Abstract
Deficits in memory performance have been linked to a wide range of neurological and neuropsychiatric conditions. While many studies have assessed the memory impacts of individual conditions, this study considers a broader perspective by evaluating how memory recall is differentially associated with nine common neuropsychiatric conditions using data drawn from 55 international studies, aggregating 15,883 unique participants aged 15–90. The effects of dementia, mild cognitive impairment, Parkinson’s disease, traumatic brain injury, stroke, depression, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, and bipolar disorder on immediate, short-, and long-delay verbal learning and memory (VLM) scores were estimated relative to matched healthy individuals. Random forest models identified age, years of education, and site as important VLM covariates. A Bayesian harmonization approach was used to isolate and remove site effects. Regression estimated the adjusted association of each clinical group with VLM scores. Memory deficits were strongly associated with dementia and schizophrenia (p < 0.001), while neither depression nor ADHD showed consistent associations with VLM scores (p > 0.05). Differences associated with clinical conditions were larger for longer delayed recall duration items. By comparing VLM across clinical conditions, this study provides a foundation for enhanced diagnostic precision and offers new insights into disease management of comorbid disorders.
Keywords: verbal learning, memory, dementia, depression, Parkinson’s disease, schizophrenia, stroke, traumatic brain injury, bipolar disorder, attention-deficit/hyperactivity disorder
1. Introduction
Memory performance is a core cognitive function and a key determinant of overall health and quality of life [1,2]. Numerous neurological, neurodevelopmental, and neuropsychiatric disorders have been linked to memory deficits, including, but not limited to, dementia [3], mild cognitive impairment (MCI) [4], Parkinson’s disease (PD) [5], traumatic brain injury (TBI) [6], stroke [7], depression [8], attention-deficit/hyperactivity disorder (ADHD) [9], schizophrenia [10], and bipolar disorder (BD) [11]. However, comprehensive evaluations of cognitive measures have typically been limited to meta-analyses of distinct clinical groups. Little work has directly assessed the relative impact of various neurological and neuropsychiatric disorders on verbal learning and memory (VLM) within one unified framework, in part due to the complexities of examining heterogeneous clinical populations with distinct research priorities and treatment gaps [12,13]. Understanding the differential impact(s) of clinical conditions on VLM could yield tangible benefits, including enhanced diagnostic precision and new insights into disease management for individuals with comorbid neurological and neuropsychiatric disorders [14,15].
1.1. Neurological Disorders and VLM
This study aggregated assessments of VLM based on single-word list learning tasks. VLM assessments involve immediate, short-term, and long-term memory items, which reflect different temporal stages or types of memory processing related to the encoding, storage, and retrieval of verbal information [16]. These stages are governed by neurological mechanisms with varying susceptibility to clinical conditions.
This study focused on VLM specifically because they are directly relevant to many everyday activities, such as conversation and instruction, and they are also sensitive measures of the cognitive impact of many diseases. Unlike disease-specific measures, VLM tools are also versatile and can be used with validity across a range of distinct clinical conditions. For example, Alzheimer’s disease and related dementias (AD/RD) have profound and extensively studied effects on various aspects of VLM [17], particularly impaired encoding abilities [18]. Amnestic MCI, which can be a prodromal phase of AD/RD [19], also exerts well-documented effects on VLM [20]. Yet, even within these well-characterized diseases, complex and heterogeneous memory performance is often observed across individuals and disease subtypes [21,22], and some individuals with AD have relatively preserved memory in the early disease stages [23]. Dementia subtypes, such as vascular dementia (VaD) and frontotemporal dementia (FTD) can demonstrate variation in VLM across affected individuals [24]. Some individuals with FTD may have difficulties with the storage, consolidation, and later recall of verbal information [25,26], whereas VaD associations with VLM can depend on the extent and location of vascular pathology [27]. PD and related neurodegenerative disorders often present with memory difficulties in the later stages of disease progression [28], while some studies have found VLM changes in newly diagnosed PD patients [29].
TBI along the severity spectrum has been linked to cognitive changes, including VLM deficits [30,31]. Research also suggests an increased risk for dementia among TBI survivors [32]. A wide range of conditions can emerge and persist after TBI, although the cognitive effects of TBI depend on injury severity and recency [33,34]. VLM impairments also occur in other acquired brain injury populations, such as stroke, in which various aspects of memory are affected depending on the location and severity of injury [35].
1.2. Neuropsychiatric Disorders and VLM
Despite intensive research, the precise impacts of various common neuropsychiatric disorders on cognitive functioning remain unclear [36]. For example, prior work found that memory deficits were not consistently associated with depression [37], while other studies found that major depressive disorder (MDD) and depression-related symptoms negatively affect memory [38]. These findings are further complicated by the comorbid relationship between depression and other conditions that may have confounding effects on memory performance, such as TBI [39]. Impairments in working memory are a core feature of ADHD, and individuals with ADHD may experience reduced performance on long-term memory tasks, while short-term memory deficits, when detected, tend to be less pronounced [40,41].
Schizophrenia is associated with decreased cognitive performance, and patients with schizophrenia consistently show substantial deficits in VLM, which has been supported by imaging studies [42,43,44,45]. Schizophrenia has also been associated with disproportionate episodic memory impairments [46]. Similarly, impaired relational memory (recall of concept and object associations) is considered a core deficit in schizophrenia [47] and has been linked to the early stages of psychosis [48]. BD has also been associated with VLM impairments [49], particularly in late-stage BD [50], which is likely due to its relation to the number of previous manic episodes and psychiatric hospitalizations [51].
The heterogeneous pathologies, disease courses, and consequences of these neurological and neuropsychiatric disorders strongly suggest a differential effect among patient populations on VLM scores. To date, comprehensive evaluations of cognitive measures have typically been limited to meta-analyses of single clinical groups. This study considered a broader perspective by quantifying item-level VLM disruptions across a range of common neurological and neuropsychiatric disorders in a large sample using a standardized analysis. This was achieved by taking advantage of new methods for the harmonization of distinct VLM assessments [52], as well as methods to isolate and remove the influence of site on VLM scores. We hypothesized that neurological and neuropsychiatric disorders would display differential relationships with VLM performance. Score declines per group were further broken out into age- and sex/gender-stratified models. By aggregating a large sample of item-level assessments drawn from diverse settings, this study reveals both absolute and comparative insights across neurological and neuropsychiatric disorders.
2. Materials and Methods
2.1. Data Source
Following prior work [53], we aggregated secondary de-identified data, drawing from 55 studies of adults contributed by collaborators in the Psychiatric Genomics Consortium (PGC), the Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA) working groups (including the ENIGMA Brain Injury working group), and the Long-term Impact of Military-relevant Brain Injury Consortium–Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC). A world map of the locations of participating institutions is shown in Figure 1.
Figure 1.
World map showing the locations of participating collaborator institutions (triangles). Details for each sub-study are provided in Supplementary Table S1.
2.2. Clinical Disorders
The inclusion of clinical disorders was based on available samples with clear diagnostic classifications. Comprehensive details for the exclusion and inclusion criteria for each group per site are detailed in Supplementary Table S1, including pharmacological considerations. AD/RD, MCI, PD, TBI, stroke, MDD, ADHD, schizophrenia/psychosis, and BD were included. Each clinical condition group was aggregated by including participants who had the same primary diagnoses across studies. For example, the ‘Schizophrenia/Psychosis’ group was defined to include any participants who had a primary diagnosis listed as one of the following eight terms: Schizophrenia, Psychosis, Clinical High Risk for Psychosis, Early Course Schizophrenia, First Episode Psychosis, SCZ Patient, SZ, Schizoaffective, and Ultra High Risk of Psychosis. Similarly, TBI included individuals with primary diagnosis terms that included Concussion, mild TBI, moderate/severe TBI, and penetrating TBI. Controls were defined as those with a primary classification listed as one of the following: Healthy control, Control, Unexposed, Negative, HN controls, and HC. This process excluded 727 individuals with ambiguous diagnoses.
