Abstract
Anuran metamorphosis is perhaps the most drastic developmental change regulated by thyroid hormone (T3) in vertebrate. It mimics the postembryonic development in mammals when many organs/tissues mature into adult forms and plasma T3 level peaks. T3 functions by regulating target gene transcription through T3 receptors (TRs), which can recruit corepressor or coactivator complexes to target genes in the absence or presence of T3, respectively. By using molecular and genetic approaches, we and others have investigated the role of corepressor or coactivator complexes in TR function during the development of two highly related anuran species, the pseudo-tetraploid Xenopus laevis and diploid Xenopus tropicalis. Here we will review some of these studies that demonstrate a critical role of coactivator complexes, particularly those containing steroid receptor coactivator (SRC) 3, in regulating metamorphic rate and ensuring the completion of metamorphosis.
1. Introduction
Ever since the discovery that one or more compounds in the thyroid could accelerate anuran metamorphosis over a century ago (Gudernatsch, 1912), anuran metamorphosis has been used as a model for studying postembryonic development in vertebrates, particularly the role of thyroid hormone (T3) during metamorphosis, and developmental regulation of organ regeneration (Dodd & Dodd, 1976; Gilbert & Frieden, 1981; Gilbert, Tata, & Atkinson, 1996; Li, Zhang, & Amaya, 2016; Marshall et al., 2019; Shi, 1999, 2013a; Tata, 1993; Wang & Shi, 2021; Yakushiji, Yokoyama, & Tamura, 2009). Metamorphosis in anurans, such as the highly related diploid Xenopus tropicalis and pseudo-tetraploid Xenopus laevis, resembles postembryonic development in mammals, a period around birth when plasma T3 level peaks and many organs/tissues mature into their adult form (Fig. 1A) (Shi, 1999; Tata, 1993). While it is difficult to study mammalian postembryonic development due to maternal influence, it is much easier to manipulate anuran metamorphosis. T3 plays a causative role in this process and its level can be easily altered by adding exogenous T3 or T3 synthesis inhibitors to tadpole rearing water to affect metamorphosis. In addition, T3 can even induce organ-specific metamorphic changes when added to organ cultures of premetamorphic tadpoles, indicating that individual tissues/organs are genetically programmed for specific T3-dependent changes. Furthermore, essentially all tissues/organs in an anuran tadpole are transformed during metamorphosis, involving drastic changes ranging from de novo formation of adult tissues such as the limb, remodeling of organs present in both tadpoles and frogs to adapt for post-metamorphic living habitat, to complete resorption of larval specific organs such as the tail (Fig. 1B). This makes it possible to use anuran metamorphosis to study diverse cellular processes during vertebrate development. The advantage of the anuran model is further strengthened by transgenic and gene-editing tools for genetic studies (Blitz, Biesinger, Xie, & Cho, 2013; Fu, Buchholz, & Shi, 2002; Kroll & Amaya, 1996; Lei et al., 2012; Lei, Guo, Deng, Chen, & Zhao, 2013; Nakade et al., 2014; Nakayama et al., 2013; Shi et al., 2015; Wang et al., 2015; Young et al., 2011), which had been a weakness for amphibian and fish models compared to the mouse model for vertebrate studies.
Fig. 1.
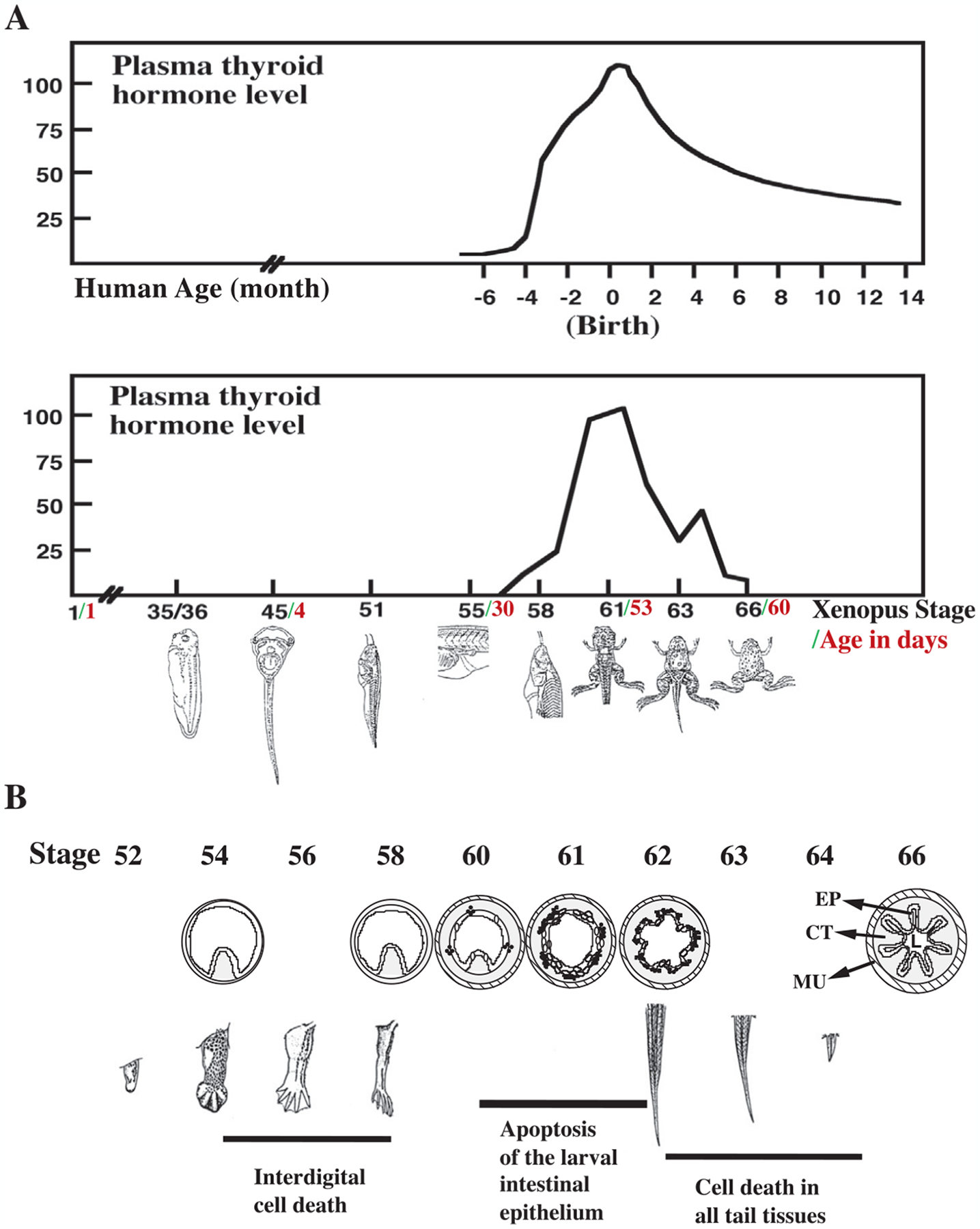
T3 and postembryonic development. (A) Plasma T3 levels peak during postembryonic development in human and anuran metamorphosis. Postembryonic development in mammals refers to the perinatal period when many organs mature into their adult forms, i.e., about 4 months before to several months after birth in human (Tata, 1993). This period corresponds to metamorphosis in anurans such as Xenopus laevis (Leloup & Buscaglia, 1977; Nieuwkoop & Faber, 1965). (B) Xenopus metamorphosis involves three major types of transformations: de novo development of adult organs such as limbs, complete degeneration of larval organs such as the tail, and remodeling of organs that are present in both tadpoles and frogs such as the intestine. Note that at least some T3-induced apoptosis occurs in these examples of all three types. EP, epithelium; CT, connective tissue; MU, muscle.
