Abstract
Background:
There is growing concern in sub-Saharan Africa that poor-quality antimicrobial medicines may negate management of infectious diseases of public health importance should they fail to meet the set criteria of quality, safety and efficacy.
Objectives:
The objective was to ascertain the quality of antiretroviral, antimalarial and antituberculosis medicines supplied and available in the public health sector in Zambia.
Design:
A descriptive cross-sectional study was conducted involving the analysis of data from the continuous routine in-country post-marketing surveillance programme in Zambia that assessed the quality of antiretroviral, antimalarial and antituberculosis medicines supplied to public healthcare facilities between January 2018 and June 2023.
Methods:
Data were extracted from laboratory quality analysis results from samples collected as part of routine post-marketing surveillance by the Zambia Medicines Regulatory Authority between January 2018 and June 2023. The samples were collected from various levels of the pharmaceutical supply chain across Zambia. Samples were analysed according to their respective pharmacopoeia standards at the Medicines Control Authority of Zimbabwe Quality Control Laboratory, a World Health Organization prequalified laboratory. Data were extracted using a structured Excel database and analysed using Microsoft Excel, and GraphPad Prism Software was used for visualizations.
Results:
Of the 198 samples, 86 (43.43%) were antiretrovirals, 54 (27.27%) antimalarials and 58 (29.29%) antituberculosis medicines. Of these 198 samples, 171 (86.36%) originated from Asia, 19 (9.60%) Africa and 8 (4.04%) Europe. All sampled medicines met their respective quality specifications with respect to tests, which included appearance, identification, assay, uniformity of mass, weight variation, disintegration, dissolution, pH and specific gravity, giving a compliance rate of 100%.
Conclusion:
Antiretrovirals, antimalarials and antituberculosis medicines obtained from public healthcare facilities in Zambia through routine post-marketing surveillance met their quality standards. This might positively impact treatment outcomes for HIV/AIDS, malaria and tuberculosis. There is a need for large-scale continuous monitoring of the quality of medicines in order to ensure quality is maintained and substandard products removed from the pharmaceutical supply chain.
Keywords: Antimicrobial medicines, quality, substandard, falsified, post-marketing surveillance, Zambia
Plain Language Summary
Outcomes from testing of antiretroviral, antimalarial and antituberculosis medicines in the Zambian public health facilities to establish if they are of good quality, safe and effective for treatment of HIV, malaria and tuberculosis
Why was the study done? Management of diseases require the medicines used are able to treat specific ailments for them to be beneficial to the patient. Bad quality medicines may not be able to treat infections and sometimes may be harmful to the patient taking them. Reports of infections that are resistant to treatment are increasing, partly due to poor-quality medicines. This increasing trend disturbs programmes that aim at eradication of these diseases, and frustrates governments. This study aimed to understand the quality of medicines for diseases that cause a lot of hospital admissions and deaths in Zambia. What did the researchers do? The authors studied results of antiretrovirals, antimalarials and antituberculosis medicines collected across Zambia over a five-and-a-half-year period and tested in a quality control laboratory to understand the quality of these products. Quality of these products can affect treatment outcomes of HIV, malaria and tuberculosis. Knowing the quality of medicines in circulation helps generate evidence for decision-making by medicine regulatory bodies as they protect public health. What did the researchers find? Of the 198 results, the majority (n = 86) were antiretrovirals, followed by antituberculosis medicine (n = 58) and antimalarials (n = 54). All medicines that were tested passed with respect to the tests, giving a compliance rate of 100%. This should give confidence to the public pharmaceutical supply chain, with respect to how they procure their pharmaceuticals and how they are distributed and stored. What do the findings mean? The results show that medicines procured for the public sector in Zambia largely meet their quality requirements. This might positively impact treatment outcomes for HIV/AIDS, malaria and tuberculosis. More similar studies are required to establish a true picture of the quality of these medicines.
Introduction
Zambia is a low-middle income country (LMIC) in Sub-Saharan Africa (SSA) with a population of about 21 million inhabitants.1,2 In all ages and genders, HIV/AIDS and Sexually Transmitted Infections (STIs), tuberculosis (TB) and malaria rank among the top 10 leading causes of mortality in Zambia. 3 In addition, the country is faced with a growing threat of Antimicrobial Resistance (AMR), one of the most significant global public health threats facing humanity,4,5 driven in part by the availability of substandard and falsified antimicrobials, especially in LMICs.6,7 This escalating threat is associated with extensive health, economic and societal implications, 8 including morbidity, mortality and increased costs, especially in SSA. 9 It is estimated that AMR will cost over US$100 trillion in output loss by 2050. 10
Substandard medical products as defined by the World Health Organization (WHO) are ‘out of specification (OOS)’, authorized/registered/licenced medical products that fail to meet their quality standards, specifications or both. 11 Substandard products frequently have low active pharmaceutical ingredients (APIs) or have problems with other performance-related parameters, for example, dissolution properties for solid dosage forms. 11 Unlike falsified medical products, which deliberately misrepresent their source or identity and are produced and distributed with criminal intent, substandard medical products are manufactured by genuine manufacturers but are OOS for one reason or the other which can include manufacturer error or poor handling during transportation or storage. 11
The quality of anti-infectives, mainly antiretrovirals (ARVs), antimalarials and anti-TB medicines, has an immediate health impact on disease burden and drug resistance. 12 Poor-quality antimicrobials pose significant risks to national disease control programmes such as eradication and control of HIV/AIDS, malaria and TB, and multilateral initiatives. 13 In addition, poor-quality antimicrobials contribute to the development of AMR. 14
According to a WHO global analysis, 11% of antimicrobials contain subtherapeutic concentrations of APIs. 15 Of these, the proportion for ARVs is 4.2%. 15 People living with HIV (PLWHIV) exposed to substandard ARVs are at increased risk of developing HIV resistance. Wallis et al. 16 reviewed key determinants of HIV drug resistance in LMICs and observed that in addition to other determinants, the quality of ARVs available in regimens merits attention.
