Abstract
Simple Summary
Enterococcus faecium, a lactic acid bacterium, is approved for use as a direct-fed microbial by the Ministry of Agriculture (MOA) in China. However, some research reports indicate that certain strains of Enterococcus faecium have developed antibiotic resistance. Moreover, some strains of Enterococcus faecium carry virulence genes and are considered to be conditional pathogens. Therefore, selecting safe and reliable strains of Enterococcus faecium as probiotics for livestock and poultry is particularly important. This experiment used Enterococcus faecium isolated from the intestinal contents of healthy minks to evaluate the effects of Enterococcus faecium on the growth performance, antioxidant capacity, immunity, and gut microbiota composition of growing male minks.
Abstract
The purpose of this experiment was to explore the effects of dietary Enterococcus faecium (EF) on the growth performance, antioxidant capacity, immunity, and intestinal microbiota of growing male minks. A total of 60 male Regal White minks at 12 weeks of age were randomly assigned to two groups, each with 15 replicates of two minks per replicate. The minks in two groups were fed the basal diets and the basal diets with viable Enterococcus faecium (more than 107 cfu/kg of diet), respectively. Compared with the minks in control, Enterococcus faecium minks had heavier body weight (BW) at week 4 and week 8 of the study (p < 0.05), greater average daily gain (ADG), and a lower feed/gain ratio (F/G) of male minks during the initial 4 weeks and the entire 8-week study period (p < 0.05). Furthermore, Enterococcus faecium increased the apparent digestibility of crude protein (CP) and dry matter (DM) compared to the control (p < 0.05). Moreover, Enterococcus faecium enhanced the serum superoxide dismutase (SOD) activity and decreased the malondialdehyde (MDA) contents (p < 0.05). The results also confirmed that Enterococcus faecium increased the levels of serum immunoglobulin A (IgA), immunoglobulin G (IgG), and the concentrations of secretory immunoglobulin A (SIgA) in the jejunal mucosa while decreasing the interleukin-8 (IL-8) and interleukin-1β (IL-1β) levels in the jejunal mucosa (p < 0.05). Intestinal microbiota analysis revealed that Enterococcus faecium increased the species numbers at the OUT level. Compared with the control, Enterococcus faecium had significant effects on the relative abundance of Paraclostridium, Brevinema, and Comamonas (p < 0.05). The results showed that Enterococcus faecium could improve the growth performance, increase the antioxidant capacity, improve the immunity of growing male minks, and also modulate the gut microbiota.
Keywords: mink, Enterococcus faecium, growth performance, antioxidant capacity, immunity, intestinal flora
1. Introduction
Probiotics, as defined by the FAO/WHO, are live microorganisms that confer health benefits to the host when administered in adequate amounts [1,2]. Lactic acid bacteria are widely utilized as probiotics across various applications. These heterotrophic anaerobes ferment carbohydrates into lactic acid, an antibacterial compound that inhibits the growth of acid-sensitive harmful bacteria by lowering the pH value [3]. In addition to lactic acid, lactic acid bacteria also produce bacteriocins, mannitol, fatty acids, and exopolysaccharides, all of which are associated with beneficial physiologic effects [4]. Recognized as safe for consumption (GRAS), lactic acid bacteria have been extensively used as probiotics in the livestock and poultry industries [5,6,7].
Enterococcus faecium, a type of lactic acid bacterium, is naturally present in the digestive tracts of animals, soil, and water. It is resistant to heat, gastric acids, and bile salts [8]. In China, it was approved for use as a direct-fed microorganism by the Ministry of Agriculture (MOA) in 2003. Previous studies on pigs and chickens have verified that Enterococcus faecium can improve growth performance, enhance immunity, and regulate intestinal flora [9,10].
The employment of Enterococcus faecium as a probiotic is still controversial. Enterococcus faecium exhibits essential probiotic properties, including heat resistance, acid tolerance, bile salt resistance, adhesion capabilities, and the ability to inhibit bacterial growth [11,12]. However, its strong adaptability increases the potential for Enterococcus faecium to develop antibiotic resistance. Particularly, overuse of antibiotics has caused a serious problem of antibiotic resistance in some strains of Enterococcus faecium [13,14]. Furthermore, the resistance is encoded on plasmids, which makes it susceptible to the potential for spread through horizontal gene transfer [15]. Amaral et al. [16] suggested that Enterococcus faecium strains isolated from food, commensal organisms, and the environment carry a low risk. As a normal component of the intestinal flora in animals, Enterococcus faecium should not be entirely ruled out as a probiotic candidate due to the pathogenicity of a few strains [17]. Therefore, identifying reliable sources for the isolation of potential probiotic strains is crucial. Considering that probiotic properties are related to host specificity, strains isolated from the host species may exhibit more advantageous probiotic effects compared to those derived from other origins [18,19].
This experiment utilized the Enterococcus faecium strain isolated from the intestinal contents of healthy minks to explore its effects on the growth performance, antioxidant capacity, immunity, and gut microbiota composition of growing male minks.
2. Material and Methods
2.1. Ethics
The Animal Care and Use Committee of Animal Science and Technology at Qingdao Agricultural University reviewed and approved the experimental protocol (approval number DKY20230524-1, date: 24 May 2023).
2.2. Enterococcus faecium
Enterococcus faecium (EF) was previously isolated from the rectal contents of minks, identified through 16S rRNA gene sequence analysis, and is preserved in the China General Microbiological Culture Collection Center (Accession No. 29262). The isolated strain of Enterococcus faecium was inoculated into the De Man, Rogosa, and Sharpe (MRS) substrate and cultivated at 37 °C for 24 h. The viable Enterococcus faecium in the suspension was not less than 109 cfu/mL. The prepared bacterial suspension was stored at 4 °C for use in the present study, prepared once every 20 days to ensure that the viable count of Enterococcus faecium remained above 107 cfu/mL.
2.3. Experimental Design and Animal Management
The research was performed at a commercial mink farm located in Haiyang, Yantai, Shandong Province. A total of 60 male Regal White minks at the age of 12 weeks with similar body weight (BW) were randomly assigned to two groups, each with 15 replicates of two minks. The minks in two groups were fed the basal diets and the basal diets with viable Enterococcus faecium probiotics (containing viable Enterococcus faecium at more than 107 cfu/kg of diet), respectively. The paste diets were composed of sea fish and byproducts, chicken byproducts, and egg products. The diets were formulated on the farm based on commercial recommendations. The detailed composition of the experimental diets and the nutrient levels are presented in Table 1. Following a one-week adaptation period, the experiment was conducted for a duration of eight weeks.
Table 1.