2.3. Measures
The California Verbal Learning Test, 2nd edition (CVLT-II) [54] is a validated tool for assessing VLM performance. The CVLT-II uses a 16-word list drawn from 4 categories that is presented verbally over 5 consecutive learning trials. The CVLT-II includes an assessment of verbal learning using immediate (no delay), short- (following a distractor task), and long- (20 min) delay free verbal recall trials and a distractor list. Free recall scores range from 0 to 16 on each of the five immediate recall/learning trials, as well as the short- and long-delay trials. A total score across the five learning trials (Trials 1–5) ranges from 0 to 80 words recalled. Raw scores from the first immediate recall trial (T1), Trials 1–5, short-delay free recall (SDFR), and long-delay free recall (LDFR) were chosen as the four primary outcomes of interest. While some studies administered measures at multiple visits, only data collected during initial assessments were included.
Prior to analysis, we performed a harmonization of scores across the CVLT-II, Rey Auditory Verbal Learning Task (RAVLT) [55], and Hopkins Verbal Learning Task–Revised (HVLT-R) [56,57] using a previously validated score conversion table tool (available at https://verbal-learning.halfpipe.group, accessed on 25 June 2024) [53]. This process ensured assessments were aligned to CVLT-II item scales; therefore, all measures were harmonized prior to analysis. To briefly describe the two underlying measures, the HVLT-R is a validated and relatively short measure of VLM deficits. The HVLT-R uses 12-word lists, which are drawn from three semantic categories, and it uses a small (N = 24) total pool of words for scoring. HVLT-R does not use a distractor list for immediate recall, and it does not assess short-delay recall performance. The HVLT-R has three consecutive learning trials. The RAVLT is a validated measure of VLM deficit. The RAVLT draws from random, semantically unrelated words and employs a 15-word list length and a distractor list, along with short- and 30-min delay recall trials.
2.4. Data Processing
Datasets from all sources were cleaned, standardized, and aggregated. Nearest neighbor imputation was used to infer missing data, and overall missingness was low (< 5%). In a study involving many data sources, individual source-specific effects can reduce statistical power and lead to inaccurate results. Therefore, following prior work, an empirical Bayes method was used to isolate and remove source-specific effects from scores while preserving covariate effects [53]. Using harmonization, we can assure uniformity above that of any single study by aggregating and comparing many datasets. This process allowed us to identify and remove biases inherent in each single data source, e.g., due to the influence of specific inclusion criteria on scores. Only controls were used to estimate site effects.
2.5. Design
Participants were assigned to clinical groups by primary clinical diagnosis. Imbalance across clinical groups was mitigated using a two-step design that included both matching and covariate adjustment [58]. Using a nearest neighbor algorithm, each clinical group was matched 1:1 with healthy controls using covariates of age, sex/gender, years of education, and geographic region. This was required because the total control group was not representative of all clinical groups. For example, controls were on average younger than participants in the AD/RD cohort. After matching, linear regression was used to estimate group differences in T1, Trials 1–5, SDFR, and LDFR scores, adjusting for covariates.
2.6. Covariates
Covariates included age, sex/gender, years of education, geographical region, language of assessment, and site/study source. The raw demographic information available varied across sites. For example, some studies recorded sex while others recorded gender; these data were aggregated into a single sex/gender variable. The data included eight languages (Czech, English, German, Italian, Korean, Norwegian, Portuguese, and Spanish) that were tested for relative impact on scores. Prior research in this sample found no significant racial differences associated with VLM scores [53], so we elected to not include race variables in our analysis.
2.7. Statistical Analysis
All analyses were conducted in Python 3. Geographical region, language of assessment, and site are highly interrelated factors. Therefore, to preliminarily screen for important covariates that may require subsequent adjustment, random forest models were used due to their robustness against collinearity [59]. The sklearn python package was used to estimate variable importances with random forest models. Linear regression was used to test for significance across groups after adjusting for the covariates identified with high feature importances in random forest models. Percentage differences in words recalled between clinical groups and matched controls were calculated by converting adjusted coefficients to percentages of words recalled. In age- and sex/gender-stratified models, confidence bounds for significance were estimated by random sampling and averaging effects, comparing within control groups.
3. Results
The total dataset consisted of 15,883 participants drawn from 55 sites; 36.8% were female, and the median age was 42 years. At the time of assessment, the cohort had received an average of 13.7 ± 3.0 years of education. As shown in Table 1, the clinical groups with the largest sample sizes were TBI (n = 4867), schizophrenia (n = 1962), and MDD (n = 1063), while the MCI (n = 233) and ADHD (n = 126) groups had the smallest sample sizes. Controls (n = 5713) were drawn from 43 out of 55 studies. Table 1 shows clear trends across groups, but additional adjustments were required. The AD/RD, MCI, and PD clinical groups tended to be older and had a higher percentage of females. The TBI and SZ groups were younger and included more males. The TBI, BD, and ADHD groups were the most educated clinical groups, on average.
Table 1.
Characteristics and average CVLT-II scores for the nine clinical groups and controls. Average VLM performance of each group (i.e., mean score) is shown as raw scores and as percentage differences with respect to (% w.r.t.) the control group.
| Clinical Group | Control | AD/RD | MCI | PD | TBI | Stroke | MDD | ADHD | SZ | BD |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 5713 | 276 | 233 | 628 | 4867 | 273 | 1063 | 126 | 1962 | 742 |
| # Studies | 43 | 5 | 4 | 3 | 23 | 3 | 7 | 3 | 19 | 12 |
| Age, % | ||||||||||
| 16–35 | 43.5 | 0.0 | 0.9 | 0.0 | 25.7 | 3.3 | 49.8 | 56.3 | 64.6 | 40.5 |
| 35–65 | 42.1 | 9.1 | 12.9 | 36.3 | 67.0 | 31.1 | 49.2 | 42.9 | 34.9 | 56.5 |
| 65+ | 14.4 | 90.1 | 86.2 | 63.7 | 7.3 | 65.6 | 1.0 | 0.8 | 0.5 | 3.0 |
| Sex/Gender, % | ||||||||||
| Male | 49.0 | 38.8 | 49.8 | 32.6 | 90.3 | 71.1 | 48.0 | 48.4 | 61.7 | 42.7 |
| Female | 51.0 | 61.2 | 50.2 | 67.4 | 9.7 | 28.9 | 62.0 | 51.6 | 38.3 | 57.3 |
| Region, % | ||||||||||
| Americas | 54.8 | 79.7 | 70.8 | 0.0 | 100.0 | 0.0 | 3.2 | 40.5 | 17.9 | 39.2 |
| Europe | 41.5 | 18.1 | 21.5 | 37.7 | 0.0 | 51.3 | 96.8 | 58.7 | 79.6 | 60.8 |
| Asia | 3.7 | 2.2 | 7.7 | 62.3 | 0.0 | 58.7 | 0.0 | 0.8 | 2.5 | 0.0 |
| Education, % | ||||||||||
| Primary | 10.0 | 50.2 | 42.5 | 51.6 | 0.7 | 30.6 | 7.6 | 15.9 | 25.4 | 21.3 |
| Secondary | 25.3 | 34.0 | 37.8 | 18.1 | 27.0 | 26.4 | 46.1 | 22.2 | 25.4 | 27.8 |
| Tertiary | 64.7 | 15.6 | 19.7 | 30.3 | 72.3 | 43.0 | 46.3 | 61.9 | 42.0 | 50.9 |
| Mean score | ||||||||||
| T1 | 6.5 | 3.0 | 4.1 | 4.2 | 5.8 | 4.6 | 7.2 | 6.4 | 5.4 | 6.2 |
| % w.r.t. controls | 0% | −54% | −37% | −35% | −11% | −29% | 11% | −2% | −17% | −5% |
| Trials 1–5 | 52.4 | 21.6 | 31.7 | 29.4 | 47.7 | 41.3 | 55.8 | 53.2 | 43.8 | 48.9 |
| % w.r.t. controls | 0% | −59% | −40% | −44% | −9% | −21% | 6% | −2% | −16% | −7% |
| SDFR | 11.5 | 1.2 | 3.9 | 6.7 | 9.9 | 8.5 | 12.2 | 11.8 | 8.9 | 10.2 |
| % w.r.t. controls | 0% | −90% | −66% | −42% | −14% | −26% | 6% | −3% | −12% | −11% |
| LDFR | 11.8 | 1.2 | 3.8 | 6.4 | 10.2 | 8.9 | 12.8 | 12.2 | 9.1 | 10.7 |
| % w.r.t. controls | 0% | −90% | −68% | −46% | −14% | −25% | 8% | 3% | −23% | −9% |
AD/RD = Alzheimer’s disease and related dementias. MCI = mild cognitive impairment. PD = Parkinson’s disease. TBI = traumatic brain injury. MDD = major depressive disorder. ADHD = attention-deficit/hyperactivity disorder. SZ = schizophrenia/psychosis. BD = bipolar disorder. T1 = first immediate recall trial. SDFR = short-delay free recall. LDFR = long-delay free recall.
