Abstract
The Epstein-Barr Virus (EBV) immediate-early protein BRLF1 is one of two transactivators which mediate the switch from latent to lytic replication in EBV-infected cells. DNA viruses often modulate the function of critical cell cycle proteins to maximize the efficiency of virus replication. Here we have examined the effect of BRLF1 on cell cycle progression. A replication-deficient adenovirus expressing BRLF1 (AdBRLF1) was used to infect normal human fibroblasts and various epithelial cell lines. BRLF1 expression induced S phase entry in contact-inhibited fibroblasts and in the human osteosarcoma cell line U-2 OS. AdBRLF1 infection produced a dramatic increase in the level of E2F1 but not E2F4. In contrast, the levels of Rb, p107, and p130 were decreased in AdBRLF1-infected cells. Electrophoretic mobility shift assays confirmed an increased level of free E2F1 in the AdBRLF1-infected human fibroblasts. Consistent with the previously described effect of E2F1, AdBRLF1-infected fibroblasts had increased levels of p53 and p21 and died by apoptosis. BRLF1-induced activation of E2F1 may be required for efficient EBV lytic replication, since at least one critical viral replication gene (the viral DNA polymerase) is activated by E2F (C. Liu, N. D. Sista, and J. S. Pagano, J. Virol. 70:2545–2555, 1996).
Infection by the human herpesvirus Epstein-Barr virus (EBV) occurs in most individuals. EBV is the causative agent of infectious mononucleosis and is associated with a variety of malignant disorders, including Burkitt’s lymphoma and nasopharyngeal carcinoma (38, 52). EBV infects both B lymphocytes and epithelial cells. Upon infection of epithelial cells, the virus enters the lytic cycle, where it actively replicates and produces new virus particles (52). In contrast, infection of B lymphocytes usually results in a latent state of infection, with the virus expressing only a small subset of genes (52). The EBV immediate-early proteins, BZLF1 and BRLF1, are able to disrupt latency in EBV-infected cells and initiate lytic viral replication (3, 6, 51, 53, 61, 69).
During productive EBV infection, expression of BRLF1 and BZLF1 occurs simultaneously within the cell (38). Both BZLF1 and BRLF1 are transcriptional activators of early EBV genes. BZLF1 binds to Ap-1-like sequences, while BRLF1 binds directly to a GC-rich motif (13, 19, 20, 22, 37). BZLF1 and/or BRLF1 binding sites are present upstream of most early EBV promoters (7, 15, 23, 31, 43, 50, 64). BRLF1 also activates the transcription of several other genes, including the EBV DNA polymerase and c-myc genes, through an indirect mechanism (16, 21). Both the viral polymerase and c-myc genes have upstream E2F binding sites (27, 44); the E2F site in the viral polymerase promoter is required for BRLF1 activation of this promoter (44), suggesting that BRLF1 may activate certain genes through modulation of E2F1.
Frequently, viruses encode regulatory proteins which modulate cell cycle progression within the infected cell. The smaller DNA tumor viruses (adenovirus, papillomavirus, and papovaviruses) all encode regulatory proteins (E1A, E7, and T antigen, respectively) (9, 11, 67) which interact with and inhibit retinoblastoma (Rb) function. Each of these viruses likewise encodes a protein (E1B, E6, and T antigen, respectively) which inhibits p53 function (40, 42, 55, 66). Inhibition of Rb and p53 function leads to S phase induction, thereby promoting maximally efficient replication of these viruses.
In herpesviruses, lytic viral replication is associated with a block in cell cycle progression rather than with induction of S phase (2, 8, 33, 45, 56). Nevertheless, the immediate-early proteins of both cytomegalovirus (CMV) and EBV inhibit Rb and p53 function (17, 47, 68, 70). In addition, both herpes simplex virus and CMV modulate E2F function(s) (28, 48, 49). Thus, efficient herpesvirus replication probably requires the regulation of critical cell cycle proteins.
The BRLF1 protein was recently shown to interact directly with Rb (68), but its effect on cell cycle progression is unknown. We have used a BRLF1-expressing adenovirus to examine the effect of BRLF1 on a variety of cell cycle regulatory proteins. We show that BRLF1 expression is sufficient to induce contact-inhibited, quiescent human fibroblasts to enter S phase and dramatically increases the level of E2F1. Consistent with the increased levels of E2F1, BRLF1-expressing cells have increased levels of p53 and p21 and rapidly die by apoptosis. BRLF1 expression is also associated with decreased levels of Rb, p107, and p130. Thus, in addition to its role as a transcriptional activator, the EBV immediate-early protein BRLF1 has profound effects on key cell cycle regulatory proteins.
MATERIALS AND METHODS
Cell culture.
A secondary culture of normal human fibroblasts (NHF5-neo, referred to here as NHF [4, 36]) was derived from neonatal foreskin. The fibroblasts were maintained in Eagle’s minimal essential medium, supplemented with 10% fetal bovine serum (FBS) and nonessential amino acids. U-2 OS (ATCC HTB 96) and Saos-2 (ATCC HTB 85), both human osteosarcoma cell lines, were maintained in McCoy’s 5a medium supplemented with 15% FBS. HeLa, a human cervical carcinoma cell line, and NPC-KT, an EBV-positive nasopharyngeal carcinoma fusion cell line (63), were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% FBS. 293, a transformed primary human embryonal kidney cell line expressing the transforming genes of adenovirus type 5 (18), was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. B95-8, an EBV-infected marmoset B-cell line, was maintained in RPMI medium supplemented with 10% FBS. All media contained penicillin (100 U/ml) and streptomycin (100 μg/ml). The cells were maintained at 37°C in a humidified atmosphere containing 10% CO2. To induce B95-8 cells into lytic EBV infection, TPA (12-O-tetradecanoylphorbol-13-acetate; 30 ng/ml) and sodium butyrate (5 mM) were added to the culture medium 72 h prior to harvest.
Plasmid DNAs and adenovirus vectors.
E2F1 pcDNA3, which was kindly provided by Joseph Nevins, contains the human E2F1 cDNA in the pcDNA3 vector (Invitrogen). pRTS 15, kindly provided by Diane Hayward, contains a genomic BRLF1 fragment expressed by the simian virus 40 (SV40) promoter in the pSG5 vector (Stratagene).
The BRLF1 recombinant adenovirus (AdBRLF1) was constructed by cloning the 2,188-bp DraI-XbaI fragment from pEBV-RIE (31), containing the BRLF1 gene, into the adenovirus shuttle vector pAdLox (24). The AdBRLF1 recombinant adenovirus was produced by cre-lox-mediated recombination of the pAdLox-BRLF1 vector with a replication-deficient, E1- and E3-deleted type 5 adenovirus. BRLF1 gene expression is driven by the CMV immediate-early promoter in AdBRLF1. The control adenovirus, AdLacZ, is identical to AdBRLF1, except that it contains the bacterial β-galactosidase gene rather than BRLF1. AdBRLF1 and AdLacZ adenovirus stocks were generated in 293 cells and prepared by the Gene Therapy Core Facility, University of North Carolina.
Adenovirus infections.