Composition and nutrient levels of diets (air-dry basis, %).
| Items | 0–4 Weeks | 5–8 Weeks |
|---|---|---|
| Sea fish and byproducts | 32 | 32 |
| Unhatched fertilized egg | 32 | 32 |
| Chicken head | 20 | 20 |
| Extruded corn | 10 | 10 |
| Lard | 1 | 2 |
| Soybean meal | 2 | 2 |
| premix 1 | 3 | 2 |
| Total | 100 | 100 |
| Nutrient levels | ||
| ME (MJ/kg) 2 | 15.98 | 17.04 |
| Ether extract | 16.65 | 19.85 |
| Crude protein | 31.81 | 31.26 |
| Calcium | 2.47 | 2.59 |
| phosphorus | 1.59 | 1.64 |
1 The premix provided the following per kg of the diets: VA 9000 IU, VC 40 mg, VE 20 mg, VK3 0.5 mg, VB1 5 mg, VB2 3 mg, VB6 2.5 mg, VB12 1 mg, VD3 2000 IU, VB3 20 mg, VB5 6 mg, VB9 0.5 mg, VB7 0.5 mg, Fe 30 mg, Zn 25 mg, Mn 10 mg, Cu 5 mg, I 0.25 mg, Se 0.2 mg. 2 The metabolic energy values are derived from calculations, whereas the remaining values are obtained through direct measurement.
All minks were housed in metallic cages arranged within an open-sided double-row shed. Each cage was made of identical wire mesh, measured 30 × 75 × 45 cm (width × length × height), and housed a pair of minks. The minks were fed twice daily at 6:30 a.m. and 3:30 p.m., respectively, throughout the study period. Each cage was equipped with a water drinker, ensuring minks enjoyed unrestricted access to drinking water. The recorded ambient temperature was 26.24 ± 3.31 °C, and the relative humidity was 65.27 ± 3.54% throughout the study. The light schedule adhered to the natural light regime.
2.4. Sample and Data Collection
2.4.1. Growth Performance
All minks were individually weighed at the beginning (week 0), week 4, and week 8 of the study to determine the initial (week 0), week 4, and final (week 8) body weight. In addition, the feed provided and the leftovers were accurately weighed and recorded over three consecutive days each week throughout the experimental period. The average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (F/G) for the minks were calculated.
2.4.2. Apparent Digestibility of Nutrients
A digestive experiment was conducted at week 7 of the study, using the endogenous indicator method to determine the apparent digestibility of nutrients. The digestive experiment lasted for 3 days and involved the collection of both diet and fecal samples. In each group, six fecal samples were collected, with each 3-day cumulative fecal sample being mixed and weighing approximately 200 g. Concurrently, diets for each group were sampled daily over the 3 days prior to feeding the minks, and these samples were then pooled to obtain representative diet samples. All diet and fecal samples were dried to a constant weight in a draft oven at 65 °C to obtain air-dried samples and determine the initial moisture content. The air-dried samples were ground in a 1 mm screen mill. The dry matter (DM), crude ash, hydrochloric acid insoluble ash, crude protein (CP), and ether extract (EE) in these diet and fecal samples were analyzed according to GB/T 6435-2014, GB/T 6438-2007, GB/T 23742-2009, GB/T 6432-2018, and GB/T 6433-2006, respectively.
| Nutrient apparent digestibility (%) = 100% − (A1/A2) × (B2/B1) |
A1: the content of hydrochloric acid-insoluble ash in the diet; A2: the content of hydrochloric acid-insoluble ash in the feces samples; B1: the content of a certain nutrient in the diet; B2: the content of a certain nutrient in the feces sample.
2.4.3. Immunity and Antioxidant Capacity
At the end of the study, eight minks per group were randomly selected. Blood was collected via heart puncture, and the minks were subsequently euthanized to obtain 2–5 g of jejunum mucosal tissue. Serum was obtained from blood samples by centrifugation at 3000× g at 4 °C for 10 min. Serum levels of immunoglobulins (IgA, IgG, and IgM), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-pX), superoxide dismutase (SOD), and malondialdehyde (MDA) were measured. Jejunum mucosal tissue samples were diluted with 0.9% saline solution (1:9 weight/volume ratio) and then homogenized using a grinder. The homogenates were centrifuged at 3500× g for 10 min at 4 °C to obtain the supernatant, which was then used to analyze the contents of secretory immunoglobulin A (SIgA) and cytokines (IL-1β, IL-8, IL-10, IL-2, IL-6, IL-12, TNF-α, and IFN-γ). All these immune indicators were measured using ELISA kits manufactured by the Jiancheng Biological Engineering Research Institute in Nanjing, China. T-AOC and the activity of GSH-pX were determined using the colorimetric method. The activity of T-SOD was measured by the hydroxylamine method, and the MDA was determined using the thiobarbituric acid (TBA) method. All the antioxidant indicator assay kits used were manufactured by the Jiancheng Biological Engineering Research Institute in Nanjing, China.
2.4.4. Intestinal Microbiota
Concurrently with the collection of jejunal mucosal tissues, rectal mucosal swabs were obtained. DNA from rectal mucosal swabs was extracted to determine intestinal flora, employing the method delineated by Li et al. [20]. The extracted genomic DNA was detected, amplified (ABI GeneAmp®, 9700), purified, recovered (Axyprep DNA Gel Extraction Kit, Axygen, Union City, CA, USA), quantified (QuantiFluor™-ST Blue Fluorescence Quantification System, Promega, Madison, WI, USA), and sequenced (Illumina MiSeq pE 300 platform) [21].
2.5. Statistical Analysis
The data on growth performance, immunity, and antioxidant capacity were analyzed by a Student’s t-test using SPSS 25.0 (SPSS Institute, Inc., Chicago, IL, USA), and the results were presented as means ± standard deviation. p < 0.05 means a significant difference.
The data of the intestinal microbiota were analyzed on the I-Sanger cloud platform. FLASH 1.2.11 software performs paired-end sequencing. The differences in alpha diversity indices and species composition were statistically analyzed through the Student’s t-test. The Spearman correlation coefficient was used to analyze the correlation between the intestinal flora and the immune indicators of the jejunal mucosa in growing male minks.
3. Results
3.1. Growth Performance
The Enterococcus faecium had significant effects on body weight, ADG, and F/G (p < 0.05, Table 2), but the effects were not observed on ADFI (p > 0.05). Compared with the control minks, EF minks had higher BW (p < 0.05) at week 4 and week 8 of the study, greater ADG (p < 0.05), and less F/G (p < 0.05) during the first 4 weeks and the entire 8 weeks of the study.
Table 2.
