Abstract
In BALB/c mice, sensitization to respiratory syncytial virus (RSV) attachment (G) glycoprotein leads to the development of lung eosinophilia upon challenge infection with RSV, a pathology indicative of a strong in vivo induction of a Th-2-type response. In this study, we found that a strong, RSV G-specific, Th-1-type cytokine response occurred simultaneously with a Th-2-type response in G-primed mice after RSV challenge. Both Th-1 and Th-2 effector CD4+ T cells recognized a single immunodominant site on this protein, implying that the differentiation of memory CD4+ T cells along the Th-1 or Th-2 effector pathway was independent of the epitope specificity of the T cells. A similar observation was made in G-primed H-2b haplotype mice after RSV challenge, further suggesting that this process is not dependent on the peptide epitope presented. On the other hand, genes mapping to loci outside of the major histocompatibility complex region are crucial regulators of the development of a Th-2-type response and lung eosinophilia. The implication of these findings for the immune mechanisms underlying the pathogenesis of RSV is discussed.
Respiratory syncytial virus (RSV) is the most common cause of viral lower respiratory tract infection worldwide. The disease is most severe in infants, children with underlying cardiopulmonary diseases, and children who are immunodeficient (8, 36). Recent evidence also implicates RSV as an important cause of respiratory tract infection in the elderly population (13, 26). Despite its importance as a human pathogen, there is currently no effective immunoprophylactic agent for this virus. Early attempts to vaccinate children with formalin-inactivated whole virions (FI-RSV) resulted in a paradoxically severe disease in these children when they were subsequently infected with the virus (10, 24). The mechanism(s) underlying the immunopathological effect of FI-RSV is not completely understood.
Recent advances in the understanding of T-lymphocyte biology, in combination with the availability of animal models of RSV infection, have given a significant impetus to the study of the immune mechanisms operating during RSV infection. In particular, the role of cytokines produced by T cells has been clearly demonstrated. Induction of a Th-2-type response (high interleukin-4 [IL-4] and IL-5 production) has been shown to lead to the development of systemic illness (weight loss) as well as the development of pulmonary eosinophilia (1, 15–17, 29). Neutralization of IL-4, at the time of priming or viral challenge, in mice sensitized to FI-RSV results in the amelioration of systemic illness (11, 14, 37). Furthermore, the presence of eosinophils in the peripheral blood and in the lungs of children who had been vaccinated with FI-RSV and subsequently infected with RSV suggests the important role of Th-2 cytokines in the pathogenesis of this disease (24).
In a murine model of RSV infection, the induction of Th-1- or Th-2-type cytokine responses is dependent on the sensitizing RSV antigen (2, 28, 34, 35). BALB/c mice primed with vaccinia virus expressing the attachment glycoprotein of RSV (RSV G) exhibit extensive recruitment of eosinophils into the lungs upon infection with RSV, indicating a strong Th-2 response (2, 28, 34, 35). Studies have suggested that the induction of Th-2 responses by this protein may be regulated by factors including the context of the protein during priming and the presence of immunomodulatory effectors, such as CD8+ T lymphocytes, during the differentiation and activation of CD4+ T cells (4, 17, 21, 23, 34, 37). However, the influence of the intrinsic properties of RSV G glycoprotein, especially the major histocompatibility complex (MHC)-peptide ligand complex recognized by effector CD4+ T cells on the induction of polarized cytokine responses to this protein has not been examined in detail.
In the present study we analyzed in detail the cytokine profile of effector CD4+ T cells elicited by the RSV G glycoprotein in a murine model of RSV infection. We have found that RSV G glycoprotein primes for the simultaneous induction of Th-1- and Th-2-type responses after challenge infection of BALB/c (H-2d) mice with RSV and that both types of cytokine responses are produced by CD4+ T cells directed to a single immunodominant region of the G protein. This finding indicates that MHC-ligand complexes derived from RSV G glycoprotein do not direct the differentiation of CD4+ T lymphocytes towards a Th-1 or Th-2 phenotype. This view is further supported by findings with BALB/b (H-2b) mice, which differ from BALB/c mice at the MHC locus but have identical background genes. RSV G-sensitized BALB/b mice, when challenged with RSV, produce both Th-1- and Th-2-type cytokines directed to a single region of the RSV G glycoprotein. Finally, we began to evaluate the contribution of non-MHC genes to the development of pulmonary eosinophilia in this model. The implications of these findings for the immunopathogenesis of RSV and the development of vaccines are discussed.
MATERIALS AND METHODS
Mice.
Female BALB/c (H-2d) and C57BL/6 (H-2b) mice 8 to 12 weeks old were purchased from Taconic Farms Inc., Germantown, N.Y. Female BALB/b (H-2b) mice were purchased from the Jackson Laboratory, Bar Harbor, Maine. The mice were maintained in a pathogen-free condition.
Virus and infection of mice.
Recombinant vaccinia virus expressing the fusion (VF) and attachment (VG) glycoproteins of RSV were obtained from J. L. Beeler (Food and Drug Administration and National Institutes of Health). The generation and characterization of these viruses have been previously described (3, 39). Recombinant vaccinia virus expressing only β-galactosidase (VSC11) was used as a control. RSV (A2 strain) was a generous gift from P. L. Collins, (National Institute of Allergy and Infectious Diseases, National Institutes of Health). RSV was grown in HEp-2 cells and plaque purified. The virus stock was grown in HEp-2 cells and titered for infectivity. The mice were infected with 3 × 106 PFU of recombinant vaccinia virus by scarification at the base of the tail. In some experiments, the mice were given 106 PFU of RSV in 50 μl of inoculum intranasally 3 weeks after priming and sacrificed 5 days later. Cells were isolated from the lungs for in vitro culture. Lung tissue was prepared for histopathological study.