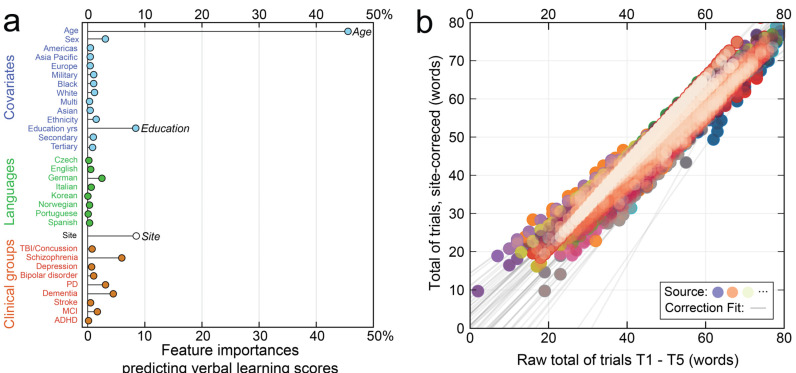
Determining the influence of language, study, and covariates on scores is challenging due to collinearity. To address this issue, Figure 2a shows a stem plot of random forest model feature importances predicting VLM scores averaged across items. The model achieved 80.7% explained variance, with age (45.6%), education (8.5%), and origin study (8.4%) estimated as the three most important features. By contrast, the eight language variables (Czech, English, German, Italian, Korean, Norwegian, Portuguese, and Spanish) were minimally predictive of scores. This analysis suggested that source correction followed by regression on covariates was sufficient to accurately estimate performance associations with clinical conditions. Correction for site effects was performed using ComBat-GAM [60], an empirical Bayes method that was specified to tolerate nonlinearities in score responses as a function of age. The fits used to correct for each site are shown as gray lines in Figure 2b. The harmonization process was performed for each item.
Figure 2.
Preliminary screening of covariates by random forest models. In (a), feature importances identified age, education, and site as the most important variables for adjustment. In (b), raw Trials 1–5 scores are plotted against Trials 1–5 scores after source correction (color indicates origin study). Gray lines show the fits used to correct for each site.
After site correction, linear regression was used to estimate covariates (Table 2). The model comparators used were: age, 16–35; sex/gender, male; geographic region, Americas; and education, primary level. Age-related differences in scores were significant across all VLM items (p < 0.001). Compared to those aged 16–35 years, the number of words recalled on both SDFR and LDFR declined by more than 20% for those aged 65+. Age-related differences were also significant for the immediate recall items, T1, and Trials 1–5 (p < 0.001). Women showed higher VLM performance across all measures compared to men, ranging from +2.9% to +7.6% (p < 0.001 for all items). Geographic region showed inconsistent associations and mixed significance across items. Education level was positively associated with VLM performance across all items (p < 0.001).
Table 2.
Blocked linear regressions estimating scores on immediate, short-, and long-delay verbal learning items for the whole cohort after site correction. Coefficients are shown as percentage associations with each item score.
| Regression | T1 | Trials 1–5 | SDFR | LDFR |
|---|---|---|---|---|
| N | 15,883 | 15,883 | 15,883 | 15,883 |
| Adj. R2 | 19.2% | 37.7% | 32.9% | 34.0% |
| Age, % (CI) | ||||
| 16–35 | – | – | – | – |
| 35–65 | −3.5 (−3.9, −3.1) † | −6.0 (−6.5, −5.5) † | −9.2 (−10.0, −8.5) † | −9.7 (−10.5, −8.9) † |
| 65+ | −8.2 (−8.9, −7.6) † | −13.6 (−14.4, −12.8) † | −21.8 (−23.1, −20.5) † | −22.2 (−23.5, −20.9) † |
| Sex/Gender, % (CI) | ||||
| Male | – | – | – | – |
| Female | 2.9 (2.5, 3.3) † | 4.9 (4.4, 5.3) † | 7.0 (6.2, 7.8) † | 7.6 (6.9, 8.4) † |
| Region, % (CI) | ||||
| Americas | – | – | – | – |
| Europe | −0.6 (−1.7, 0.5) | −7.0 (−8.3, −5.8) † | −9.1 (−11.2, −7.0) † | −8.5 (−10.6, −6.4) † |
| Asia | 1.3 (0.7, 1.8) † | 1.6 (1.0, 2.1) † | 0.7 (−0.3, 1.6) | 1.4 (0.4, 2.4) |
| Education, % (CI) | ||||
| Primary | – | – | – | – |
| Secondary | 3.2 (2.6, 3.8) † | 4.7 (3.9, 5.4) † | 6.2 (5.0, 7.5) † | 5.8 (4.6, 7.0) † |
| Tertiary | 4.5 (3.9, 5.1) † | 7.1 (6.4, 7.8) † | 10.5 (9.3, 11.6) † | 9.3 (8.1, 10.4) † |
T1 = immediate recall trial 1. Trials 1–5 = total score across immediate recall/learning trials 1–5. SDFR = short-delay free recall. LDFR = long-delay free recall. CI = confidence interval. † indicates significance at p < 0.001
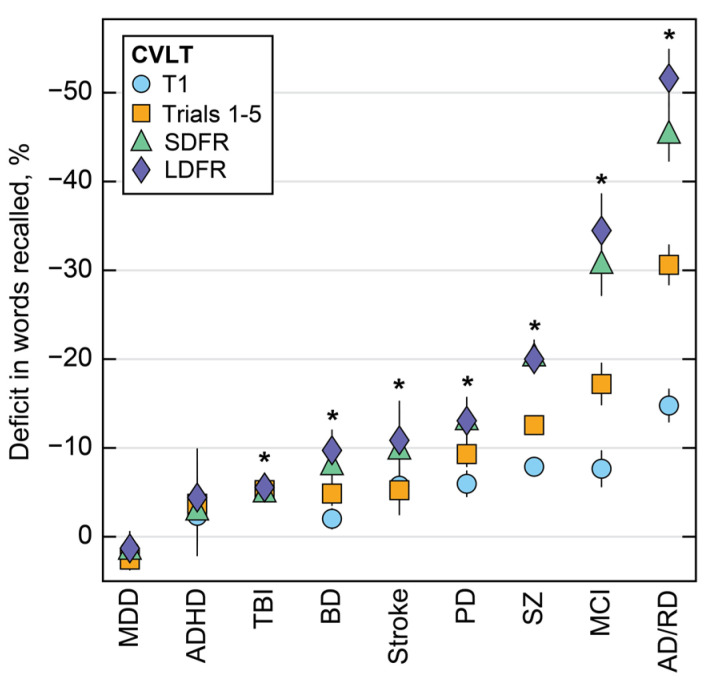
Percentage differences in words recalled between clinical groups and matched controls were estimated from regression coefficients (Figure 3). The disorder with the largest score difference relative to controls was AD/RD (T1: −14.8%, SDFR: −45.6%, LDFR: −51.6%), while ADHD and MDD showed the smallest effects overall on the percentage of words recalled. All clinical groups were significantly different from controls across all VLM items (p < 0.001), except for ADHD and MDD, which did not exhibit consistently different recall from matched controls (p > 0.05). The strength of association varied across items and delay durations. In Figure 3, a consistent pattern emerged where declines in LDFR (purple diamonds) exceeded T1 deficits (blue circles). This association was most pronounced for AD/RD, which exhibited a 37% difference between the percentage of words recalled on T1 and LDFR items compared to controls.