Normal human fibroblasts (NHF) (106) were plated on 60-mm plates and U-2 OS, Saos-2, HeLa, and 293 cells (106) were plated on 100-mm plates 24 h prior to infection. The normal growth medium was removed and replaced with adenovirus in 2% FBS in the appropriate media; a multiplicity of infection (MOI) of 500 was used for NHF, while an MOI of 50 was used for U-2 OS, Saos-2, HeLa, and 293 cells. The cells were infected in a small volume for 2 to 3 h; fresh medium containing serum was then added to the infection media to return the serum concentration to that used in the normal growth media for each cell type.
Transfections.
Plasmid DNA was purified with the Qiagen Maxi kit as specified by the manufacturer. DNA was transfected by electroporation with 5 μg of DNA and 107 cells per condition. The cells were shocked at 1,500 V with a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin, Madison, Wis.). Epithelial cells were harvested and suspended in RPMI medium supplemented with 10% FBS prior to electroporation. Protein extracts were harvested 48 h posttransfection.
Cell cycle analysis.
Cells were incubated with 10 μM bromodeoxyuridine (BrdU) for 60 min at 37°C, 24 or 48 h after infection with adenovirus. Trypsin-released cells were fixed in 1.5 ml of cold 1× phosphate-buffered saline (PBS)–3 ml of ice-cold 95% ethanol and incubated at 4°C overnight. The cells were pelleted, suspended in 3 ml of 0.8% pepsin in 0.1 N HCl, and incubated at 37°C for 20 min. Lysates were centrifuged, nuclear pellets were suspended in 1.5 ml of 2 N HCl and incubated at 37°C for 20 min, 3 ml of 0.1 M sodium borate was added, and the lysates were repelleted. The nuclear pellets were washed in IFA (10 mM HEPES [pH 7.4], 150 mM NaCl, 4% FBS, 0.1% sodium azide) containing 0.5% Tween 20. They were then stained with anti-BrdU antibody (1:50; Becton Dickinson) in IFA at room temperature (RT) for 30 min, washed with IFA containing Tween 20, counterstained with goat anti-mouse immunoglobulin G (whole molecule)-fluorescein isothiocyanate conjugate (1:100; Sigma) in IFA at RT for 30 min, and washed with IFA containing Tween 20. Nuclear pellets were suspended in IFA containing 500 μg of RNase A and 50 μg of propidium iodide per ml and incubated at 37°C for 15 min and then on ice overnight. Fluorescence was measured with a fluorescence-activated cell sorter (FACS; Becton Dickinson).
Immunofluorescence.
Cells were harvested 24 h postinfection, washed with PBS, fixed in 60% acetone on ice for 10 min, and washed with PBS–0.5% bovine serum albumin (BSA). They were then stained with anti-BRLF1 antibody (1:100; Argene) in PBS–0.5% BSA for 60 min and washed with PBS–0.5% BSA and counterstained with goat anti-mouse immunoglobulin G (whole molecule)-fluorescein isothiocyanate conjugate (1:100) in PBS–0.5% BSA for 60 min. They were then washed and suspended in PBS and analyzed with a FACS.
Apoptosis assays.
Apoptosis was determined by three methods: Hoechst staining, a DNA fragmentation assay (39, 54) and immunoblotting. In Hoechst staining, the cells were fixed in methanol-acetic acid (3:1) for 2 h, stained with Hoechst stain for 10 min, and viewed by fluorescence microscopy for apoptotic bodies. In DNA fragmentation analysis for HeLa cells, the cells were pelleted and rinsed in PBS and then suspended in 0.5 ml of DNA extraction buffer (20 mM Tris HCl [pH 7.4], 10 mM EDTA, 0.2% Triton X 100) and incubated at RT for 10 min. Debris was pelleted, and supernatant was transferred to a new tube and treated with proteinase K (100 μg/ml) at 50°C overnight. Samples were treated with RNase A (50 μg/ml) at 37°C for 2 h. DNA was extracted twice with phenol and once with an isoamyl alcohol-chloroform (1:24) mixture and precipitated with 2 volumes 95% ethanol–1/10 volume of 10 M ammonium acetate–glycogen (5 μg/ml) at −20°C overnight. The DNA was pelleted, suspended in 1× TE (10 mM Tris [pH 7.5], 1 mM EDTA), and electrophoresed on a 1.25% agarose gel. In DNA fragmentation analysis for normal human fibroblasts, floating cells were pelleted, washed in PBS, suspended in 40 μl of PBS–360 μl of lysis buffer (10 mM Tris [pH 7.5], 10 mM EDTA [pH 8], 0.6% sodium dodecyl sulfate), and incubated at 37°C for 2 h. Then 100 μl of 5 M NaCl was added to each sample, and the mixture was incubated at 4°C overnight. Samples were centrifuged to remove cell debris, and DNA was precipitated with 2 volumes of cold 95% ethanol overnight at −20°C. The DNA was pelleted, washed with 70% ethanol, suspended in 1× TE, and analyzed on a 1.25% agarose gel. Lastly, apoptosis was monitored by immunoblotting for poly(ADP-ribose) polymerase (PARP) cleavage (41) as described below.
Immunoblotting.
Cells were harvested 24 to 48 h postinfection and washed with PBS, and nuclear extracts were obtained as previously described (10). Proteins were separated by polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane. The membranes were washed with blotting buffer (1× PBS, 0.05% Tween 20, 1% milk) and incubated at RT for 60 min with primary antibody in blotting buffer. Primary antibodies included anti-BMRF1 (1:40; Capricorn Products, Inc.), anti-E2F1 (1:100; C-20 sc-193 [Santa Cruz]), anti-E2F4 (1:1,000; C-108 sc-512X [Santa Cruz]), anti-Rb (1:250; 14001A G3-245 [PharMingen]), anti-p107 (1:1,000; C-18 sc-318X [Santa Cruz]), anti-p130 (1:1,000; C-20 sc-317X [Santa Cruz]), anti-p53 (1:200; DO-1 sc-126 [Santa Cruz]), anti-p21 (1:500; C-19 sc-397 [Santa Cruz]), and anti-PARP (1:100; A-20 sc-1562 [Santa Cruz]). The membranes were washed in blotting buffer and incubated with horseradish peroxidase-conjugated secondary antibody at RT for 60 min. The membranes were washed in blotting buffer, and proteins were detected by enhanced chemiluminescence (ECL; Amersham).
Viral DNA replication assay.
Viral DNA was isolated at various times postinfection (0, 24, and 48 h) by the method of Hirt (29). Extracts were treated with DNase-free proteinase K prior to extraction, ethanol precipitation, and suspension in 1× TE. Viral DNA was digested with ClaI and electrophoresed on a 0.8% agarose–Tris-borate-EDTA (TBE) gel. The DNA was transferred to a nitrocellulose membrane by the method of Southern (57). The immobilized DNA was detected with a 32P-labeled pAdlox DNA probe and visualized by autoradiography.
Electrophoretic mobility shift assay.