Effect of Enterococcus faecium on the growth performance of mink.
| Items | Groups | p-Value | |
|---|---|---|---|
| 0 (Control) | EF | ||
| BW, g | |||
| wk 0 | 1281.15 ± 45.60 | 1280.35 ± 40.03 | 0.962 |
| wk 4 | 1736.54 ± 112.22 | 1825.38 ± 54.45 | 0.020 |
| wk 8 | 2171.73 ± 167.59 | 2282.31 ± 84.09 | 0.044 |
| ADG, g | |||
| 0–4 weeks | 16.26 ± 3.66 | 19.46 ± 1.22 | 0.010 |
| 5–8 weeks | 15.54 ± 3.79 | 16.32 ± 2.97 | 0.566 |
| 0–8 weeks | 15.90 ± 2.86 | 17.89 ± 1.51 | 0.037 |
| ADFI, g | |||
| 0–4 weeks | 268.80 ± 12.67 | 267.95 ± 15.32 | 0.880 |
| 5–8 weeks | 304.81 ± 21.54 | 295.15 ± 22.06 | 0.270 |
| 0–8 weeks | 286.80 ± 14.12 | 281.55 ± 14.50 | 0.359 |
| F/G | |||
| 0–4 weeks | 17.36 ± 4.23 | 13.80 ± 1.05 | 0.011 |
| 5–8 weeks | 20.73 ± 5.19 | 18.52 ± 2.75 | 0.188 |
| 0–8 weeks | 18.60 ± 3.54 | 15.81 ± 1.10 | 0.016 |
BW = body weight, ADG = average daily gain, ADFI = average daily feed intake, and F/G = feed-to-gain ratio.
3.2. Apparent Digestibility of Nutrients
Enterococcus faecium had effects on the apparent digestibility of DM and CP (p < 0.05, Table 3), but the effects were not observed on the apparent digestibility of EE and Ash (p > 0.05). Compared with the control, EF increased the apparent digestibility of DM and CP (p < 0.05).
Table 3.
Effect of Enterococcus faecium on the apparent digestibility of nutrients in mink.
| Items | Groups | p-Value | |
|---|---|---|---|
| 0 (Control) | EF | ||
| DM, % | 75.59 ± 1.24 | 79.30 ± 0.60 | <0.001 |
| CP, % | 86.40 ± 0.50 | 88.75 ± 0.59 | <0.001 |
| EE, % | 96.78 ± 0.88 | 96.91 ± 1.56 | 0.867 |
| Ash, % | 19.26 ± 4.42 | 22.71 ± 5.76 | 0.320 |
DM = dry matter; CP = crude protein; EE = ether extract.
3.3. Serum Antioxidant Indexes
The Enterococcus faecium had effects on serum SOD activity and MDA level (p < 0.05, Table 4), but the effects were not observed on serum T-AOC level and GSH-pX activity (p > 0.05). Compared with the control, EF increased SOD activity (p < 0.05) and decreased MDA levels (p < 0.05).
Table 4.
Effect of Enterococcus faecium on the antioxidant capacity of mink.
| Items | Groups | p-Value | |
|---|---|---|---|
| 0 (Control) | EF | ||
| T-AOC, U/mL | 17.28 ± 2.70 | 17.88 ± 3.24 | 0.713 |
| MDA, nmol/mL | 10.92 ± 1.41 | 9.24 ± 0.96 | 0.037 |
| GSH-pX, μmol/L | 1609.30 ± 219.50 | 1610.81 ± 221.78 | 0.991 |
| SOD, U/mL | 112.89 ± 24.63 | 144.18 ± 12.54 | 0.011 |
T-AOC = total antioxidant capacity; MDA = malondialdehyde; GSH-pX = glutathione peroxidase; SOD = superoxide dismutase.
3.4. Serum Immune Indexes
Enterococcus faecium had effects on serum IgA and IgG levels (p < 0.05, Table 5), but the effects were not observed on serum IgM (p > 0.05). Compared with the control, EF increased serum IgA and IgG levels (p < 0.05).
Table 5.
Effect of Enterococcus faecium on serum immune indexes of mink.
| Items | Groups | p-Value | |
|---|---|---|---|
| 0 (Control) | EF | ||
| IgA, μg/mL | 57.38 ± 2.96 | 61.93 ± 1.90 | 0.008 |
| IgG, g/L | 6.58 ± 0.31 | 7.15 ± 0.31 | 0.005 |
| IgM, μg/mL | 504.51 ± 28.53 | 502.03 ± 51.31 | 0.907 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M.
3.5. Jejunum Mucosal Immune Indexes
The Enterococcus faecium had significant effects on the levels of IL-8, IL-1β, and SIgA in the jejunum mucosal (p < 0.05, Table 6), but the effects were not observed on other indexes (p > 0.05). Compared with the control, EF significantly increased SIgA levels (p < 0.05) while decreasing IL-8 and IL-1β levels (p < 0.05).
Table 6.
Effect of Enterococcus faecium on the jejunum mucosal immune system of mink.
| Items | Groups | p-Value | |
|---|---|---|---|
| 0 (Control) | EF | ||
| IL-2, p g/mL | 340.43 ± 18.77 | 329.75 ± 39.14 | 0.592 |
| IL-6, p g/mL | 32.07 ± 4.74 | 35.72 ± 3.94 | 0.197 |
| IL-8, p g/mL | 116.58 ± 3.35 | 105.87 ± 2.79 | 0.001 |
| IL-10, p g/mL | 86.40 ± 9.73 | 84.82 ± 7.05 | 0.754 |
| IL-1β, p g/mL | 333.44 ± 27.23 | 299.47 ± 14.71 | 0.04 |
| SIgA, p g/mL | 2467.64 ± 100.81 | 3096.28 ± 154.19 | <0.001 |
| IFN-γ, p g/mL | 1305.58 ± 295.82 | 1016.93 ± 77.63 | 0.094 |
| TNF-α, p g/mL | 764.83 ± 38.05 | 758.62 ± 86.23 | 0.875 |
IL-2 = interleukin-2; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-1β = interleukin-1β; SIgA = secretory immunoglobulin A; IFN-γ = interferon-gamma; TNF-α = tumor necrosis factor-alpha.
3.6. Intestinal Microbiota
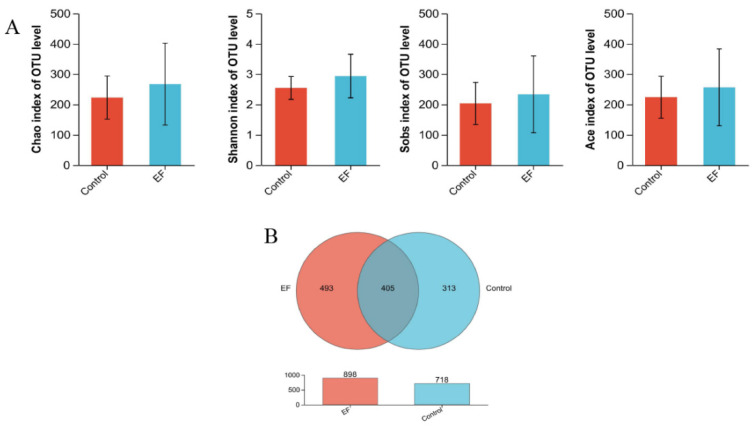
As shown in Figure 1, the sequencing saturation curve tends to flatten out at the end, indicating that all samples have sufficient sequencing depth. There were no differences among the two groups in the ACE, Chao, Shannon, and Sobs indices (p > 0.05, Figure 2A). However, as depicted in Figure 2B, the control group contained 718 species at the OUT level, while the EF group had 898 species at the OUT level, and 405 species were found to co-occur across both groups.
Figure 1.
Coverage index of the two groups on the OUT level.