2. Transcriptional regulation by TR and its dual functions in Xenopus development
Extensive in vitro and in vivo studies have shown that the T3 nuclear receptors (TRs) mediate the major biological effects of T3 (Buchholz, Paul, Fu, & Shi, 2006; Forrest, 1994; Laudet & Gronemeyer, 2002; Lazar, 1993; Shi, Wong, Puzianowska-Kuznicka, & Stolow, 1996; Yen, 2001). TRs are encoded by two highly conserved TR genes, TRα and TRβ genes, in all vertebrates, including the diploid anuran Xenopus tropicalis and pseudo-tetraploid anuran Xenopus laevis, which has two duplicated TRα and TRβ genes (Laudet & Gronemeyer, 2002; Shi, Yaoita, & Brown, 1992; Wang, Matsuda, & Shi, 2008; Yaoita, Shi, & Brown, 1990). TRs regulates target gene transcription in a T3-dependent manner. For T3-inducible genes, they bind to T3 response elements (TREs) in the target genes as heterodimers with 9-cis retinoic acid receptors (RXRs) to repress their transcription in the absence of T3 and activate them when T3-bound by recruiting corepressor and coactivator complexes, respectively (Bulynko & O’Malley, 2011; Grimaldi, Buisine, Miller, Shi, & Sachs, 2013; McKenna et al., 2009; O’Malley, Malovannaya, & Qin, 2012; Perissi, Jepsen, Glass, & Rosenfeld, 2010; Shi, Matsuura, Fujimoto, Wen, & Fu, 2012).
Frog development offers an opportunity to study the developmental functions of both unliganded and liganded TRs. During premetamorphosis, TRs, especially TRα, are expressed but there is little T3 (Kanamori & Brown, 1992; Leloup & Buscaglia, 1977; Shi et al., 1992; Wang & Brown, 1993; Wong & Shi, 1995; Yaoita & Brown, 1990), suggesting that most TRs are likely unliganded and function as repressors of T3-inducible genes to prevent premature metamorphosis (Fig. 2). During metamorphosis, T3 level rises due to endogenous synthesis, causing TRs to be liganded, thereby activating T3-inducible genes to cause metamorphosis (Fig. 2). Thus, TRs have dual functions during frog development (Sachs et al., 2000). Indeed, extensive genetic studies have supported such a dual function model. Earlier studies include transient and transgenic overexpression of wild type or mutant TRs in Xenopus laevis, demonstrating that blocking TR function inhibits T3-inducible gene expression and metamorphosis while enhancing TR function upregulates T3 target genes and accelerate metamorphosis (Buchholz et al., 2006; Buchholz, Hsia, Fu, & Shi, 2003; Buchholz, Tomita, Fu, Paul, & Shi, 2004; Das, Schreiber, Huang, & Brown, 2002; Nakajima & Yaoita, 2003; Puzianowska-Kuznicka, Damjanovski, & Shi, 1997; Schreiber & Brown, 2003; Schreiber, Das, Huang, Marsh-Armstrong, & Brown, 2001). The roles of endogenous TRα and TRβ have also been revealed through gene knockout studies in the diploid Xenopus tropicalis (Buchholz & Shi, 2018; Choi et al., 2015; Choi, Ishizuya-Oka, & Buchholz, 2017; Nakajima, Tanizaki, Luu, Zhang, & Shi, 2020; Nakajima, Tazawa, & Shi, 2019; Nakajima, Tazawa, & Yaoita, 2018; Sachs, 2015; Sakane et al., 2018; Shi, 2021; Shibata et al., 2021; Shibata, Tanizaki, & Shi, 2020; Shibata, Wen, Okada, & Shi, 2020; Tanizaki, Shibata, Zhang, & Shi, 2021, 2022; Tanizaki, Zhang, Shibata, & Shi, 2022; Wen et al., 2017; Wen & Shi, 2015, 2016; Yen, 2015). These studies not only support the dual functions of TR during development but also offer details on TR-subtype-specific and developmental stage- and organ-dependent functions of TRs in regulating metamorphic timing and rate. As predicted by the dual function model, premetamorphic tadpoles lacking both TRα and TRβ fail to respond to exogenous T3, and initiate metamorphosis prematurely relative to wild type siblings, but fail to complete metamorphosis (Shibata, Wen, et al., 2020). Interestingly, many adult organs appear to form in the absence of any TRs and the tadpoles can develop up to the climax of metamorphosis, while larval tissue resorption is inhibited, likely contributing to the death of the TR-double knockout animals at the climax of metamorphosis (Shi, 2021; Shibata, Wen, et al., 2020).
Fig. 2.
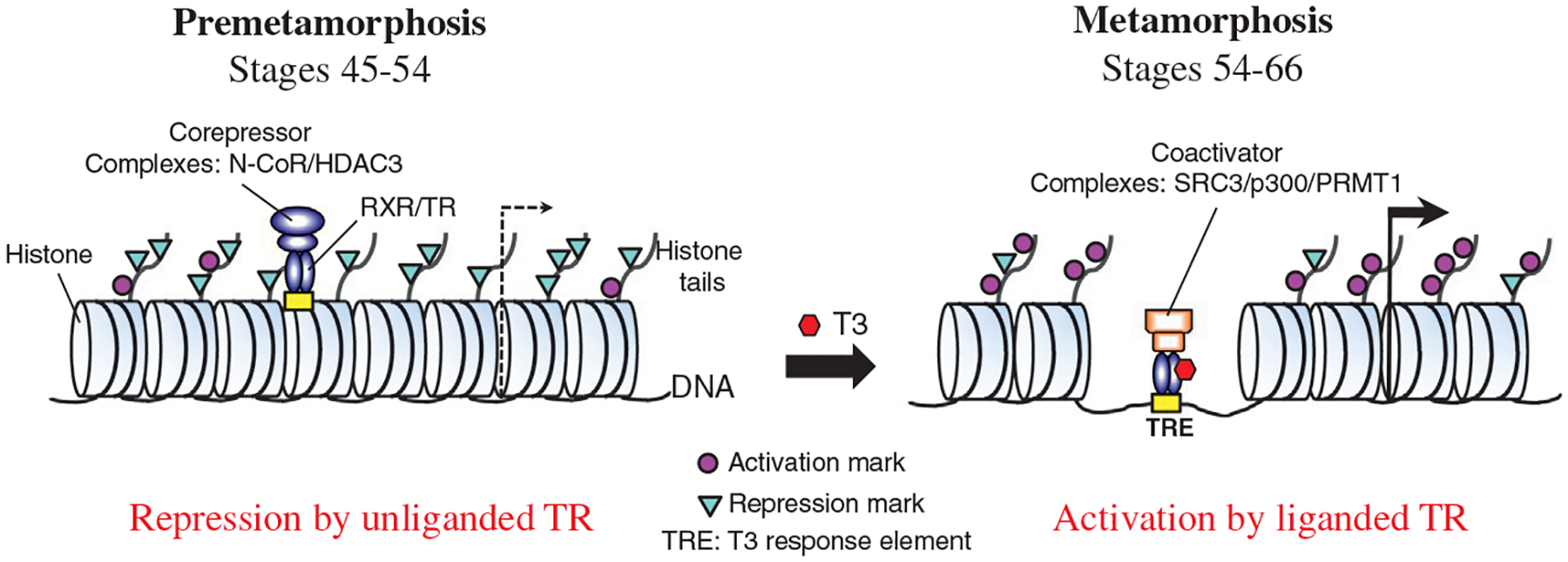
Dual function of TR during Xenopus metamorphosis. TR can repress and activate T3-inducible genes in the absence and presence of T3, respectively. The presence of little or no T3 in premetamorphic tadpoles enables most or all TRs remaining in the unliganded state and TR/RXR heterodimers recruit HDAC-containing corepressors complexes to target genes. This in turn reduces the levels of activation histone marks and increases the levels of repression histone marks, resulting in gene repression. With rising levels of T3 during metamorphosis, TRs become T3-bound and TR/RXR heterodimers recruit coactivator complexes. This then causes chromatin remodeling, including the loss of 2–3 nucleosomes around the TRE and histone modifications, particularly an increase in activation histone marks and a decrease in repression histone marks, and eventually transcriptional activation. N-CoR, nuclear corepressor; HDAC, histone deacetylase; SRC3, steroid receptor coactivator 3; p300, a histone acetyltransferase; PRMT1, protein arginine methyltransferase 1.