Figure 3.
Percentage changes in words recalled are shown for each clinical group relative to matched controls. Numerical data for this figure are available in Supplementary Table S2. MDD = major depressive disorder. ADHD = attention-deficit/hyperactivity disorder. TBI = traumatic brain injury. BD = bipolar disorder. PD = Parkinson’s disease. SZ = schizophrenia. MCI = mild cognitive impairment. AD/RD = Alzheimer’s disease and related dementias. T1 = immediate recall trial 1. Trials 1–5 = total score across immediate recall/learning trials 1–5. SDFR = short-delay free recall. LDFR = long-delay free recall. * significant at p < 0.01 for all four items.
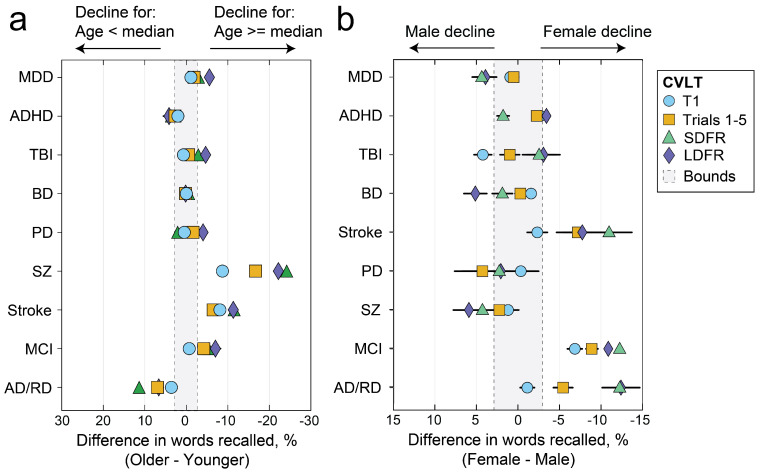
We additionally tested for sex/gender- and age-related differences in percentage change in VLM scores for each clinical group relative to matched controls. In Figure 4, declines for each clinical group relative to controls are shown with additional age and sex/gender stratification across older/younger and female/male groups. Median age per group was used as the stratification cutoff. Relative to matched controls, older patients with schizophrenia show greater declines than younger patients with schizophrenia. Females with stroke, MCI, and AD/RD showed worse declines than males with the same diagnoses.
Figure 4.
Age- (a) and sex/gender- (b) stratified percentage score differences across female/male and above/below median age groups relative to matched controls. MDD = major depressive disorder. ADHD = attention-deficit/hyperactivity disorder. TBI = traumatic brain injury. BD = bipolar disorder. PD = Parkinson’s disease. SZ = schizophrenia. MCI = mild cognitive impairment. AD/RD = Alzheimer’s disease and related dementias. T1 = immediate recall trial 1. Trials 1–5 = total score across immediate recall/learning trials 1–5. SDFR = short-delay free recall. LDFR = long-delay free recall.
4. Discussion
Neurological and neuropsychiatric disorders have heterogeneous impacts on cognitive functioning [12,13]. Even in clinical diseases with well-established mechanisms of memory disruption, appreciable variations have been observed across studies and populations [8,10,17,18,21,26,36,40,49]. Understanding the differential impacts of clinical conditions on VLM performance may have tangible benefits, including new insights into disease management for individuals with comorbid neurological concerns with compounding memory effects. Drawing from a large repository of 15,883 unique participant assessments aggregated across 55 studies from multiple international consortia, this study investigated the impact of nine common neurological and neuropsychiatric disorders on VLM. We used existing harmonization tools to align scores drawn from different geographical regions and VLM assessments within a single unified analysis.
Our findings suggest that some, but not all, conditions were associated with abnormalities in VLM, confirming our a priori hypothesis. A pattern emerged in which the impact of clinical conditions on scores tended to increase with recall delay duration. This association was most pronounced for AD/RD, which exhibited a 37% difference in words recalled between the immediate and long-delay assessments. These data recapitulate that LDFR is an effective differentiator of disorders, particularly for medial temporal lobe pathology and hippocampal-dependent disorders. Our findings revealed that AD/RD, MCI, and schizophrenia were most strongly associated with VLM differences relative to controls, even after adjusting for covariates, including age. Although AD/RD and MCI are broad diagnostic categories, both have profound and heterogeneous effects on memory. Complex and heterogeneous memory performance is often observed across individuals and disease subtypes, such as amnestic MCI and non-amnestic MCI, amongst others [21].
Participants with TBI of any severity scored 5.1–5.5% lower on the VLM compared to controls, and while AD/RD showed large differences across items of different delay duration, TBI was associated with a narrower range of deficits across items. In the current analyses, all TBI severities were grouped together, and findings could vary across injury severity.
Relative to controls, individuals with ADHD showed lower VLM scores on all items, but these differences were not consistently significant. Similarly, MDD effects were small (1–2%) compared to the associations of AD/RD and MCI (30–50%), and MDD was not consistently significantly associated with VLM. These mixed findings are consistent with the mixed literature on the impacts of MDD and ADHD on cognitive functioning [41].
Age and education have well-established associations with memory performance, and this study identified both as important covariates of VLM. Random forest models also identified origin site of assessment as the third most important determinant of VLM scores. Site effects cannot be observed or mitigated in single-site studies, but in this study, data were aggregated from a large number of studies so that harmonization models could isolate and remove individual site effects. In a secondary analysis stratifying clinical group associations with VLM by age and sex/gender, older patients with schizophrenia showed greater declines than younger patients with schizophrenia. Females with stroke, MCI, and AD/RD showed worse declines than males with the same diagnoses.
The strengths of this study include a large sample size of more than 15,000 participants drawn from 55 diverse international data sources of VLM assessment. Random forest models that are robust against collinearity were used to identify key covariates requiring further adjustment. A large and diverse sample of over 5000 healthy control participants facilitated a good comparison pool for each clinical group.