Figure 2.
(A) Alpha diversity indices of the growing male minks in different groups, including the Chao index, Shannon index, Sobs index, and ACE index. (B) Venn diagram of species numbers in two groups at the OUT level.
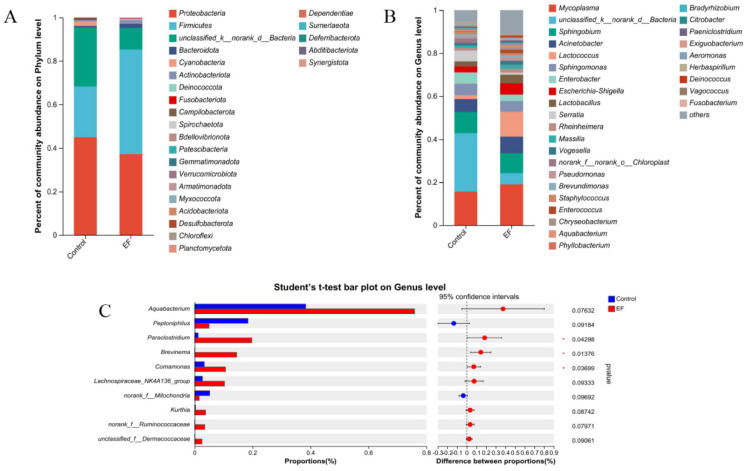
At the phylum level, Proteobacteria, unclassified_k__norank_d__Bacteria, Firmicutes, Cyanobacteria, and Bacteroidota were the top five dominant phyla in the control group (Figure 3A). Firmicutes, Proteobacteria, unclassified_k__norank_d__Bacteria, Bacteroidota, and Actinobacteriota were the top five dominant phyla in the EF group. At the genus level, unclassified_k__norank_d__Bacteria, Mycoplasma, Sphingobium, Acinetobacter, and Enterobacter were the top five dominant genera in the control group (Figure 3B). Mycoplasma, Lactococcus, Sphingobium, Acinetobacter, and unclassified_k__norank_d__Bacteria were the top five dominant genera in the EF group. Compared with the control, EF increased the relative abundance of Paraclostridium, Brevinema, and Comamonas (p < 0.05, Figure 3C).
Figure 3.
(A) Distribution of bacterial community structure at phylum level. (B) Distribution of bacterial community structure at the genus level. (C) The significance of differences among the two groups of the same species at the genus level (* represents p < 0.05). The result was statistically analyzed through the Student’s t−test.
3.7. Correlation Analysis
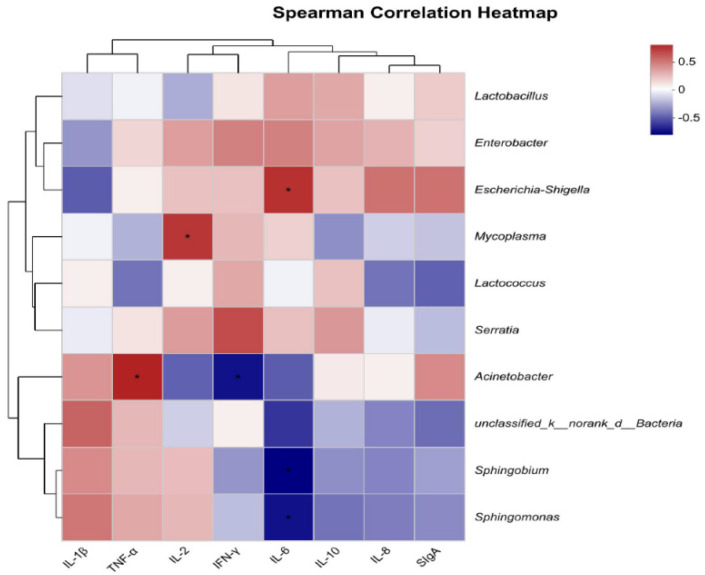
A Spearman correlation analysis was conducted to assess the relationship between the top 10 bacterial genera and the intestinal immune status of developing male minks (Figure 4). Escherichia-Shigella was positively correlated with IL-6. Mycoplasma was positively correlated with IL-2. Acinetobacter was positively correlated with TNF-α and negatively correlated with INF-γ. Sphingobium and Sphingomonas were negatively correlated with IL-6.
Figure 4.
Heatmap shows the correlation between intestinal flora (genus level) and intestinal immune indicators. The X-axis and Y-axis are intestinal immune indicators and species, respectively, and the correlation R−values and p−values are obtained through calculation. R−values are displayed in different colors in the figure. If the p−values are less than 0.05, they are marked with *. The legend on the right is the color range of different R−values; the left and upper sides present the species and immune indicator cluster trees; * represents p < 0.05.
4. Discussion
One of the major findings of the current study was that Enterococcus faecium improved ADG in mink aged 12–16 weeks. It appears that the improvement in ADG was only evident during the initial 4 weeks of the study. Between week 5 and week 8, there was no difference in ADG between the control minks and those fed with Enterococcus faecium, indicating the short-term effects of Enterococcus faecium on growth performance. However, this short-term effect on ADG resulted in minks fed with Enterococcus faecium having a greater BW at both week 4 and week 8 of the study. Additionally, the increased ADG was associated with an improvement in the feed/gain ratio (F/G), but there was no associated change in average daily feed intake (ADFI). This suggests that minks fed Enterococcus faecium exhibited efficient dietary utilization. The results were consistent with those of previous studies on pigs [22,23], which found that supplementation with Enterococcus faecium increased growth performance and feed efficiency. Similarly, a study on rabbits [24] showed that supplementation with Enterococcus faecium could enhance the live weight and ADG. In the current study, Enterococcus faecium increased the apparent digestibility of DM and CP. The results are consistent with previous research on pigs, which has shown that Enterococcus faecium enhances nutrient digestibility [25,26]. It suggests that the improvement in the growth performance of minks is attributed to Enterococcus faecium enhancing the apparent digestibility of nutrients. Chen et al. [27] have demonstrated that Enterococcus faecium has a positive influence on the digestibility of DM in pigs. As a lactic acid bacterium, Enterococcus faecium is capable of producing short-chain fatty acids and some bioactive substances [28]. These bioactive compounds enhance the activity of digestive enzymes [29], stimulate the development of intestinal villi [30], and limit the proliferation of pathogenic bacteria [31]. By facilitating these processes, Enterococcus faecium has enhanced the nutritional digestibility of mink, thereby improving the growth performance of the animals.
