Dear Editor,
Bird flu, primarily caused by avian influenza A viruses, poses significant pandemic threats due to antigenic drift and shift. 1 The highly pathogenic avian influenza (HPAI) A(H5N1) virus has caused global outbreaks in birds, killing millions of wild birds and poultry, with sporadic cases in humans often being fatal with a case fatality rate of 52% (893 cases in 24 countries between 2003 and 2024, with 463 deaths) 2 reaching all continents. 3 The 2009 H1N1 pandemic demonstrated the risks of reassortment between human, avian and swine influenza viruses. 4 Recent H5N1 outbreaks in mammals raise concerns about potential human transmission. Effective cooking is one step toward critical control. In an experiment cooking study cooking ground beef at 120°F (48.9°C), the temperature of a rare steak was not able to inactivate the virus, but it was not detected at 145°F–160°F (62.8°C–71.1°C), suggesting sufficient cooking temperatures are required to inactivate HPAI A(H5N1) virus in meat. 5 Although cows routinely contract other forms of influenza viruses, one study had shown that they could potentially contract A(H5N1) virus following an experiment in four calves inoculated with A(H5N1) virus isolated from a cat. 6 It is concerning that for the first time, HPAI A(H5N1) virus, clade 2.3.4.4b has been identified in 145 dairy herds across 12 US states (Colorado, Idaho, Iowa, Kansas, Michigan, Minnesota, New Mexico, North Carolina, Ohio, South Dakota, Texas, Wyoming; as of July 8 2024; Figure 1). 7 Cows show symptoms of infection including sharply reduced milk supply, changes in milk thickness and lethargy. Of particular concern is the recent detection of the A(H5N1) virus in humans who had direct contact with dairy cattle, marking the first recorded instances of transmission from cows to humans. On 1 April 2024, a case was reported in Texas, followed by two cases in Michigan on 22 May and 30 May 2024 and one case in Colorado on 3 July 2024 (Figure 1).8–11 Previously, in 2022, a single case in the United States involved transmission from poultry, bringing the total number of human A(H5N1) cases in the country to five. In 2024, the first two cases reported conjunctivitis (clade 2.3.4.4b), the third case (clade 2.3.4.4b) reported typical symptoms of acute respiratory illness, and, the recent case in Colorada only reported conjunctivitis (clade not known yet). 12 These four recent cases have raised concerns about viral mutations enhancing infectivity in mammals and humans. Infected cattle increase the risk due to higher human–animal interactions. Genetic analysis of the Texas case shows mammalian adaptation with the PB2 E627K mutation. The Michigan case lacks changes in hemagglutinin but has a PB2 M631L mutation, indicating mammalian adaptation and suggests direct cow-to-human transmission. These mutations are known to enhance replication and pathogenicity. This does not imply that an outbreak will occur, but the presence of infected cattle increases the risk due to more frequent human–animal interactions compared to wildlife. As per USDA, out of 96 muscle samples from culled dairy cows, only 1 tested positive for viral particles and no contaminated meat has entered the food supply. 5
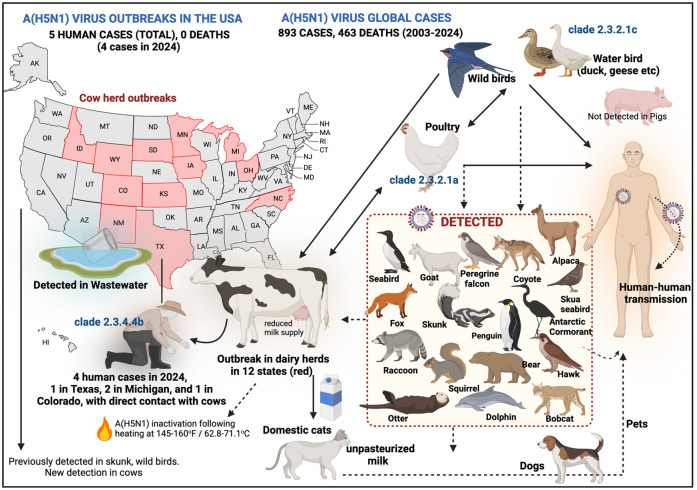
Figure 1.
Is H5N1 the next pandemic? Since 2003, A(H5N1) has accounted for 893 global human cases with 463 deaths in 24 countries, including 11 cases in 2024. Antarctica has seen the first-ever cases of A(H5N1) virus in animals. In 2024, a man in Texas, two in Michigan, and one in Colorado, contracted H5N1 after contact with dairy cattle. H5N1 has been detected in several states across United States in wastewater, and there have been 145 cowherd outbreaks in 12 states across the United States (as of 8 July 2024), as shown. Cats contracted H5N1 from infected cow’s milk, and hunting dogs have also been found to be positive after retrieving wild waterfowl. The virus’s ability to infect pets is concerning, as wild birds transmit it to poultry and cattle. Other animals, as shown in the inset, have also been detected with A(H5N1) virus infection, but their role in transmission remains unclear. Solid lines depict confirmed transmission routes, while dotted lines depict uncertain transmission pathways.