Peptides.
Synthetic peptides were made by standard 9-fluoroenylmethoxycarbonyl chemistry with a model AMS422 peptide synthesizer (Gilson Co. Inc., Middleton, Wis.).
Histopathology.
The lungs of the mice were harvested and fixed in 10% formalin in phosphate-buffered saline. The specimens were processed, embedded, and sectioned by American Histolabs Inc. (Gaithersburg, Md.). Sections of the lungs were prepared and stained for eosinophils by the Leinert-Giemsa technique. Morphometric analysis of lung eosinophilia was performed as previously described (34, 35). In brief, cells with characteristic eosinophil staining in and around blood vessel walls were enumerated. The lengths of the vessels were estimated with a micrometer attached to the eyepiece of a microscope. The results are expressed as the number of eosinophils present per millimeter of blood vessel. Three to five blood vessels were examined in each section from individual mice.
Lymphocyte culture.
Cells were isolated from lung tissue as previously described (35). Briefly, the lungs were minced in Iscove’s medium (Gibco, Gaithersburg, Md.) containing 10% fetal calf serum. Lung tissue was tapped through a wire screen. Particulate matter was removed by quick centrifugation at 1,000 rpm. The cell suspension was layered over a Ficoll-Hypaque density gradient (Lymphocyte-M; Cedarlane, Ontario, Canada) and centrifuged at 400 × g for 20 min. The mononuclear cells at the interface were isolated and used in in vitro culture. Single-cell suspensions were isolated from a spleen by grinding the spleen through a wire screen followed by a quick centrifugation. The cells were cultured at the indicated cell numbers with irradiated naive spleen cells infected with RSV at a multiplicity of infection of 0.1. The ratio of responders to stimulators was in general 5:1. In some experiments, the cells were stimulated with the indicated synthetic peptide (1 μg/ml).
Detection of cytokines in culture supernatants.
Supernatants from in vitro culture of cells isolated from spleens or lungs were collected at 48 h after stimulation and kept at −70°C until analyzed. The concentrations of IL-2, IL-4, IL-5, and gamma interferon (IFN-γ) in these supernatants were measured with commercial enzyme-linked immunosorbent assay (ELISA) reagents under conditions recommended by the manufacturer (Pharmingen, San Diego, Calif.).
Flow cytometric analysis.
Staining of lung mononuclear cells for surface molecules and intracellular cytokine was performed by a method previously described (27). Cells were resuspended at 105 to 106/ml and stimulated with RSV-infected splenocytes or synthetic peptides (1 μg/ml). The cells were harvested 48 h after stimulation with RSV-infected spleen cells or 6 h after stimulation with peptides. Six hours prior to harvest, brefeldin A was added at 10 μg/ml to enhance intracellular accumulation of cytokines. The cells were washed twice with phosphate-buffered saline and stained for surface expression of CD4 by using rat anti-mouse CD4-Tricolor (Caltag Laboratories, Burlingame, Calif.). The cells were then fixed with Cytofix solution (Pharmingen), permeabilized, and stained for intracellular IFN-γ and IL-4 with rat anti-mouse IFN-γ-phycoerythrin and rat antimouse IL-4-fluorescein isothiocyanate conjugates (Pharmingen) according to the protocol recommended by the manufacturer. The stained cells were analyzed on a fluorescence-activated cell sorter (Becton Dickson & Co., Mountain View, Calif.) with Cellquest software. Ten thousand gated events with forward and side scatters characteristic of mononuclear leukocytes were collected and analyzed.
Assays for cell-mediated cytotoxicity.
The Cr51 release cytotoxicity assay was performed as previously described. The MC57G cell line expressing MHC class I (H-2b) or the P815 cell line expressing MHC class I (H-2d) was used as a target for these assays. Target cells, either uninfected or infected with appropriate virus, were incubated with Cr51 overnight at room temperature and then washed twice and plated at 5 × 103 per well in a 96-well, flat-bottom tissue culture plate. Effector cells were added at variable cell numbers to appropriate wells in quadruplicate. The plates were incubated at 37°C in 10% CO2 for 6 h. One hundred microliters of supernatant was harvested from each well and counted on a gamma counter (Isomedic; ICN Biomedicals Inc., Costa Mesa, Calif.). The percent lysis was calculated as previously described (34).
RESULTS
RSV G elicits both Th-1- and Th-2-type cytokines.
