Abstract
Objective: The prognosis of malignant tumors with peritoneal metastases and cancerous ascites has generally been poor, with limited treatment options. The PRaG regimen, which comprised of hypofractionated radiotherapy, programmed cell death-1 (PD-1) inhibitor, and granulocyte-macrophage colony-stimulating factor (GM-CSF), showed a survival advantage in patients with advanced solid tumors who failed at least the first line of standard systemic treatment. Intraperitoneal infusion of PD-1 inhibitors may be a novel therapeutic strategy for managing malignant ascites. Integrating the PRaG regimen with intraperitoneal perfusion of a PD-1 inhibitor might control malignant ascites and provide further survival benefits in these patients. This proposed study aims to investigate the safety and efficacy of intraperitoneal infusion of serplulimab in combination with the PRaG regimen in patients with simultaneous advanced solid tumors and cancerous ascites who fail at least the first-line treatment. Methods: This proposed study is a prospective, single-arm, open-label, multicenter clinical trial. All eligible patients will receive 2 cycles of intensive treatment, a combination of PRaG regimen with an intraperitoneal infusion of PD-1 inhibitor. The patients who are beneficially treated with intensive treatment will receive consolidation treatment every 2 weeks until ascites disappear, disease progression occurs, intolerable toxicity occurs, or for up to 1 year. Phase I of this study will be conducted using a modified 3 + 3 design. The dose of intraperitoneal infusion of PD-1 inhibitor for phase II will be determined according to dose-limiting toxicity evaluation in the phase I study. Conclusion: This prospective, open-label, multicenter study will potentially lead to intraperitoneal perfusion of a PD-1 inhibitor being a new strategy for malignant ascites patients and provide a meaningful efficacy and safety of the combination of PRaG regimen with an intraperitoneal infusion of PD-1 inhibitor for these patients.
Keywords: cancerous ascites, intraperitoneal infusion of PD-1 inhibitor, hypofractionated radiotherapy, immunotherapy, GM-CSF
Background
Peritoneal metastatic (PM) carcinoma results from cancer cells metastasizing to the peritoneum via blood circulation or direct peritoneal implantation. 1 PM is mostly secondary to ovarian cancers (46%), followed by gastric cancers (14%), colorectal tumors (7%), gastrointestinal neuroendocrine tumors (6%), and peritoneal cancers of unknown origin (3%–5%). 2 Malignant ascites (MA) is the abnormal accumulation of peritoneal fluid resulting from primary peritoneal tumors or other malignant tumors entering the peritoneum. 3 The life expectancy of patients with refractory MA was poor, with the overall survival (OS) ranging from 1 to 6 months in nonovarian cancers. MA secondary to ovarian cancer had a longer life expectancy than MA originated from other types, and the OS was about 10–24 months.4,5 In a retrospective study by Mackey et al, 6 76 patients with MA had a median OS of 11.1 weeks. Significant predictors of poor prognosis were edema, decreased serum albumin, and liver metastasis. 6
Current Treatment Options for PM Carcinoma
Chemotherapy was recommended for patients with ovarian cancers and gastric cancers, but there were few reports supporting the efficacy of chemotherapy for massive ascites in other cancer types. 7 Due to the presence of a plasma-peritoneal barrier and inadequate blood supply, systemic treatment has limitations in treating PM. 8
Other treatment options for PM cancer were cytoreductive surgery (CRS), intraperitoneal chemotherapy (IPEC), hyperthermic IPEC (HIPEC), and pressurized intraperitoneal aerosol chemotherapy. 9 Surgery was generally preferred in patients with limited peritoneal metastasis with a peritoneal cancer index (PCI) of <20. 10 IPEC was previously the preferred option for patients with extensive peritoneal metastases, however, its efficacy was limited with severe side effects. The National Particle Component Toxicity study demonstrated that postoperative use of paclitaxel peritoneal infusion compared with the intravenous injection did not significantly increase the efficiency in gastric cancer patients with a high risk of peritoneal recurrence. 11 The PRODIGE 7 study revealed that CRS combined with HIPEC for colorectal peritoneal metastases did not improve the OS compared with CRS alone. Furthermore, CRS combined with HIPEC resulted in an increased rate of late complications. 12
Compared to other treatments, HIPEC remains the primary option for patients with a high PCI index (>20). However, research indicated that HIPEC did not improve the survival rate in patients with advanced ovarian cancers. 13 In summary, the evidence supporting HIPEC's efficacy in treating peritoneal metastasis is not compelling, and effective treatment for extensive peritoneal metastases remains elusive.