Source: The bio-render (https://www.biorender.com/) was used for developing this figure.
Wild aquatic birds (geese, ducks) are regarded as the original reservoir of A(H5N1) virus, which was first detected in 1996 in China, which spread to domestic chickens in 1997. The clade 2.3.2.1c of A(H5N1) has led several outbreaks in poultry and humans in Southeast Asian countries, including infections to five humans in Cambodia and one in Vietnam in 2024. The clade 2.3.2.1a has been identified in poultry and in a returned traveller to Australia from India. A(H5N1) virus was also detected in unpasteurized clinical samples of milk. 13 However, a recall is not required for commercial milk supplies as pasteurization inactivates the virus, and consumption of raw milk is generally not recommended. It is unclear if pigs can contract A(H5N1) virus, but if they do, it is particularly concerning as they can serve as mixing vessels for avian and human influenza viruses. This can lead to the emergence of new, potentially more virulent strains capable of human-to-human transmission, significantly increasing the risk of a pandemic. Since 2022, A(H5N1) has also been identified in 20 mammal species including, mink, goats, bears, foxes, sea lions, mountain lions, dolphins, seals, coyotes, otters, raccoons, skunks and squirrels. 14 To add to the concern, wastewater testing in several states across the United States identified H5N1 avian flu in 9 different Texas cities, 15 and A(H5N1) virus was detected in 59 wastewater treatment plants across the United States (from 190 wastewater treatment plants located in 41 states), 16 including California, Idaho, Iowa, Minnesota, Michigan and Texas. This suggests that A(H5N1) virus may spread in wild or domestic animals (cats, dogs) and enter residential areas. Several domestic cats have recently died due to H5N1 infections in a Texas farm and in Kansas, which had consumed unpasteurized raw milk from symptomatic cows. 17 Hunting dogs in Washington state, particularly those frequently in contact with waterfowl in regions experiencing A(H5N1) outbreaks, have been detected with A(H5N1) virus. 18 Given the infection in cats and dogs, there is a high possibility of developing other intermediate hosts. This could lead to the virus circulating among domestic pets, which might then spill over to humans and potentially result in a pandemic.
In 2024, A(H5N1) virus has been detected in several mammals in the United States including, 19 mountain lion (Montana), skunks (Washington, Idaho, California), bobcats (Washington, Vermont, New York), domestic cats (Texas, New Mexico, Ohio, Michigan, Montana, South Dakota), American mink (Kentucky), raccoons (New York, Colorado), red fox (Missouri, New York, Michigan), Virginia opossum (Michigan), farmed goats (Minnesota) and dolphins (Florida). In addition, in New York city, Canada geese, red-tailed hawk, peregrine falcon and a chicken, have been reported to be infected with A(H5N1) virus clade 2.3.4.4b 20 (Figure 1); A(H5N1) has also been reported in thousands of poultry and birds in 2024, as well as, in Alpacas in a farm in Idaho. In February 2024, A(H5N1) virus was detected in mainland Antarctica for the first time. It was first detected in two brown skua seabirds, on the western side of Antarctica, close to South America. 21 Subsequently, H5N1 was detected in 12 Antarctic skua seabird carcasses on Beak Island, with further cases in Hope Bay and Devil and Paulet islands of Antarctica.22,23 In March 2024, nine Adelie penguins and one Antarctic cormorant tested positive for H5N1, posing a significant threat to the local wildlife. So far, Australia and New Zealand have not reported any cases of A(H5N1) in animals.