Nayyar et al. 17 estimates that 35% of antimalarial medicines in SSA are substandard while 20% are falsified. Furthermore, Renschler et al. 18 reports that consumption of such poor-quality antimalarial medicines may cause up to 120,000 under-five deaths annually. Bate et al. 19 conducted a field analysis of substandard and falsified anti-TB medicines in 19 cities in Africa, India and some middle-income countries and found that 9.1% of the drugs sampled failed basic quality control tests. The failure rate was 16.6% in Africa, 10.1% in India and 3.9% in other middle-income countries.
Access to quality-assured medicines is an essential prerequisite for universal health coverage, and pharmaceutical distributors play an important role in ensuring the quality of medicines along the supply chain. 20 With less than 30% of WHO Member States having a National Medicines Regulatory Authority (NMRA) able to adequately enforce quality standards, the quality of medicines remains at risk in LMICs.21,5,11
Procurement, storage and distribution of medicines are managed by several public, private and non-governmental actors in LMICs, including importers, procurement agencies, distributors and retailers.22–25 In Zambia, medicines for HIV/AIDS, malaria and TB in the public healthcare supply chain are procured with support from development partners such as the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund (GF), respectively. 26 Procurement through PEPFAR and GF is done in compliance with the WHO quality standards, that is, the Model Quality Assurance System for Procurement Agencies (MQAS) 27 or Good Distribution Practices (GDP). 28 Under these conditions, commodities procurement is performed under a stringent quality assurance policy to ensure product quality, safety and efficacy. 27 On the contrary, most private for-profit distributors in LMICs, Zambia included, have very weak quality assurance systems for pharmaceutical products to prevent harm caused by poor-quality medicines. Low compliance for quality assurance in this sector is a commercial interest. 29
The Zambia Medicines Regulatory Authority (ZAMRA) conducts active Post-Marketing Surveillance (PMS) of medicines in circulation in the pharmaceutical supply chain. 30 During this, samples of medicines are collected and analysed for quality control purposes to ascertain their quality, safety and efficacy. Despite this, there is still a general lack of data relating to quality of medicines which poses challenges for effective and timely regulatory decision-making.
The overall objective of the survey was to assess and report the quality of ARVs, antimalarials and anti-TB medicines supplied in Zambia that represent a high public health risk for populations should they not meet the criteria of quality, safety and efficacy.
Methodology
Study design
This was a descriptive analysis of results from the continuous routine in-country PMS programme assessing the quality of ARVs, antimalarial and anti-TB medicines by ZAMRA between January 2018 and June 2023.
Medicines selection criteria
The selected medicines included in the surveillance were all available products used as treatment options for HIV, malaria and TB, respectively, supplied to and provided by Zambia’s public healthcare facilities.
Sampling sites and sample collection – sampling framework
Samples were collected from across Zambia using a risk-based random sampling approach as follows: For each district visited, sampling consideration was made with preference for closeness to the point of care, poor storage conditions and transportation and stock availability. Where samples for the selected products were not available or considered inadequate, sampling was performed from the nearest site with adequate stocks.
Samples were collected from the public-sector healthcare facilities from levels fully representative of the supply chain in the country as shown in Figure 1.
Figure 1.
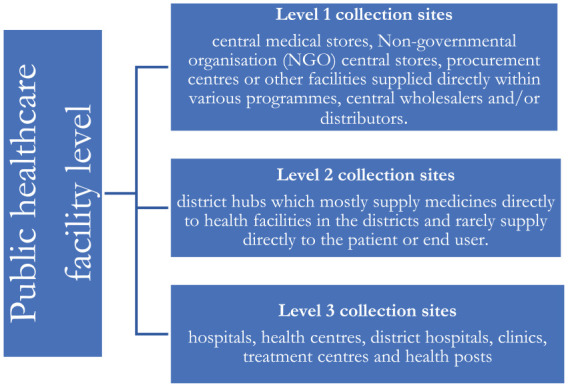
Pharmaceutical supply chain levels from which ARVs, antimalarial and antituberculosis medicines were sampled across Zambia.
Samples were collected by trained ZAMRA inspectors according to standard operating procedures for sampling. At the collection site, one batch of the product of interest was collected. During sampling, not less than 100 tablets were collected per individual sample for solid dosage forms and 50 mL for solutions.
All samples were collected in their original packages. The following information was recorded for each sample in the Sampling Form: Brand and generic name, dosage form, strength, batch or lot number, date of manufacturing and expiration, name and address of the manufacturer, country of manufacture, packaging and pack size, sampling site and sampling date.
Quality evaluation
The assessment and evaluation of samples were conducted at two levels:
Visual assessment at the point of collection: this involved visual examination of labelling and containers, presentation, assessment of conditions or environment in which the product was stored or offered, and any other objective or subjective considerations.
Compendial testing: tests were performed at the laboratory to determine if the medicine specifications as defined in the recognized pharmacopoeia for identity, strength and performance-related parameters (disintegration, dissolution, content/weight uniformity, etc.) were met. Laboratory testing of all samples was performed according to the testing protocols adopted by the testing laboratory.