The Potential Rationale of Intraperitoneal Infusion of Immunotherapy for Peritoneal Metastasis Patients
T-cell activation depends on effective antigen processing and presentation by antigen-presenting cells in lymph nodes, as well as in the peritoneal fluid. The immune cells can enter the underlying terminal lymphatic lacunas, which further can drain into the main lymphatic channels through the mediastinal lymph nodes. 14 In the tumor microenvironment of ovarian cancers with MA, aggregates containing activated CD4+T cells and CD8+T cells were present. 15 Programmed cell death-1 (PD-1) inhibitors can interact with activated cytolytic lymphocytes, killing tumor cells, which provides a theoretical basis for the intraperitoneal infusion of PD-1 inhibitors. Previous studies considered the peritoneum to be a lymphoid organ, with the double-layered greater omentum possessing peritoneal composites. This structure contained clusters of immune cells, known as milky spots, comprising 67.9% macrophages, 10.1% B cells, 10.2% T cells, and a few dendritic cells (DCs). 16 The research studies found that PM tumor cells were more prone to infiltration into these milky spots. Among macrophages, tumor-associated macrophages (TAMs) exhibited plasticity and polarized toward M1/M2 phenotypes when stimulated by corresponding cytokines. In animal models of colon cancer, more than 50% of TAMs within tumors expressed PD-1 on their surface. These PD-1-positive TAMs were M2 types, known to promote tumor growth. Furthermore, the function of PD-1+ TAMs was reversed by blocking the PD-1 pathway. 17 This finding provided additional theoretical support for the function of intraperitoneal injection of PD-1 inhibitors.
The New Treatment Model of Combining PD-1 Inhibitors, Radiotherapy, and Granulocyte-Macrophage Colony-Stimulating Factor (PRaG Therapy)
Hypofractionated radiotherapy (HFRT) or stereotactic body radiotherapy (SBRT) is routinely performed in clinical practice, which induces immunogenic cell death, creating the “in situ tumor vaccine.” Radiotherapy also alters the tumor microenvironment, promoting DC and T-cell functions and activating immune effects. This response can be enhanced by combining radiotherapy with systemic immunotherapy, such as PD-1 inhibitor or granulocyte-macrophage colony-stimulating factor (GM-CSF). 18
GM-CSF promotes the differentiation of monocytes/M1-type macrophages and DCs, enhances their activity, amplifies antigen presentation, and boosts the immune effects. 19 The GM-CSF improved the efficacy of PD-1 inhibitors and boosted the radiotherapy's immune effects in a clinical study. 20
We combined a PD-1 inhibitor, radiotherapy, and GM-CSF (PRaG therapy) 21 and designed a prospective clinical trial (ChiCTR1900026175) that utilized the PRaG regimen to rescue patients with advanced solid tumors who failed at least the first-line chemotherapy. Preliminary results revealed that this triple therapy improved the efficacy of PD-1 inhibitors and broadened the spectrum of PD-1 immunotherapy against cancers.22–25 Prior research suggested that PRaG treatment may be a promising salvageable treatment for advanced multiple metastatic solid tumors.
Based on recent research findings, we propose that intraperitoneal infusion of PD-1 inhibitor can produce antitumor effects and hypothesize that the combination of PD-1 inhibitor intraperitoneal infusion treatment and the PRaG therapy may offer survival benefits to patients with advanced malignant PM and simultaneous MA.
Methods
Objectives of the Proposed Study
This proposed study is a prospective, single-arm, open-label, multicenter clinical trial and was registered on ClinicalTrials.gov under the trial number NCT05501340. Our study was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University (approval number JD-LK-2022-033-01).
This study will investigate the safety of combined PRaG therapy and intraperitoneal infusion of a PD-1 inhibitor through a phase I clinical trial. Furthermore, the effectiveness of this combined therapy will be verified through a phase II clinical trial.
The main objective of phase I of the PRaG 4.0P study is to assess the safety of combined PRaG therapy and intraperitoneal infusion of a PD-1 inhibitor in the treatment of patients with advanced solid tumors and simultaneous cancerous ascites. Safety will be evaluated from dose-limiting toxicity (DLT) response, adverse events (AEs), and serious AEs (SAEs).
The main objective of phase II (single-arm prospective trial) of the PRaG 4.0P study is to evaluate the safety and efficacy of combined PRaG therapy and intraperitoneal infusion of a PD-1 inhibitor in the treatment of advanced solid tumors and simultaneous cancerous ascites.
Furthermore, this proposed research will analyze the mechanism of combined therapy and will screen potential biomolecular markers for predicting the efficacy. This will be achieved by detecting lymphocyte subsets, cytokines, and metabolite levels in peripheral blood and malignant ascitic fluid.