Priming of BALB/c mice with the RSV G glycoprotein leads, on intranasal challenge with RSV, to the development of lung inflammation characterized by the recruitment of eosinophils. Mice sensitized to the fusion glycoprotein of RSV (RSV F) or to the other proteins of RSV do not develop lung eosinophilia upon challenge with RSV (23, 28, 35). In models of parasitic infection, such as by Leishmania species, the development of eosinophil-rich inflammation has been shown to be strongly linked to Th-2 polarization of cytokine response, with the production of both IL-4 and IL-5 and little or no production of IFN-γ (30). To further define the mechanism underlying the eosinophilic lung inflammation and cytokine response of RSV G-specific effectors, we examined histologic sections and the ex vivo cytokine response of lung effector cells from the lungs of RSV G-primed mice after infection with live RSV. Groups of mice were primed with either a vaccinia virus recombinant expressing the RSV G glycoprotein (VG) or the control vaccinia virus (VSC11), which expresses no RSV proteins. Three weeks following priming, the mice were challenged intranasally with live RSV. At day 5 postchallenge, portions of their lungs were taken for histology and morphometric analysis. The remaining lung tissue was used for isolation of infiltrating lung mononuclear cells. These isolated lung mononuclear cells were stimulated in vitro with infectious RSV, and the cytokine secretion was quantitated.
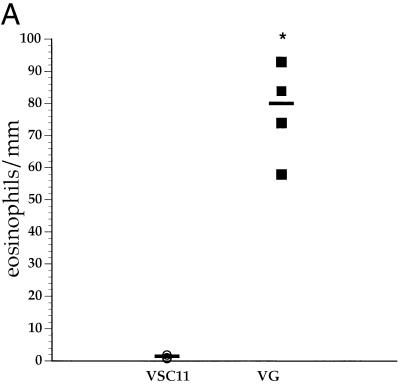
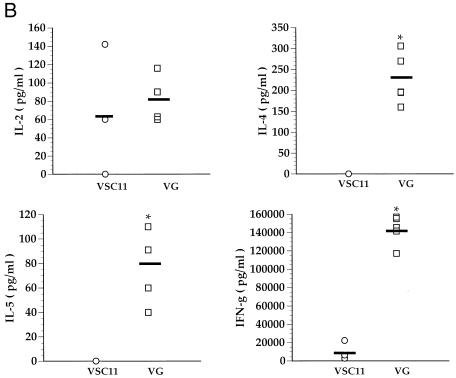
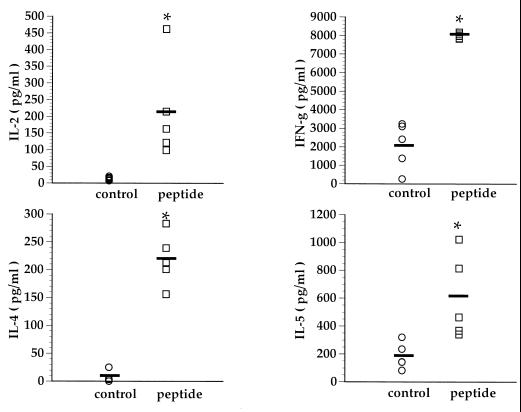
As previously reported, all the RSV G-sensitized mice had marked lung eosinophilia following RSV challenge (Fig. 1A). The development of lung eosinophilia required RSV G priming, as mice primed with the control vaccinia virus (VSC11) did not demonstrate morphometric evidence of enhanced eosinophilia. In addition, the levels of IL-4 and IL-5 produced by infiltrating lung mononuclear cells correlated well with the degree of eosinophilia (Fig. 1B). However, after RSV challenge lung mononuclear cells from G-primed mice also simultaneously produced the Th-1-type cytokines, namely, IFN-γ (Fig. 1B), in RSV G-sensitized mice.
FIG. 1.
Lung eosinophilia and cytokine production in BALB/c mice. (A) Mice were primed with recombinant vaccinia virus expressing RSV G glycoprotein (VG) or control vaccinia virus (VSC11). Three weeks after priming, the mice were infected with RSV, and their lungs were harvested 5 days postchallenge. Sections of lung were stained for eosinophils, and the eosinophils present around blood vessels were enumerated. Each data point represents an average of eosinophils per millimeter of blood vessel length in an individual animal. (B) Mononuclear cells isolated from these lungs were stimulated with RSV in vitro, and the cytokines in the supernatants were analyzed by ELISA. Each data point represents an individual mouse. ∗, P < 0.05 (compared to VSC11-primed mice) by Student’s t test.
During viral infection several cell types are potential sources of IFN-γ, e.g., natural killer cells, CD8+ T cells, and CD4+ Th-1 cells. Since during challenge infection with RSV the extents of primary CD8+-T-cell response in the lungs of VG- and VSC11-primed animals are likely to be comparable, IFN-γ production by primary RSV-specific effector cytotoxic T lymphocytes does not readily account for the elevated levels of IFN-γ produced by infiltrating mononuclear cells from the lungs of VG-primed mice. This enhanced IFN-γ production in VG-primed mice is unlikely to be due to the activation of G-specific memory CD8+ T cells, since we and others have shown that sensitizing with the RSV G glycoprotein does not prime a memory CD8+-T-cell response (2, 34). Thus, the elevated production of IFN-γ by the infiltrating lung mononuclear cells after RSV challenge in VG-primed mice most likely reflects the response of memory CD4+ T cells specific to the RSV G glycoprotein with a Th-1-type cytokine phenotype.