The purpose of identification test was to verify the identity of the API of the respective medicine. An assay test was used to determine the strength (content) of the API in the pharmaceutical formulation. Disintegration test was applied to measure the time a solid dosage form breaks into particles. Dissolution – a process by which a solid enters a solution – was applied as a tool for predicting bioavailability. Uniformity of active ingredients in a formulation was measured as weight variation and content uniformity. This test ensures a constant dose of drug between individual drugs.
The samples with their collection forms were kept in labelled sampling bags and sealed. Storage and handling of samples during collection, transportation and before analysis complied with the manufacturer’s instructions. All collected samples were subjected to quality analysis at the Medicines Control Authority of Zimbabwe (MCAZ) Quality Control Laboratory, which is a WHO-prequalified laboratory for full quality control analysis.
Laboratory testing
All medicines selected for this survey were in oral solid dosage forms, except for two antimalarials (artesunate) which were in injection form and one ARV (nevirapine) in oral solution form. Typical quality parameters such as physical appearance, identification, disintegration, dissolution, assay and weight uniformity were performed on the samples according to respective pharmacopoeial (e.g. Ph. Int., BP and USP) and manufacturer’s methods.
Data management and statistical analysis
The collected data were checked for any inconsistencies. The data were double-entered into a Microsoft Excel 2021 database and GraphPad Prism Software (GraphPad6, La Jolla, CA, USA). Descriptive statistics was used wherever appropriate and results presented as frequencies and percentages.
Results
Number of samples
A total of 198 samples, of which 86 (43%) were ARVs, 54 (27%) antimalarials and 58 (29%) anti-TB medicines, were collected and analysed. All samples met the acceptance criteria with respect to labelling information and package integrity before they were submitted for compendial analysis.
Sample source by country of manufacture
Table 1 shows manufacturing countries and the percentage distribution of the antimicrobials sampled across Zambia. Of the 198 samples, 164 (82.83%) were manufactured in India; 19 (9.60%) in Uganda; 8 (4.04%) in European countries such as Switzerland, Germany, Cyprus and Turkey; 4 (2.02%) in China; and 3 (1.52%) in South Korea.
Table 1.
Sample country of origin.
| Antimicrobial | India, n = 164 (%) | China, n = 4 (%) | Uganda, n = 19 (%) | South Korea, n = 3 (%) | Europe, a n = 8 (%) |
|---|---|---|---|---|---|
| ARVs | 77 (46.95) | – | 4 (21.05) | 2 (66.67) | 3 (37.50) |
| Antimalarials | 31 (18.90) | 4 (100) | 15 (78.95) | – | 4 (50.00) |
| Antituberculosis | 56 (34.15) | – | – | 1 (33.33) | 1 (12.50) |
| Overall % | 82.83 | 2.02 | 9.60 | 1.52 | 4.04 |
Country of origin of samples from Europe: Switzerland (2), Germany (3), Cyprus (1), Turkey (2).
Sample source by supply chain level
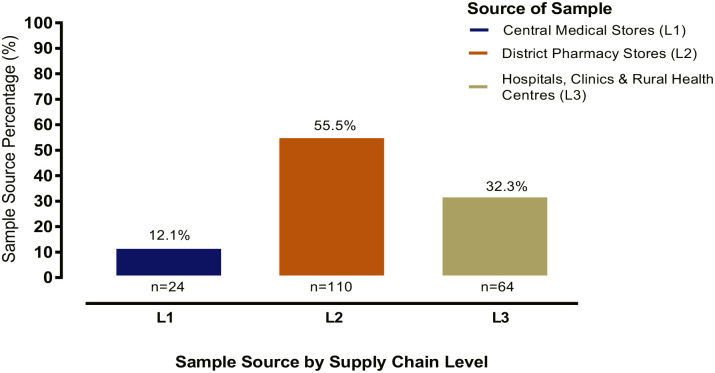
Figure 2 shows the level of the supply chain where samples were collected. Majority of samples (110 (55.55%)) were collected from district pharmacy stores (L2), followed by 64 (32.32%) which were collected from hospitals, clinics and rural health centres (L3), while 24 (12.12%) came from central medical stores (L1).
Figure 2.
Supply chain level of antiretrovirals, antimalarial and antituberculosis medicines collected from health facilities and storage areas across the 10 provinces of Zambia between January 2018 and June 2023.
Quality assessment
Table 2 shows that all (100%) samples of antimalarials, ARVs and anti-TB medicines complied with specific quality tests undertaken.
Table 2.
Quality of different antimalarials, antiretrovirals and antituberculosis medicines obtained from health facilities and storage areas across the 10 provinces of Zambia between January 2018 and June 2023.