Overview of Study Design
Phase I Clinical Trial: Sample Size and Safe Dose Decision
A data review panel, comprising investigators, medical supervisors, physicians, clinical representatives, and statisticians, will determine whether phase I dose should be reduced, based on the occurrence of DLT. The data review panel will assess all data related to AEs, including non-DLT, laboratory results, and other safety parameters. Any other stipulations specified in the dose reduction plan will be examined. Quality control measures for critical safety data, including ongoing study monitoring parameters, clinical database review reports, and data accuracy and completeness by site investigators will be detailed. Decisions and rationale on dose reduction will be finalized by study personnel and documented, and copies will be maintained at each study site.
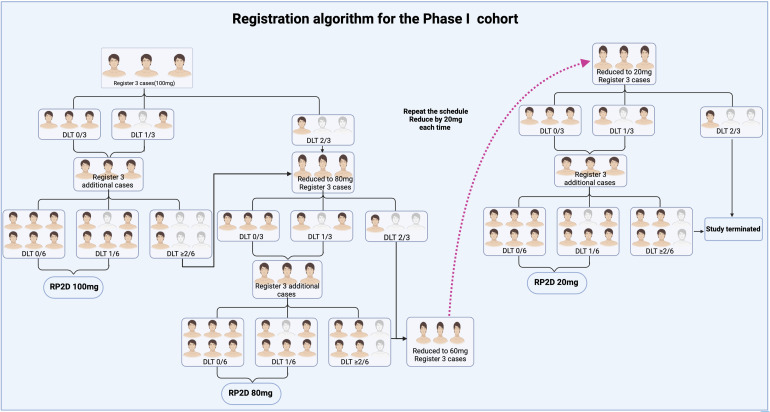
In phase I trial, serplulimab will be administered as intraperitoneal infusions from a starting dose of 100 mg. If no one among the first 3 patients develops DLT, the original dose will be continued, and 3 more cases will be enrolled. If there is still no one or one develops DLT, the dose of 100 mg intraperitoneal infusion will be used as the recommended phase 2 dose (RP2D) trial. If 1 of the first 3 patients, experiences DLT, then 3 additional patients will be treated with the same dose; if 2 or more cases in all 6 patients experience DLT, then the dose will be considered to exceed the maximum tolerated dose (MTD), then the dose will be reduced to 80 mg intraperitoneal infusion. Likewise, if DLT occurs in 2 or more patients, then the dose will be reduced to 60 mg. Accordingly, with each subsequent reduction being 20 mg a minimum dose will be 20 mg. If a trial dose surpasses the MTD, and 6 patients are administered with the next lower dose, this dose will be considered MTD. If the dose is reduced to 20 mg and 2 or more patients still experience DLT, the trial will be terminated.
The overview of the treatment design is shown in Figure 1.
Figure 1.
Registration model for the proposed study. Phase I of this study will be conducted using a modified 3 + 3 design. RP2D of intraperitoneal infusion of PD-1 inhibitor will be verified according to the DLT evaluation in the phase I study.
Abbreviations: DLT, dose-limiting toxicity; RP2D, recommended phase 2 dose.
Definition of DLT Reaction by Intraperitoneal Infusion of PD-1 Inhibitor
Severe diarrhea, abdominal infection/peritoneal infection, intestinal obstruction and perforation, gastrointestinal and abdominopelvic organ hemorrhage, acute kidney injury (AKI), liver failure, allergic reactions, and other hematologic abnormalities within 2 weeks of intraperitoneal infusion will be considered PD-1 inhibitor-related toxic reactions. The definition of DLT reaction related to intraperitoneal infusion of PD-1 inhibitor is outlined in Table 1.
Table 1.
Definition of DLT Reactions.
| PD-1-related toxic events within 2 weeks of intraperitoneal infusion | Diarrhea | Increased number of defecations compared to baseline, ≥7 per day; requiring hospitalization; |
| Abdominal/peritoneal infection | Post-treatment procalcitonin (PCT) level of >2.0 ng/mL; exclusion of spontaneous peritonitis and preexisting peritoneal infections; positive post-treatment ascites pathogenic microbial culture, requiring intravenous antibiotics, antifungal or antiviral agents; requiring invasive therapy | |
| Intestinal obstruction | Gastrointestinal decompression with no relief from conventional noninvasive treatment and requirement of surgical intervention. | |
| Gastrointestinal, hepatobiliary, or pancreatic perforation | Requiring invasive intervention or hospitalization | |
| Gastrointestinal and abdominopelvic organ hemorrhage | Requiring blood transfusion; requiring invasive intervention or hospitalization. | |
| AKI | Requiring hospitalization, with occurrence of AKI within 3 days of infusion | |
| Liver failure | Fluttering tremor; mild hepatic encephalopathy; presence of drug-induced liver damage | |
| Allergic reactions | Symptomatic bronchospasm with or without urticaria; requiring parenteral therapy; allergic reaction-related edema/angioedema; hypotension. | |
| Hematologic adverse events | Grade ≥3 neutropenia and grade ≥4 anemia and thrombocytopenia | |
| Other systemic toxic reactions | ≥Grade 3 adverse events according to the CTCAE 5.0 |
Abbreviations: DLT, dose-limiting toxicity; PD-1, programmed cell death-1; AKI, acute kidney injury;
Phase II Clinical Trial Dose Selection
Upon completion of the phase I trial, all available data, including information on the subjects who discontinued treatment will be compiled and summarized. The determination of dose for the phase II trial will be determined by a comprehensive analysis of all data from the phase I trial, including assessing safety, tolerability, and efficacy.