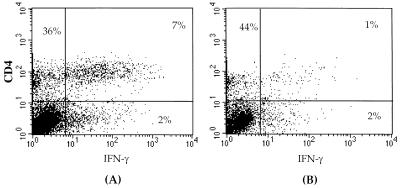
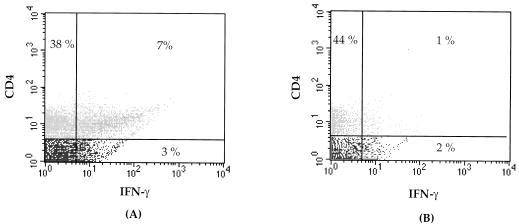
To directly demonstrate the presence of RSV-specific Th-1 effector cells in VG-primed mice, mononuclear cells from the lungs of these animals were isolated at day 5 post-RSV challenge and stimulated in vitro with either uninfected, irradiated splenocytes or RSV-infected, irradiated splenocytes. Forty-eight hours post-stimulation, these cells were simultaneously stained for surface expression of CD4 and intracellular IFN-γ expression (Fig. 2). As Fig. 2A demonstrates, the majority of IFN-γ-containing cells infiltrating the lungs were CD4+. Intracellular accumulation of IFN-γ required specific stimulation of these CD4+ T cells by RSV-infected splenocyte stimulators, as in vitro stimulation with uninfected splenocytes resulted in minimal IFN-γ production by CD4+ T cells (Fig. 2B). Because of the low level of IFN-γ produced in vitro by infiltrating mononuclear cells in the lungs of VSC11-primed mice undergoing primary RSV infection (Fig. 1B), intracellular IFN-γ production by lung mononuclear cells of these animals was not examined. During primary RSV infection the frequency of intracellular IFN-γ-staining CD4+ T cells infiltrating the lungs has been previously reported to be low at day 5 postinfection (22). Our finding of low levels of IFN-γ production by lung mononuclear cells from VSC11-primed mice after challenge infection with RSV is consistent with this earlier data.
FIG. 2.
RSV G induces RSV-specific CD4+ T cells which secrete IFN-γ. Mononuclear cells were isolated form the lungs of VG-primed mice 5 days after challenge. The cells were exposed to RSV-infected spleen cells (A) or mock-infected spleen cells (B) and then stained for surface expression of CD4 and for intracellular IFN-γ.
Th-1 and Th-2 responses are directed to a single peptide epitope of RSV G glycoprotein.
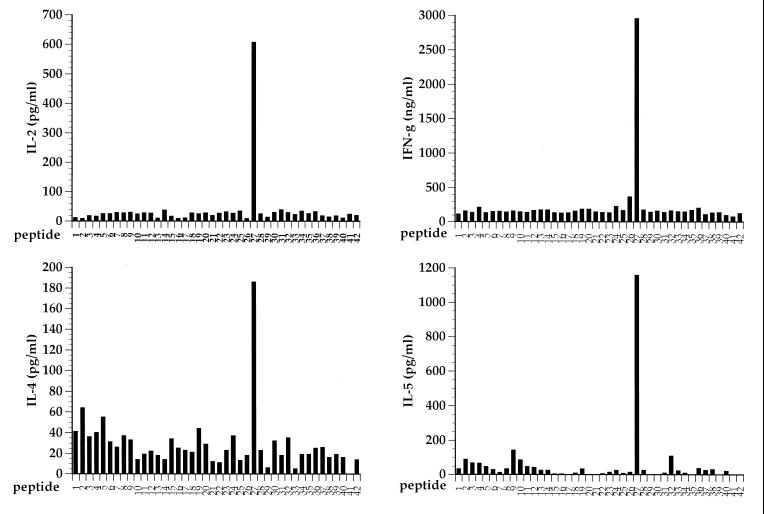
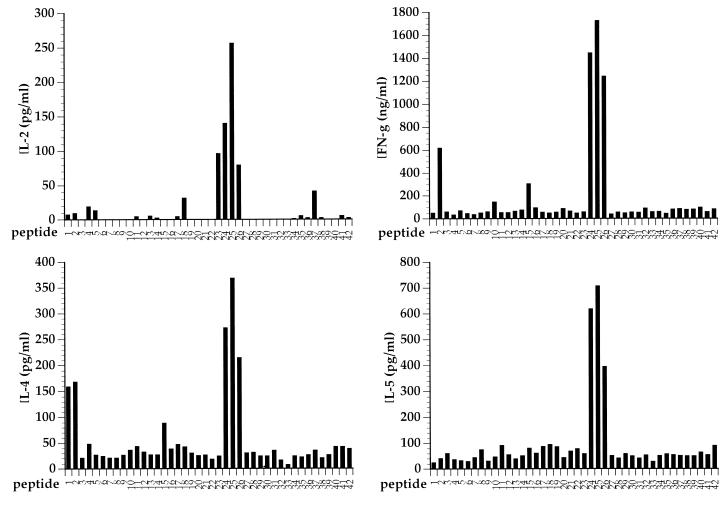
Several studies have suggested that the differentiation of CD4+ T cells along a Th-1 or Th-2 pathway might be dictated by the MHC-peptide ligand specificity of these cells (7, 9, 25). Since the RSV G glycoprotein appears to prime for both Th-1- and Th-2-type CD4+-effector-cell responses after RSV challenge, it is of interest to characterize the peptide epitope(s) contained within this protein which are recognized by Th-1 or Th-2 effector cells. To define potential epitopes, we made a series of overlapping peptides spanning the entire RSV G protein. These peptides were 15 amino acids in length, with an 8-amino-acid overlap. Individual peptides were then tested for the ability to stimulate cytokine release from heterogeneous populations of RSV G-specific T cells generated by two or three rounds of in vitro stimulation of immune splenocytes from individual VG-primed mice with RSV. As shown in Fig. 3 for a representative bulk culture, only one synthetic peptide was recognized by these bulk cultures of RSV G-specific CD4+ effector T cells. This peptide defines an epitope spanning residues 183 to 197 of the RSV G molecule. Identical results were obtained with two independently derived RSV G-specific splenocyte cultures from G-primed BALB/c mice. No additional epitopes were identified with this panel of synthetic peptides. These results suggest that this epitope spanning residues 183 to 197 represents the dominant epitope recognized by RSV G-specific CD4+ T cells in mice with the H-2d haplotype. Interestingly, this single-peptide epitope simultaneously stimulated the production of the Th-1-type cytokines, IL-2 and IFN-γ, as well as the Th-2-type cytokines, IL-4 and IL-5.