The infection of several US cattle herds with A(H5N1) virus has raised alarms about its potential spread to humans. Previously circulating primarily in birds, the virus now infects mammals that regularly interact with humans. The development of a vaccine against A(H5N1) virus is essential for control measures. 24 As such, H5N1 adjuvanted cell-based monovalent vaccine, AUDENZ™, was approved by the FDA in September 2020 to help protect people over the age of 6 months against A(H5N1) infection in the event of a pandemic. 25 This is important for pandemic preparedness and the company Sequiris, a subsidiary of CSL Limited in Australia, has stockpile of the vaccine. GlaxoSmithKline and Sanofi have seasonal influenza production capacity and would be able to scale up production of H5N1 vaccines including AUDENZ, if needed. Previously, egg-based H5N1 vaccines were approved for Sanofi Pasteur’s vaccine in April 2007 in the United States, GlaxoSmithKline’s Prepandrix vaccine was approved by the EU in May 2008, and CSL Limited Panvax vaccine was approved in Australia in June 2008. Further, efforts should be made to expand use of poultry A(H5N1) viral vaccines despite challenges such as inter-flock deployment and restrictions on selling vaccinated poultry products abroad. 26 Cows are vaccinated against several diseases, but there are currently no approved H5N1 avian influenza vaccines for cattle, and companies have started to work on a A(H5N1) vaccine for cows. The current situation in the United States with the two infected humans from milk cows suggests the dynamic nature of zoonotic viruses and their potential to disrupt scientific and economic systems. 27 The detection of HPAI A(H5N1) in four dairy farm workers is a crucial alert for the global health community, urging a reassessment of our understanding of viral spread and human infection. This case not only enhances our knowledge of A(H5N1) viral transmission but also influences our strategy for emerging infectious diseases. Immediate action is essential to improve surveillance, refine diagnostics and strengthen public health infrastructure. Strengthening these areas will better protect frontline food system workers and the general public from the persistent threat of emerging zoonoses.
Acknowledgments
None.
Footnotes
ORCID iDs: Alfonso J. Rodriguez-Morales  https://orcid.org/0000-0001-9773-2192
https://orcid.org/0000-0001-9773-2192
Andres F. Henao-Martinez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Ranjit Sah  https://orcid.org/0000-0002-2695-8714
https://orcid.org/0000-0002-2695-8714
Contributor Information
Vasso Apostolopoulos, Institute for Health and Sport, Victoria University, Werribee Campus, Biomedical Innovation Group, 235 Hoppers Lane, Werribee, Melbourne, VIC 3030, Australia.
Vivek P. Chavda, Department of Pharmaceutics and Pharmaceutical Technology, L M College of Pharmacy, Ahmedabad, Gujarat, India
Rachana Mehta, Dr. Lal PathLabs Nepal, Chandol, Kathmandu, Nepal; Department of Medical Laboratories Techniques, Al-Mustaqbal University, Hillah Babil, Iraq; Clinical Microbiology, RDC, Manav Rachna International Institute of Research and Studies, Faridabad, Haryana, India.
Alfonso J. Rodriguez-Morales, Faculty of Health Sciences, Universidad Científica del Sur, Lima, Peru; Gilbert and Rose-Marie Chagoury School of Medicine, Lebanese American University, Beirut, Lebanon.
Andrés F Henao-MartÍnez, Department of Medicine, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Ranjit Sah, SR Sanjeevani Hospital, Kalyanpur, Siraha, Nepal; Department of Microbiology, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India; Department of Public Health Dentistry, Dr. D. Y. Patil Dental College and Hospital, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Vasso Apostolopoulos: Conceptualization; Supervision; Writing – original draft; Writing – review & editing.
Vivek P. Chavda: Writing – original draft; Writing – review & editing.
Rachana Mehta: Writing – review & editing.
Alfonso J. Rodriguez-Morales: Writing – review & editing.
Andrés F Henao-Martínez: Writing – review & editing.
Ranjit Sah: Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Ahmad F, Haque S, Tawil S, et al. Avian influenza spillover to humans: are we prepared to deal with another potential pandemic? Travel Med Infect Dis 2023; 55: 102634. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhary RK, Ananthesh L, Patil P, et al. System biology approach to identify the hub genes and pathways associated with human H5N1 infection. Vaccines (Basel) 2023; 11: 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charostad J, Rezaei Zadeh Rukerd M, Mahmoudvand S, et al. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: an imminent threat at doorstep. Travel Med Infect Dis 2023; 55: 102638. [DOI] [PubMed] [Google Scholar]
- 4. Cheng P, Zhai K, Han W, et al. Characterizing the antigenic evolution of pandemic influenza A (H1N1) pdm09 from 2009 to 2023. J Med Virol 2024; 96: e29657. [DOI] [PubMed] [Google Scholar]
- 5. Animal and Plant Health Inspection Service, The United States Department of Agriculture (USDA). Updates on H5N1 beef safety studies, https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock/h5n1-beef-safety-studies (2024, accessed 20 May 2024).
- 6. Kalthoff D, Hoffmann B, Harder T, et al. Experimental infection of cattle with highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008; 14: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Animal and Plant Health Inspection Service, The United States Department of Agriculture (USDA). Highly pathogenic avian influenza (HPAI) detections in livestock, https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock (2024, accessed 30 May 2024).