Nuclear extracts (12 μg) were incubated at RT for 30 min in a 20-μl reaction volume in 20 mM HEPES (pH 7.9)–20% glycerol–0.1 M KCl–0.2 mM EDTA–0.5 mM dithiothreitol supplemented with 20 μg of BSA and 2 μg of denatured salmon sperm DNA. When applicable, competing antibodies (1 μl of E2F1 [Santa Cruz]) or DNA (2 pmol of cyclic AMP-responsive element binding protein [CREB; Promega] or E2F [a 24-bp oligonucleotide containing the E2F binding site from the dihydrofolate reductase promoter; 5′ CCACAATTTCGCGCCAAACTTGAC 3′]) was added to the nuclear extracts prior to the 20-min incubation. A radiolabeled E2 oligonucleotide probe (2 × 104 cpm) spanning nucleotides −31 to −74 of the adenovirus E2 promoter (32) was added to each sample, and the mixtures were incubated at RT for 20 min. A 2-μl volume of 1.5× loading dye (glycerol; 1× TE [1:1]) was added to each sample, and the mixtures were electrophoresed on a 4.5% polyacrylamide–0.11% bisacrylamide gel in 0.5× TBE at 4°C. The gel was dried, and complexes were visualized by autoradiography.
RESULTS
AdBRLF1 induces lytic EBV infection.
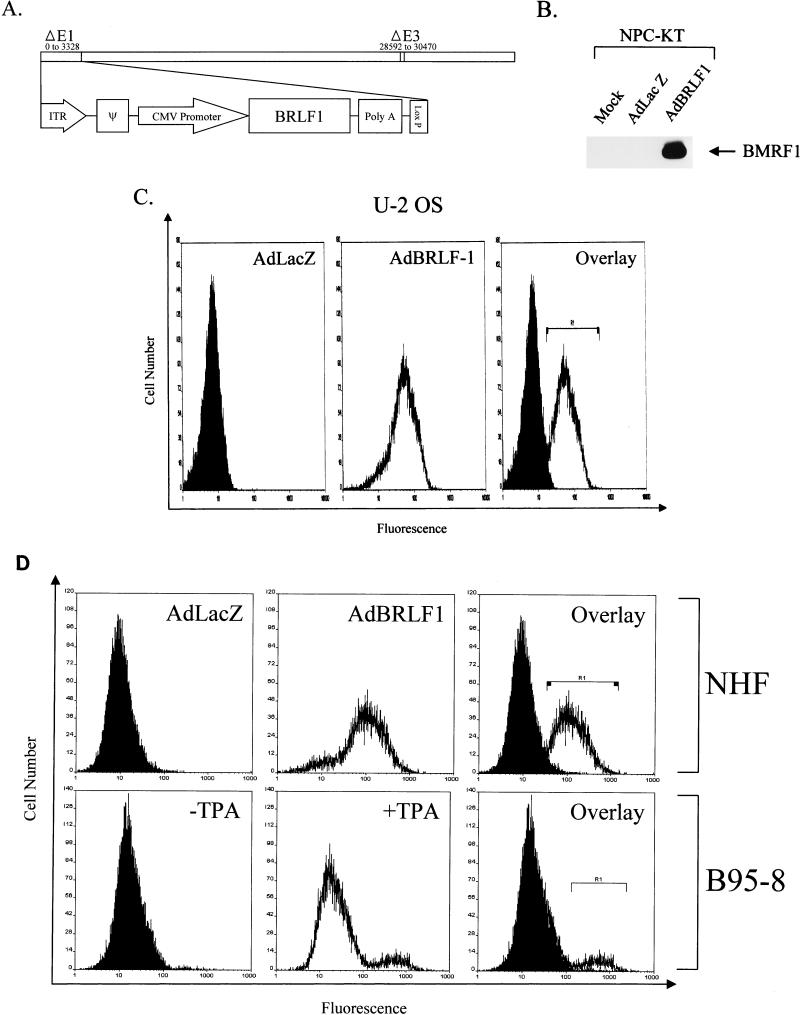
A recombinant adenovirus, AdBRLF1, was constructed to express BRLF1 in a majority of cells for these studies. The recombinant adenovirus (in which BRLF1 is driven by the CMV IE promoter) has E1 and E3 deleted and therefore is replication deficient (Fig. 1A). To confirm the expression of functional BRLF1 protein from AdBRLF1, NPC-KT cells (which harbor latent EBV) were either mock infected or infected with the control AdLacZ vector or AdBRLF1 and assayed for expression of an early EBV protein, BMRF1, used as an index of viral replication. Immunoblot analysis indicated that neither mock-infected nor AdLacZ-infected cells expressed the BMRF1 protein. However, AdBRLF1 was able to induce lytic EBV infection, as indicated by the expression of BMRF1 (Fig. 1B). These results confirm that the AdBRLF1 vector produces functional BRLF1 protein.
FIG. 1.
The AdBRLF1 recombinant adenovirus efficiently infects cells. (A) Schematic representation of the recombinant adenovirus expressing BRLF1 (AdBRLF1). AdBRLF1 was produced by cre-loxP-mediated recombination of the pAdLox-BRLF1 shuttle vector with a replication-deficient, E1- and E3-deleted type 5 adenovirus (24). ITR, inverted terminal repeat, ψ, packaging signal. (B) NPC-KT cells (with a latent EBV infection) were mock, AdLacZ, or AdBRLF1 infected at an MOI of 50 and immunoblotted 24 h after infection by using a monoclonal antibody directed against the early lytic EBV protein BMRF1. (C) U-2 OS cells were infected with AdLacZ (left) or AdBRLF1 (middle) (MOI, 50) and analyzed for BRLF1 expression by immunofluorescence staining and FACS analysis 24 h after infection. The right panel represents an overlay of the AdLacZ and AdBRLF1 histograms; cells within the R1 bracket were considered positive for BRLF1 expression. (D) NHF, either AdLacZ or AdBRLF1 infected (MOI, 500), were analyzed for BRLF1 expression 24 h postinfection. In a parallel experiment, B95-8 cells, in the presence (+) or absence (−) of the inducing agent, TPA, were also examined for BRLF1 expression. The R1 bracket indicates cells which were considered positive for BRLF1 expression.
The infection efficiency of AdBRLF1 was analyzed by FACS analysis (with a BRLF1-specific monoclonal antibody) in a variety of cell types. As shown in Fig. 1C, approximately 90% of U-2 OS cells expressed the BRLF1 protein when infected with an MOI of 50. Similar results were obtained in experiments with HeLa and Saos-2 cells (data not shown); however, an MOI of 500 was required to infect a similar percentage of NHF. To confirm that AdBRLF1 expresses a level of BRLF1 which is similar to that found in cells lytically infected with EBV, FACS analysis was used to compare the level of BRLF1 expression in AdBRLF1-infected fibroblasts and lytically infected B95-8 cells (Fig. 1D). Although only 10% of B95-8 cells expressed BRLF1 in this experiment upon TPA induction, while 78% of the fibroblasts expressed BRLF1, the level of BRLF1 expression per cell in B95-8 cells was similar to or slightly higher than the level observed in AdBRLF1-infected fibroblasts. The mean fluorescence units of the BRLF1-expressing population in induced B95-8 cells and AdBRLF1-infected fibroblasts were 587 and 143, respectively (Fig. 1D). These experiments indicate that the level of BRLF1 expression induced by AdBRLF1 infection is biologically relevant and that the efficiency of AdBRLF1 infection in the above cell lines is sufficient to examine the effect of BRLF1 on cell cycle progression.
AdBRLF1 induces cells to enter S phase.