The Sample Size for Phase II Clinical Trial
According to retrospective reports, the OS of a substantial number of patients with cancerous ascites varies from 1 to 6 months. Some literature reports a median OS of 5.7 months from the time of diagnosis. 26 In the phase II study, the doses found to be safe in the phase I study will be utilized. The sample size of the enrolled patients will be calculated based on OS data from the analysis of the phase I study.
Treatment Protocol
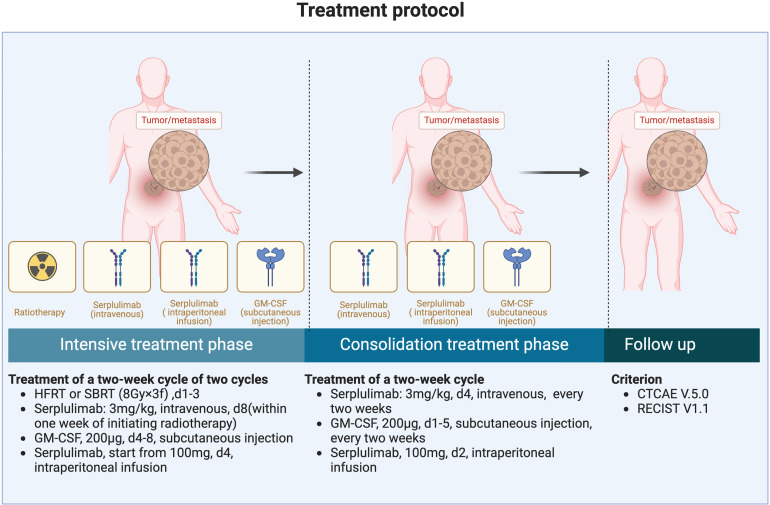
The treatment program consists of 2 distinct stages, the intensive treatment phase and the consolidation treatment phase (Figure 2).
Figure 2.
Treatment plan of this proposed study. The treatment includes an intensive treatment phase and a consolidat ion treatment phase.
Intensive Treatment Phase
Patients will be enrolled and treated with HFRT or SBRT (8Gy × 3f) to particular cancer lesions (appropriate peritoneal metastases which can be localized or other organ metastatic lesions), and serplulimab at a dose of 3 mg/kg will be administered intravenously within 1 week of radiotherapy onset, and a GM-CSF (Molgramostim; Xiamen Amoytop Biotech Co., Ltd, Xiamen, China) at a dose of 200 μg will be administered by subcutaneous injection daily for 5 days, and serplulimab (HLX10; Shanghai Henlius Biotech, China) will be administered by intraperitoneal infusion after the end of radiotherapy. This treatment constitutes a 2-week cycle, and every cycle will be performed on different target lesions. A total of 2 cycles of triple therapy will be completed.
Consolidation Treatment Phase
For patients who are effective after the intensive treatment, serplulimab at a dose of 3 mg/kg IV every 2 weeks and a GM-CSF at a dose of 200 μg subcutaneously for a total of 5 days, every 2 weeks will be administered until ascites disappears, disease progression occurs, intolerable toxicity arises, or for up to 1 year.
Furthermore, intraperitoneal infusion of serplulimab every 2 weeks will be given, until the disappearance of ascites, disease progression, intolerable toxicity occurrence, or for up to 1 year.
Patient Eligibility
This study will enroll patients diagnosed with malignant metastases and simultaneous MA. Participants will be pathologically confirmed for a malignant solid tumor, as well as validated by either peritoneal lesions or the presence of positive exfoliated tumor cells in ascitic fluid. The specific inclusion and exclusion criteria are detailed in Table 2. Written informed consent will be obtained for the enrolled patients.
Table 2.
Inclusion and Exclusion Criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients must fulfill all the following criteria | Patients should not be included if one of the following exclusion criteria is found. |
|
|
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NYHA, New York Heart Association.