This study also had several limitations. While 13 countries and 8 languages were represented in our dataset, our data were skewed toward English-speaking samples from the Western hemisphere. Our ability to tease out the influence of disorders on VLM scores may also be confounded by unmeasured considerations, such as substance use disorder and other diseases that are highly comorbid in patients with neurocognitive disorders. We elected to frame the study using primary diagnosis groups and a matched design, but common comorbid concerns for TBI and depression, such as post-traumatic stress disorder, may contribute to the association between clinical conditions and VLM deficits. The effects of comorbidities on VLM is a standing limitation that would require deeper medical histories not consistently documented in each underlying study. Future work could explore whether there are meaningful interaction effects observed for patients with overlapping clinical conditions. While recognition test items are an important part of VLM testing, the harmonization of recognition memory indices was not implemented in existing harmonization models [53], and therefore, recognition memory items were not considered. Harmonization models, although previously validated, might introduce some bias for sources with low sample sizes. The data were aggregated from multiple sources with different instruments, settings, inclusion criteria, and assessment procedures [53]. However, a detailed understanding of study effects was not necessary to remove these effects in aggregate using Bayesian harmonization procedures. Data were largely drawn from studies of mild TBI, and the magnitude of associations with VLM may not reflect findings for more severe forms of TBI.
5. Conclusions
This study highlights the benefit of aggregating large sample sizes to determine how VLM deficits vary across the spectrum of neurological and neuropsychiatric disorders. Our findings suggest that some, but not all, conditions were associated with abnormalities in VLM. A pattern emerged suggesting that the impact of clinical conditions on scores tended to increase with recall delay duration. VLM performance was not consistently associated with a diagnosis of depression or ADHD on all items relative to matched controls, but it was significantly associated with all other conditions assessed. In a secondary analysis stratifying clinical group associations with VLM by age and sex/gender, older patients with schizophrenia showed greater declines than younger patients with schizophrenia. Females with stroke, MCI, and AD/RD showed worse declines than males with the same diagnoses. Beyond cognitive assessment, these findings may provide a means to identify novel diagnostic and mechanistic neuroimaging biomarkers of cognitive performance from large-scale, global open neuroscience initiatives with greater sensitivity and statistical power.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci14070669/s1, Table S1: Inclusion/exclusion criteria for each data source; Table S2: Deficit in words recalled for each clinical condition relative to matched controls. Refs. [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, E.K., S.W.L., H.M.L., P.-W.L., C.E., M.J.P., P.M.T., F.G.H., D.F.T., E.A.W. and E.L.D.; methodology, E.K. and P.-W.L.; software, E.K. and S.V.; validation, E.K.; formal analysis, E.K. and S.V.; investigation, M.M.A., M.A. (Martin Alda), S.A.-L., T.J.A., C.A., R.F.A., M.A. (Mihai Avram), R.A.-A., T.B., N.B., L.J.B., S.B., A.B., K.B., K.C., V.D.C., N.D.C., D.X.C., B.C.-F., J.C.D.-A., K.D.-O., U.D., D.D., N.D., J.D., C.M.D.-C., S.G.D., E.D., S.E., C.E., F.F., L.E.F., C.E.F., P.F.-C., H.G., C.C.G., J.G., D.G., M.G., A.G.-Z., M.H., J.H., C.H., K.R.H., D.H., A.I., A.J., M.K. (Michael Kaess), X.K., K.K. (Kimbra Kenney), B.K., M.S.K., M.K. (Minah Kim), J.K. (Jochen Kindler), T.K. (Tilo Kircher), K.K. (Karolina Knížková), K.K.K., D.K., W.S.K., T.K. (Taylor Kuhn), V.K., J.K. (Junsoo Kwon), R.L., S.L., J.L. (Jungha Lee), J.L. (Jean Lengenfelder), S.W.L., V.L.-J., S.M.L., M.L., A.J.L., C.M. (Cassandra Marotta), C.A.M., P.M., A.M., C.R.M., S.M., T.R.M., J.M.-N., C.M. (Chantal Michel), R.A.M., B.M., D.J.M., I.N., M.R.N., A.N., T.O., V.O., J.O., A.O., V.O.G.d.l.F., M.O., H.P., M.P., F.P. (Fabrizio Piras), F.P. (Federica Piras), E.P.-C., J.R. (Jonathan Repple), G.R., J.R. (Jonathan Rodriguez), M.R., K.R.-M., J.R. (Jared Rowland), N.P.R., R.S., A.-M.S., A.S. (Andre Schmidt), J.C.S., G.S., F.Š., A.S. (Alena Stasenko), F.S., B.S., A.T., F.T.-O., S.I.T., E.B.T., I.T., M.T., J.A.T., K.M.U., G.U., E.V., L.V., W.C.W., E.W., L.T.W., K.W., A.W., M.-J.W., G.R.W., L.N.Y., G.B.Z.-S., M.J.P., P.M.T, D.F.T., F.G.H. and E.A.W.; data curation, H.M.L. and E.L.D.; writing—original draft preparation, E.K. and S.W.L.; writing—review and editing, H.M.L., P.M.T., M.J.P., D.F.T., F.G.H., E.A.W. and E.L.D.; visualization, E.K.; supervision, P.M.T., M.J.P., D.F.T., F.G.H. and E.A.W.; project administration, E.K. and E.L.D.; funding acquisition, M.M.A., M.A. (Martin Alda), S.A.-L., T.J.A., C.A., N.B., A.B., K.C., V.D.C., N.D.C., B.C.-F., J.C.D.-A., K.D.-O., J.D., C.M.D.-C., S.G.D., E.D., S.E., C.E., F.F., C.E.F., P.F.-C., H.G., C.C.G., J.H., C.H., A.I., A.J., M.K. (Michael Kaess), E.K., M.K. (Minah Kim), T.K. (Tilo Kircher), K.K.K., D.K., W.S.K., T.K. (Taylor Kuhn), J.K. (Jochen Kindler), R.L., J.L. (Jean Lengenfelder), C.R.M., T.R.M., D.J.M., I.N., A.N., T.O., J.O., A.O., F.P. (Fabrizio Piras), F.P. (Federica Piras), E.P.-C., G.R., R.S., A.-M.S., S.R.S., G.S., A.S. (Alena Stasenko), B.S., A.T., S.I.T., J.A.T., K.M.U., D.V., E.V., L.V., L.T.W., G.R.W., P.M.T., D.F.T., F.G.H., E.A.W. and E.L.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article and code used for analysis will be made available by the authors on reasonable request pending appropriate study approvals and data transfer agreements between participating institutions.