In the current study, Enterococcus faecium increased the activity of SOD and effectively reduced the MDA content in the serum of minks. SOD serves as the first line of defense against oxygen-derived free radicals, catalyzing the conversion of superoxide to oxygen and hydrogen peroxide [32]. MDA is generated during the process of lipid peroxidation of unsaturated fatty acids within the phospholipids of the cell membrane [6]. A previous study has demonstrated the potential of Enterococcus faecium to increase the serum T-AOC level and SOD activity, as well as to decrease the serum MDA concentration in broilers [33]. In vitro studies have also found that Enterococcus faecium and its metabolites possess antioxidant activity [34,35,36]. Maintaining redox balance is essential for the health of cells and animals [37]. The antioxidant capacity of the organism represents its ability to resist oxidative damage [38]. Enterococcus faecium may enhance antioxidant function by inducing the secretion of bilirubin, which inhibits serum lipid peroxidation and the formation of reactive oxygen species (ROS) [39]. Furthermore, it may stimulate the expression of enzymes in the antioxidant defense system by activating and transferring nuclear factors, thereby promoting the removal of ROS and improving antioxidant capacity [40].
The immune response is an important defense mechanism in animals [41]. Immunoglobulins in serum are proteins produced by plasma cells and possess antibody activity [38]. They constitute the primary immune molecule of animal humoral immunity and are integral to the immune system [42]. The findings of the present study demonstrated that Enterococcus faecium increased IgA and IgG contents in the serum of growing male minks. Similarly, previous studies have found that Enterococcus faecium increases the concentration of IgG in the serum of broilers and increases the concentration of IgA in pigeon milk [10,43]. These results suggest that Enterococcus faecium has the potential to increase the concentration of various immunoglobulins (IgA, IgG, and IgM), thereby improving the humoral immune status of animals. It is possible that the heterologous antigens present in Enterococcus faecium stimulate the immune system, enhancing the production of immunoglobulins and improving humoral immunity [44].
In the present study, Enterococcus faecium also enhanced the level of SIgA in the jejunum mucosa, consistent with previous studies on broilers, which showed that dietary supplementation with Enterococcus faecium increased intestinal SIgA concentration during Salmonella and Campylobacter infection [45,46]. The mucosal system, with SIgA as its primary defense, is the first line of immune defense [47,48]. SIgA plays a crucial role in maintaining the integrity of the mucosal barrier and preventing pathogen invasion by promoting immune exclusion in the intestinal tract [49,50,51]. The observed increase in SIgA may be attributed to the induction of polymeric immunoglobulin receptor (pIgR) expression by Enterococcus faecium, which activates pattern recognition receptors and subsequently enhances SIgA secretion in the intestine [50].
Furthermore, Enterococcus faecium decreased IL-8 and IL-1β levels in the jejunal mucosa, as found in previous studies on porcine epithelial cells and broilers [52,53]. Both IL-1β and IL-8 are pro-inflammatory and play a key role in intestinal resistance to foreign pathogens [54,55]. Mi et al. [56] also reported in a study on broilers that Enterococcus faecium could reduce the production and secretion of IL-1β by preventing the expression of pro-Caspase-1. Cytokines are closely related to mucosal immune responses. Classified by their opposing effects on the inflammatory process, cytokines are divided into pro-inflammatory cytokines, which amplify the response, and anti-inflammatory cytokines, which mitigate it [47]. Disruption of the balance between pro-inflammatory and anti-inflammatory cytokines leads to the occurrence of inflammatory reactions [57]. Enterococcus faecium’s unique peptidoglycan composition, processed by peptidoglycan hydrolase secreting antigen A (SagA), produces muropeptides that trigger nucleotide-binding oligomerization domain-containing protein 2, thereby activating innate immunity and creating a micro-environment conducive to immunological therapy [58]. This mechanism may contribute to the immune-modulating effects of Enterococcus faecium on growing male minks.
Numerous studies have demonstrated that probiotics can exert beneficial effects by regulating the intestinal microbiota [59,60,61]. However, in this study, Enterococcus faecium had no effects on alpha diversity indices in minks. In contrast to this finding, a previous study in piglets reported that Enterococcus faecium increased various alpha diversity indices from day 1 to 14 [9]. Similarly, another study on broilers found that Enterococcus faecium had significant effects on the alpha diversity index at day 39 but not at day 21 [62]. These discrepancies may be due to the varying effects of Enterococcus faecium on different animal species and at different growth stages. We speculate that the increase in microbial species could lead to improved intestinal health [63]. The results of the Venn diagram in the current study have shown that Enterococcus faecium increased the species numbers at the OTU level, confirming its regulatory effect on the intestinal flora of growing male minks.
At the phylum level, Proteobacteria and Firmicutes were the most dominant on the rectal mucosa in male minks, which is consistent with previous studies on minks [64,65]. Firmicutes, known for their short-chain fatty acid (SCFA) production, can effectively regulate the intestinal immune system and maintain the balance of the intestinal flora [66]. In contrast, Proteobacteria, often associated with intestinal flora imbalance due to their production of lipopolysaccharide and flagellin, can lead to inflammatory responses [67].
At the genus level, Enterococcus faecium increased the relative abundance of Paraclostridium, Brevinema, and Comamonas, whose increase was also reported in a previous study on hybrid snakeheads [68]. Comamonas, an opportunistic pathogen found in various environments, including animal intestines [69], and Brevinema, a spirochaete involved in lignocellulose breakdown and nitrogen fixation [70]. In addition, Paraclostridium and Brevinema were the dominant bacteria in the intestinal flora of healthy fish [71,72]. These results suggest that Enterococcus faecium may promote metabolism and growth by regulating the relative abundance of intestinal flora. Moreover, our analysis of the correlation between the intestinal microbiota at the genus level and gut immune indicators revealed that Enterococcus faecium could act as gut microbiota modulators, enhancing the immune function of growing male minks and thereby improving the host’s defense against pathogenic microorganisms.
5. Conclusions
In conclusion, dietary supplementation with Enterococcus faecium (containing viable Enterococcus faecium at more than 107 cfu/kg of diet) promotes growth performance, enhances the apparent digestibility of nutrients, and improves antioxidant capacity in growing male minks. Additionally, Enterococcus faecium also improves immunity and regulates the intestinal microbiota. However, this current study is a preliminary feeding experiment to investigate the probiotic effects of the isolated Enterococcus faecium on minks. Further research is necessary for an accurate supplementation evaluation and a comprehensive safety assessment of the isolated strain of Enterococcus faecium. This includes screening for virulence genes and antibiotic resistance to determine its potential as a probiotic candidate.