To determine the effect of BRLF1 on cell cycle progression, cells were either mock infected or infected with the control AdLacZ virus or the AdBRLF1 virus and were labeled with BrdU for 60 min 1 or 2 days after infection to determine the percentage of cells in S phase. BrdU incorporation was quantitated by using a BrdU-specific antibody in FACS analysis. As shown in two independent experiments (Table 1), AdBRLF1-infected confluent U-2 OS and NHF cultures had many more cells in S phase than did the mock-infected or AdLacZ-infected cultures. In confluent U-2 OS cultures, 70 to 75% of AdBRLF1-infected cells were in S phase, versus only 32 to 45% of cells infected with the control adenovirus vector. More strikingly, AdBRLF1 was able to overcome the G0 block imposed by contact inhibition in the NHF, with the percentage of cells in S phase increasing from 7 to 32% following BRLF1 expression; similar results were obtained in a second independent experiment (Table 1). In contrast to the results found with U-2 OS osteosarcoma cells (which have wild-type p53 and Rb), no increase in the percentage of cells in S phase was evident after AdBRLF1 expression in the Saos-2 osteosarcoma cell line (which has no Rb or p53). These results indicate that BRLF1 is able to induce S phase entry, possibly in a p53- and/or Rb-dependent manner.
TABLE 1.
BRLF1 activates S phase progression
| Infection | % of S phase cellsa
|
|||||
|---|---|---|---|---|---|---|
| NHF
|
U-2 OS
|
Saos-2
|
||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | Expt 1 | Expt 2 | |
| Mock | 4 | 3 | 44 | 55 | 39 | 44 |
| AdLacZ | 7 | 2 | 32 | 45 | 44 | 46 |
| AdBRLF1 | 32 | 36 | 75 | 70 | 35 | 48 |
Percentage of S phase cells as determined by BrdU incorporation. Results are shown for two independent experiments.
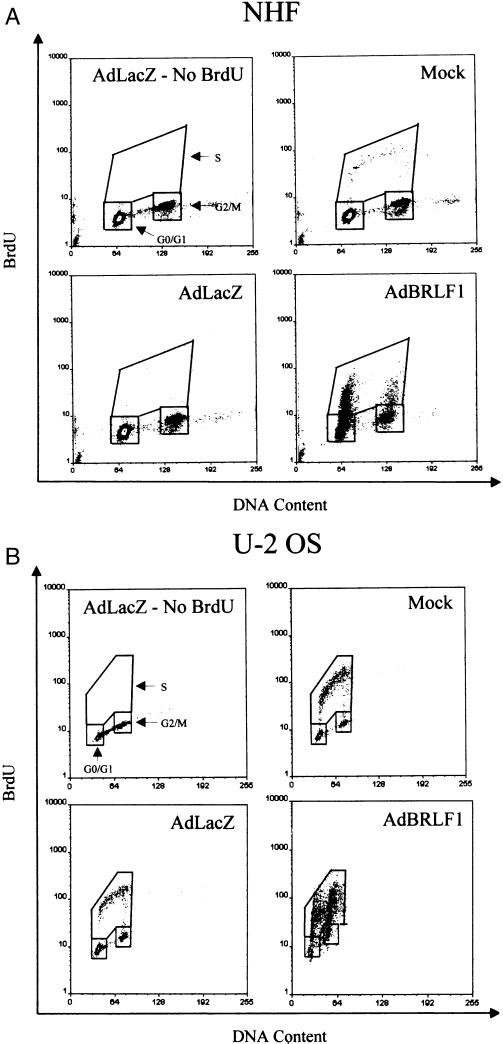
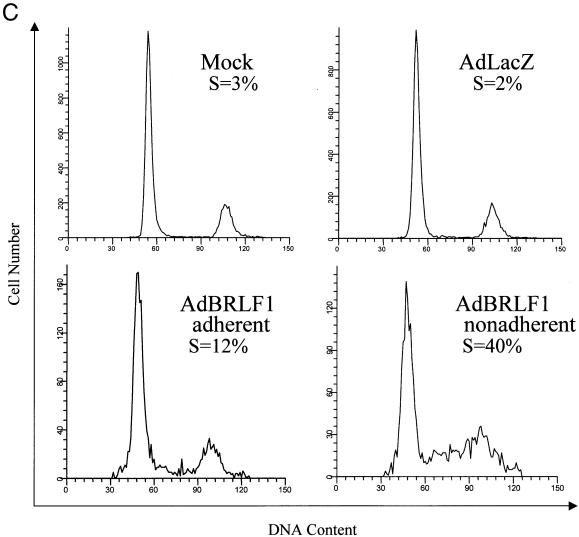
To further examine the effect of BRLF1 expression on the cell cycle, AdBRLF1-infected cells were stained with propidium iodide after BrdU labeling and analyzed by FACS. Cell cycle profiles of mock-infected, AdLacZ-infected, and AdBRLF1-infected cells 24 h postinfection are shown in Fig. 2A and B. The DNA content is represented on the x axis, and BrdU fluorescence is represented on the y axis. Cells which are entering or in S phase incorporate BrdU and are represented in the box labeled S. As suggested by the previous experiments, BRLF1 expression in NHF (Fig. 2A) and U-2 OS cells (Fig. 2B) increased the number of cells in S phase. Interestingly, contact-inhibited BRLF1-expressing NHF which had become detached from the plate (Fig. 2C, AdBRLF1 nonadherent) exhibited an even greater percentage of cells in S phase. In contrast, BRLF1 did not increase S phase progression in Saos-2 cells (data not shown).
FIG. 2.
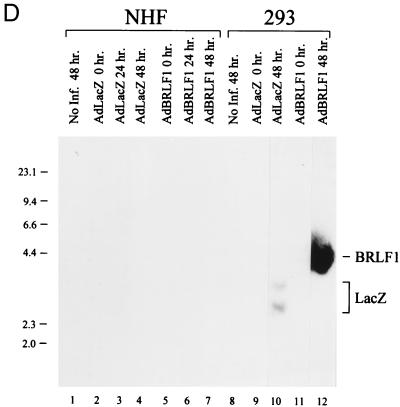
Cell cycle analysis of AdBRLF1-infected cells. (A) NHF were mock, AdLacZ, or AdBRLF1 infected. Prior to harvest, the cells were labeled for 1 h with BrdU, stained with propidium iodide and an anti-BrdU antibody, and then subjected to FACS analysis. The DNA content (propidium iodide staining) is represented on the x axis, and BrdU fluorescence is represented on the y axis. An AdLacZ-infected control, not labeled with BrdU, was included to determine the background fluorescence for each cell type. The locations of the G0/G1, G2/M, and S phases are indicated. The apparent increased fraction of 4N S phase cells in BRLF1-infected cells probably reflects S phase initiation by tetraploid fibroblasts (14). (B) U-2 OS cells were infected with the various adenoviruses and analyzed as in panel A. (C) NHF were mock, AdLacZ or AdBRLF1 infected, stained with propidium iodide, and subjected to FACS analysis; adherent and nonadherent AdBRLF1-infected cells were analyzed separately. DNA content is represented on the x axis, and cell number is represented on the y axis. (D) NHF (lanes 1 to 7) and 293 cells (lanes 8 to 12) were infected with AdLacZ or AdBRLF1, and viral DNA was harvested at various times postinfection (0, 24, and 48 h) by the Hirt method (29). DNA was digested, separated by electrophoresis, and detected with a linearized 32P-labeled pAdLox probe. Samples were visualized by autoradiography. The bands representing replicated BRLF1 and AdLacZ adenovirus DNA are indicated on the right.