FIG. 3.
RSV G-specific Th-1- and Th-2-type responses are directed to the same epitope. A panel of peptides spanning the RSV G molecule was tested for the ability of individual peptides to elicit cytokine production by an RSV G-specific T-cell line. Only one peptide, spanning residues 183 to 197, was found to stimulate T cells to produce both Th-1- and Th-2-type cytokines.
Cytokine profiles of RSV G peptide-specific CD4+ effector T cells.
The above results suggested that RSV G-specific CD4+ effector T cells could produce both Th-1 and Th-2 cytokines in response to a dominant RSV G epitope. Since these results were obtained with RSV G-specific effector T cells generated from splenocytes after several rounds of stimulation in vitro, the specificity and cytokine profile of these cells could represent a unique property, an oligoclonal-T-cell population expanded in vitro, and thus may not accurately reflect the specificity of effector T cells found in vivo in the lungs after RSV infection. This is unlikely, since the effector cells used for this analysis were generated in a relatively short-term in vitro culture. To confirm that the peptide epitope we defined was recognized by lung effector cells, infiltrating mononuclear cells from the lungs of VG-primed mice were isolated 5 days after intranasal RSV challenge. These cells were stimulated ex vivo with the immunogenic RSV G 183-to-197 peptide epitope or a control nonstimulatory peptide (RSV G 1-to-15); the cytokine secretion by these cells was then analyzed 48 h later. As shown in Fig. 4, the lung mononuclear cell population from individual RSV G-sensitized mice produced IL-2, IL-4, IL-5, and IFN-γ in response to stimulation with the RSV G 183-to-197 peptide. Mononuclear cells stimulated with the control peptide did not show any cytokine responses. This result indicates that the epitope defined by the RSV G 183-to-197 peptide is recognized by RSV G-specific effector T cells generated in vivo in response to RSV challenge of RSV G-primed mice and both Th-1- and Th-2-type responses are simultaneously elicited against a single-peptide epitope of the G protein.
FIG. 4.
Recognition of RSV G peptide by lung mononuclear cells. Mononuclear cells were isolated from the lungs of VG-primed mice 5 days after intranasal challenge with the live RSV. The cells were stimulated with a control peptide or the RSV G 183-to-197 peptide, and the cytokines produced were analyzed by ELISA. Each data point represents an individual mouse. ∗, P < 0.05 (compared to control peptide) by Student’s t test. Horizontal lines indicate the mean value of each group.
To directly enumerate the cytokine profiles of CD4+ T cells present during RSV infection in the lungs of mice sensitized to RSV G glycoprotein, lung mononuclear cells were isolated from VG-primed mice on day 5 post-RSV challenge and analyzed for intracellular cytokine after stimulation by the G 183-to-197 peptide. The cells were triple stained for CD4, IL-4, and IFN-γ and analyzed by flow cytometry. CD4+, IFN-γ-producing T cells which recognize the defined peptide are present in the lungs of VG-sensitized mice (Fig. 5A). The cytokine response of these cells is specific for the G 183-to-197 peptide. No response to the control G 1-to-15 peptide was detected (Fig. 5B). The percentages of CD4+ T cells which were stained positive for either IFN-γ or IL-4 were approximately equal (data not shown). These findings further support the view that Th-1 and Th-2 responses are elicited from memory CD4+ T cells directed to the RSV G glycoprotein G 183-to-197 epitope.
FIG. 5.
Peptide-specific Th-1 effector CD4+ T cells in the lungs of RSV G-sensitized mice. The flow cytometric analysis of the lung effector population from VG-primed mice during RSV infection is shown. Lung cells were stimulated with either RSV G peptide (A) or control peptide (B) and then stained for cell surface CD4 and intracellular IFN-γ.
RSV G elicitation of Th-1 and Th-2 type cytokines is not MHC dependent.
If the epitope and T-cell specificities do not play a central role in the regulation of CD4+-T-cell differentiation, then the pattern of cytokine responses in BALB/c mice should be observed in congenic mice genetically identical to BALB/c mice at all loci except the MHC. To test this possibility, we analyzed the pattern of cytokines produced by mononuclear cells isolated from congenic BALB/b mice (H-2b haplotype) which had been primed with VG. These cells were stimulated with individual peptides as shown in Fig. 3, and the cytokines in the supernatants were analyzed. As shown in Fig. 6, a single dominant region of this protein (spanning residues 162 to 190) elicits the production of IL-2 and IFN-γ (Th-1 cytokines) as well as IL-4 and IL-5 (Th-2 cytokines) by immune splenocytes from BALB/b mice. Thus, despite the allelic difference in the MHC molecules expressed by BALB/b and BALB/c mice, and hence in epitopes selected by these MHC class II alleles, the patterns of cytokine responses to the RSV G protein in these two mouse strains are similar. This result strongly implies that the induction of a Th-2-type response to RSV G after challenge infection is not due to a unique Th-2 epitope in RSV G.