| API (international non-proprietary name) | n | Quality test result |
||
|---|---|---|---|---|
| Pass | Fail | Overall pass, n (%) | ||
| Antimalarials | ||||
| Artemether/Lumefantrine | 50 | 50 | 0 | 50 (100) |
| Sulphadoxine/Pyrimethamine | 2 | 2 | 0 | 2 (100) |
| Artesunate | 2 | 2 | 0 | 2 (100) |
| Antiretrovirals | ||||
| Tenofovir/Lamivudine/Efavirenz | 11 | 11 | 0 | 11 (100) |
| Tenofovir/Lamivudine | 8 | 8 | 0 | 8 (100) |
| Efavirenz | 9 | 9 | 0 | 9 (100) |
| Lamivudine | 4 | 4 | 0 | 4 (100) |
| Lopinavir/Ritonavir | 7 | 7 | 0 | 7 (100) |
| Atazanavir/Ritonavir | 5 | 5 | 0 | 5 (100) |
| Abacavir/Lamivudine | 3 | 3 | 0 | 3 (100) |
| Dolutegravir/Lamivudine/Tenofovir | 15 | 15 | 0 | 15 (100) |
| Dolutegravir | 3 | 3 | 0 | 3 (100) |
| Tenofovir/Emtricitabine | 10 | 10 | 0 | 10 (100) |
| Abacavir | 3 | 3 | 0 | 3 (100) |
| Dolutegravir/Emtricitabine/Tenofovir | 1 | 1 | 0 | 1 (100) |
| Nevirapine | 1 | 1 | 0 | 1 (100) |
| Lamivudine/Zidovudine | 6 | 6 | 0 | 6 (100) |
| Antituberculosis drugs | ||||
| Rifampicin/Isoniazid/Pyrazinamide/Ethambutol | 12 | 12 | 0 | 12 (100) |
| Rifampicin/Isoniazid/Pyrazinamide | 6 | 6 | 0 | 6 (100) |
| Rifampicin/Isoniazid/Ethambutol | 2 | 2 | 0 | 2 (100) |
| Rifampicin/Isoniazid | 8 | 8 | 0 | 8 (100) |
| Isoniazid | 24 | 24 | 0 | 24 (100) |
| Ethambutol | 5 | 5 | 0 | 5 (100) |
| Cycloserine | 1 | 1 | 0 | 1 (100) |
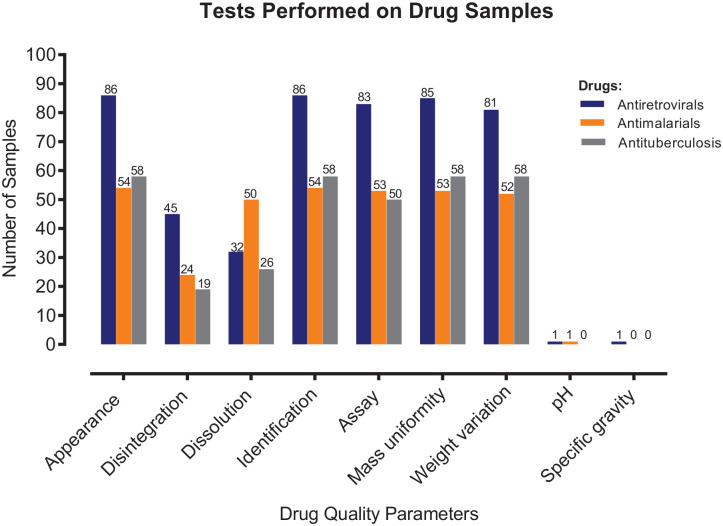
Figure 3 shows that all 198 (100%) samples were positively identified to contain their label-claimed APIs. All 186 (100%) samples that were subjected to assay tests met the tolerance limits set by various pharmacopoeias used. With respect to disintegration and dissolution, 88 and 108 samples, respectively, underwent these tests with a compliance rate of 100%. Other parameters the samples underwent include uniformity of mass, weight variation, and specific gravity and pH (for oral solution and injectable) with a compliance rate of 100%.
Figure 3.
Summary of tests conducted on samples of ARVs, antimalarial and antituberculosis medicines from public health facilities in Zambia between January 2018 and June 2023.
Methods of assay
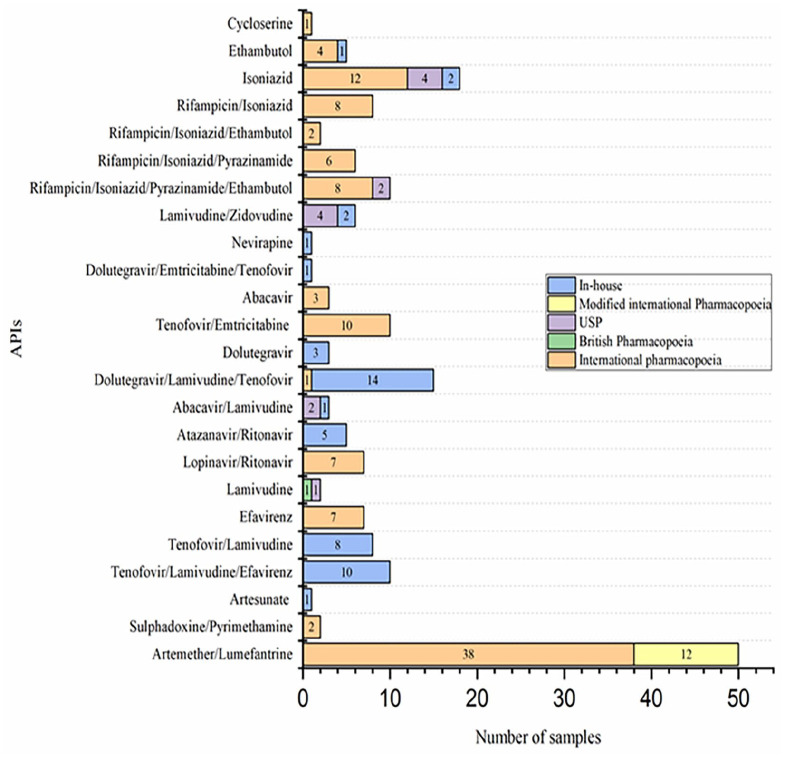
Figure 4 shows assay methods used in the analysis of samples of antimalarials, ARVs and anti-TB medicines that underwent this test. All (100%) samples complied with assay tests.
Figure 4.
Methods of analysis for determination of assay of different antimalarials, antiretrovirals and antituberculosis medicines obtained from health facilities and storage areas across the 10 provinces of Zambia between January 2018 and June 2023.