Endpoints of this Proposed Study
The tolerability of intraperitoneal infusion of serplulimab remains unexplored. Consequently, this phase I study will assess the safety and tolerability of this combination therapy. The primary endpoints will be the incidence rates of AEs and SAEs, including DLTs, and will be evaluated according to the Common Terminology Criteria for AE (CTCAE) V.5. In the phase II study, the primary endpoint will be l OS, which will be calculated from the enrollment date to the date of death or last known alive. Secondary endpoints will include objective response rates (ORRs), which will be based on the Response Evaluation Criteria in Solid Tumors, version 1.1, progression-free survival (PFS), disease control rate (DCR), and ascites control rate. ORR will be defined as the proportion of patients with complete response (CR) or partial response (PR). DCR will be the percentage of patients with CR, PR, or stable disease (SD) from enrollment. PFS will be calculated from enrollment to disease progression, death, or censored at the last clinical follow-up.
Ascites will be assessed using abdominal computed tomography (CT) or ultrasound. The immediate outcome of MA will be assessed through ultrasound. Complete remission (CR) will be defined as the complete disappearance of ascites, lasting more than 4 weeks, and partial remission (PR) as a >50% reduction of ascites lasting over 4 weeks. SD will be defined as a decrease in ascitic fluid volume of <50% or an increase of ≤25%, lasting over 4 weeks, and progression disease (PD) will be defined as an increase of >25% in ascitic fluid volume on ultrasound. The recent outcome of MA will be assessed based on CT and will be categorized as none (grade I), mild (grade II, limited to the pelvis), moderate (grade III, beyond the pelvis), and massive (grade IV, entire abdomen).10,27,28 Furthermore, peripheral blood and ascitic fluid will be sampled before and after the treatment. As an exploratory objective, these samples will be evaluated to determine correlations between individual immune responses and prognosis-related biomarkers. The endpoints are summarized in Table 3.
Table 3.
The Endpoints of the Study.
| Phase I | Phase II | |
|---|---|---|
| Main endpoints | DLT AEs SAEs |
OS |
| Secondary endpoints | OS PFS DCR ORR Ascites improvement rate |
PFS DCR ORR Ascites improvement rate |
| Observational parameters | T-cell activation and cytokines change in ascitic fluid QOL score FACIT-AI Biomarkers in ascitic fluid and peripheral blood |
Abbreviations: DLT, dose-limiting toxicity; AE, adverse event; SAE, serious adverse event; OS, overall survival; PFS, progression-free survival; DCR, disease control rate; ORR, objective response rate; QOL, quality of life; FACIT-AI, functional assessment of chronic illness therapy-ascites index.
Statistical Analysis
The statistical analysis will be performed using SPSS version 25.0 software (IBM Corp, Armonk, NY, USA). The frequencies of DLT will be calculated in all patients who receive at least 1 cycle of treatment. The period for evaluating DLTs will extend from the start of treatment until 92 days thereafter. DLTs will be summarized at 30 and 92 days after the initiation of the carbon-ion radiotherapy treatment. The AEs/SAEs will also be summarized and listed according to their severity and frequency. The PFS and OS endpoints in the intention-to-treat patients will be estimated using the Kaplan–Meier method. A p-value of <.05 (two-sided) will be considered statistically significant. The study will compare changes in the white blood cell, granulocyte, and lymphocyte counts, as well as differential cells (CD45+CD3+, CD45+CD3+CD4+CD8−, CD45+CD3+CD8+CD4−, CD3+CD8−CD45RA−CD197+CD38+HLA-DR+, CD3+CD8+CD39+PD-1+, CD3+CD8−CD45RA−CD197−, CD3+CD8−CD45RA−D197+, CD3+CD8+CD45RA−CD197−, CD3+CD8+CD45RA−CD197+, CD3+CD8−CD45RA−CD197−CD38+HLA-DR+, CD3+CD8+CD45RA−CD197−CD38+HLA-DR+, CD3+CD8+CD45RA−CD197+CD38+HLA-DR+, CD3+CD4+CD25highCD127±, CD3−CD19−CD14+CD16−HLA−DRhighCD40+, CD3−CD19−CD14−HLA−DR+CD11c+CD123−, CD3+CD8+OX40+CD40L+, CD8+PD-1+, CD8+CD122+, CD8+CD27+, CD8+CD39+, CD4+CD38+HLA-DR+, and CD8+CD38+HLA-DR+) and cytokines (interleukin [IL]-2, IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor, interferon-γ) before and after radiotherapy both in peripheral blood and ascitic fluid, and will determine if any of the differences are statistically significant. Cox regression analysis will be performed, combining changes in these counts with the patient's survival time to assess their influence on the survival rate. Additionally, the study will employ the Kaplan–Meier method to analyze survival, focusing on the relationship between survival rate and changes in cytokines and lymphocyte counts in both peripheral blood and ascitic fluid.