FIG. 6.
Identification of peptide epitopes recognized by BALB/b mice. Experiments were done as in Fig. 3 except that the RSV G-specific T-cell line was derived form BALB/b mice. Both Th-1- and Th-2-type cytokines were produced in response to stimulation with peptides spanning residues 162 to 190.
Non-MHC genes are crucial in the development of pulmonary eosinophilia.
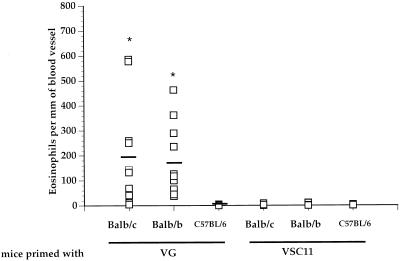
So far, we have shown that the induction of RSV G-specific Th-1 and Th-2 CD4+ effector T cells is not influenced by their ligand specificity and therefore is both T-cell receptor (TCR) and MHC independent. Several studies have shown that polarized cytokine responses to microbial infection are linked to the genetic background of the host strain. To further examine the contributions of MHC and non-MHC genes in the induction of lung pathology in this model, we compared the development of lung eosinophilia in several strains of mice which differ at either the MHC or non-MHC gene loci. C57BL/6 (H-2b), BALB/c (H-2d), and BALB/b (H-2b) mice were primed with VG and challenged 3 weeks later with RSV intranasally. Their lungs were taken 5 days after challenge and examined for the presence of eosinophils. Figure 7 shows a morphometric analysis of eosinophil numbers in histologic lung sections taken from mice of these three strains. Both BALB/c and BALB/b strains developed extensive lung eosinophilia. The phenomenon is dependent on RSV G-specific recognition, as the VSC11-primed mice do not generate an eosinophilic response. Importantly, C57BL/6 mice, which are identical to the BALB/b strain at the MHC loci but different at other gene loci, did not develop the eosinophilic response seen in BALB/b mice. The lungs of VG-primed C57BL/6 mice did exhibit extensive mononuclear cell infiltration not found in mice primed with control vaccinia virus, indicating that they were successfully primed against RSV G (data not shown). This data strongly implicates strain-specific, non-MHC genes as major determinants in the development of a Th-2 response in this model.
FIG. 7.
Development of pulmonary eosinophilia is dependent on non-MHC gene loci of the host. Mice differing at the MHC or non-MHC gene loci were primed with VG or control vaccinia virus. The mice were then infected with RSV, and their lungs were analyzed for eosinophilia. Three to five blood vessels in each lung section were examined for the presence of eosinophils, and the eosinophils in and around the vessels were enumerated and expressed as the number of eosinophils per unit length (in millimeters) of vessel wall. Each data point represents the number of eosinophils present around individual blood vessels in the lungs of the mice (four to five mice per group). ∗, P < 0.05 (compared to VG-primed C57BL/6 mice) by Student’s t test. There is no statistical difference between VG-primed BALB/c and BALB/b mice (P = 0.698).
DISCUSSION
In this study, we have clearly demonstrated that BALB/c mice sensitized to the RSV G glycoprotein produced in both Th-1- and Th-2-type cytokines when challenged with RSV. The concurrent induction of Th-1- and Th-2-type responses was shown both at the level of bulk T-cell cultures and at a single-cell level. Importantly, these polarized CD4+ effector T cells from BALB/c mice recognized the same amino acid epitope, suggesting that the specificity of T cells is not the crucial factor in directing the differentiation pathway of CD4+ T cells and the production of cytokines by the activated CD4+ effector T cells. This finding was further supported by the observation that T cells from H-2b haplotype BALB/b mice previously sensitized to RSV G glycoprotein likewise secrete both Th-1- and Th-2-type cytokines in response to a single, distinct region of the RSV G molecule. Genes unlinked to the MHC loci appeared to play a crucial role in the development of polarized cytokine responses in this model, since BALB/b mice, which share non-MHC background genes with BALB/c mice, but not H-2b haplotype C57BL/6 mice developed lung eosinophilia when sensitized to the RSV G glycoprotein.
Polarized cytokine responses by CD4+ effector T cells have been implicated in the pathogeneses of many diseases. In most models of microbial infection, the cytokine responses usually deviate towards either a Th-1 (high IL-2 and IFN-γ with low IL-4 and IL-5) or Th-2 (high IL-4 and IL-5 with low IFN-γ) pole (5, 6). In this regard, the immune response to the RSV G glycoprotein is notable in that both Th-1- and Th-2-type responses are concurrently induced. Indeed, analysis of the cytokine profile exhibited by lung mononuclear cells in VG-primed mice revealed that the frequencies of Th-1 and Th-2 effector cells are roughly equal (data not shown). Our results are in agreement with recent studies on cytokine responses during experimental viral infection in RSV and lymphocytic choriomeningitis virus models, which showed a concurrent induction of Th-1- and Th-2-type cytokines (33, 40).