3. A role of histone acetylation in gene regulation by TR
For T3-inducible genes, TR/RXR heterodimers bind to TREs in the target genes to repress their transcription in the absence of T3 and activate them when liganded by recruiting corepressor and coactivator complexes, respectively (Bulynko & O’Malley, 2011; Grimaldi, Buisine, Miller, et al., 2013; McKenna et al., 2009; O’Malley et al., 2012; Perissi et al., 2010; Shi et al., 2012). The best-studied TR-corepressor complexes are those containing either one of the two highly related proteins N-CoR (nuclear corepressor) and SMRT (silencing mediator of retinoid and thyroid hormone receptors). These complexes also contain histone deacetylases such as HDAC3 (Burke & Baniahmad, 2000; Chen & Evans, 1995; Glass & Rosenfeld, 2000; Guenther et al., 2000; Horlein et al., 1995; Ishizuka & Lazar, 2003; Jones, Sachs, Rouse, Wade, & Shi, 2001; Jones & Shi, 2003; Li et al., 2000, 2002; Perissi et al., 2010; Stewart, Li, & Wong, 2005; Stewart, Tomita, Shi, & Wong, 2006; Tomita, Buchholz, & Shi, 2004; Yoon et al., 2003; Zhang, Kalkum, Chait, & Roeder, 2002; Zhang & Lazar, 2000). On the other hand, many coactivator complexes have histone acetyltransferase activities. In particular, the steroid receptor coactivators (SRCs: SRC1, SRC2, and SRC3), which bind liganded TR directly and have histone acetyltransferase activities, form large complexes that contain histone acetyltransferases p300/CBP, and the histone methyltransferases PRMT1 and CARM1 (Chakravarti et al., 1996; Chen et al., 1997, 1999; Demarest et al., 2002; Hong, Kohli, Trivedi, Johnson, & Stallcup, 1996; Koh, Chen, Lee, & Stallcup, 2001; Li, Gomes, & Chen, 1997; Li, O’Malley, & Wong, 2000; Ogryzko, Schiltz, Russanova, Howard, & Nakatani, 1996; Onate, Tsai, Tsai, & O’Malley, 1995; Sheppard, Harries, Hussain, Bevan, & Heery, 2001; Takeshita, Cardona, Koibuchi, Suen, & Chin, 1997; Voegel et al., 1998; Voegel, Heine, Zechel, Chambon, & Gronemeyer, 1996). Thus, histone acetylation like plays a critical role in target gene regulation by T3 during amphibian metamorphosis.
Indeed, analyses of in vivo histone acetylation levels at T3-induced genes by using chromatin immunoprecipitation (ChIP) assay with antibodies against acetylated histones have revealed that treatment of premetamorphic Xenopus tadpoles with T3 or an HDAC inhibitor increases histone acetylation, mimicking that during frog metamorphosis (Matsuura, Fujimoto, Fu, & Shi, 2012; Sachs, Amano, Rouse, & Shi, 2001; Sachs, Amano, & Shi, 2001; Sachs & Shi, 2000). Furthermore, treatment of premetamorphic tadpoles with a histone deacetylase inhibitor also upregulates T3 response genes in the absence of T3, resembling T3 treatment, although it does not induce metamorphosis (Sachs, Amano, Rouse, & Shi, 2001; Sachs, Amano, & Shi, 2001). In addition, ChIP assays with antibodies against corepressors have revealed that TRs recruit corepressor complexes to T3-target genes in different organs of premetamorphic Xenopus laevis tadpoles (Buchholz et al., 2003; Sachs et al., 2002; Tomita et al., 2004), and transgenic overexpression of a dominant negative form of the corepressor N-CoR containing only the TR-interacting domain causes precocious upregulation of T3-target genes and initiation of metamorphosis in Xenopus laevis (Sato, Buchholz, Paul, & Shi, 2007). Such findings support an important role of histone deacetylation in gene repression via recruitment of HDAC-containing corepressor complexes by unliganded TR in premetamorphic tadpoles and are consistent with the dual function model of TR in development.
4. Critical involvement of steroid-receptor coactivator complexes in regulating metamorphosis by liganded TR
The dual function model for TR implies that during metamorphosis when T3 is present, liganded TR recruits coactivator complexes. Indeed, ChIP assays have shown that at least the complexes containing histone acetyltransferases SRC3 and p300 and histone methyltransferase PRMT1 (protein arginine methyltransferase 1) are recruited by TR during natural or T3-induced metamorphosis (Havis, Sachs, & Demeneix, 2003; Matsuda, Paul, Choi, Hasebe, & Shi, 2009; Paul, Buchholz, Fu, & Shi, 2005, 2007; Paul, Fu, Buchholz, & Shi, 2005). This is accompanied by increased local histone acetylation at the T3-target genes and upregulation of target gene expression.
Of particular interest among the coactivators is the TR-binding histone acetyltransferase SRC3. During Xenopus laevis development, SRC3 is upregulated by T3 and more importantly recruited by liganded TR in a gene- and tissue-specific manner (Paul, Buchholz, et al., 2005; Paul & Shi, 2003). A functional role of SRC3-containing coactivator complexes in frog metamorphosis was first demonstrated by transgenic studies with a dominant negative form of Xenopus laevis SRC3 (F-dnSRC3) (Paul, Fu, et al., 2005). F-dnSRC3 contains only the TR-binding domain of SRC3 (Fig. 3) and its binding to liganded TR is expected to prevent the formation of a normal functional coactivator complex due to the lack of other domains, such as the one binding to the histone acetyltransferase p300. In addition, it will also compete against the binding of other coactivators, including the endogenous wild type SRC3, to liganded TR during metamorphosis, thus inhibiting coactivator recruitment by liganded TR.
Fig. 3.