The reporting of this study conforms to SPIRIT guidelines. 29
Ethics and Dissemination
Our study was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University (Approval No. JD-LK-2022-033-01). Findings will be disseminated through national and international conferences. We also plan to publish our findings in a high-impact peer-reviewed journal.
Consent to Participate
All patients provided written informed consent prior to enrollment in the study. This consent form must be signed by the subject and the investigator-designated research professional obtaining the consent. Consent will be obtained by the study coordinator or investigator of the study at each site. Consent will include provisions for collection and use of participant data and biological specimens. Consent will be documented in the medical record.
Discussion
As of now, several intraperitoneal immunotherapy strategies are under investigation. For example, Catumaxomab, a bispecific (anti-epithelial cell adhesion molecule and anti-CD3) trifunctional antibody, was approved by the European Union in 2009 for intraperitoneal treatment of patients with MA. 8 Other immunotherapy, such as immune stimulators, cytokines, and cancer vaccines are considered potential strategies for intraperitoneal immunotherapy. 30
Given the lack of a previous dose reference for intraperitoneal infusion of PD-1 inhibitors, we referred to data from intravenous infusion when selecting the perfusion dose. The dose–exposure–effect relationship (D–E–R) was outlined for the safety and efficacy of serplulimab (HLX10). The probability of various AEs such as immune-related AE, ≥grade 3 AE, ≥grade 2 AE, and ≥grade 3 immune-related AE were used as safety indices. The maximum serum concentration at steady state, the minimum serum concentration at steady state and the area under the curve normalized to a week (WAUC) served as drug exposure indices. This suggested that the D–E–R curves were consistent for 3 mg/kg once every 2 weeks, 3 mg/kg once every 3 weeks (Q3W), 4.5 mg/kg Q3W, and 6 mg/kg Q3W administration of HLX10 dosing regimens. The correlation curves between HLX10 exposure and the probability of immune-related AE were found to be horizontal, aligning with the results of pembrolizumab and nivolumab studies. It was suggested that the likelihood of immune-related AE occurrence remained unchanged under any of the four HLX10 dosing regimens, indicating that all four regimens had a favorable safety profile. Therefore, we utilized a dose-decreasing model to swiftly determine the feasible intraperitoneal infusion dose. A prior case report highlighted that a patient with peritoneal metastasis from pancreatic cancer, accompanied by massive ascites, experienced a notable reduction in ascites after bi-weekly intraperitoneal infusions of 20 mg nivolumab. 31 As such, PD-1 inhibitor intraperitoneal infusion may emerge as a novel therapy, but more evidence from prospective clinical trials is requisite.
The therapeutic efficacy of PD-1 inhibitors as monotherapy is limited. Based on the cancer-immunity cycle, certain treatments, such as GM-CSF and radiotherapy, might augment the effectiveness of PD-1 inhibitors. Various phase I/II clinical trials have confirmed the safety and efficacy of PD-1 inhibitors when combined with HFRT or SBRT for advanced metastatic tumors. GM-CSF, which can enhance antigen presentation by amplifying dendritic cell function, was safe and tolerable with a dose of 150 µg/m2 per day or 200 µg per day for 14 consecutive days.24,32 Furthermore, prior phase II trials have demonstrated that 12% of the patients experienced grade 3 or above treatment-related adverse effects of the triple combination (PRaG) therapy,22–25 which was acceptable and tolerable.
Peripheral lymphocytes, based on blood circulation, can partially reflect a patient's immune status and predict the efficacy of immunotherapy. In this trial,we explored the subsets of lymphocyte subsets and cytokines biomarkers in both peripheral blood and ascitic fluid. T cells can be differentiated into naïve T cells (TN), effector T cells (TEff), and memory T cells (TM). 33 Further, TM can be divided into stem cell memory T cells (TSCM), central memory T cells (TCM), and effector memory T cells (TEM) according to the different effector functions, proliferation, and migration abilities. 34 TN cells can respond to immune mobilization reserve capacity. The TCM/TEff ratio and the increase of TCM and TEM after immune checkpoint therapy were correlated with outcomes. We tested the CD8+TN(CD45RA+CD3+CD8+CD4−), CD4+TN(CD45RA+CD3+CD4+CD8−), CD4+TCM(CD3+CD8−CD45RA-D197+), CD4+TEM(CD3+CD8−CD45RA−CD197−), CD8+TEM(CD3+CD8+CD45RA−CD197−), and CD8+TCM(CD3+ CD8+CD45RA−CD197+) in each cycle. The T-cell activation phenotype, which responds to the activated proliferation and functional status of T cells, positively predicts the efficacy of immune checkpoint inhibitor treatment. T-cell activation phenotype often expresses costimulatory molecule receptors, and chemokine receptors, such as CD39, HLA-DR, CD38, and OX40.35–40 So we also sought to explore the different phenotypes within treatment. Myeloid-derived cells (CD3−CD19−CD14+CD16−HLA−DRhighCD40+ and CD3−CD19−CD14−HLA−DR+CD11c+CD123−) were also explored in this study. As we know, this is the first prospective study to combine systemic anti-PD-1 therapy with intraperitoneal anti-PD-1 therapy. We will evaluate the safety and efficacy of combining intraperitoneal infusion of a PD-1 inhibitor with the PRaG therapy model.