Author Contributions
Validation, L.W. and Y.L.; formal analysis, F.S.; investigation, Q.R. and J.C.; data curation, L.C. and Z.J.; writing—original draft preparation, L.C.; writing—review and editing, L.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Animal Care and Use Committee of Animal Science and Technology at Qingdao Agricultural University reviewed and approved the experimental protocol (approval number DKY20230524-1, date: 24 May 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Shandong Province Agricultural Innovation Team (SDAIT-21).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marco M.L., Sanders M.E., Gänzle M., Arrieta M.C., Cotter P.D., De V.L., Hill C., Holzapfel W., Lebeer S., Merenstein D., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021;18:196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Reviews. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 3.Fayol-Messaoudi D., Berger C.N., Coconnier-Polter M.-H., Moal V.L.-L., Servin A.L. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005;71:6008–6013. doi: 10.1128/AEM.71.10.6008-6013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y., Cui Y., Yue F., Liu L., Shan Y., Liu B., Zhou Y., Lü X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019;94:475–499. doi: 10.1016/j.foodhyd.2019.03.032. [DOI] [Google Scholar]
- 5.Deng Z., Hou K., Zhao J., Wang H. The Probiotic Properties of Lactic Acid Bacteria and Their Applications in Animal Husbandry. Curr. Microbiol. 2021;79:22. doi: 10.1007/s00284-021-02722-3. [DOI] [PubMed] [Google Scholar]
- 6.Yang C., Wang S., Li Q., Zhang R., Xu Y., Feng J. Effects of Probiotic Lactiplantibacillus plantarum HJLP-1 on Growth Performance, Selected Antioxidant Capacity, Immune Function Indices in the Serum, and Cecal Microbiota in Broiler Chicken. Animals. 2024;14:668. doi: 10.3390/ani14050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Zhang M., Su L., Zhao L., Sun L., Jin Y., Guo Y. Effect of different levels of Lactobacillus added to diets on fat deposition and meat quality of Sunit lambs. Meat Sci. 2024;213:109470. doi: 10.1016/j.meatsci.2024.109470. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z.-l., Chen Y.-j., Meng Q.-l., Zhang X., Wang X.-l. Progress in the application of Enterococcus faecium in animal husbandry. Front. Cell. Infect. Microbiol. 2023;13:1168189. doi: 10.3389/fcimb.2023.1168189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu C., Xing W., Liu X., Zhang X., Li K., Liu J., Deng B., Deng J., Li Y., Tan C. Effects of dietary supplementation of probiotic Enterococcus faecium on growth performance and gut microbiota in weaned piglets. AMB Express. 2019;9:33. doi: 10.1186/s13568-019-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Effects of dietary Enterococcus faecium NCIMB 11181 supplementation on growth performance and cellular and humoral immune responses in broiler chickens. Poult. Sci. 2019;98:150–163. doi: 10.3382/ps/pey368. [DOI] [PubMed] [Google Scholar]
- 11.Kasimin M.E., Shamsuddin S., Molujin A.M., Sabullah M.K., Gansau J.A., Jawan R. Enterocin: Promising Biopreservative Produced by Enterococcus sp. Microorganisms. 2022;10:684. doi: 10.3390/microorganisms10040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento L.C.S., Casarotti S.N., Todorov S.D., Penna A.L.B. Probiotic potential and safety of enterococci strains. Ann. Microbiol. 2019;69:241–252. doi: 10.1007/s13213-018-1412-5. [DOI] [Google Scholar]
- 13.Coll F., Gouliouris T., Blane B., Yeats C.A., Raven K.E., Ludden C., Khokhar F.A., Wilson H.J., Roberts L.W., Harrison E.M., et al. Antibiotic resistance determination using Enterococcus faecium whole-genome sequences: A diagnostic accuracy study using genotypic and phenotypic data. Lancet Microbe. 2024;5:e151–e163. doi: 10.1016/S2666-5247(23)00297-5. [DOI] [PubMed] [Google Scholar]
- 14.Suvorov A. What Is Wrong with Enterococcal Probiotics? Probiotics Antimicrob. Proteins. 2020;12:1–4. doi: 10.1007/s12602-020-09633-y. [DOI] [PubMed] [Google Scholar]
- 15.Arredondo-Alonso S., Top J., McNally A., Puranen S., Pesonen M., Pensar J., Marttinen P., Braat J.C., Rogers M.R.C., van Schaik W., et al. Plasmids Shaped the Recent Emergence of the Major Nosocomial Pathogen Enterococcus faecium. mBio. 2020;11:03284–03319. doi: 10.1128/mBio.03284-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral D.M.F., Silva L.F., Casarotti S.N., Nascimento L.C.S., Penna A.L.B. Enterococcus faecium and Enterococcus durans isolated from cheese: Survival in the presence of medications under simulated gastrointestinal conditions and adhesion properties. J. Dairy Sci. 2017;100:933–949. doi: 10.3168/jds.2016-11513. [DOI] [PubMed] [Google Scholar]
- 17.Hanchi H., Mottawea W., Sebei K., Hammami R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update. Front. Microbiol. 2018;9:1791. doi: 10.3389/fmicb.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanifeh M., Spillmann T., Huhtinen M., Sclivagnotis Y.S., Grönthal T., Hynönen U. Ex-Vivo Adhesion of Enterococcus faecalis and Enterococcus faecium to the Intestinal Mucosa of Healthy Beagles. Animals. 2021;11:3283. doi: 10.3390/ani11113283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed N.A., Khattab R.A., Ragab Y.M., Hassan M. Safety assessment of Enterococcus lactis strains complemented with comparative genomics analysis reveals probiotic and safety characteristics of the entire species. BMC Genom. 2023;24:667. doi: 10.1186/s12864-023-09749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Zhen S., Cao L., Sun F., Wang L. Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals. 2023;13:2958. doi: 10.3390/ani13182958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y., Yu Y., Shen Y., Li Q., Lan J., Wu Y., Zhang R., Cao G., Yang C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021;100:101358. doi: 10.1016/j.psj.2021.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y.H., Zhang C.Y., Wang L.X., Shang Q.H., Zhang G.G., Yang W.R. Effects of dietary supplementation of Enterococcus faecium on growth performance, intestinal morphology, and selected microbial populations of piglets. Livest. Sci. 2018;210:111–117. doi: 10.1016/j.livsci.2018.02.010. [DOI] [Google Scholar]
- 23.Sato Y., Kuroki Y., Oka K., Takahashi M., Rao S., Sukegawa S., Fujimura T. Effects of Dietary Supplementation with Enterococcus faecium and Clostridium butyricum, Either Alone or in Combination, on Growth and Fecal Microbiota Composition of Post-weaning Pigs at a Commercial Farm. Front. Vet. Sci. 2019;6:26. doi: 10.3389/fvets.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonová M.P., Chrastinová Ľ., Lauková A. Effect of beneficial strain Enterococcus faecium EF9a isolated from Pannon White rabbit on growth performance and meat quality of rabbits. Ital. J. Anim. Sci. 2020;19:650–655. doi: 10.1080/1828051X.2020.1781553. [DOI] [Google Scholar]