Conflicts of Interest
Celso Arango has been a consultant to or has received honoraria or grants from Abbot, Acadia, Ambrosetti, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, Takeda, and Teva. Amy Brodtmann serves on the editorial boards of Neurology and International Journal of Stroke. Benedicto Crespo-Facorro has been a consultant to or has received honoraria or grants from Angelini, Boehringer, Johnson, Lundbeck, Otsuka, and Rovi. Covadonga M. Diaz-Caneja has received honoraria from Angelini and Viatris. Christopher C. Giza has been a consultant to the NBA, NFL, NHLPA, and Los Angeles Lakers, he has served on the advisory board for Highmark Interactive, Novartis, MLS, NBA, and USSF, and he handles 1–2 medicolegal cases annually. Jonathan Repple has received speaking honoraria from Janssen and Hexal. Jair C. Soares has been a consultant to, has received honoraria or grants from, or has stock in Alkermes, Allergan, Asofarma, ATAI, Boehringer Ingelheim, Compass, Johnson & Johnson, Livanova, Pfizer, Pulvinar Neuro LLC, Relmada, Sanofi, and Sunovian. Paul M. Thompson received partial research support from Biogen, Inc., for research unrelated to this manuscript. Lucy Vivash received partial research support for this project by an investigator-initiated research grant from Biogen (US). Biogen had no role in the analysis or writing of this manuscript. Additionally, Lucy Vivash received support from Eisai (JP) and Life Molecular Imaging for research unrelated to this manuscript. Glenn R. Wylie has received research support from the NJ Commission for Brain Injury Research, the Dept of Veterans Affairs, Biogen, Bristol, Myers, Squibb, and Genetech, and he has served on advisory boards for the CDMRP and the VA; all of these activities are unrelated to this research. Lakshmi N. Yatham has been on speaker or advisory boards for, or has received research grants from, Alkermes, Abbvie, Canadian Institutes of Health Research, Sumitomo Dainippon Pharma, GlaxoSmithKline, Intracellular Therapies, Merck, Sanofi, Sequiris, Servier, and Sunovion, all outside this work. David F. Tate received funding from the Defense and Veterans Brain Injury Centers, the U.S. Army Medical Research and Materiel Command, and the Chronic Effects of Neurotrauma Consortium (CENC). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors declare no conflicts of interest. The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Funding Statement
This research was funded by the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military Relevant Brain Injury Consortium (LIMBIC), Grant/Award Numbers: W81XWH18PH, TBIRPLIMBIC under Awards Numbers: W81XWH1920067 and W81XWH1320095; US Department of Defense, Grant/Award Number: AZ150145; US Department of Veterans Affairs, Grant/Award Numbers: I01 CX002097, I01 CX002096, I01 HX003155, I01 RX003444, I01 RX003443, I01 RX003442, I01 CX001135, I01 CX001246, I01 RX001774, I01 RX001135, I01 RX002076, I01 RX001880, I01 RX002172, I01 RX002173, I01 RX002171, I01 RX002174, I01 RX002170, 1I01 RX003444; National Institutes of Health (NIH), Grant/Award Number(s): RF1NS115268, RF1NS128961, U01NS086625, U01MH124639, P50MH115846, R01MH113827, R25MH080663, K08MH068540, R01NS100973, R01EB006841, P20GM103472, RO1MH083553, T32MH019535, R01 HD061504, RO1MH083553, R01AG050595, R01AG076838, R01AG060470, R01AG064955, P01AG055367, K23MH095661, R01MH094524, R01MH121246, T32MH019535, R01NS124585, R01NS122827, R61NS120249, R01NS122184, U54EB020403, R01MH116147, R56AG058854, P41EB015922, R01MH111671, P41RR14075, M01RR01066, R01EB006841, R01EB005846, R01 EB000840, RC1MH089257, U24 RR021992, and NCRR 5 month-RR001066 (MGH General Clinical Research Center); National Institute of Mental Health (NIMH), Grant/Award Number: 1P20RR021938; Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, Grant/Award Numbers: PI15-00852, PI18-00945, JR19-00024, PI17-00481, PI20-00721; Sara Borrell contract, Grant/Award Number: CD19-00149; German Research Foundation DFG grant FOR2107, Grant/Award Numbers: JA 1890/7-1, JA 1890/7-2, NE2254/1-2, NE2254/2-1, NE2254/3-1, NE2254/4-1, KI588/14-1, KI588/14-2, DA1151/5-1, DA1151/5-2, SFB-TRR58, Projects C09 and Z02; European Union, NextGenerationEU, Grant/Award Numbers: PMP21/00051, PI19/01024; Structural Funds; Seventh Framework Program; H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking: Project PRISM-2, Grant/Award Number: 101034377; Project AIMS-2-TRIALS, Grant/Award Number: 777394; Horizon Europe; NSF, Grant/Award Number: 2112455; Madrid Regional Government, Grant/Award Number: B2017/BMD-3740 AGES-CM-2; Dalhousie Medical Research Foundation; Research Nova Scotia, Grant/Award Number: RNS-NHIG-2021-1931; NJ Commission on TBI Research Grants, Grant/Award Numbers: CBIR11PJT020, CBIR13IRG026; Department of Psychology, University of Oslo; Sunnaas Rehabilitation Hospital, Grant/Award Number: HF F32NS119285; Canadian Institutes of Health Research, Grant/Award Number: 166098; Neurological Foundation of New Zealand, Grant/Award Number: 2232 PRG; Canterbury Medical Research Foundation, University of Otago; Biogen US Investigator-initiated grant; Italian Ministry of Health, Grant/Award Number: RF-2019-12370182 and Ricerca Corrente RC 23; National Institute on Aging; National Health and Medical Research Council, Investigator Grant/Award Number: APP1176426; PA Health Research, Grant/Award Number: 4100077082; La Caixa Foundation, Grant/Award Number: 100010434, fellowship code: LCF/BQ/PR22/11920017; Research Council of Norway, Grant/Award Number: 248238; Health Research Council of New Zealand Sir Charles Hercus Early Career Development, Grant/Award Numbers: 17/039 and 14-440; Health Research Council of New Zealand, Grant/Award Numbers: 20/538 and 14/440; Research and Education Trust Pacific Radiology, Grant/Award Number: MRIJDA; South-Eastern Norway Regional Health Authority, Grant/Award Number: 2018076; Norwegian ExtraFoundation for Health and Rehabilitation, Grant/Award Numbers: 2015/FO5146; South-Eastern Norway Regional Health Authority, Grant/Award Number: 2015044; Stiftelsen K.G. Jebsen, Grant/Award Number: SKGJ MED-02; The Liaison Committee between Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU), Grant/Award Number: 2020/39645; National Health and Medical Research Council, Grant/Award Number: APP1020526; Brain Foundation; Wicking Trust; Collie Trust; Sidney and Fiona Myer Family Foundation; U.S. Army Medical Research and Materiel Command (USAMRMC), Grant/Award Number: 13129004; Department of Energy, Grant/Award Number: DE-FG02-99ER62764; Mind Research Network; National Association for Research in Schizophrenia and Affective Disorders, Young Investigator Award; Blowitz Ridgeway and Essel Foundations; Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster; NOW ZonMw TOP, Grant/Award Number: 91211021; UCLA Easton Clinic for Brain Health; UCLA Brain Injury Research Center; Stan and Patty Silver; Clinical and Translational Research Center, Grant/Award Numbers: UL1RR033176, UL1TR000124; Mount Sinai Institute for NeuroAIDS Disparities; VA Rehab SPIRE; CDMRP PRAP; VA RR&D, Grant/Award Number: IK2RX002922; Veski Fellowship; Femino Foundation grant; Fundación Familia Alonso; Fundación Alicia Koplowitz; CIBERSAM, Madrid Regional Government, Grant/Award Numbers: B2017/BMD-3740 AGES-CM-2, 2019R1C1C1002457, 21-BR-03-01, 2020M3E5D9079910, 21-BR-03-01; Deutsche Forschungsgemeinschaft (DFG), Grant/Award Numbers: NE2254/1-2, NE2254/2-1, NE2254/3-1, NE2254/4-1.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Katz J., Saadon-Grosman N., Arzy S. The life review experience: Qualitative and quantitative characteristics. Conscious. Cogn. 2017;48:76–86. doi: 10.1016/j.concog.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Maki Y., Yamaguchi T., Yamagami T., Murai T., Hachisuka K., Miyamae F., Ito K., Awata S., Ura C., Takahashi R., et al. The impact of subjective memory complaints on quality of life in community-dwelling older adults. Psychogeriatrics. 2014;14:175–181. doi: 10.1111/psyg.12056. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis Z., Shah R.C., Bennett D.A. Diagnosis and Management of Dementia: Review. JAMA. 2019;322:1589–1599. doi: 10.1001/jama.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jongsiriyanyong S., Limpawattana P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimers Dis. Other Demen. 2018;33:500–507. doi: 10.1177/1533317518791401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faglioni P., Saetti M.C., Botti C. Verbal learning strategies in Parkinson’s disease. Neuropsychology. 2000;14:456–470. doi: 10.1037/0894-4105.14.3.456. [DOI] [PubMed] [Google Scholar]