Although BRLF1 increased the total number of diploid fibroblasts in early S phase, the number of diploid fibroblasts in mid-S phase did not increase, even after 48 h, suggesting that BRLF1 may induce a block in S phase progression (Fig. 2A and B). To confirm that the increase in the number of S phase cells evident in BRLF1-expressing cells was due to cellular DNA replication and not adenovirus DNA replication, we performed a viral DNA replication assay with NHF and 293 cells (Fig. 2D). As expected, in the presence of adenovirus E1A protein, adenovirus DNA replication occurred for both AdLacZ and AdBRLF1 in 293 cells (Fig. 2D, lanes 8 to 12). However, essentially no viral DNA replication was evident in AdLacZ- or AdBRLF1-infected NHF (lanes 1 to 7). Furthermore, BRLF1-infected NHF treated with hydroxyurea, an inhibitor of semiconservative DNA replication but not of DNA repair synthesis (5), exhibited diminished BrdU incorporation per cell (data not shown). These results support the conclusion that the increase in the number of S phase cells evident for BRLF1-infected NHF is due to cellular DNA replication.
AdBRLF1 induces an increase in E2F1.
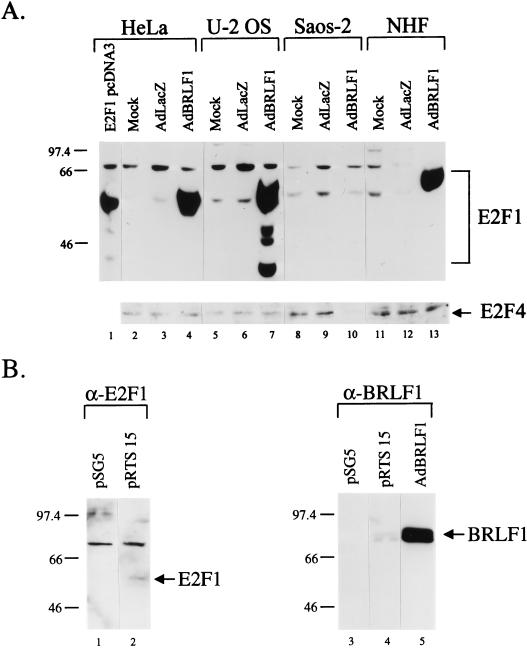
Entry into S phase is controlled, in part, by the level of transcriptionally active E2F (34, 39, 65). Therefore, the effect of BRLF1 expression on two different E2F family members was examined (Fig. 3A). Infection with the AdBRLF1 vector dramatically increased the level of E2F1 in NHF, HeLa and U-2 OS cells. However, consistent with the cell cycle results, BRLF1 expression did not increase E2F1 level in Saos-2 cells. In contrast to its effect on E2F1, BRLF1 did not increase the level of E2F4 in any cell type (Fig. 3A).
FIG. 3.
BRLF1 induces an increase in E2F1 protein. (A) HeLa, U-2 OS, Saos-2, and NHF cells were mock, AdLacZ, or AdBRLF1 infected or transfected with 5 μg of the E2F1 expression vector E2F1 pcDNA3 (HeLa cells only) and analyzed for E2F1 and E2F4 expression by immunoblotting. (B) HeLa cells were transfected (5 μg) with the control vector pSG5 or the BRLF1-expression vector pRTS 15 and analyzed for E2F1 expression by immunoblotting (left). The level of BRLF1 expression in HeLa cells infected with AdBRLF1 or transfected with the pRTS 15 expression vector was determined by immunoblotting (right).
To determine whether the BRLF1-induced increase in E2F1 occurs only within the context of the adenovirus system, HeLa cells were transfected with a BRLF1 expression plasmid, pRTS 15, or a control vector and assayed for E2F1 expression (Fig. 3B). A small increase in the E2F1 level was evident in the BRLF1-transfected cells compared to the control vector (Fig. 3B, lanes 1 and 2). Although the increase in the E2F1 level was much less dramatic in the BRLF1-transfected cells (lanes 1 and 2) than that seen in AdBRLF1-infected cells (Fig. 3A, lanes 3 and 4), the level of BRLF1 expression by the transfected plasmid was much less than that by the AdBRLF1 vector (Fig. 3B, lanes 4 and 5). These results indicate that BRLF1 is capable of inducing E2F1 in both transfection and recombinant adenovirus environments.
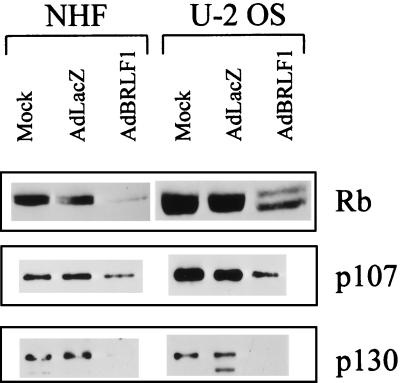
BRLF1 reduces the level of Rb, p107, and p130.
Since BRLF1 has previously been shown to interact directly with Rb (68), we also examined the effect of BRLF1 expression on the level of Rb, p107, and p130. Interaction between a number of viral proteins (including BK virus and SV40 T antigens and papillomavirus E7) and Rb family members has been shown to diminish the overall protein levels of Rb family members (25, 35, 58, 59). As shown in Fig. 4, AdBRLF1-infected NHF and U-2 OS cells had a decreased level of Rb, p107, and p130 in comparison to AdLacZ-infected cells. Thus, the ability of BRLF1 to induce S phase progression may reflect not only increased levels of E2F1 but also decreased levels of Rb family members.
FIG. 4.
BRLF1 decreases the level of Rb family members. NHF and U-2 OS cells were mock, AdLacZ, or AdBRLF1 infected and analyzed for the level of Rb, p107, and p130 at 24 h postinfection.
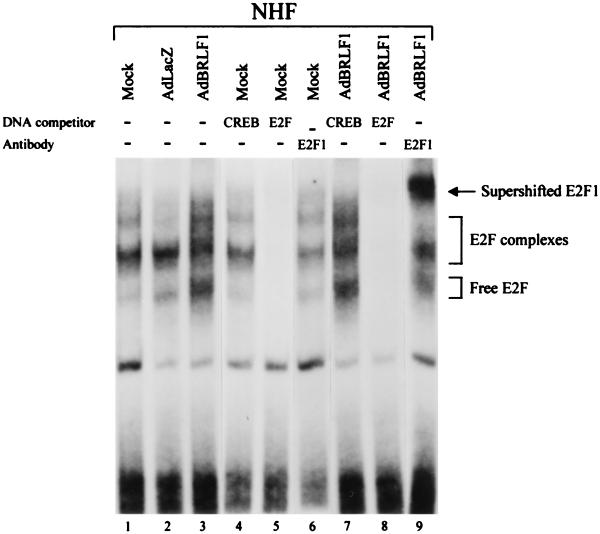
AdBRLF1 induces increased levels of free E2F.