Discussion
Quality control is a key issue in supply chain monitoring, regulatory enforcement and ensuring consumer protection against substandard or falsified medical products. 31 In achieving this, manufactured medicines should comply with specific quality parameters that guarantee their safety, effectiveness and quality. However, this is not always the case as medicine quality problems arise due to a plethora of reasons, including non-compliance to Good Manufacturing Practices (GMP) and Good Distribution and Storage Practices (GDP). 32 To our knowledge, this is the first paper to report the quality of ARVs, antimalarials and anti-TB medicines supplied in Zambia’s public healthcare facilities using routine PMS data.
Quality failures of medicines can result in patient harm and have been linked to deaths worldwide.33,34 They contribute to AMR driven to a large extent by the administration of subtherapeutic doses derived from poor-quality antimicrobials,5–7 poisoning, treatment failure and adverse drug reactions. 35 In addition to causing patient harm, the use of poor-quality medicines can have an enormous economic impact on individuals and the healthcare system in general.33,36 Eventually, these outcomes jeopardize the entire healthcare system leading to loss of confidence in the healthcare systems and governments. This necessitates regular monitoring of the quality of medicines to prevent any potential public health disaster.
Our overarching finding showed that the ARVs, antimalarials and anti-TB medicines utilized in public healthcare facilities in Zambia meet the specified quality standards assessed. All of the 198 samples of ARVs, antimalarials and anti-TB medicines passed the tests for appearance, identification, assay, uniformity of mass and weight variation, disintegration, dissolution and pH including specific gravity, respectively (Figure 3). Methods for determination of assay for the respective APIs included the International Pharmacopoiea (Ph. Int), United States Pharmacopoiea (USP), British Pharmacopoiea (BP) and manufacturers In-house (IH) methods (Figure 4). For pharmacopoeia methods, the latest version was used according to the year each sample was analysed. According to Ahmed et al., 37 these tests are considered as some of the universal tests for pharmaceutical products. The findings could serve as a useful dashboard for quality assurance of ARVs, antimalarial and anti-TB medicines in the public healthcare sector.
In this survey, visual inspection of the dosage units did not reveal any inconsistencies or signs of degradation or contamination. Labelling information regarding dosage form, brand name, active ingredient/strength, batch number, name of manufacturer and expiry dates was provided for samples (100%). Labelling and packaging details are crucial as they play a significant role in ensuring drug safety, promoting treatment adherence, facilitating rational drug usage and facilitating the reporting of adverse drug reactions. 38 Our findings revealed that all examined products had sufficient labelling and included informational leaflets in their packaging. Some studies have reported inadequate compliance with labelling requirements for ARV and antimalarial medicines in countries such as Tanzania and Malawi. 39
Substandard and falsified medical products of all therapeutic classes have been found in many countries.11,40 The prevalence of substandard and falsified medicines seems to differ between countries and regions. A study of the quality of antimalarial medicines in Africa by the WHO 41 revealed that the prevalence of poor-quality medicines was much higher in West Africa than in East Africa. The study also, similar to findings in this survey, found no substandard or falsified antimalarial medicines in several countries, including Ethiopia and Kenya. 41
In contrast to the findings of this survey, several similar surveys have shown trends of poor-quality antimalarial and other antimicrobial medicines circulating in many African countries.17,42 However, the trends seem to differ between medicines selected from the public and private for-profit sectors. For instance, in Malawi, Chikowe et al. 43 found that despite 100% compliance to visual inspection tests, 88.4% (n = 112) of antimalarial medicines collected from private markets (licenced and unlicensed outlets) failed quality tests with respect to API content. However, another study in Malawi revealed a low prevalence of substandard and falsified medicines found in public and faith-based health facilities. 44
According to Do et al., 45 there is little data in the public domain on the quality of ARVs in supply chains and the proportion of substandard and falsified ARVs is relatively low in comparison with other classes of essential medicines. However, it must be understood that even a low proportion of poor-quality ARVs in the supply chain can make a large difference in the HIV/AIDS international landscape.
A quality survey of ARV medicines conducted by the WHO Prequalification Team (WHO-PQT) in cooperation with the National Medicines Regulatory Authorities/Ministries of Health in five countries in SSA, namely, Burkina Faso, Democratic Republic of the Congo (DRC), Nigeria, Rwanda and Zambia, found that the quality of ARV medicines at official public- and private-sector procurement and treatment centres was of good quality, with 125/126 (99.2%) of samples tested, fully compliant with the specifications set for the survey. 46
The survey by the WHO-PQT also revealed that in all quality surveys conducted, across product categories, 113/682 (16.6%) non-prequalified product samples failed to comply with specifications, compared with only 7/464 (1.51%) WHO-prequalified product samples. 46 These results demonstrate that WHO prequalification reliably assures uniform quality standards and reconfirms its positive impact in making ARVs of consistently good quality available for procurement in countries.
The prevalence of substandard and falsified anti-TB drug products in LMICs is well established.19,47 A study of the quality of two main first-line anti-TB medicines, isoniazid and rifampicin, in 19 cities of LMICs revealed a 9.1% (65/713) failure rate for requisite levels of API or disintegration. 19 The failure rate was 16.6% in Africa, 10.1% in India and 3.9% in other middle-income countries. Furthermore, Bate et al. 19 observed that while some of the failed products could have been attributed to poor formulation, some of the under-dosed products may have been properly manufactured but lost API due to degradation in poor storage conditions.48,49
Notwithstanding the fact that some reports have revealed the presence of substandard pharmaceutical products on the Zambian market.30,50,51 For instance, between 2018 and 2021, the ZAMRA recalled 83 pharmaceutical products which included one batch of locally manufactured anti-TB medicine, isoniazid tablets, for failing assay test and eight batches of ARVs atazanavir and ritonavir for an out-of-trend impurity after a 24-month shelf-life point. 30 The recalled batches of atazanavir and ritonavir were manufactured in India.