- 25.Zhang Z.F., Lee J.M., Kim I.H. Effects of Enterococcus faecium DSM 7134 on weanling pigs were influenced by dietary energy and crude protein density. Livest. Sci. 2014;169:106–111. doi: 10.1016/j.livsci.2014.09.022. [DOI] [Google Scholar]
- 26.Xin J.L., Sang I.L., Kwang Y.L., Dinh H.N., In H.K. Effects of a blend of organic acids and medium-chain fatty acids with and without Enterococcus faecium on growth performance, nutrient digestibility, blood parameters, and meat quality in finishing pigs. Can. J. Anim. Sci. 2018;98:852–859. [Google Scholar]
- 27.Chen Y.J., Min B.J., Cho J.H., Kwon O.S., Son K.S., Kim I.H., Kim S.J. Effects of Dietary Enterococcus faecium SF68 on Growth Performance, Nutrient Digestibility, Blood Characteristics and Faecal Noxious Gas Content in Finishing Pigs. Asian-Australas. J. Anim. Sci. 2006;19:406–411. doi: 10.5713/ajas.2006.406. [DOI] [Google Scholar]
- 28.Sonei A., Dovom M.R.E., Yavarmanesh M. Evaluation of probiotic, safety, and technological properties of bacteriocinogenic Enterococcus faecium and Enterococcus faecalis strains isolated from lighvan and koozeh cheeses. Int. Dairy J. 2024;148:105807. doi: 10.1016/j.idairyj.2023.105807. [DOI] [Google Scholar]
- 29.Liu J., Cao S.C., Liu J., Xie Y.N., Zhang H.F. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-Australas. J. Anim. Sci. 2018;31:1660–1669. doi: 10.5713/ajas.17.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Wu T., Chen Z., Meng Y., Zhu Z., Wang Q., Tian J., Yi D., Wang L., Zhao D., et al. Dietary Supplementation with Enterococcus faecium R1 Attenuates Intestinal and Liver Injury in Piglets Challenged by Lipopolysaccharide. Animals. 2021;11:1424. doi: 10.3390/ani11051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teame T., Wang A., Xie M., Zhang Z., Yang Y., Ding Q., Gao C., Olsen R.E., Ran C., Zhou Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020;7:570344. doi: 10.3389/fnut.2020.570344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 33.Song D., Duan T., Cheng J., Qiao L., Wang L., Chen L., Li A., Wang W. Effects of Embedded Lactobacillus plantarum and Enterococcus faecium on Growth Performance, lmmune and Antioxidant Function and lntestinal Microflora of Broilers. Chin. J. Anim. Nutr. 2022;34:877–887. [Google Scholar]
- 34.Tilwani Y.M., Lakra A.K., Domdi L., Yadav S., Jha N., Arul V. Optimization and physicochemical characterization of low molecular levan from Enterococcus faecium MC-5 having potential biological activities. Process Biochem. 2021;110:282–291. doi: 10.1016/j.procbio.2021.08.021. [DOI] [Google Scholar]
- 35.Kim K., Kim J., Kim S., Kim S., Nguyen T.H., Kang C. Antioxidant and Anti-Inflammatory Effect and Probiotic Properties of Lactic Acid Bacteria Isolated from Canine and Feline Feces. Microorganisms. 2021;9:1971. doi: 10.3390/microorganisms9091971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahnama Vosough P., Habibi Najafi M.B., Edalatian Dovom M.R., Javadmanesh A., Mayo B. Evaluation of antioxidant, antibacterial and cytotoxicity activities of exopolysaccharide from Enterococcus strains isolated from traditional Iranian Kishk. J. Food Meas. Charact. 2021;15:5221–5230. doi: 10.1007/s11694-021-01092-5. [DOI] [Google Scholar]
- 37.Noruzi H., Aliabadi F.A., Imari Z.K. Effects of different levels of pistachio (Pistachia vera) green hull aqueous extract on performance, intestinal morphology and antioxidant capacity in Eimeria challenged broilers. Poult. Sci. 2024;103:103667. doi: 10.1016/j.psj.2024.103667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y., Leng X., Zhao Y., Zhao Y., Wang Q. Effects of dietary Artemisia annua supplementation on growth performance, antioxidant capacity, immune function, and gut microbiota of geese. Poult. Sci. 2024;103:103594. doi: 10.1016/j.psj.2024.103594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capcarova M., Weiss J., Hrncar C., Kolesarova A., Pal G. Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010;94:e215–e224. doi: 10.1111/j.1439-0396.2010.01010.x. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M.S., Choi Y.H., Choi Y.S., Alam M.B., Lee S.H., Yoo J.C. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018;239:502–510. doi: 10.1016/j.foodchem.2017.06.106. [DOI] [PubMed] [Google Scholar]
- 41.Li B., Wu K., Duan G., Yin W., Lei M., Yan Y., Ren Y., Zhang C. Folic Acid and Taurine Alleviate the Impairment of Redox Status, Immunity, Rumen Microbial Composition and Fermentation of Lambs under Heat Stress. Animals. 2024;14:998. doi: 10.3390/ani14070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y., Chen Y., Li J., Qu H., Zhao Y., Wen C., Zhou Y. Dietary β-sitosterol regulates serum lipid level and improves immune function, antioxidant status, and intestinal morphology in broilers. Poult. Sci. 2020;99:1400–1408. doi: 10.1016/j.psj.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma H., Li Y., Han P., Zhang R., Yuan J., Sun Y., Li J., Chen J. Effects of Supplementing Drinking Water of Parental Pigeons with Enterococcus faecium and Bacillus subtilis on Antibody Levels and Microbiomes in Squabs. Animals. 2024;14:178. doi: 10.3390/ani14020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciszewski A., Jarosz Ł., Marek A., Michalak K., Grądzki Z., Kaczmarek B., Rysiak A. Effect of combined in ovo administration of zinc glycine chelate (Zn-Gly) and a multistrain probiotic on the modulation of cellular and humoral immune responses in broiler chickens. Poult. Sci. 2023;102:102823. doi: 10.1016/j.psj.2023.102823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaffová V., Bobíková K., Husáková E., Levkut M., Herich R., Revajová V., Levkutová M., Levkut M. Interaction of TGF-β4 and IL-17 with IgA secretion in the intestine of chickens fed with E. faecium AL41 and challenged with S. enteritidis. Res. Vet. Sci. 2015;100:75–79. doi: 10.1016/j.rvsc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Letnická A., Karaffová V., Levkut M., Revajová V., Herich R. Influence of oral application of Enterococcus faecium AL41 on TGF-β4 and IL-17 expression and immunocompetent cell distribution in chickens challenged with Campylobacter jejuni. Acta Vet. Hung. 2017;65:317–326. doi: 10.1556/004.2017.031. [DOI] [PubMed] [Google Scholar]
- 47.Han D., Yang H., Li J., Zhang C., Ye L., Dong J., Zhang C., Guo R., Xin J. Macleaya cordata extract improves growth performance, immune responses and anti-inflammatory capacity in neonatal piglets. Vet. Microbiol. 2024;293:110090. doi: 10.1016/j.vetmic.2024.110090. [DOI] [PubMed] [Google Scholar]