S phase progression is mediated by free E2F (not bound to Rb family members) (34, 39, 65). An electrophoretic mobility shift assay was performed to compare the E2F DNA binding activity of NHF which were either mock infected, AdLacZ infected, or AdBRLF1 infected (Fig. 5, lanes 1 to 3). Addition of an E2F DNA competitor demonstrated that the majority of the DNA complexes contain E2F (lanes 5 and 8). Preliminary experiments involving deoxycholate treatment and a variety of antibodies directed against Rb, p107, p130, E2F1, and E2F4 were performed to identify the positions of free E2F and various E2F complexes (data not shown). Mock-infected or AdLacZ-infected NHF had predominantly E2F4-composed complexes (data not shown). In AdBRLF1-infected cells, there was an increase in the level of free E2F, and this increase was due to E2F1 (Fig. 5, lanes 3 and 9). Although it was not always possible to differentiate between Rb-E2F and p107-E2F complexes, in most experiments there was a decrease in the level of the mixed Rb-E2F and p107-E2F complexes. These results indicate that BRLF1 increases the level of free E2F1 in cells, consistent with its ability to increase the total level of E2F1 while decreasing the level of Rb.
FIG. 5.
BRLF1 increases free E2F1 levels. NHF were mock, AdLacZ, or AdBRLF1 infected. Nuclear extracts were harvested at 24 h postinfection and analyzed for DNA binding to a labeled probe containing an E2F binding site by the electrophoretic mobility shift assay (lanes 1 to 3). DNA competitors (CREB [lanes 4 and 7] and E2F [lanes 5 and 8]) and competing E2F1 antibody (lanes 6 and 9) were included in the binding-reaction mixture to determine the composition of various DNA binding complexes. The positions of free E2F, E2F complexes, and supershifted E2F1 are indicated. The decrease in the level of the uppermost E2F complex evident in the AdLacZ sample was unusual; these complexes were typically visible for this sample.
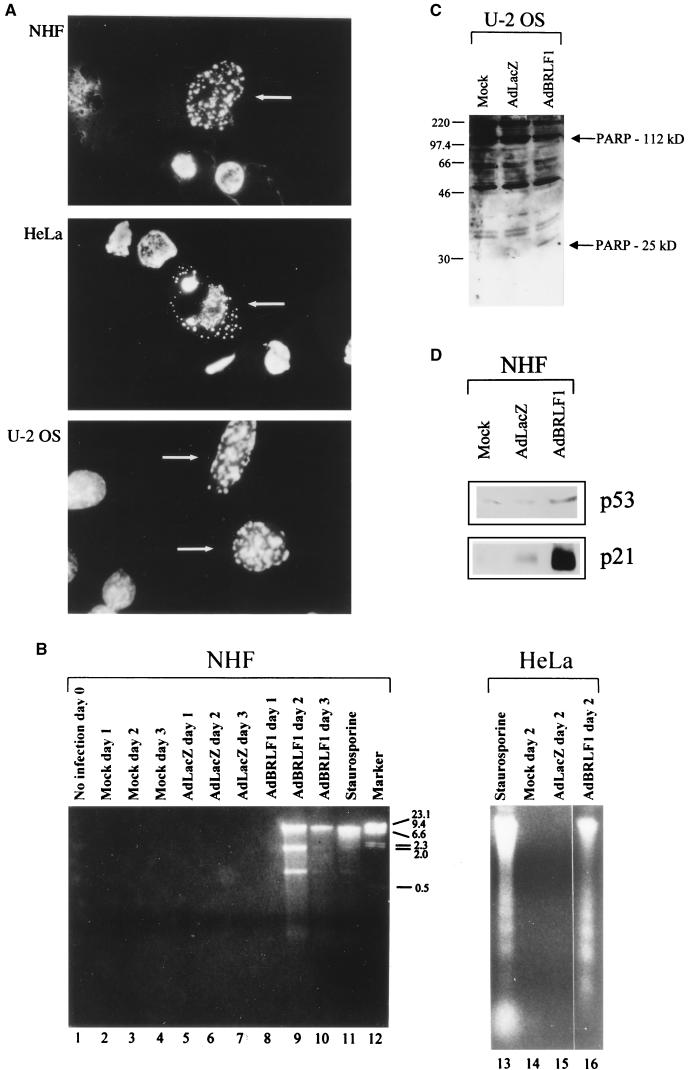
AdBRLF1 induces apoptosis.
In spite of the ability of BRLF1 to induce S phase progression, AdBRLF1-infected cells died within 3 days after infection (data not shown). Propidium iodide staining of AdBRLF1-infected cells showed a large number of cells containing less than 1× DNA, suggesting that BRLF1-induced cell death is due to apoptosis (data not shown; such cells are excluded from the cell cycle analysis in Fig. 2). Hoechst staining of BRLF1-infected NHF, HeLa and U-2 OS cells revealed more apoptotic bodies (Fig. 6A) than in AdLacZ-infected cells (data not shown). To further study BRLF1-induced apoptosis, we analyzed DNA fragmentation in NHF released into the supernatant at various times postinfection. This method was previously used to demonstrate that E2F-1 expression induces apoptosis in rat fibroblasts (39). As shown in Fig. 6B, AdBRLF1-infected NHF had fragmented DNA similar to that produced by the apoptotic drug staurosporine in these cells (lanes 9 to 11). AdBRLF1 expression also induced apoptosis in HeLa cells as evidenced by DNA fragmentation and laddering (Fig. 6B) and in U-2 OS cells as evidenced by PARP cleavage (Fig. 6C). These results indicate that BRLF1 induces apoptosis in at least some cell types.
FIG. 6.
BRLF1 induces apoptosis. (A) NHF, HeLa, and U-2 OS cells were infected with AdBRLF1. The cells were fixed in methanol-acetic acid (3:1) for 2 h, stained with Hoechst stain for 10 min, and visualized by fluorescence microscopy. Apoptotic cells are indicated by the arrows. (B) NHF and HeLa cells were mock, AdLacZ, and AdBRLF1 infected. Apoptosis in HeLa cells was measured 2 days after infection, while apoptosis in NHF was examined on days 1, 2, and 3 postinfection by using a DNA ladder assay (39, 54). Staurosporine-treated cells served as the positive control in both assays. DNA size markers (in kilobases) are indicated on the right of the NHF lanes. (C) U-2 OS cells were mock, AdLacZ, or AdBRLF1 infected and analyzed for PARP cleavage by immunoblotting. (D) NHF were mock, AdLacZ, or AdBRLF1 infected, and the levels of p53 and p21 were determined by immunoblotting 24 h postinfection.
E2F1 induces apoptosis by activating p53 (39). Similarly, BRLF1 (by inducing E2F1) might be expected to increase the level of p53. As shown in Fig. 6D, this is indeed the case. The p53 induced by BRLF1 appears to be functional, since BRLF1 expression is also associated with increased p21 (which is transcriptionally activated by p53) (12). The findings that BRLF1 induces p53 and that apoptosis is reduced in p53-deficient Saos-2 cells, suggests that BRLF1-induced apoptosis is at least partially mediated through p53.