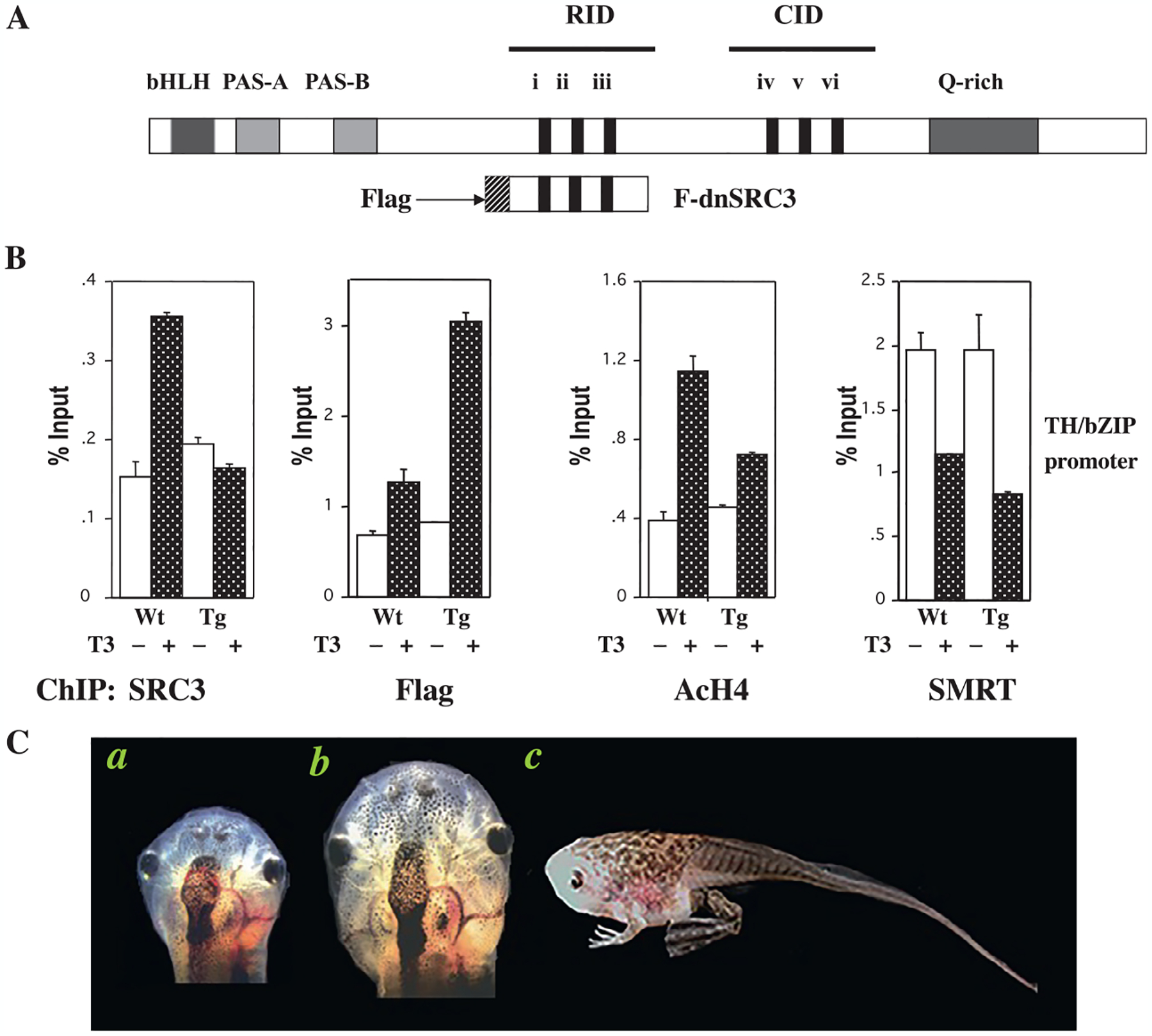
Transgenic overexpression of a Flag-tagged dominant negative steroid receptor coactivator 3 (F-dnSRC3) inhibits metamorphosis. (A) A schematic representation of the full-length SRC3. bHLH/PAS, basic helix–loop–helix and PAS dimerization domains; RID, receptor interaction domain; CID, CBP/p300 interaction domain. The LXXLL motifs present in the protein are numbered from i to vi. A glutamine (Q)-rich region is present toward the C-terminal end of the protein. The Flag-tagged dominant negative form (F-dnSRC3) used in transgenesis is shown below the full length SRC3. It covers aa 600 to aa 751, which comprise the LXXLL motifs i to iii, forming the RID and fused to an N-terminal peptide containing the Flag tag and nuclear localization sequences. (B) Transgenic expression of F-dnSRC3 does not affect T3-induced release of corepressor SMRT but inhibits the recruitment of endogenous wild type SRC3 to the T3-regulated TH/bZIP promoter, thus reducing histone acetylation. Wild type (WT) and transgenic (Tg) animals at stage 54 were treated with 10 nM T3 for 2 days. Intestinal nuclei were isolated and ChIP assays performed by using anti-SRC3 (for endogenous wild type SRC3), anti-acetylated histone H4 (AcH4), anti-SMRT (for endogenous corepressor SMRT), and anti-Flag (for transgenic F-dnSRC3) antibodies. The TRE region of the T3-response gene TH/bZIP was analyzed by real time PCR after immunoprecipitation with the indicated antibodies. Note that F-dnSRC3 was recruited to the promoter upon T3-treatment, competing against the recruitment of endogenous SRC3 recruitment and reducing local AcH4 level in the transgenic animals. (C) F-dnSRC3 inhibits metamorphosis. Wild type (a) and transgenic Xenopus laevis tadpoles (b) at stage 54 after treatment with 5 nM T3 for 3 days, with the resorption of gills and development of the Meckel’s cartilage (leading to protrusion of the jaw) in the wild type but not transgenic tadpole after T3 treatment. (c) A transgenic tadpole which failed to undergo tail resorption during natural development and eventually died. See Paul, Fu, et al. (2005) for more details.
Indeed, transgenic overexpression of F-dnSRC3 in Xenopus laevis inhibited the recruitment of endogenous wild type SRC3 to the promoter regions of T3-target genes, but expectedly did not influence the release of corepressors in the presence of T3 (Fig. 3) (Paul, Fu, et al., 2005). Furthermore, it also reduced histone acetylation at target gene promoters and T3-induction of target gene expression (Paul, Fu, et al., 2005). Most importantly, the expression of F-dnSRC3 delayed/inhibited both T3-induced and natural metamorphosis (Fig. 3) (Paul, Fu, et al., 2005). Some of the transgenic animals developed to the climax of metamorphosis but failed to complete tail resorption and eventually died (Fig. 3) (Paul, Fu, et al., 2005). These phenotypes resemble those of Xenopus tropicalis animals with both TR genes knocked out (Shi, 2021; Shibata, Wen, et al., 2020), highlighting the importance of liganded TR to recruit coactivators to ensure proper rate of metamorphic progression and complete resorption of larval tissues.