There are many reasons that could explain the results observed in this survey. One such reason could be practices around sourcing the medicines. ARVs, antimalarial and anti-TB medicines procured by donors or government-funded are typically subject to quality policies that require them to be WHO-prequalified or approved for use in a stringent regulatory environment. 46 In the Zambian public health sector, ARVs, antimalarials and anti-TB medicines are procured through multinational cooperating partners such as Global Fund and are provided for free in public health facilities, including mission hospitals and other faith-based NGOs. The survey by the WHO-PQT also revealed that Zambia and Nigeria applied more rigorous registration policies, further ensuring products in circulation were quality-assured. 46 Therefore, the high compliance of these products in the public sector can further be attributed to the MQAS for Procurement Agencies 27 and GDP 28 from selection, procurement, distribution and storage applied by the procuring agencies. The MQAS also supports continuous monitoring of the medicines down the supply chain until their end of shelf-life.
Findings on the quality of ARVs, antimalarial and anti-TB medicines in the public health sector in Zambia are encouraging. Unfortunately, the same may not apply to medicines imported and distributed by the private pharmaceutical sector. Like in many LMICs, the private sector in Zambia is subject to the effect of market forces, which so far do not seem to prioritize investments in quality control systems. 52
In Zambia, some pharmaceutical companies are international subsidiaries capable of implementing quality management systems to source quality products from prequalified suppliers. On the contrary, other wholesalers do not have the same degree of quality assurance infrastructure in place. 53 Therefore, it is clear that some wholesalers in Zambia may not typically have the capacity to conduct their own audits of manufacturers, and instead may rely upon other information less accurate for ascertaining quality which may lead to the inclusion of low-quality products in Zambia. 53 Furthermore, even though control and monitoring of storage conditions may not be complex, it may be expensive and time-consuming for some wholesalers. 29
Therefore, there is a need to invest more in the quality assurance and GDP for medical products imported, stored and distributed by both the public and private for-profit actors for a more holistic approach to safeguarding public health from substandard and falsified medical products.
Limitations of the study
It must be noted that the survey did not quantify the percentage of ARVs, antimalarial and anti-TB medicines distributed in the private-for-profit sector. It was limited to ARVs, antimalarial and anti-TB medicines in public healthcare facilities. Therefore, our findings cannot be generalized to all distribution chains operating in Zambia which include the private pharmaceutical sector. The survey was also limited by the set of districts, specific selection of medicines and a limited number of samples taken from storage areas and health facilities. In addition, some quality tests were not conducted on all samples. For example, an assay test was performed on 96.5% of ARVs, 98.1% of antimalarial and 86.2% of anti-TB medicines. Further, dissolution test was performed on 37.2% of ARVs, 96.1% of antimalarial and 44.8% of anti-TB medicines. Furthermore, the tests conducted were for routine quality parameters to the exclusion of tests such as related substances, impurities, microbial enumeration tests for solid dosage forms and water content.
Recommendations
To ensure effective regulation of complex, multi-stakeholder pharmaceutical supply chains, robust systems are required to ensure that quality products are procured, and quality is maintained in storage and distribution. Furthermore, strengthened PMS with modern field screening technologies for on-spot identification of substandard and falsified medicines and sustained investment in laboratory capacity at ZAMRA is a must. This should be supplemented by a medicine traceability programme providing end-to-end visibility of products. Future surveys should be conducted on antimicrobials found in the private-for-profit sector and be compared with the public sector.
Conclusion
The quality of ARVs, antimalarials and anti-TB medicines in the public health supply chain largely meet the quality standards assessed. We found no concerns regarding the quality of ARVs, antimalarials and anti-TB medicines in the public healthcare facilities. This should give some confidence in the management of diseases of public health importance in Zambia. The high quality of these commodities can be attributed to the WHO MQAS and GDP standards applied by GF, the main supplier of these products in the Zambian public health system. It is a well-known fact that quality systems for pharmaceutical importers and distributors in LMICs are non-compliant with WHO quality standards. It is, therefore, important that countries urgently incorporate WHO quality standards into national pharmaceutical regulations as a baseline, in addition to strong regulatory oversight and reliance on stringent regulatory authorities in making decisions. We will continue to monitor the PMS data for ARVs, antimalarials and anti-TB medicines in both the public and private-for-profit sector in Zambia in the future.
Acknowledgments
The authors would like to thank the Director General and Management at ZAMRA for providing permission to publish this study.
Footnotes
ORCID iDs: Elimas Jere  https://orcid.org/0009-0004-6816-915X
https://orcid.org/0009-0004-6816-915X
Billy Chabalenge  https://orcid.org/0000-0003-4864-887X
https://orcid.org/0000-0003-4864-887X
Declarations
Ethical approval and consent to participate: Official permission to access routine surveillance data was obtained from the ZAMRA management. Due to the nature of the study, ethics approval and informed consent to participate were not applicable. Confidentiality was guaranteed by not making any sampling facilities and product manufacturers identifiable.
Consent for publication: Official permission to access and publish routine post-marketing surveillance data was obtained from the ZAMRA management.
Author contributions: Elimas Jere: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing original draft and Writing review editing.
Derick Munkombwe: Data curation, Formal analysis, Visualization and Writing review editing.
Moses Mukosha: Formal analysis, Validation and Writing review editing.
Steward Mudenda: Formal analysis, Validation and Writing review editing.
Aubrey Chichonyi Kalungia: Formal analysis, Validation and Writing review editing.
Billy Chabalenge: Writing original draft, Methodology, Formal analysis, Validation and Writing review editing.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: The first and sixth authors are Pharmacists currently working under Zambia Medicines Regulatory Authority, Department of Medicines Control and are involved in post-marketing surveillance activities. Zambia Medicines Regulatory Authority had no role in the design of this study and collection, analysis and interpretation of data. Any views and opinions expressed are personal and belong solely to the individuals and do not represent any individual institutions, or organizations that the individuals are associated with in a personal or professional capacity. The remaining authors declare that they have no conflict of interest.
Availability of data and materials: The data sets for the current study can be made available from the corresponding author on reasonable request.
References
- 1. Hobson RH, Andrew RD, Williams GJ. Zambia. Encyclopedia Britannica (8 May 2024), https://www.britannica.com/place/Zambia.
- 2. Worldometers. Zambia Population, 2024, https://www.worldometers.info/world-population/zambia-population/
- 3. Institute for Health Metrics and Evaluation, https://www.healthdata.org/research-analysis/health-by-location/profiles/zambia.
- 4. World Health Organization. Global action plan on antimicrobial resistance 2015, https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1. [DOI] [PubMed]
- 5. World Health Organization. Antimicrobial resistance 2020, Https://Www.Who.Int/En/News-Room/Fact-Sheets/Detail/Antimicrobial-Resistance.
- 6. Gulumbe BH, Adesola RO. Revisiting the blind spot of substandard and fake drugs as drivers of antimicrobial resistance in LMICs. Ann Med Surg 2023; 85(2): 122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sulis G, Sayood S, Gandra S. Antimicrobial resistance in low-and middle-income countries: current status and future directions. Expert Rev Anti Infect Ther 2022; 20(2): 147–160. [DOI] [PubMed] [Google Scholar]
- 8. Essack SY, Desta AT, Abotsi RE, et al. Antimicrobial resistance in the WHO African region: current status and roadmap for action. J Public Health 2017; 39(1): 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moyo P, Moyo E, Mangoya D, et al. Prevention of antimicrobial resistance in sub-Saharan Africa: what has worked? What still needs to be done? J Infect Public Health 2023; 16(4): 632–639. [DOI] [PubMed] [Google Scholar]
- 10. Joshua IA, Bobai M, Woje CS. (eds). Managing antimicrobial resistance beyond the hospital antimicrobial stewardship: the role of one health. In: The Global Antimicrobial Resistance Epidemic: Innovative Approaches and Cutting-edge Solutions. London: IntechOpen, 2022, p. 39 [Google Scholar]
- 11. WHO Global Surveillance and Monitoring System for substandard and falsified medical products, 2017, https://iris.who.int/bitstream/handle/10665/326708/9789241513425-eng.pdf?sequence=1
- 12. Akpobolokemi T, Martinez-Nunez RT, Raimi-Abraham BT. Tackling the global impact of substandard and falsified and unregistered/unlicensed anti-tuberculosis medicines. J Med Access 2022; 6: 23992026211070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nayyar GML, Breman JG, Mackey TK, et al. Falsified and substandard drugs: stopping the pandemic. Am J Trop Med Hyg 2019; 100(5): 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chokshi A, Sifri Z, Cennimo D, et al. Global contributors to antibiotic resistance. J Glob Infect Dis 2019; 11(1): 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. A study on the public health and socioeconomic impact of substandard and falsified medical products. Geneva: WHO Press, 2017. [Google Scholar]
- 16. Wallis CL, Godfrey C, Fitzgibbon JE, et al. Key factors influencing the emergence of human immunodeficiency virus drug resistance in low-and middle-income countries. J Infect Dis 2017; 216(Suppl. 9): S851–S856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nayyar GM, Breman JG, Newton PN, et al. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis 2012; 12(6): 488–496. [DOI] [PubMed] [Google Scholar]
- 18. Renschler JP, Walters KM, Newton PN, et al. Estimated under-five deaths associated with poor-quality antimalarials in sub-Saharan Africa. Am J Trop Med Hyg 2015; 92(Suppl. 6): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bate R, Jensen P, Hess K, et al. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis 2013; 17(3): 308–311. [DOI] [PubMed] [Google Scholar]
- 20. SDG indicators. Metadata Repository, https://unstats.un.org/sdgs/metadata/.
- 21. Vaz A, Roldão Santos M, Gwaza L, et al. WHO collaborative registration procedure using stringent regulatory authorities’ medicine evaluation: reliance in action? Expert Rev Clin Pharmacol 2022; 15(1): 11–17. [DOI] [PubMed] [Google Scholar]
- 22. Yadav P, Tata HL, Babaley M. The World Medicines Situation. Storage and supply chain management. Geneva: World Health Organization, 2011, pp. 31–22. [Google Scholar]
- 23. Nebot Giralt A, Schiavetti B, Meessen B, et al. Quality assurance of medicines supplied to low-income and middle-income countries: poor products in shiny boxes? BMJ Glob Health 2017; 2(2): e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhattacharya B, Lam F. Overcoming shortages of essential medicines: perspectives from industrial and systems engineering and public health practice. In: Smith K, Ram P. (eds) Transforming Global Health: Interdisciplinary Challenges, Perspectives, and Strategies. Cham: Springer, 2020, pp. 179–191. [Google Scholar]
- 25. Subramanian L. Effective demand forecasting in health supply chains: emerging trend, enablers, and blockers. Logistics 2021; 5(1): 12. [Google Scholar]
- 26. Ehrenkranz P, Grimsrud A, Holmes CB, et al. Expanding the vision for differentiated service delivery: a call for more inclusive and truly patient-centred care for people living with HIV. J Acquir Immune Defic Syndr 1999. 2021; 86(2): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Annex 3 model quality assurance system for procurement agencies. WHO Technical Report Series 2014; 48: 137–291. [Google Scholar]
- 28. World Health Organization. Global benchmarking tool (GBT) for evaluation of national regulatory systems, https://www.who.int/medicines/regulation/benchmarkingtool/en/ (accessed 24 March 2020).