The finding that RSV G induces both Th-1- and Th-2-type effector cells led us to examine the peptide epitopes recognized by these cells. Several studies have indicated that the affinity of the interaction between the MHC-peptide ligand and the TCR may determine the eventual cytokine phenotype exhibited by CD4+ T cells (7, 9, 25). According to this view, certain MHC-peptide ligand complexes will preferentially induce a Th-1 or Th-2 response, so-called Th-1 and Th-2 epitopes. A recent study by Sparer et al. has suggested that RSV G glycoprotein may contain a region corresponding to G protein residues 193 to 203 which represents such a Th-2-type epitope, since virus with a mutant RSV G lacking this region failed to elicit lung eosinophilia in BALB/c mice (32). We have independently identified a single dominant epitope in RSV G spanning residues 183 to 197 which maps to the same region as a previously identified Th-2 epitope. This epitope, however, is recognized by both Th-1 and Th-2 effector cells. If, as our data suggests, this is the dominant epitope recognized by CD4+ T cells of H-2d haplotype mice, then deletion of the portion of RSV G containing this epitope will significantly reduce the immunogenicity of this molecule and the subsequent development of lung eosinophilia. In support of our result, a recent study has identified the same peptide region of the RSV G molecule which elicited both IFN-γ and IL-5 responses in RSV G-primed BALB/c mice (38). The concurrent induction of Th-1- and Th-2-type responses to this epitope suggests that the peptide ligand specificity of the TCR displayed by RSV G-specific CD4+ T cells may not play a critical role in determining the polarized cytokine response and the phenotype of activated effector CD4+ T cells. Studies demonstrating that CD4+ T cells expressing identical TCRs can be driven to become Th-1 or Th-2 effector cells support this view (19, 31). An alternative explanation for our observation is that differences in the affinities of the TCRs on individual G-specific CD4+-T-cells to their MHC-peptide ligands may influence the cytokine profile and the polarization of CD4+ T cells. In this instance, the TCR repertoire of RSV G-specific CD4+ T cells in the two strains of mice (BALB/c and BALB/b) examined in this study must exhibit a sufficient range of affinities for their respective G epitopes to drive the differentiation of both Th-1 and Th-2 effector cells directed to a single G epitope. Further studies will be required to more precisely define the contribution of TCR affinity to the induction of polarized CD4+-T-cell responses in the RSV model.
The epitope specificities of CD4+ T cells appear to play a minor role in the selective induction of polarized cytokine responses in the murine RSV model. In support of this view, we found that neither the generation of RSV G-specific CD4+ Th-1 and Th-2 effector cells nor the development of lung eosinophilia was dependent on the MHC haplotype of the host. BALB/b mice, which differ at MHC loci from BALB/c mice, predictably recognize an RSV G epitope(s) distinct from the epitope recognized by BALB/c mice. Despite the difference in epitopes recognized by these two strains of animals, the pattern of cytokine response in BALB/b mice is similar to that in BALB/c mice. Th-1- and Th-2-type cytokine responses are induced in both strains of mice, and these dominant epitopes elicit both Th-1- and Th-2-type cytokine responses. The magnitudes of Th-2 responses in these two strains of animals are quite comparable, as judged by the extent of eosinophil recruitment into the lungs following RSV infection. Importantly, VG-primed C57BL/6 mice, which have the same MHC haplotype as BALB/b mice but differ in non-MHC genetic loci, do not develop lung eosinophilia after challenge with RSV. In sum, these results strongly suggest that for these mouse strains the induction of Th-1 and Th-2 cytokine responses to the RSV G protein is primarily regulated by non-MHC-linked genetic loci.
We have previously shown that the induction of virus-specific cytolytic CD8+ T cells is associated with a reduction in Th-2-type cytokine production and lung eosinophilia (34). The absence of lung eosinophilia in RSV G-sensitized C57BL/6 mice might then be related to their ability to mount a cytolytic-CD8+-T-cell response to RSV G. This explanation is unlikely, since to date neither C57BL/6 nor BALB/b mice have exhibited a detectable RSV G-specific, MHC class I-restricted cytotoxic T-lymphocyte response to this protein (7a). The absence of lung eosinophilia in C57BL/6 mice in spite of the apparent inability of these mice to mount a cytolytic-CD8+-T-cell response to this antigen does not necessarily negate the role of CD8+ T cells in the down-regulation of Th-2 cytokine production. Recent studies of RSV G-primed C57BL/6 mice have shown that elimination of CD8+ T cells in vivo during viral challenge led to the development of lung eosinophilia (20). It is possible that noncytolytic CD8+ T cells which are capable of the down-regulation of a Th-2 response exist in vivo. Furthermore, CD8+ T cells are likely not the only factors that regulate the induction of Th-2 responses. As shown in this study, the ability of the host to mount a strong Th-2-type response clearly depends on other factors, particularly the non-MHC genetic makeup of the host. Candidates for non-MHC genes which may participate in the generation of Th-1–Th-2 responses include the genes encoding cytokines known to have a polarizing effect on T-cell differentiation, cytokine receptors, and signaling molecules along the cytokine signal transduction pathway. Recent studies of humans and mice have demonstrated polymorphism in cytokine and cytokine receptor genes which may be linked to susceptibility to the development of allergic diseases (12, 18).