Since F-dnSRC3 is expected to compete against the binding of all TR-interacting coactivators, not just SRCs, by liganded TR, the transgenic studies with F-dnSRC3 suggest but do not prove that SRC-containing complexes are critical for metamorphosis. On the other hand, a separate transgenic study with a dominant negative form of p300, which binds to SRC to form a large coactivator complex, provided strong evidence for an essential role of SRC-containing complexes during Xenopus laevis metamorphosis (Paul et al., 2007). This dominant negative p300 contained only the domain that binds to SRCs. It can bind to SRCs but does not affect the direct binding of liganded TR to any coactivators, including SRC3. However, the lack of other domains in p300 prevents the formation of a functional SRC-containing coactivator complex when the dominant negative p300 binds to SRCs. When this dominant negative p300 was overexpressed in transgenic Xenopus laevis tadpoles, it inhibited the recruitment of endogenous wild type p300 to T3-target promoters by liganded TR and reduced local histone acetylation and gene activation (Paul et al., 2007). More importantly, it also delayed/inhibited both T3-induced and natural metamorphosis, resembling the effects of the transgenic expression of F-dnSRC3. These observations indicate that coactivator complexes containing SRC-p300 are essential for the control of amphibian metamorphosis by liganded TR.
Studies on yet another component of the SRC-p300 complexes, PRMT1, a histone arginine methyltransferase, provides further support for the critical role of such complexes in TR function during metamorphosis. PRMT1 was found to be recruited by TR in a T3-dependent manner to T3 target genes during Xenopus laevis metamorphosis (Matsuda et al., 2009). More importantly, transgenic overexpression of wild type PRMT1 enhanced TR function, including increased binding of TR to endogenous target genes and increased gene activation by T3 (Matsuda et al., 2009). It also accelerated metamorphic progression (Matsuda et al., 2009). These findings thus support a role of SRC-containing coactivator complexes in regulating the rate of metamorphosis by liganded TR.
With the development of gene-editing technologies that can knockout genes in Xenopus laevis and tropicalis (Lei et al., 2013; Shi et al., 2015; Wang et al., 2015), it has become possible to investigate the role of endogenous cofactors during metamorphosis. Indeed, the SRC3 gene was recently knocked out in Xenopus tropicalis by using CRISPR-mediated gene knockout technology (Tanizaki, Bao, Shi, & Shi, 2021). Consistent with SRC3 being a histone acetyltransferase, knocking SRC3 resulted in a reduction in overall histone acetylation in tadpoles (Fig. 4). Interestingly, removing both copies of the endogenous SRC3 gene had little effect on either the external morphology of the animals or overall developmental rate, with all three genotypes (wild type, SRC heterozygous and homozygous) completing metamorphosis (when tail is completely resorbed) at similar ages (Tanizaki, Bao, et al., 2021). On the other hand, there was a delay in the remodeling of the animal intestine, where SRC3 is upregulated during metamorphosis (Paul & Shi, 2003), accompanied by reduced expression of T3 target genes in the intestine at the climax of metamorphosis (Fig. 4) (Tanizaki, Bao, et al., 2021). Furthermore, when premetamorphic tadpoles were treated with physiological concentration of T3 to induce precocious metamorphosis, the SRC3 knockout caused a delay as reflected by the morphology of the hindlimbs, which is one of the first and the most T3-sensitive morphological changes during metamorphosis (Fig. 4). In addition, intestinal remodeling induced by T3 was also delayed/inhibited by the SRC3 knockout, including reduced apoptosis of larval epithelial cells and adult intestinal stem cell proliferation (Fig. 4) (Tanizaki, Bao, et al., 2021). Thus, endogenous SRC3 is important for temporal control of metamorphosis by functioning as a TR coactivator.
Fig. 4.
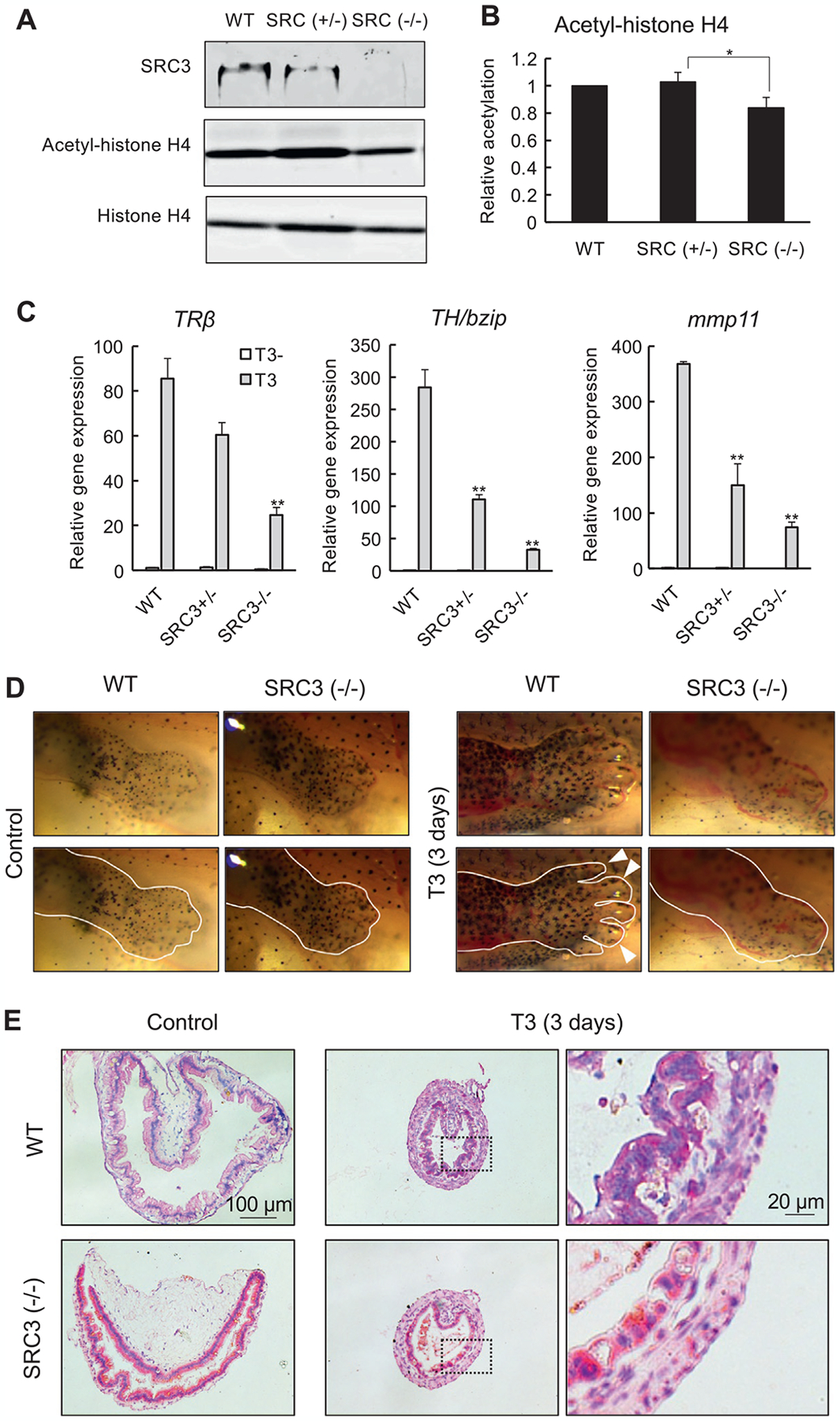
SRC3 knockout reduces histone H4 acetylation and inhibits T3-induced metamorphosis. (A)/(B) Western blots showing that homozygous SRC3 knockout tadpoles have no detectable SRC3 protein and reduced histone H4 acetylation. Total protein was isolated from whole body of wild type (WT), heterozygous (SRC3+/−), and compound heterozygous (functionally homozygous, SRC3−/−) SRC3 mutant tadpoles at stage 46 and subjected to Western blot analyses with antibodies against SRC3, acetyl-histone H4, or histone H4 (A). The signal intensity of the bands was measured by image j and presented as the mean ± standard deviation of n = 3 (B). Statistical analysis: ANOVA one-way followed by Tukey analysis. *P <0.05. (C) SRC3 knockout reduces gene activation by T3 in the intestine. The intestine was isolated from wild type (WT), SRC3+/− and SRC3−/− tadpoles at stage 54 with or without 18 h T3 treatment before RNA isolation and qRT-PCR analysis of the indicated TR target genes. **P < 0.01 for T3 treated vs. WT intestine. (D) SRC knockout inhibits T3-induced limb metamorphosis. Wild type (WT) and SRC3 knockout (SRC3−/−) tadpoles at stage 54 were treated with 10 nM T3 for 3 days at 25°C and hindlimb region was photographed. The lower panels were the same as the top panels except the added white line marking the approximate boundary of the outer limb epithelium and/or the boundaries of the digits (white arrowheads indicate some of the digits of the wild type animal treated with T3). (E) Adult intestinal epithelium development is inhibited by SRC3 knockout. Cross-sections of the intestine in WT and SRC3−/− tadpoles as in (D) were stained with methyl green-pyronin Y (staining DNA blue and RNA red). Note that the adult epithelium folds began to form after 3 days of T3 treatment in WT but not SRC3−/− tadpoles. See Tanizaki, Bao, et al. (2021) for more details.