- 48.Karaffová V., Tóthová C., Szabóová R., Revajová V., Lauková A., Ševčíková Z., Herich R., Žitňan R., Levkut M., Levkut M., et al. The Effect of Enterococcus faecium AL41 on the Acute Phase Proteins and Selected Mucosal Immune Molecules in Broiler Chickens. Life. 2022;12:598. doi: 10.3390/life12040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaetzel C.S. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host–microbial mutualism. Immunol. Lett. 2014;162:10–21. doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Z., Yang G., Zhang M., Yang R., Wang Y., Guo P., Zhang J., Wang C., Liu Q., Gao Y. Dietary Supplementation of Mixed Organic Acids Improves Growth Performance, Immunity, and Antioxidant Capacity and Maintains the Intestinal Barrier of Ira Rabbits. Animals. 2023;13:3140. doi: 10.3390/ani13193140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian Z., Liu X., Dai R., Xiao Y., Wang X., Bi D., Shi D. Enterococcus faecium HDRsEf1 Protects the Intestinal Epithelium and Attenuates ETEC-Induced IL-8 Secretion in Enterocytes. Mediat. Inflamm. 2016;2016:7474306. doi: 10.1155/2016/7474306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalifa A., Ibrahim H.I.M. Enterococcus faecium from chicken feces improves chicken immune response and alleviates Salmonella infections: A pilot study. J. Anim. Sci. 2023;101:16. doi: 10.1093/jas/skad016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Q.J., Zhu D.D., Wang D.D., Zhang B.B., Ren A., Zhang Z.B. Effects of dietary supplementation with glutamine on the lymphocyte proliferation and intestinal immune gene expression in broiler chickens infected with Salmonella enteritidis. Res. Vet. Sci. 2021;139:18–24. doi: 10.1016/j.rvsc.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z., Xu P., Liu C., Chen J., Ren B., Du E., Guo S., Li P., Li L., Ding B. Effect of Tannic Acid on Antioxidant Function, Immunity, and Intestinal Barrier of Broilers Co-Infected with Coccidia and Clostridium perfringens. Animals. 2024;14:955. doi: 10.3390/ani14060955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi J., He T., Hu X., Wang Z., Wang T., Qi X., Li K., Gao L., Liu C., Zhang Y., et al. Enterococcus faecium C171: Modulating the Immune Response to Acute Lethal Viral Challenge. Int. J. Antimicrob. Agents. 2023;62:106969. doi: 10.1016/j.ijantimicag.2023.106969. [DOI] [PubMed] [Google Scholar]
- 57.Chen P., Lv H., Liu W., Wang Y., Zhang K., Che C., Zhao J., Liu H. Effects of Lactobacillus plantarum HW1 on Growth Performance, Intestinal Immune Response, Barrier Function, and Cecal Microflora of Broilers with Necrotic Enteritis. Animals. 2023;13:3810. doi: 10.3390/ani13243810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim B., Wang Y.-C., Hespen C.W., Espinosa J., Salje J., Rangan K.J., Oren D.A., Kang J.Y., Pedicord V.A., Hang H.C. Enterococcus faecium secreted antigen A generates muropeptides to enhance host immunity and limit bacterial pathogenesis. eLife. 2019;8:45343. doi: 10.7554/eLife.45343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T., Teng K., Liu Y., Shi W., Zhang J., Dong E., Zhang X., Tao Y., Zhong J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019;10:90. doi: 10.3389/fmicb.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao M., Liang X., Meng Y., Lu H., Lin K., Gong P., Liu T., Yi H., Pan J., Zhang Y., et al. Probiotics induce intestinal IgA secretion in weanling mice potentially through promoting intestinal APRIL expression and modulating the gut microbiota composition. Food Funct. 2024;15:4862–4873. doi: 10.1039/D4FO00962B. [DOI] [PubMed] [Google Scholar]
- 61.Wu R., Chang S., Zhang H., Yang X., Gu R., Wang S., Liu X., Liu X., Ochir M., Wu J. Compound probiotics microcapsules improve milk yield and milk quality of dairy cows by regulating intestinal flora. Food Bioeng. 2024;3:110–125. doi: 10.1002/fbe2.12084. [DOI] [Google Scholar]
- 62.Suvorov A., Zhao S., Leontieva G., Alekhina G., Yang J., Tsapieva A., Karaseva A., Smelova V., Guo D., Chen L. Evaluation of the Efficacy of Enterococcus faecium L3 as a Feed Probiotic Additive in Chicken. Probiotics Antimicrob. Proteins. 2022;15:1169–1179. doi: 10.1007/s12602-022-09970-0. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Wang C., Su W., Jiang Z., He H., Gong T., Kai L., Xu H., Wang Y., Lu Z. Co-fermented yellow wine lees by Bacillus subtilis and Enterococcus faecium regulates growth performance and gut microbiota in finishing pigs. Front. Microbiol. 2022;13:1003498. doi: 10.3389/fmicb.2022.1003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu X., Li D., Wang L., Guo Z., Wang G., Wang L. Effects of Antimicrobial Peptide on Growth Performance, Nutrient Apparent Digestibilities and lntestinal Flora of Growing Female Minks. Chin. J. Anim. Nutr. 2022;34:1194–1204. [Google Scholar]
- 65.Bahl M.I., Hammer A.S., Clausen T., Jakobsen A., Skov S., Andresen L. The gastrointestinal tract of farmed mink (Neovison vison) maintains a diverse mucosa-associated microbiota following a 3-day fasting period. MicrobiologyOpen. 2017;6:e00434. doi: 10.1002/mbo3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Houtman T.A., Eckermann H.A., Smidt H., de Carolina W. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022;12:3140. doi: 10.1038/s41598-022-07176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo S., Yu Y., Wan J., Mao Y., Zhang H., Zhang J., Tian X., Zhao Q. Progress in research on the relationship between Proteobacteria and the imbalance of mammalian colonic intestinal flora. Chin. J. Microecol. 2022;34:479–484. [Google Scholar]
- 68.Mi O., Xu S., Chen K., Luo Q., Liu H., Liang X., Zhao J. Effects of Lactic Acid Bacteria on lntestinal Microflora and Growth Performance of Hybrid Snakehead. J. Agric. Biotechnol. 2022;30:138–150. [Google Scholar]
- 69.Ryan M.P., Sevjahova L., Gorman R., White S. The Emergence of the Genus Comamonas as Important Opportunistic Pathogens. Pathogens. 2022;11:1032. doi: 10.3390/pathogens11091032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M., Li L., Huang T., Liu Y., Lei A., Ma C., Chen F., Chen M. Effects of Attenuated S. agalactiae Strain YM001 on Intestinal Microbiota of Tilapia Are Recoverable. Front. Microbiol. 2018;9:3251. doi: 10.3389/fmicb.2018.03251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z., Li A., Wang Y., Iqbal M., Zheng A., Zhao M., Li Z., Wang N., Wu C., Yu D. Comparative analysis of microbial community structure between healthy and Aeromonas veronii-infected Yangtze finless porpoise. Microb. Cell Factories. 2020;19:123. doi: 10.1186/s12934-020-01383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwatsuki T., Kanazawa T., Ogasawara T., Hosotani K., Tsuchiya K., Watanabe S., Suzuki T., Moriuchi R., Kanesaki Y., Dohra H. 16S rRNA Gene Amplicon Sequencing of Gut Microbiota in Three Species of Deep-Sea Fish in Suruga Bay, Japan. Microbiol. Resour. Announc. 2021;10:1128. doi: 10.1128/MRA.01260-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.