Conclusion
This prospective, open-label, single-arm, multicenter study will potentially lead to intraperitoneal perfusion of a PD-1 inhibitor being a new strategy for MA. And will provide a safe reference dose for intraperitoneal infusion of serplulimab in the Phase I study. Furthermore, we aim to evaluate the efficacy and safety of a combination of PRaG regimen with an intraperitoneal infusion of PD-1 inhibitor for MA patients. Experimental biomarkers of cytokines and lymphocyte subsets will be explored in this study.
Acknowledgments
The authors thank the patients and their families as well as the investigators, coinvestigators, the study teams at each of the participating centers, and Shanghai Henlius Biotech, Inc.
Abbreviations
- AE
adverse event
- AKI
acute kidney injury
- CR
complete response
- CRS
cytoreductive surgery
- CTCAE
common terminology criteria for adverse event
- DCR
disease control rate
- DC
dendritic cell
- D–E–R
dose–exposure–effect relationship
- DLT
dose-limiting toxicity
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HFRT
hypofractionated radiotherapy
- HIPEC
hyperthermic intraperitoneal chemotherapy
- IL
interleukin
- IPEC
intraperitoneal chemotherapy
- MA
malignant ascites
- MTD
maximum tolerated dose
- ORR
objective response rate
- OS
overall survival
- PCI
peritoneal cancer index
- PD-1
programmed cell death-1
- PFS
progression-free survival
- PM
peritoneal metastatic
- PR
partial response
- Q3W
once every 3 weeks
- RP2D
recommended phase 2 dose
- SAE
serious adverse event
- SBRT
stereotactic body radiotherapy
- SD
stable disease
- TAM
tumor-associated macrophage
- TCM
central memory T cells
- TEM
effector memory T cells
- TEff
effector T cells
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work received funding through the National Natural Science Foundation of China (82171828), Key Medical Discipline Construction Unit of Jiangsu Province for the 14th Five-year plan (JSDW202236), the Key R&D plan of Jiangsu Province (Social Development) (BE2021652), Suzhou Clinical Key Diseases Diagnosis and Treatment Technology Special Project (LCZX201808), Open Project of Provincial Key Laboratory of Soochow University (KJS1961), the Subject construction support project of the Second Affiliated Hospital of Soochow University (XKTJ-RC202001, XKTJ-HRC20210011), Foundation of Chinese Society of Clinical Oncology (Y-XD202002/zb-0015) and Wu Jieping Medical Foundation (320.6750.2021-01-12), Suzhou Radiotherapy Clinical Medical Center (Szlcyxzx202103), the Project of State Key Laboratory of Radiation Medicine and Protection (GZN1202302).