- 6.Paterno R., Folweiler K.A., Cohen A.S. Pathophysiology and Treatment of Memory Dysfunction after Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2017;17:52. doi: 10.1007/s11910-017-0762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews G., Halford G.S., Shum D.H., Maujean A., Chappell M., Birney D.P. Verbal learning and memory following stroke. Brain Inj. 2014;28:442–447. doi: 10.3109/02699052.2014.888758. [DOI] [PubMed] [Google Scholar]
- 8.Kizilbash A.H., Vanderploeg R.D., Curtiss G. The effects of depression and anxiety on memory performance. Arch. Clin. Neuropsychol. 2002;17:57–67. doi: 10.1093/arclin/17.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Skodzik T., Holling H., Pedersen A. Long-Term Memory Performance in Adult ADHD. J. Atten. Disord. 2017;21:267–283. doi: 10.1177/1087054713510561. [DOI] [PubMed] [Google Scholar]
- 10.Antoniades M., Schoeler T., Radua J., Valli I., Allen P., Kempton M.J., McGuire P. Verbal learning and hippocampal dysfunction in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev. 2018;86:166–175. doi: 10.1016/j.neubiorev.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miskowiak K.W., Mariegaard J., Jahn F.S., Kjaerstad H.L. Associations between cognition and subsequent mood episodes in patients with bipolar disorder and their unaffected relatives: A systematic review. J. Affect. Disord. 2022;297:176–188. doi: 10.1016/j.jad.2021.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Cintron D.W., Adler N.E., Gottlieb L.M., Hagan E., Tan M.L., Vlahov D., Glymour M.M., Matthay E.C. Heterogeneous treatment effects in social policy studies: An assessment of contemporary articles in the health and social sciences. Ann. Epidemiol. 2022;70:79–88. doi: 10.1016/j.annepidem.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Y.Y., Papez V., Chang W.H., Mueller S.H., Denaxas S., Lai A.G. Comparing clinical trial population representativeness to real-world populations: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3:e674–e689. doi: 10.1016/S2666-7568(22)00186-6. [DOI] [PubMed] [Google Scholar]
- 14.Alsaadi T., Kassie S., Mohamed Ali O., Mozahem K., Al Fardan S., Ahmed A.M. Psychiatric Comorbidity in Neurological Disorders: Towards a Multidisciplinary Approach to Illness Management in the United Arab Emirates. Front. Psychiatry. 2019;10:263. doi: 10.3389/fpsyt.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neal M.A., Baslet G. Treatment for Patients with a Functional Neurological Disorder (Conversion Disorder): An Integrated Approach. Am. J. Psychiatry. 2018;175:307–314. doi: 10.1176/appi.ajp.2017.17040450. [DOI] [PubMed] [Google Scholar]
- 16.Brem A.K., Ran K., Pascual-Leone A. Learning and memory. In: Lozano A.M., Hallett M., editors. Handbook of Clinical Neurology. Volume 116. Elsevier; Amsterdam, The Netherlands: 2013. pp. 693–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris R.G., Kopelman M.D. The memory deficits in Alzheimer-type dementia: A review. Q. J. Exp. Psychol. A. 1986;38:575–602. doi: 10.1080/14640748608401615. [DOI] [PubMed] [Google Scholar]
- 18.Martin A., Brouwers P., Cox C., Fedio P. On the nature of the verbal memory deficit in Alzheimer’s disease. Brain Lang. 1985;25:323–341. doi: 10.1016/0093-934X(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 19.Cooper C., Sommerlad A., Lyketsos C.G., Livingston G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry. 2015;172:323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro F., Guerreiro M., De Mendonca A. Verbal learning and memory deficits in Mild Cognitive Impairment. J. Clin. Exp. Neuropsychol. 2007;29:187–197. doi: 10.1080/13803390600629775. [DOI] [PubMed] [Google Scholar]
- 21.Khan Z.U., Martin-Montanez E., Navarro-Lobato I., Muly E.C. Memory deficits in aging and neurological diseases. Prog. Mol. Biol. Transl. Sci. 2014;122:1–29. doi: 10.1016/B978-0-12-420170-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 22.Bailey H.R., Zacks J.M., Hambrick D.Z., Zacks R.T., Head D., Kurby C.A., Sargent J.Q. Medial temporal lobe volume predicts elders’ everyday memory. Psychol. Sci. 2013;24:1113–1122. doi: 10.1177/0956797612466676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntley J.D., Howard R.J. Working memory in early Alzheimer’s disease: A neuropsychological review. Int. J. Geriatr. Psychiatry. 2010;25:121–132. doi: 10.1002/gps.2314. [DOI] [PubMed] [Google Scholar]
- 24.Karantzoulis S., Galvin J.E. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert. Rev. Neurother. 2011;11:1579–1591. doi: 10.1586/ern.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bott N.T., Radke A., Stephens M.L., Kramer J.H. Frontotemporal dementia: Diagnosis, deficits and management. Neurodegener. Dis. Manag. 2014;4:439–454. doi: 10.2217/nmt.14.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poos J.M., Jiskoot L.C., Papma J.M., van Swieten J.C., van den Berg E. Meta-analytic Review of Memory Impairment in Behavioral Variant Frontotemporal Dementia. J. Int. Neuropsychol. Soc. 2018;24:593–605. doi: 10.1017/S1355617718000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 28.Fang C., Lv L., Mao S., Dong H., Liu B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Park. Dis. 2020;2020:2076942. doi: 10.1155/2020/2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyer M.K., Bronnick K.S., Hwang K.S., Bergsland N., Tysnes O.B., Larsen J.P., Thompson P.M., Somme J.H., Apostolova L.G. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2013;84:23–28. doi: 10.1136/jnnp-2012-303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geary E.K., Kraus M.F., Pliskin N.H., Little D.M. Verbal learning differences in chronic mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2010;16:506–516. doi: 10.1017/S135561771000010X. [DOI] [PubMed] [Google Scholar]
- 31.Lindsey H.M., Lalani S.J., Mietchen J., Gale S.D., Wilde E.A., Faber J., MacLeod M.C., Hunter J.V., Chu Z.D., Aitken M.E., et al. Acute pediatric traumatic brain injury severity predicts long-term verbal memory performance through suppression by white matter integrity on diffusion tensor imaging. Brain Imaging Behav. 2020;14:1626–1637. doi: 10.1007/s11682-019-00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy E., Panahi S., Stewart I.J., Tate D.F., Wilde E.A., Kenney K., Werner J.K., Gill J., Diaz-Arrastia R., Amuan M., et al. Traumatic Brain Injury and Early Onset Dementia in Post 9–11 Veterans. Brain Inj. 2022;36:620–627. doi: 10.1080/02699052.2022.2033846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haarbauer-Krupa J., Pugh M.J., Prager E.M., Harmon N., Wolfe J., Yaffe K. Epidemiology of Chronic Effects of Traumatic Brain Injury. J. Neurotrauma. 2021;38:3235–3247. doi: 10.1089/neu.2021.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh M.J., Kennedy E., Prager E.M., Humpherys J., Dams-O’Connor K., Hack D., McCafferty M.K., Wolfe J., Yaffe K., McCrea M., et al. Phenotyping the Spectrum of Traumatic Brain Injury: A Review and Pathway to Standardization. J. Neurotrauma. 2021;38:3222–3234. doi: 10.1089/neu.2021.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Qazzaz N.K., Ali S.H., Ahmad S.A., Islam S., Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: A post-stroke memory assessment. Neuropsychiatr. Dis. Treat. 2014;10:1677–1691. doi: 10.2147/NDT.S67184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriesche D., Woll C.F.J., Tschentscher N., Engel R.R., Karch S. Neurocognitive deficits in depression: A systematic review of cognitive impairment in the acute and remitted state. Eur. Arch. Psychiatry Clin. Neurosci. 2023;273:1105–1128. doi: 10.1007/s00406-022-01479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammar A., Isaksen L., Schmid M., Ardal G., Strand M. Patients with major depression show intact memory performance--given optimal conditions. Appl. Neuropsychol. 2011;18:191–196. doi: 10.1080/09084282.2011.595445. [DOI] [PubMed] [Google Scholar]