- 29. Nebot Giralt A, Bourasseau A, White G, et al. Quality assurance systems of pharmaceutical distributors in low-income and middle-income countries: weaknesses and ways forward. BMJ Glob Health 2020; 5(10): e003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chabalenge B, Jere E, Nanyangwe N, et al. Substandard and falsified medical product recalls in Zambia from 2018 to 2021 and implications on the quality surveillance systems. J Med Access 2022; 6: 27550834221141767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yabré M, Ferey L, Sakira AK, et al. Green analytical methods of antimalarial artemether-lumefantrine analysis for falsification detection using a low-cost handled NIR spectrometer with DD-SIMCA and drug quantification by HPLC. Molecules 2020; 25(15): 3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Newton PN, Amin AA, Bird C, et al. The primacy of public health considerations in defining poor quality medicines. PLoS Med 2011; 8(12): e1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caudron JM, Ford N, Henkens M, et al. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health 2008; 13(8): 1062–1072. [DOI] [PubMed] [Google Scholar]
- 34. Beargie SM, Higgins CR, Evans DR, et al. The economic impact of substandard and falsified antimalarial medications in Nigeria. PLoS ONE 2019; 14(8): e0217910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelesidis T, Kelesidis I, Rafailidis PI, et al. Counterfeit or substandard antimicrobial drugs: a review of the scientific evidence. J Antimicrob Chemother 2007; 60(2): 214–236. [DOI] [PubMed] [Google Scholar]
- 36. Newton PN, Green MD, Fernández FM, et al. Counterfeit anti-infective drugs. Lancet Infect Dis 2006; 6(9): 602–613. [DOI] [PubMed] [Google Scholar]
- 37. Ahmed S, Islam S, Ullah B, et al. A review article on pharmaceutical analysis of pharmaceutical industry according to pharmacopoeias. Orient J Chem 2020; 36(1): 1–10. [Google Scholar]
- 38. Sillo HB, Masota NE, Kisoma S, et al. Conformity of package inserts information to regulatory requirements among selected branded and generic medicinal products circulating on the East African market. PLoS ONE 2018; 13(5): e0197490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Newton PN, Hanson K, Goodman C. Do anti-malarials in Africa meet quality standards? The market penetration of non-quality-assured artemisinin combination therapy in eight African countries. Malar J 2017; 16(1): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newton PN, Bond KC. and Oxford Statement signatories. Global access to quality-assured medical products: the Oxford statement and call to action. Lancet Glob Health 2019; 7(12): e1609–e1611. [DOI] [PubMed] [Google Scholar]
- 41. World Health Organization. Survey of the quality of selected antimalarial medicines circulating in six countries of sub-Saharan Africa. Geneva: World Health Organization, 2011. [Google Scholar]
- 42. Osei-Safo D, Agbonon A, Konadu DY, et al. Evaluation of the quality of artemisinin-based antimalarial medicines distributed in Ghana and Togo. Malar Res Treat 2014; 2014: 806416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chikowe I, Osei-Safo D, Harrison JJ, et al. Post-marketing surveillance of anti-malarial medicines used in Malawi. Malar J 2015; 14(1): 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khuluza F, Kigera S, Heide L. Low prevalence of substandard and falsified antimalarial and antibiotic medicines in public and faith-based health facilities of southern Malawi. Am J Trop Med Hyg 2017; 96(5): 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Do NT, Boupha P, Newton PN, et al. The quality of antiretroviral medicines: an uncertain problem. BMJ Glob Health 2023; 8(3): e011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization. Survey of the quality of selected antiretroviral medicines circulating in five African countries. World Health Organization, 2019, https://iris.who.int/bitstream/handle/10665/330965/DI312-162-165-eng.pdf.
- 47. Laserson KF, Kenyon AS, Kenyon TA, et al. Substandard tuberculosis drugs on the global market and their simple detection. Int J Tuberc Lung Dis 2001; 5(5): 448–454. [PubMed] [Google Scholar]
- 48. Battini S, Mannava MKC, Nangia A. Improved stability of tuberculosis drug fixed-dose combination using isoniazid-caffeic acid and vanillic acid cocrystal. J Pharm Sci 2018; 107(6): 1667–1679. [DOI] [PubMed] [Google Scholar]
- 49. Bhutani H, Mariappan TT, Singh S. The physical and chemical stability of anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int J Tuberc Lung Dis 2004; 8(9): 1073–1080. [PubMed] [Google Scholar]
- 50. Jackson KD, Higgins CR, Laing SK, et al. Impact of substandard and falsified antimalarials in Zambia: application of the SAFARI model. BMC Public Health 2020; 20: 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mweemba W. Evaluation of the quality of anti-Tuberculosis drugs in Lusaka, Zambia. Med J Zambia 2015; 42(4): 144–149. [Google Scholar]
- 52. McCabe A. Private sector pharmaceutical supply and distribution chains: Ghana, Mali and Malawi. Health systems for outcomes publication. Washington, DC: World Bank, 2009. [Google Scholar]
- 53. USAID Global Health Supply Chain Program. Quality considerations of Zambian wholesalers and regulatory authorities to increase availability and access to quality maternal, newborn, and child health products in Zambia, 2020. [Google Scholar]