DISCUSSION
BRLF1 plays a critical role in EBV regulation, since its expression is sufficient to induce the switch from latent to lytic infection (51, 69). As is the case for other critical viral regulatory proteins, such as SV40 T antigen and adenovirus E1A, BRLF1 may also regulate the cell through multiple different mechanisms, thereby creating the most hospitable environment for lytic viral replication. Here we demonstrate that BRLF1 dramatically increases the level of cellular E2F1 while simultaneously decreasing the expression of Rb family members. The induction of E2F1 is accompanied by S phase entry and subsequent apoptosis. Thus, in addition to its ability to activate early EBV genes through a direct DNA binding mechanism, BRLF1 profoundly affects the host cell environment.
Although BRLF1 has been recently shown to interact directly with Rb (68), the functional consequences of this interaction have not been well studied. Our results show an overall reduction in the level of Rb family members in BRLF1-expressing cells. Although it has been suggested that the Rb-BRLF1 interaction may act to disrupt the Rb-E2F1 complex (68), a major consequence of the BRLF1-Rb interaction may instead be to reduce the stability of Rb family members. Similar results have recently been reported with other viral proteins (including SV40 T antigen, BK virus T antigen, and papillomavirus E7) which interact directly with Rb (25, 35, 58, 59).
Somewhat surprisingly, the most dramatic effect of BRLF1 expression is the induction of E2F1. In fact, the BRLF1-induced activation of S phase entry may be due primarily to E2F1 induction rather than to inactivation of Rb. As previously observed with E2F1 expression (39), BRLF1 is likewise striking in its ability to override signals of quiescence in NHF. Like E2F1, BRLF1 also induces massive apoptosis (39). Consistent with the ability of BRLF1 to increase the level of total cellular E2F1, we also observed an increase in the level of free E2F1 binding activity. Thus, activation of E2F1 (perhaps in conjunction with the loss of Rb activity) is probably responsible for much of the phenotype observed in AdBRLF1-infected cells. Interestingly, the CMV IE protein IE-72 was also recently shown to activate E2F1 function, in this case through direct phosphorylation (46, 48), and herpes simplex virus infection results in increased levels of free E2F as well as of E2F-p107 complexes (28). Thus, modulation of E2F function by IE proteins may be a requirement for efficient lytic replication of herpesviruses.
Lytic EBV infection occurs in postmitotic, differentiated epithelial cells (52), which presumably have little if any free E2F. However, it has already been shown that an E2F site is essential for BRLF1 activation of the EBV DNA polymerase (44), suggesting that free cellular E2F is required for replication of viral DNA. Consistent with this model, the level of E2F1 is increased during induction of lytic EBV infection in Akata cells (68). In addition to activating the EBV polymerase, E2F1 induction would be expected to activate the transcription of many proteins involved in cellular DNA synthesis and cell cycle progression. E2F-induced cellular proteins (which would normally be expressed at the late G1/S interphase) may be required for efficient lytic EBV replication in differentiated epithelial cells.
Currently, we are not certain how BRLF1 induces E2F1 levels. Although BRLF1 is a transcriptional activator, we have not found that AdBRLF1 infection in NHF increases the level of E2F1 mRNA (60). Therefore, BRLF1 more probably increases the stability of E2F1 protein. Ubiquitination of E2F1 targets it for degradation by the proteosome pathway; since the interaction between Rb and E2F1 protects E2F1 from degradation (1, 30), the decreased level of Rb in AdBRLF1-infected cells might have been expected to lower E2F1 levels. It will be important to determine if BRLF1 can interact directly with E2F1 (preventing degradation) or if it stabilizes the Rb-E2F interaction. In support of the latter hypothesis, we did not observe an BRLF1-induced increase in E2F1 level in Saos-2 cells, the only cell line studied which is lacking Rb. We cannot absolutely exclude the possibility that an adenovirus-encoded protein collaborates with BRLF1 to increase E2F1 levels, since the increase was much more dramatic in cells infected with the AdBRLF1 vector than in BRLF1-transfected cells.
Our data suggest that BRLF1 induces apoptosis, most probably through induction of E2F1. The observed increase in the level of p53 (and of the p53-responsive protein, p21) no doubt reflects the cellular response to unscheduled S phase entry. In the context of the intact virus, either BZLF1 (which we have shown interacts directly with and inhibits the function of p53) (70) or BHRF1 (a bcl2 homolog) (26) may act to limit BRLF1-induced apoptosis.
Our results are somewhat paradoxical, since we show that BRLF1 activates S phase entry and yet lytic EBV infection occurs in the presence of agents (such as sodium butyrate) known to inhibit S phase progression (56) and may in fact be less efficient during S phase (62). We can reconcile this paradox by assuming that E2F1 induction is the primary role of BRLF1 in this process whereas induction of S phase reflects the absence of an additional EBV protein(s) which normally counteracts the E2F1-induced S phase entry in EBV-infected cells. Expression of the EBV IE protein BZLF1 (which is expressed simultaneously with BRLF1 during disruption of viral latency) arrests cells in G0/G1 (2). Thus, in the context of the entire virus, BZLF1 may arrest cells in G0/G1 to prevent cellular DNA replication, while BRLF1 may aid viral replication by E2F1 induction. Consistent with this hypothesis, our preliminary results indicate that BZLF1 does not prevent BRLF1-induced E2F1 accumulation (data not shown). It will be important to determine how BZLF1 and BRLF1 interact in cells to create and maintain a cellular environment favorable for lytic EBV replication.
ACKNOWLEDGMENTS
We thank Joseph Nevins, Diane Hayward, and Erik Flemington for reagents. We thank Cheryl Cistulli for microscopy assistance. We thank Erik Flemington, Jane Azizkhan, Steve Bachenheimer, and Xue Xiong for helpful discussions. We thank Joseph Pagano for critical reading of the manuscript. We thank Doug McCarty, Jude Samulski, and the UNC Gene Therapy Core Facility for adenovirus preparation.
This work was supported by Public Health Service grants 2T32AI07001 (J.J.S.) and PO1-CA19014 and RO1-CA58853 (S.C.K.) from the National Institutes of Health.