5. Conclusion
The biphasic nature of amphibian metamorphosis has long made it an excellent model to study postembryonic development in vertebrates, particularly regarding the roles of T3 and TRs in tissue maturation and remodeling. The recent advancement in molecular and genetic tools has further enhanced the value of the Xenopus model (Brown & Cai, 2007; Buchholz, 2015; Buchholz & Shi, 2018; Grimaldi, Buisine, Bilesimo, & Sachs, 2013; Shi, 2013b, 2021; Shi et al., 2012). Studies on amphibian metamorphosis has revealed dual involvement of TR in development, in the unliganded form, before sufficient T3 is available, to repress T3-inducible genes and thus prevent premature adult organ development, and later in the liganded state to activate target genes and ensure metamorphosis of different organs/tissues. Such metamorphic changes share many conserved features with postembryonic mammalian development (Buchholz & Shi, 2018; Sachs & Buchholz, 2017; Shi, 2021). Furthermore, the dependence of metamorphosis in everyone organ/tissue on T3 in anurans has made it possible to investigate the roles of TR-binding cofactors in metamorphosis. While many of the cofactors are involved in signaling by different hormones, no hormone other than T3 has such a necessary and sufficient effect on anuran metamorphosis, making it easy to correlate the effects of altering any cofactors on metamorphosis with the role of such cofactors in TR signaling. The studies on SRC3 during Xenopus metamorphosis have provided some of the strongest pieces of evidence for SRC-containing histone acetyltransferase complexes in the regulation of vertebrate development by any nuclear hormone receptors. Interestingly, inhibiting SRC-complex function via transgenic expression of a dominant negative SRC3 has much bigger effects than knocking out SRC3 during Xenopus metamorphosis, likely reflecting redundancies among SRC3 family members or among different TR-binding coactivators during Xenopus development. This appears to be quite similar to findings in mouse knockout studies. SRC3 knockout in mouse also has only mild effects, including retarded growth and development, and decreased reproductive function (Xu et al., 2000), and SRC1 knockout has even less effect (Xu et al., 1998). In contrast, double knockout of both SRC1 and SRC3 in mouse causes embryonic lethality (Chen, Liu, & Xu, 2010), highlighting SRC redundancies and compensation during development. Such findings support evolutional conservations in the function of SRC-containing histone acetyltransferase complexes and the validity of the anuran model for studying the roles of such complexes in postembryonic vertebrate development.
Acknowledgment
This research in the authors’ laboratory was supported by the intramural Research Program of NICHD, NIH.
References
- Blitz IL, Biesinger J, Xie X, & Cho KW (2013). Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis, 51, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, & Cai L (2007). Amphibian metamorphosis. Developmental Biology, 306, 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR (2015). More similar than you think: Frog metamorphosis as a model of human perinatal endocrinology. Developmental Biology, 408, 188–195. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia VS-C, Fu L, & Shi Y-B (2003). A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Molecular and Cellular Biology, 23, 6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, & Shi YB (2006). Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. General and Comparative Endocrinology, 145, 1–19. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, & Shi YB (2018). Dual function model revised by thyroid hormone receptor alpha knockout frogs. General and Comparative Endocrinology, 265, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, & Shi Y-B (2004). Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Molecular and Cellular Biology, 24, 9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulynko YA, & O’Malley BW (2011). Nuclear receptor coactivators: structural and functional biochemistry. Biochemistry, 50, 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LJ, & Baniahmad A (2000). Co-repressors 2000. The FASEB Journal, 14, 1876–1888. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, et al. (1996). Role of CBP/P300 in nuclear receptor signalling. Nature, 383, 99–103. [DOI] [PubMed] [Google Scholar]
- Chen JD, & Evans RM (1995). A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature, 377, 454–457. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, et al. (1997). Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell, 90, 569–580. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu Z, & Xu J (2010). The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Molecular Endocrinology, 24, 1917–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, et al. (1999). Regulation of transcription by a protein methyltransferase. Science, 284, 2174–2177. [DOI] [PubMed] [Google Scholar]
- Choi J, Ishizuya-Oka A, & Buchholz DR (2017). Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinology, 158, 1623–1633. [DOI] [PubMed] [Google Scholar]
- Choi J, Suzuki KI, Sakuma T, Shewade L, Yamamoto T, & Buchholz DR (2015). Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology, 156, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H, & Brown DD (2002). Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America, 99, 12230–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, et al. (2002). Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature, 415, 549–553. [DOI] [PubMed] [Google Scholar]
- Dodd MHI, & Dodd JM (1976). The biology of metamorphosis. In Lofts B (Ed.), Physiology of the amphibia (pp. 467–599). New York: Academic Press. [Google Scholar]
- Forrest D (1994). The erbA/thyroid hormone receptor genes in development of the central nervous system. Seminars in Cancer Biology, 5, 167–176. [PubMed] [Google Scholar]
- Fu L, Buchholz D, & Shi YB (2002). Novel double promoter approach for identification of transgenic animals: A tool for in vivo analysis of gene function and development of gene-based therapies. Molecular Reproduction and Development, 62, 470–476. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, & Frieden E (1981). Metamorphosis: A problem in developmental biology (2nd ed.). New York: Plenum Press. [Google Scholar]
- Gilbert LI, Tata JR, & Atkinson BG (1996). Metamorphosis: Post-embryonic reprogramming of gene expression in amphibian and insect cells. New York: Academic Press. [Google Scholar]
- Glass CK, & Rosenfeld MG (2000). The coregulator exchange in transcriptional functions of nuclear receptors. Genes & Development, 14, 121–141. [PubMed] [Google Scholar]
- Grimaldi AG, Buisine N, Bilesimo P, & Sachs LM (2013). High-throughput sequencing will metamorphose the analysis of thyroid hormone receptor function during amphibian development. Current Topics in Developmental Biology, 103, 277–303. [DOI] [PubMed] [Google Scholar]
- Grimaldi A, Buisine N, Miller T, Shi YB, & Sachs LM (2013). Mechanisms of thyroid hormone receptor action during development: lessons from amphibian studies. Biochimica et Biophysica Acta, 1830, 3882–3892. [DOI] [PubMed] [Google Scholar]
- Gudernatsch JF (1912). Feeding experiments on tadpoles. I. The influence of specific organs given as food on growth and differentiation: a contribution to the knowledge of organs with internal secretion. Arch Entwicklungsmech Org, 35, 457–483. [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, & Shiekhattar R (2000). A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes & Development, 14, 1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Havis E, Sachs LM, & Demeneix BA (2003). Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports, 4, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Kohli K, Trivedi A, Johnson DL, & Stallcup MR (1996). GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proceedings of the National Academy of Sciences of the United States of America, 93, 4948–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, et al. (1995). Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature, 377, 397–404. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, & Lazar MA (2003). The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Molecular and Cellular Biology, 23, 5122–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Sachs LM, Rouse N, Wade PA, & Shi YB (2001). Multiple N-CoR complexes contain distinct histone deacetylases. The Journal of Biological Chemistry, 276, 8807–8811. [DOI] [PubMed] [Google Scholar]