- 8. Texas Health and Human Services (THHS). Health alert: first case of novel influenza A(H5N1) in Texas, March 2024, https://www.dshs.texas.gov/news-alerts/health-alert-first-case-novel-influenza-h5n1-texas-march-2024 (2024, accessed 22 May 2024).
- 9. Sah R, Srivastava S, Kumar S, et al. Concerns on H5N1 avian influenza given the outbreak in US dairy cattle. Lancet Reg Health Americas 2024; 35: 100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Centers for Disease Control and Prevention (CDC). CDC reports second human case of H5 bird flu tied to dairy cow outbreak, https://www.cdc.gov/media/releases/2024/s0522-human-case-h5.html (2024, accessed 23 May 2024).
- 11. Uyeki TM, Milton S, Webb CR, et al. Highly pathogenic avian influenza A(H5N1) virus infection in a dairy farm worker. N Engl J Med 2024; 390(21): 2028–2029. [DOI] [PubMed] [Google Scholar]
- 12. The Centers for Disease Control and Prevention (CDC). CDC confirms second human H5 bird flu case in Michigan; third case tied to dairy outbreak, https://www.cdc.gov/media/releases/2024/p0530-h5-human-case-michigan.html (2024, accessed 30 May 2024).
- 13. The United States Food and Drug Administration (FDA). Questions and answers regarding milk safety during highly pathogenic avian influenza (HPAI) outbreaks, https://www.fda.gov/food/milk-guidance-documents-regulatory-information/questions-and-answers-regarding-milk-safety-during-highly-pathogenic-avian-influenza-hpai-outbreaks (2024, accessed 22 May 2024).
- 14. The Centers for Disease Control and Prevention (CDC). Influenza flu. Bird flu in pets and other animals. https://www.cdc.gov/flu/avianflu/avian-in-other-animals.htm (2024, accessed 20 May 2024).
- 15. Center for Infectious Disease Research & Policy (CIDRP). University of Minnesota. Wastewater testing finds H5N1 avian flu in 9 Texas cities, https://www.cidrap.umn.edu/avian-influenza-bird-flu/wastewater-testing-finds-h5n1-avian-flu-9-texas-cities (2024, accessed 20 May 2024).
- 16. Wolfe M, Duong D, Shelden B, et al. Detection of hemagglutinin H5 influenza A virus sequence in municipal wastewater solids at wastewater treatment plants with increases in influenza A in Spring 2024. Environ Sci Technol Lett 2024; 11(6): 526–532. [Google Scholar]
- 17. Burrough ER, Magstadt DR, Petersen B, et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis 2024; 30(7): 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown JD, Black A, Haman KH, et al. Antibodies to influenza A(H5N1) virus in hunting dogs retrieving wild fowl, Washington, USA. Emerg Infect Dis 2024; 30(6): 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Animal and Plant Health Inspection Service, The United States Department of Agriculture (USDA). Detections of highly pathogenic avian influenza in mammals, https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (2024, accessed 30 May 2024).
- 20. Meade PS, Bandawane P, Bushfield K, et al. Detection of clade 2.3.4.4b highly pathogenic H5N1 influenza virus in New York City. J Virol 2024; 98(6): e0062624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong C. Bird-flu threat disrupts Antarctic penguin studies. Nature. Epub ahead of print March 2024. DOI: 10.1038/d41586-024-00807-0. [DOI] [PubMed] [Google Scholar]
- 22. Munoz G, Mendieta V, Ulloa M, et al. Lack of highly pathogenic avian influenza H5N1 in the South Shetland Islands in Antarctica, early 2023. Animals (Basel) 2024; 14(7): 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stokstad E. In Antarctica, scientists track a dangerous bird flu. Science 2024; 383: 1281. [DOI] [PubMed] [Google Scholar]
- 24. Li F, Liu B, Xiong Y, et al. Enhanced downstream processing for a cell-based avian influenza (H5N1) vaccine. Vaccines (Basel) 2024; 12(2): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Food and Drug Administration (FDA). AUDENZ, https://www.fda.gov/vaccines-blood-biologics/audenz (2024, accessed 22 May 2024).
- 26. Kong D, He Y, Wang J, et al. A single immunization with H5N1 virus-like particle vaccine protects chickens against divergent H5N1 influenza viruses and vaccine efficacy is determined by adjuvant and dosage. Emerg Microbes Infect 2024; 13(1): 2287682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maartens LH, Frizzo da Silva L, Dawson S, et al. The efficacy of an inactivated avian influenza H5N1 vaccine against an African strain of HPAI H5N8 (clade 2.3.4.4 B). Avian Pathol 2023; 52: 176–184. [DOI] [PubMed] [Google Scholar]