REFERENCES
- 1.Campanero M, Flemington E. Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRb tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:2221–2226. doi: 10.1073/pnas.94.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cayrol C, Flemington E. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 1998;15:2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 3.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Dallie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cistulli C, Kaufmann W. p53-dependent signaling sustains DNA replication and enhances clonogenic survival in 254 nm ultraviolet-irradiated human fibroblasts. Cancer Res. 1998;58:1993–2002. [PubMed] [Google Scholar]
- 5.Cleaver J E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969;37:334–348. [PubMed] [Google Scholar]
- 6.Countryman J, Miller G. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox M, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bruyn Kops A, Knipes D. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988;55:868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 9.DeCaprio J, Ludlow J, Figge J, Shew J, Huang C, Lee W, Marsilio E, Paucha E, Livingston D. SV40 large T antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 10.Dignam J, Lebovitz R, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 12.el-Deiry W, Tokino T, Velculescu V, Levy D, Parsons R, Trent J, Lin D, Mercer W, Kinzler K, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 13.Farrell P J, Rowe D T, Rooney C M, Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to consensus Ap1 site and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filatov L, Golubovskaya V, Hurt J C, Byrd L, Phillips J, Kaufmann W. Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 protein. Oncogene. 1998;16:1825–1838. doi: 10.1038/sj.onc.1201711. [DOI] [PubMed] [Google Scholar]
- 15.Flemington E K, Borras A M, Lytle J P, Speck S H. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J Virol. 1992;66:922–929. doi: 10.1128/jvi.66.2.922-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemington E K, Lytle J P, Cayrol C, Borras A M, Speck S H. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol Cell Biol. 1994;14:3041–3052. doi: 10.1128/mcb.14.5.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortunato E A, Sommer M H, Yoder K, Spector D H. Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J Virol. 1997;71:8176–8185. doi: 10.1128/jvi.71.11.8176-8185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grand R, Smiley J, Russel W C, Narin R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1997;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 19.Gruffat H, Duran N, Buisson M, Wild F, Buckland R, Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992;66:46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutsch D, Marcu K B, Kenney S. The Epstein-Barr virus BRLF1 gene product transactivates the murine and human c-myc promoters. Cell Mol Biol. 1994;40:747–760. [PubMed] [Google Scholar]
- 22.Hardwick J M, Lieberman P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardwick J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy S, Kitamura M, Harns-Stansil T, Dai Y, Pines J. Construction of adenovirus vectors through cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris K, Christensen J, Radany E, Imperiale M J. Novel mechanisms of E2F induction by BK virus large-T antigen: requirement of both the pRb-binding and the J domains. Mol Cell Biol. 1998;18:1746–1756. doi: 10.1128/mcb.18.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A B. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiebert S, Lipp M, Nevins J. E1A-dependent transactivation of the human myc promoter is mediated by the E2F factor. Proc Natl Acad Sci USA. 1989;86:3594–3598. doi: 10.1073/pnas.86.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilton M, Mounghane D, McLean T, Contractor N, O’Neil J, Carpenter K, Bachenheimer S L. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1998;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- 29.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann F, Martelli F, Livingston D, Wang Z. The retinoblastoma gene product protects E2F1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996;10:2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]
- 31.Holley-Guthrie E A, Quinlivan E B, Mar E C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imperiale M J, Nevins J R. Adenovirus 5 E2 transcription unit: an E1A-inducible promoter with an essential element that functions independently of position or orientation. Mol Cell Biol. 1984;4:875–882. doi: 10.1128/mcb.4.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jault F M, Jault J M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 35.Jones D L, Munger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann W, Schwartz J, Hurt J, Byrd L, Galloway D, Levedakou E, Pedneault L. Inactivation of G2 checkpoint function and chromosomal destabilization are linked in human fibroblasts expressing human papillomavirus type 16 E6. Cell Growth Differ. 1997;8:1105–1114. [PubMed] [Google Scholar]
- 37.Kenney S, Holley-Guthrie E, Mar E C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, Hirsch M, Chanock R, Melnick J, Monath T, Roizman B, Streib J E, editors. Fields virology. 3rd ed. New York, N.Y: Lippincott-Raven; 1996. pp. 2343–2395. [Google Scholar]
- 39.Kowalik T F, DeGregori J, Schwarz J K, Nevins J R. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane D, Crawford L. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- 41.Lazebnik Y, Kaufmann S, Desnoyers S, Poirier G, Earnshaw W. Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 42.Levine A. p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990;177:419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman P M, Hardwick J M, Hayward S D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type specific manner by two independent signal regions. J Virol. 1989;63:3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Sista N D, Pagano J S. Activation of the Epstein-Barr virus DNA polymerase promoter by the BRLF1 immediate-early protein is mediated through USF and E2F. J Virol. 1996;70:2545–2555. doi: 10.1128/jvi.70.4.2545-2555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. Virology. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margolis M, Pajovic S, Wong E, Wade M, Jupp R, Nelson J, Azizkhan J. Interaction of the 72-kilodalton human cytomegalovirus IE1 gene product with E2F1 coincides with E2F-dependent activation of dihydrofolate reductase transcription. J Virol. 1995;69:7759–7767. doi: 10.1128/jvi.69.12.7759-7767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muralidhar S, Doniger J, Mendelson E, Araujo J, Kashanchi F, Azumi N, Brady J, Rosenthal L. Human cytomegalovirus mtrII oncoprotein binds to p53 and down-regulates p53-activated transcription. J Virol. 1996;70:8691–8700. doi: 10.1128/jvi.70.12.8691-8700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pajovic S, Wong E, Black A, Azizkhan J. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol Cell Biol. 1997;17:6459–6464. doi: 10.1128/mcb.17.11.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poma E, Kowalik T, Zhu L, Sinclair J H, Huang E. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J Virol. 1996;70:7867–7877. doi: 10.1128/jvi.70.11.7867-7877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinlivan E B, Holley-Guthrie E A, Norris M, Gutsch D, Bachenheimer S L, Kenney S C. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;21:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, Chanock R, Melnick J, Monath T, Roizman B, Straus S, editors. Fields virology. New York, N.Y: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 53.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandstrom P, Buttke T. Autocrine production of extracellular catalase prevents apoptosis of the human CEM T-cell line in serum-free medium. Proc Natl Acad Sci USA. 1993;90:4708–4712. doi: 10.1073/pnas.90.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarnow P, Ho Y, Williams J, Levine A. Adenovirus E1b-58 kd tumor antigen and SV40 large T antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 56.Shandan F F, Cowsert L M, Billarreal L P. n-Butyrate, a cell cycle blocker, inhibits the replication of polyomaviruses and papillomaviruses but not that of adenoviruses and herpesviruses. J Virol. 1994;68:4785–4796. doi: 10.1128/jvi.68.8.4785-4796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 58.Stubdal H, Zalvide J, Campbell K, Schweitzer C, Roberts T, DeCaprio J. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1998;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stubdal H, Zalvide J, DeCaprio J. Simian virus 40 large T antigen alters the phosphorylation state of the Rb-related proteins p130 and p107. J Virol. 1998;70:2781–2788. doi: 10.1128/jvi.70.5.2781-2788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swenson, J. J. 1998. Unpublished data.
- 61.Takada K, Shimizu N, Sakuma S, Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EB DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takase K, Kelleher C A, Terada N, Jones J F, Lucas J J, Gelfand E W. Dissociation of EBV genome replication and host cell proliferation in anti-IgG-stimulated Akata cells. Clin Immunol Immunopathol. 1996;81:168–174. doi: 10.1006/clin.1996.0173. [DOI] [PubMed] [Google Scholar]
- 63.Takimoto T, Kamide M, Umeda R. Establishment of Epstein-Barr virus (EBV) associated nuclear antigen (EBNA) positive nasopharyngeal carcinoma hybrid cell line (NPC-KT) Arch Oto-Rhino-Laryngol. 1984;239:87–92. doi: 10.1007/BF00454266. [DOI] [PubMed] [Google Scholar]
- 64.Urier G, Buisson M, Chambard P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg R. E2F and cell proliferation: a world turned upside down. Cell. 1995;85:457–459. doi: 10.1016/s0092-8674(00)81244-1. [DOI] [PubMed] [Google Scholar]
- 66.Werness B, Levine A, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 67.Whyte P, Williamson N, Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989;56:67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- 68.Zacny V, Wilson J, Pagano J S. The Epstein-Barr virus immediate-early gene product, BRLF1, interacts with the retinoblastoma protein during the viral lytic cycle. J Virol. 1998;72:8043–8051. doi: 10.1128/jvi.72.10.8043-8051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Gutsch D, Kenney S. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol Cell Biol. 1994;14:1929–1938. doi: 10.1128/mcb.14.3.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]