The immunological and pathological consequences of the simultaneous induction of Th-1 and Th-2 responses are poorly understood at present. It is conceivable that Th-1 and Th-2 effector T cells may counterregulate each other, resulting in the suppression of cytokine production by one or both populations. Studies which have examined the cytokine response of differentiated Th-1 and Th-2 effector cells when these cells were simultaneously activated in vitro have failed to demonstrate this counterregulation (27). Whether fully differentiated CD4+ effector T cells are likewise refractory to counterregulation in vivo awaits further investigation. However, IFN-γ and IL-4 produced by Th-1 and Th-2 effector cells could influence the differentiation of naive and memory CD4+ T cells present at the site of inflammation. These cytokines may also exert synergistic or opposing effects on other steps of the inflammatory process, such as the up-regulation of adhesion molecules and the recruitment of inflammatory cells (including T cells and eosinophils) to the site of inflammation. In this connection, we have previously reported that the severity of lung eosinophilia in RSV G-sensitized mice was more pronounced in the absence of IFN-γ (35). An in-depth understanding of the roles of cytokines secreted by different types of effector T cells in the clearance of RSV from the lungs as well as in the pathogenesis of RSV-mediated lung injury will be critical for the development of an effective vaccine against this virus.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases. A.S. is a recipient of an American Lung Association Research Grant.
We thank J. Beeler of FDA for providing recombinant vaccinia virus expressing the RSV F and G genes under the auspices of the WHO-UNDP program for vaccine development.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 3.Ball L A, Young K K, Anderson K, Collins P L, Wertz G W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci USA. 1986;83:246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bembridge G P, Garcia B R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. [PubMed] [Google Scholar]
- 5.Bretscher B A, Wu G, Menon N, Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 6.Brown D R, Fowell D J, Corry D B, Wynn T A, Moskowitz N H, Cheever A W, Locksley R M, Reiner S L. Beta 2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carballido J M, Faith A, Carballido-Perrig N, Blaser K. The intensity of T cell receptor engagement determines the cytokine pattern of human allergen-specific T helper cells. Eur J Immunol. 1997;27:515–521. doi: 10.1002/eji.1830270224. [DOI] [PubMed] [Google Scholar]
- 7a.Chang, W. Unpublished data.
- 8.Channock R M, Parrott R H, Connors M, Collins P L, Murphy B R. Serious respiratory tract disease caused by respiratory syncytial virus: prospects for improved therapy and effective immunization. Pediatrics. 1992;90:137–143. [PubMed] [Google Scholar]
- 9.Chaturvedi P, Yu Q, Southwood S, Sette A, Singh B. Peptide analogs with different affinities for MHC alter the cytokine profile of T helper cells. Int Immunol. 1996;8:745–755. doi: 10.1093/intimm/8.5.745. [DOI] [PubMed] [Google Scholar]
- 10.Chin J, Magoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 11.Connors M, Giese N A, Kulkarni A B, Firestone C Y, Morse H C, III, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deichmann K A, Heinzmann A, Forster J, Dischinger S, Mehl C, Brueggenolte E, Hildebrandt F, Moseler M, Kuehr J. Linkage and allelic association of atopy and markers flanking the IL4-receptor gene. Clin Exp Allergy. 1998;28:151–155. doi: 10.1046/j.1365-2222.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 13.Falsey A R, Walsh E E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis. 1998;177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 14.Fischer J E, Johnson J E, Kuli Z R, Johnson T R, Aung S, Parker R A, Graham B S. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol. 1997;71:8672–8677. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham B S. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–293. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 16.Graham B S, Davis T H, Tang Y W, Gruber W C. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr Res. 1993;34:167–172. doi: 10.1203/00006450-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 18.Hershey G K, Friedrich M F, Esswein L A, Thomas M L, Chatila T A. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–1725. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh C S, Heimberger A B, Gold J S, O’Garra A, Murphy K M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussel T, Georgiou A, Spearer T E, Matthews S, Pala P, Openshaw P J M. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- 21.Hussell T, Baldwin C J, O’Garra A, Openshaw P J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 22.Hussell T, Spender L C, Georgiou A, O’Garra A, Openshaw P J. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 23.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 25.Murray J S. How the MHC selects Th1/Th2 immunity. Immunol Today. 1998;19:157–163. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson K G, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community; comparative, prospective, population based study of disease burden. Br Med J. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O’Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Openshaw P J, Clarke S L, Record F M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 29.Openshaw P J, Hussell T. The effect of IL-12 treatment on vaccine-enhanced illness during infection with respiratory syncytial virus. Dev Biol Stand. 1998;92:179–185. [PubMed] [Google Scholar]
- 30.Scott P, Pearce E, Cheever A W, Coffman R L, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 31.Seder R A, Paul W E, Davis M M, Fazekas de St. Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Melero J A, Openshaw P J. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spender L C, Hussell T, Openshaw P J. Abundant IFN-gamma production by local T cells in respiratory syncytial virus-induced eosinophilic lung disease. 1998. J. Gen. Virol. 1751–1758. [DOI] [PubMed] [Google Scholar]
- 34.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srikiatkhachorn A, Braciale T J. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stott E, Taylor G. Respiratory syncytial virus; brief review. Arch Virol. 1985;84:1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y W, Graham B S. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Investig. 1994;94:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tebbey P W, Hagen M, Hancock G E. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wertz G W, Stott E J, Young K K, Anderson K, Ball L A. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987;61:293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmire J K, Asano M S, Murali K K, Suresh M, Ahmed R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J Virol. 1998;72:8281–8288. doi: 10.1128/jvi.72.10.8281-8288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]