- Jones PL, & Shi Y-B (2003). N-CoR-HDAC corepressor complexes: Roles in transcriptional regulation by nuclear hormone receptors. In Workman JL (Ed.), Current Topics in Microbiology and Immunology: Protein Complexes that Modify Chromatin (pp. 237–268). Berlin: Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- Kanamori A, & Brown DD (1992). The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. The Journal of Biological Chemistry, 267, 739–745. [PubMed] [Google Scholar]
- Koh SS, Chen DG, Lee YH, & Stallcup MR (2001). Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. The Journal of Biological Chemistry, 276, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Kroll KL, & Amaya E (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development, 122, 3173–3183. [DOI] [PubMed] [Google Scholar]
- Laudet V, & Gronemeyer H (2002). The nuclear receptor FactsBook. San Diego: Academic Press. [Google Scholar]
- Lazar MA (1993). Thyroid hormone receptors: multiple forms, multiple possibilities. Endocrine Reviews, 14, 184–193. [DOI] [PubMed] [Google Scholar]
- Lei Y, Guo X, Deng Y, Chen Y, & Zhao H (2013). Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell & Bioscience, 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, et al. (2012). Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proceedings of the National Academy of Sciences of the United States of America, 109, 17484–17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup J, & Buscaglia M (1977). La triiodothyronine: hormone de la métamorphose des amphibiens. CR Academy of Sciences, 284, 2261–2263. [Google Scholar]
- Li H, Gomes PJ, & Chen JD (1997). RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proceedings of the National Academy of Sciences of the United States of America, 94, 8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Q, Yoon HG, Huang ZQ, Strahl BD, Allis CD, et al. (2002). Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Molecular and Cellular Biology, 22, 5688–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, O’Malley BW, & Wong J (2000). p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Molecular and Cellular Biology, 20, 2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, et al. (2000). Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. The EMBO Journal, 19, 4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang S, & Amaya E (2016). The cellular and molecular mechanisms of tissue repair and regeneration as revealed by studies in Xenopus. Regeneration, 3, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LN, Vivien CJ, Girardot F, Péricard L, Scerbo P, Palmier K, et al. (2019). Stage-dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability. Proceedings of the National Academy of Sciences of the United States of America, 116, 3614–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Hasebe T, & Shi Y-B (2009). Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Molecular and Cellular Biology, 29, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Fujimoto K, Fu L, & Shi Y-B (2012). Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology, 153, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, et al. (2009). Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Molecular Endocrinology, 23, 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S, Tsubota T, Sakane Y, Kume S, Sakamoto N, Obara M, et al. (2014). Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nature Communications, 5, 5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tanizaki Y, Luu N, Zhang H, & Shi YB (2020). Comprehensive RNA-Seq analysis of notochord-enriched genes induced during Xenopus tropicalis tail resorption. General and Comparative Endocrinology, 287, 113349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tazawa I, & Shi YB (2019). A unique role of thyroid hormone receptor beta in regulating notochord resorption during Xenopus metamorphosis. General and Comparative Endocrinology, 277, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tazawa I, & Yaoita Y (2018). Thyroid Hormone Receptor alpha- and beta-Knockout Xenopus tropicalis Tadpoles Reveal Subtype-Specific Roles During Development. Endocrinology, 159, 733–743. [DOI] [PubMed] [Google Scholar]
- Nakajima K, & Yaoita Y (2003). Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Developmental Dynamics, 227, 246–255. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, & Grainger RM (2013). Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis, 51, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, & Faber J (1965). Normal table of Xenopus laevis. Amsterdam: North Holland Publishing. [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, & Nakatani Y (1996). The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell, 87, 953–959. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Malovannaya A, & Qin J (2012). Minireview: nuclear receptor and coregulator proteomics- -2012 and beyond. Molecular Endocrinology, 26, 1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onate SA, Tsai SY, Tsai MJ, & O’Malley BW (1995). Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science, 270, 1354–1357. [DOI] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, & Shi Y-B (2005). Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. The Journal of Biological Chemistry, 280, 27165–27172. [DOI] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, & Shi Y-B (2007). SRC-p300 coactivator complex is required for thyroid hormone induced amphibian metamorphosis. The Journal of Biological Chemistry, 282, 7472–7481. [DOI] [PubMed] [Google Scholar]
- Paul BD, Fu L, Buchholz DR, & Shi Y-B (2005). Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Molecular and Cellular Biology, 25, 5712–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, & Shi Y-B (2003). Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis. Cell Research, 13, 459–464. [DOI] [PubMed] [Google Scholar]
- Perissi V, Jepsen K, Glass CK, & Rosenfeld MG (2010). Deconstructing repression: evolving models of co-repressor action. Nature Reviews. Genetics, 11, 109–123. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Damjanovski S, & Shi Y-B (1997). Both thyroid Hormone and 9-cis Retinoic Acid receptors are Required to Efficiently mediate the Effects of Thyroid Hormone on Embryonic Development and Specific Gene Regulation in Xenopus laevis. Molecular and Cellular Biology, 17, 4738–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM (2015). Unliganded thyroid hormone receptor function: amphibian metamorphosis got TALENs. Endocrinology, 156, 409–410. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Amano T, Rouse N, & Shi YB (2001). Involvement of histone deacetylase at two distinct steps in gene regulation during intestinal development in Xenopus laevis. Developmental Dynamics, 222, 280–291. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Amano T, & Shi YB (2001). An essential role of histone deacetylases in postembryonic organ transformations in Xenopus laevis. International Journal of Molecular Medicine, 8, 595–601. [DOI] [PubMed] [Google Scholar]
- Sachs LM, & Buchholz DR (2017). Frogs model man: In vivo thyroid hormone signaling during development. Genesis, 55. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Damjanovski S, Jones PL, Li Q, Amano T, Ueda S, et al. (2000). Dual functions of thyroid hormone receptors during Xenopus development. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 126, 199–211. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, & Shi Y-B (2002). N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Molecular and Cellular Biology, 22, 8527–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, & Shi Y-B (2000). Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. PNAS, 97, 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane Y, Iida M, Hasebe T, Fujii S, Buchholz DR, Ishizuya-Oka A, et al. (2018). Functional analysis of thyroid hormone receptor beta in Xenopus tropicalis founders using CRISPR-Cas. Biology Open, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Buchholz DR, Paul BD, & Shi Y-B (2007). A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mechanisms of Development, 124, 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, & Brown DD (2003). Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. Proceedings of the National Academy of Sciences of the United States of America, 100, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, & Brown DD (2001). Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. PNAS, 98, 10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard HM, Harries JC, Hussain S, Bevan C, & Heery DM (2001). Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1. Molecular and Cellular Biology, 21, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B (1999). Amphibian Metamorphosis: From morphology to molecular biology. New York: John Wiley & Sons, Inc. [Google Scholar]