ORCID iD: Peifeng Zhao https://orcid.org/0009-0002-0098-8154
References
- 1.Granieri S, Bonomi A, Frassini S, et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: a meta-analysis of randomized controlled trials. Eur J Surg Oncol. 2021;47(11):2757-2767. [DOI] [PubMed] [Google Scholar]
- 2.Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangisetty SL, Miner TJ. Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg. 2012;4(4):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stukan M. Drainage of malignant ascites: patient selection and perspectives. Cancer Manag Res. 2017;9:115-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge C, Badgwell BD. Palliation of malignant ascites. J Surg Oncol. 2019;120(1):67-73. [DOI] [PubMed] [Google Scholar]
- 6.Mackey JR, Venner PM. Malignant ascites: demographics, therapeutic efficacy and predictors of survival. Can J Oncol. 1996;6(2):474-480. [PubMed] [Google Scholar]
- 7.Matsusaki K, Aridome K, Emoto S, et al. Clinical practice guideline for the treatment of malignant ascites: section summary in clinical practice guideline for peritoneal dissemination (2021). Int J Clin Oncol. 2022;27(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang D, Kim IH. Molecular mechanisms and potential rationale of immunotherapy in peritoneal metastasis of advanced gastric cancer. Biomedicines. 2022;10(6):1376-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Quteimat OM, Al-Badaineh MA. Intraperitoneal chemotherapy: rationale, applications, and limitations. J Oncol Pharm Pract. 2014;20(5):369-380. [DOI] [PubMed] [Google Scholar]
- 10.Kim DW, Jee YS, Kim CH, et al. Multicenter retrospective analysis of intraperitoneal paclitaxel and systemic chemotherapy for advanced gastric cancer with peritoneal metastasis. J Gastric Cancer. 2020;20(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N, Kanda M, Yoshikawa T, et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: final results of the INPACT trial. Gastric Cancer. 2018;21(6):1014-1023. [DOI] [PubMed] [Google Scholar]
- 12.Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256-266. [DOI] [PubMed] [Google Scholar]
- 13.Vergote I, Harter P, Chiva L. Hyperthermic intraperitoneal chemotherapy does not improve survival in advanced ovarian cancer. Cancer. 2019;125(Suppl 24):4594-4597. [DOI] [PubMed] [Google Scholar]
- 14.Rathod S. T cells in the peritoneum. Int Rev Cell Mol Biol. 2022;371:15-41. [DOI] [PubMed] [Google Scholar]
- 15.Landskron J, Helland Ø, Torgersen KM, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother. 2015;64(3):337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krist LF, Eestermans IL, Steenbergen JJ, et al. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241(2):163-174. [DOI] [PubMed] [Google Scholar]
- 17.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in neutropenia. J Immunol. 2015;195(4):1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley RK, Mitchell E, Behr S, et al. Phase 2 trial of pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC): clinical outcomes and biomarker analyses. J Clin Oncol. 2018;36(15_Suppl):4087. [Google Scholar]
- 21.Kong Y, Ma Y, Zhao X, et al. Optimizing the treatment schedule of radiotherapy combined with anti-PD-1/PD-L1 immunotherapy in metastatic cancers. Front Oncol. 2021;11:638873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Xing P, Kong Y, et al. PD-1 inhibitor combined with radiotherapy and GM-CSF in MSS/pMMR metastatic colon cancer: a case report. Front Oncol. 2023;13:1078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Hong Z, Xu M, et al. PRag therapy of refractory metastatic gastric cancer: a case report. Front Immunol. 2022;13:926740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong Y, Zhao X, Xu M, et al. PD-1 Inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front Immunol. 2022;13:952066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Kong Y, Zhang L. Anti-PD-1 immunotherapy combined with stereotactic body radiation therapy and GM-CSF as salvage therapy in a PD-L1-negative patient with refractory metastatic esophageal squamous cell carcinoma: a case report and literature review. Front Oncol. 2020;10:1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18(5):945-949. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Li X, Chen D, et al. Intraperitoneal administration of cisplatin plus bevacizumab for the management of malignant ascites in ovarian epithelial cancer: results of a phase III clinical trial. Med Oncol. 2015;32(2):292. [DOI] [PubMed] [Google Scholar]
- 28.Kou F, Gong J, Li Y, et al. Phase I study of intraperitoneal bevacizumab for treating refractory malignant ascites. J Int Med Res. 2021;49(2):300060520986664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei GX, Du Y, Zhou YW, et al. Peritoneal carcinomatosis with intraperitoneal immunotherapy: current treatment options and perspectives. Expert Rev Gastroenterol Hepatol. 2022;16(9):851-861. [DOI] [PubMed] [Google Scholar]
- 31.Wang ST, Chiu CF, Bai HJ, et al. Intraperitoneal nivolumab in a patient with pancreatic cancer and refractory malignant ascites. Eur J Cancer. 2021;148:48-50. [DOI] [PubMed] [Google Scholar]
- 32.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795-803. [DOI] [PubMed] [Google Scholar]
- 33.Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Sun Z, Chen L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein Cell. 2020;11(8):549-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Gomez C, Michelas M, Scarlata CM, et al. Circulating exhausted PD-1(+)CD39(+) helper CD4T cells are tumor-antigen-specific and predict response to PD-1/PD-L1 axis blockade. Cancers (Basel). 2022;14(15):3679-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun. 2017;8(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575-579. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Hu Q, Hu K, et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Transl Med. 2019;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzaschi G, Minari R, Zecca A, et al. Soluble PD-L1 and circulating CD8+PD-1+ and NK cells enclose a prognostic and predictive immune effector score in immunotherapy treated NSCLC patients. Lung Cancer. 2020;148:1-11. [DOI] [PubMed] [Google Scholar]
- 40.Han J, Duan J, Bai H, et al. TCR Repertoire diversity of peripheral PD-1(+)CD8(+) T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res 2020. 8(1):146-154. [DOI] [PubMed] [Google Scholar]