- 38.Pan Z., Park C., Brietzke E., Zuckerman H., Rong C., Mansur R.B., Fus D., Subramaniapillai M., Lee Y., McIntyre R.S. Cognitive impairment in major depressive disorder. CNS Spectr. 2019;24:22–29. doi: 10.1017/S1092852918001207. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy E., Ozmen M., Bouldin E.D., Panahi S., Mobasher H., Troyanskaya M., Martindale S.L., Merritt V.C., O’Neil M., Sponheim S.R., et al. Phenotyping Depression after Mild Traumatic Brain Injury: Evaluating the Impact of Multiple Injury, Gender, and Injury Context. J. Neurotrauma. 2024;41:924–933. doi: 10.1089/neu.2023.0381. [DOI] [PubMed] [Google Scholar]
- 40.Alderson R.M., Kasper L.J., Hudec K.L., Patros C.H. Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: A meta-analytic review. Neuropsychology. 2013;27:287–302. doi: 10.1037/a0032371. [DOI] [PubMed] [Google Scholar]
- 41.Kofler M.J., Singh L.J., Soto E.F., Chan E.S.M., Miller C.E., Harmon S.L., Spiegel J.A. Working memory and short-term memory deficits in ADHD: A bifactor modeling approach. Neuropsychology. 2020;34:686–698. doi: 10.1037/neu0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowie C.R., Harvey P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006;2:531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rannikko I., Paavola L., Haapea M., Huhtaniska S., Miettunen J., Veijola J., Murray G.K., Barnes A., Wahlberg K.E., Isohanni M., et al. Verbal learning and memory and their associations with brain morphology and illness course in schizophrenia spectrum psychoses. J. Clin. Exp. Neuropsychol. 2012;34:698–713. doi: 10.1080/13803395.2012.668875. [DOI] [PubMed] [Google Scholar]
- 44.Tanskanen P., Haapea M., Veijola J., Miettunen J., Jarvelin M.R., Pyhtinen J., Jones P.B., Isohanni M. Volumes of brain, grey and white matter and cerebrospinal fluid in schizophrenia in the Northern Finland 1966 Birth Cohort: An epidemiological approach to analysis. Psychiatry Res. 2009;174:116–120. doi: 10.1016/j.pscychresns.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Tanskanen P., Ridler K., Murray G.K., Haapea M., Veijola J.M., Jaaskelainen E., Miettunen J., Jones P.B., Bullmore E.T., Isohanni M.K. Morphometric brain abnormalities in schizophrenia in a population-based sample: Relationship to duration of illness. Schizophr. Bull. 2010;36:766–777. doi: 10.1093/schbul/sbn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraguljac N.V., Srivastava A., Lahti A.C. Memory deficits in schizophrenia: A selective review of functional magnetic resonance imaging (FMRI) studies. Behav. Sci. 2013;3:330–347. doi: 10.3390/bs3030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepage M., Hawco C., Bodnar M. Relational Memory as a Possible Neurocognitive Marker of Schizophrenia. JAMA Psychiatry. 2015;72:946–947. doi: 10.1001/jamapsychiatry.2015.0488. [DOI] [PubMed] [Google Scholar]
- 48.Avery S.N., Armstrong K., Blackford J.U., Woodward N.D., Cohen N., Heckers S. Impaired relational memory in the early stage of psychosis. Schizophr. Res. 2019;212:113–120. doi: 10.1016/j.schres.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quraishi S., Frangou S. Neuropsychology of bipolar disorder: A review. J. Affect. Disord. 2002;72:209–226. doi: 10.1016/S0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 50.Cao B., Passos I.C., Mwangi B., Bauer I.E., Zunta-Soares G.B., Kapczinski F., Soares J.C. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. J. Psychiatr. Res. 2016;73:102–107. doi: 10.1016/j.jpsychires.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez-Aran A., Vieta E., Reinares M., Colom F., Torrent C., Sanchez-Moreno J., Benabarre A., Goikolea J.M., Comes M., Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 52.Pomponio R., Erus G., Habes M., Doshi J., Srinivasan D., Mamourian E., Bashyam V., Nasrallah I.M., Satterthwaite T.D., Fan Y., et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage. 2020;208:116450. doi: 10.1016/j.neuroimage.2019.116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy E., Vadlamani S., Lindsey H.M., Lei P.W., Jo-Pugh M., Adamson M., Alda M., Alonso-Lana S., Ambrogi S., Anderson T.J., et al. Bridging Big Data: Procedures for Combining Non-equivalent Cognitive Measures from the ENIGMA Consortium. bioRxiv. 2023 doi: 10.1101/2023.01.16.524331. [DOI] [Google Scholar]
- 54.Delis D.C., Kramer J.H., Kaplan E., Ober B.A. California Verbal Learning Test—Second Edition (CVLT–II) Pearson; London, UK: 2000. [Google Scholar]
- 55.Rey A. The Clinical Examination in Psychology. Presses Universitaries de France; Paris, France: 1958. L’examen clinique en psychologie. [Google Scholar]
- 56.Benedict R.H., Schretlen D., Groninger L., Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 57.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin. Neuropsychol. 1991;5:125–142. doi: 10.1080/13854049108403297. [DOI] [Google Scholar]
- 58.Greifer N., Stuart E.A. Matching Methods for Confounder Adjustment: An Addition to the Epidemiologist’s Toolbox. Epidemiol. Rev. 2022;43:118–129. doi: 10.1093/epirev/mxab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breiman L. Random Forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 60.Reynolds M., Chaudhary T., Eshaghzadeh Torbati M., Tudorascu D.L., Batmanghelich K., Alzheimer’s Disease Neuroimaging I. ComBat Harmonization: Empirical Bayes versus fully Bayes approaches. Neuroimage Clin. 2023;39:103472. doi: 10.1016/j.nicl.2023.103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toga A.W., Crawford K.L. The Alzheimer’s Disease Neuroimaging Initiative informatics core: A decade in review. Alzheimers Dement. 2015;11:832–839. doi: 10.1016/j.jalz.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michel C., Kaess M., Fluckiger R., Buetiger J.R., Schultze-Lutter F., Schimmelmann B.G., Gekle W., Jandl M., Hubl D., Kindler J. The Bern Early Recognition and Intervention Centre for mental crisis (FETZ Bern)—An 8-year evaluation. Early Interv. Psychiatry. 2022;16:289–301. doi: 10.1111/eip.13160. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Corcuera P., Salvador R., Monte G.C., Salvador Sarro S., Goikolea J.M., Amann B., Moro N., Sans-Sansa B., Ortiz-Gil J., Vieta E., et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J. Affect. Disord. 2013;148:170–178. doi: 10.1016/j.jad.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Aine C., Calhoun V.D., Canive J., Hanlon F., Jung R., Kiehl K., Mayer A., Perrone-Bizzozero N., Stephen J., Tesche C. Center for Biomedical Research Excellence (COBRE) [(accessed on 6 June 2024)]. Available online: http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html.
- 65.Brodtmann A., Werden E., Pardoe H., Li Q., Jackson G., Donnan G., Cowie T., