelium, coagulation and fibrinolysis is enhanced and associates with renal anti‐neutrophil cytoplasmic antibody‐associated vasculitis. Nephrol Dial Transplant. 2015;30(suppl 1):i53‐9. 10.1093/ndt/gfu379 [DOI] [PubMed] [Google Scholar]
- 79. Ma J, Zhang Y, Gong Y, Zhu Y, Li M, Zhao J. Correlation between plasma levels of D‐dimer and IL‐1, IL‐6, and TNF‐α in patients with rheumatoid arthritis. Int J Clin Exp Med. 2018;11(9):9865‐9871. [Google Scholar]
- 80. Chen S, Huang X, Huang Y, et al. Role of plasma fibrinogen in assessing disease activity of patients with gout. Clin Chim Acta. 2020;510:483‐487. 10.1016/j.cca.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 81. Cicarini WB, Duarte RCF, Ferreira KS, et al. Impact of markers of endothelial injury and hypercoagulability on systemic lupus erythematosus. Lupus. 2020;29(2):182‐190. 10.1177/0961203319899478 [DOI] [PubMed] [Google Scholar]
- 82. Oh YJ, Park EH, Park JW, Song YW, Lee EB. Practical utility of D‐dimer test for venous thromboembolism in systemic lupus erythematosus depends on disease activity: a retrospective cohort study. J Korean Med Sci. 2020;35(43):e356. 10.3346/jkms.2020.35.e356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan L, Hua L, Zhao Y, et al. Clinical study of serum markers and immune protein expression in patients with ANCA‐associated vasculitis. Acta Med Mediterr. 2020;36:1239‐1244. 10.19193/0393-6384_2020_2_194 [DOI] [Google Scholar]
- 84. Wu KL, Liang QH, Ding N, Li BW, Hao J. Sphingosine‐1‐phosphate in anti‐neutrophil cytoplasmic antibody‐associated vasculitis: coagulation‐related clinical indicators and complications. Biosci Rep. 2020;40(10):BSR20200157. 10.1042/BSR20200157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feng J, Li J, Li Y, Jin Y, Du F, Chen X. Elevated serum D‐dimer may reflect the presence of gut inflammation in spondyloarthritis. Front Med. 2022;8:8. 10.3389/fmed.2021.816422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huang Y, Chen L, Zhu B, Han H, Hou Y, Wang W. Evaluation of systemic lupus erythematosus disease activity using anti‐α‐enolase antibody and RDW. Clin Exp Med. 2021;21(1):73‐78. 10.1007/s10238-020-00657-w [DOI] [PubMed] [Google Scholar]
- 87. Roldan LP, Roldan PC, Sibbitt WL Jr., Qualls CR, Ratliff MD, Roldan CA. Aortic adventitial thickness as a marker of aortic atherosclerosis, vascular stiffness, and vessel remodeling in systemic lupus erythematosus. Clin Rheumatol. 2021;40(5):1843‐1852. 10.1007/s10067-020-05431-7 [DOI] [PubMed] [Google Scholar]
- 88. Xue L, Tao L, Li X, et al. Plasma fibrinogen, D‐dimer, and fibrin degradation product as biomarkers of rheumatoid arthritis. Sci Rep. 2021;11(1):16903. 10.1038/s41598-021-96349-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zheng CS, Qin XJ, Ni H, Chen RY, Liu JL, Wang WH. Evaluation of disease activity of systemic lupus erythematosus by D‐dimer combined with red blood cell distribution width. Clin Lab. 2021;67(9). 10.7754/Clin.Lab.2021.210118 [DOI] [PubMed] [Google Scholar]
- 90. Göğebakan H, Cerrah S. The role of plasma fibrinogen, D‐dimer and fibrinogen degradation products in diagnosis and disease activity in patients with ankylosing spondylitis. Egyptian Rheumatol. 2022;44(1):47‐51. 10.1016/j.ejr.2021.08.002 [DOI] [Google Scholar]
- 91. Qiang F, Xu H, Sheng J. Relationship between plasma fibrinogen degradation products(FDP) and D‐dimer levels and disease activity in rheumatoid arthritis: a STROBE compliant article. Medicine. 2022;101(36):e30455. 10.1097/MD.0000000000030455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang Q, Dai SM. An interaction between the inflammatory condition and the hypercoagulable condition occurs in primary Sjögren syndrome. Clin Rheumatol. 2023;42(4):1107‐1112. 10.1007/s10067-022-06498-0 [DOI] [PubMed] [Google Scholar]
- 93. Cohen J. Statistical power analysis. Current Directions Psychol Sci. 1992;1(3):98‐101. [Google Scholar]
- 94. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta‐analysis: as simple as it gets. J Clin Psychiatry. 2020;81(5):20f13681. 10.4088/JCP.20f13681 [DOI] [PubMed] [Google Scholar]
- 95. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109(5):357‐361. 10.1016/s0002-9343(00)00493-9 [DOI] [PubMed] [Google Scholar]
- 96. Tita‐Nwa F, Bos A, Adjei A, Ershler WB, Longo DL, Ferrucci L. Correlates of D‐dimer in older persons. Aging Clin Exp Res. 2010;22(1):20‐23. 10.1007/BF03324810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Righini M, Van Es J, Den Exter PL, et al. Age‐adjusted D‐dimer cutoff levels to rule out pulmonary embolism: the ADJUST‐PE study. JAMA. 2014;311(11):1117‐1124. 10.1001/jama.2014.2135 [DOI] [PubMed] [Google Scholar]
- 98. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D‐dimer cut‐off values in older patients with suspected venous thromboembolism: systematic review and meta‐analysis. BMJ. 2013;346:f2492. 10.1136/bmj.f2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA. 2012;308(13):1350‐1356. 10.1001/2012.jama.11741 [DOI] [PubMed] [Google Scholar]
- 100. Choi HK, Rho YH, Zhu Y, Cea‐Soriano L, Aviña‐Zubieta JA, Zhang Y. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population‐based outpatient cohort study. Ann Rheum Dis. 2013;72(7):1182‐1187. 10.1136/annrheumdis-2012-201669 [DOI] [PubMed] [Google Scholar]
- 101. Li L, Lu N, Avina‐Galindo AM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population‐based study. Rheumatology. 2021;60(1):188‐195. 10.1093/rheumatology/keaa262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chung WS, Peng CL, Lin CL, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. 2014;73(10):1774‐1780. 10.1136/annrheumdis-2013-203380 [DOI] [PubMed] [Google Scholar]
- 103. Conforti A, Berardicurti O, Pavlych V, Di Cola I, Cipriani P, Ruscitti P. Incidence of venous thromboembolism in rheumatoid arthritis, results from a “real‐life” cohort and an appraisal of available literature. Medicine. 2021;100(33):e26953. 10.1097/MD.0000000000026953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pieper CF, Rao KMK, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D‐dimer levels in community‐dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55(11):M649‐M657. 10.1093/gerona/55.11.m649 [DOI] [PubMed] [Google Scholar]
- 105. Khaleghi M, Saleem U, McBane RD, Mosley TH Jr., Kullo IJ. African‐American ethnicity is associated with higher plasma levels of D‐dimer in adults with hypertension. J Thromb Haemostasis. 2009;7(1):34‐40. 10.1111/j.1538-7836.2008.03215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Raffield LM, Zakai NA, Duan Q, et al. D‐dimer in African Americans: whole genome sequence analysis and relationship to cardiovascular disease risk in the Jackson Heart Study. Arterioscler Thromb Vasc Biol 2017;37(11):2220‐2227. 10.1161/ATVBAHA.117.310073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data supporting the findings of this systematic review and meta‐analysis are available from A. Z. upon reasonable request.