- Shi YB (2013a). Current Topics in Developmental Biology. Animal metamorphosis. Preface. Current Topics in Developmental Biology, 103, xv–xvi. [DOI] [PubMed] [Google Scholar]
- Shi YB (2013b). Unliganded thyroid hormone receptor regulates metamorphic timing via the recruitment of histone deacetylase complexes. Current Topics in Developmental Biology, 105, 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB (2021). Life without thyroid hormone receptor. Endocrinology, 162(bqab028), 021–012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB, Matsuura K, Fujimoto K, Wen L, & Fu L (2012). Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell & Bioscience, 2, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Wang F, Cui Y, Liu Z, Guo X, Zhang Y, et al. (2015). Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis. The FASEB Journal, 29, 4914–4923. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Wong J, Puzianowska-Kuznicka M, & Stolow MA (1996). Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thyroid hormone and its receptors. BioEssays, 18, 391–399. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Yaoita Y, & Brown DD (1992). Genomic organization and alternative promoter usage of the two thyroid hormone receptor ß genes in Xenopus laevis. The Journal of Biological Chemistry, 267, 733–788. [PubMed] [Google Scholar]
- Shibata Y, Tanizaki Y, & Shi YB (2020). Thyroid hormone receptor beta is critical for intestinal remodeling during Xenopus tropicalis metamorphosis. Cell & Bioscience, 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Tanizaki Y, Zhang H, Lee H, Dasso M, & Shi YB (2021). Thyroid hormone receptor is essential for larval epithelial apoptosis and adult epithelial stem cell development but not adult intestinal morphogenesis during Xenopus tropicalis metamorphosis. Cell, 10, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Wen L, Okada M, & Shi YB (2020). Organ-specific requirements for thyroid hormone receptor ensure temporal coordination of tissue-specific transformations and completion of xenopus metamorphosis. Thyroid, 30, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MD, Li J, & Wong J (2005). Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Molecular and Cellular Biology, 25, 2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D, Tomita A, Shi YB, & Wong J (2006). Chromatin immunoprecipitation for studying transcriptional regulation in Xenopus oocytes and tadpoles. Methods in Molecular Biology, 322, 165–181. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, & Chin WW (1997). TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. The Journal of Biological Chemistry, 272, 27629–27634. [DOI] [PubMed] [Google Scholar]
- Tanizaki Y, Bao L, Shi B, & Shi YB (2021). A role of endogenous histone acetyltransferase steroid hormone receptor coactivator 3 in thyroid hormone signaling during Xenopus intestinal metamorphosis. Thyroid, 31, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki Y, Shibata Y, Zhang H, & Shi YB (2021). Analysis of thyroid hormone receptor alpha-knockout tadpoles reveals that the activation of cell cycle program is involved in thyroid hormone-induced larval epithelial cell death and adult intestinal stem cell development during Xenopus tropicalis metamorphosis. Thyroid, 31, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki Y, Shibata Y, Zhang H, & Shi YB (2022). Thyroid hormone receptor alpha controls the hind limb metamorphosis by regulating cell proliferation and wnt signaling pathways in Xenopus tropicalis. International Journal of Molecular Sciences, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizaki Y, Zhang H, Shibata Y, & Shi YB (2022). Thyroid hormone receptor alpha controls larval intestinal epithelial cell death by regulating the CDK1 pathway. Communications Biology, 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR (1993). Gene expression during metamorphosis: An ideal model for post-embryonic development. BioEssays, 15, 239–248. [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, & Shi Y-B (2004). Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Molecular and Cellular Biology, 24, 3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJ, Tini M, Vivat V, Chambon P, & Gronemeyer H (1998). The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. The EMBO Journal, 17, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJ, Zechel C, Chambon P, & Gronemeyer H (1996). TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. The EMBO Journal, 15, 3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, & Brown DD (1993). Thyroid hormone-induced gene expression program for amphibian tail resorption. The Journal of Biological Chemistry, 268, 16270–16278. [PubMed] [Google Scholar]
- Wang X, Matsuda H, & Shi Y-B (2008). Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology, 149, 5610–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Shi Y-B (2021). Evolutionary divergence in tail regeneration between Xenopus laevis and Xenopus tropicalis. Cell & Bioscience, 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shi Z, Cui Y, Guo X, Shi YB, & Chen Y (2015). Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell & Bioscience, 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, & Shi YB (2015). Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology, 156, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, & Shi YB (2016). Regulation of growth rate and developmental timing by Xenopus thyroid hormone receptor alpha. Development, Growth & Differentiation, 58, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shibata Y, Su D, Fu L, Luu N, & Shi Y-B (2017). Thyroid hormone receptor α controls developmental timing and regulates the rate and coordination of tissue specific metamorphosis in Xenopus tropicalis. Endocrinology, 158, 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, & Shi Y-B (1995). Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. The Journal of Biological Chemistry, 270, 18479–18483. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, & O’Malley BW (2000). The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is requried for normal growth, puberty, female reproductive function, and mammary gland development. Proceedings of the National Academy of Sciences of the United States of America, 97, 6379–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, & O’Malley BW (1998). Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science, 279, 1922–1925. [DOI] [PubMed] [Google Scholar]
- Yakushiji N, Yokoyama H, & Tamura K (2009). Repatterning in amphibian limb regeneration: A model for study of genetic and epigenetic control of organ regeneration. Seminars in Cell & Developmental Biology, 20, 565–574. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, & Brown DD (1990). A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes & Development, 4, 1917–1924. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Shi YB, & Brown DD (1990). Xenopus laevis alpha and beta thyroid hormone receptors. Proceedings of the National Academy of Sciences of the United States of America, 87, 7090–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM (2001). Physiological and molecular basis of thyroid hormone action. Physiological Reviews, 81, 1097–1142. [DOI] [PubMed] [Google Scholar]
- Yen PM (2015). Unliganded TRs regulate growth and developmental timing during early embryogenesis: Evidence for a dual function mechanism of TR action. Cell & Bioscience, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H-G, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, et al. (2003). Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. The EMBO Journal, 22, 1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Cherone JM, Doyon Y, Ankoudinova I, Faraji FM, Lee AH, et al. (2011). Efficient targeted gene disruption in the soma and germ line of the frog Xenopus tropicalis using engineered zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America, 108, 7052–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kalkum M, Chait BT, & Roeder RG (2002). The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Molecular Cell, 9, 611–623. [DOI] [PubMed] [Google Scholar]
- Zhang J, & Lazar MA (2000). The mechanism of action of thyroid hormones. Annual Review of Physiology, 62, 439–466. [DOI] [PubMed] [Google Scholar]


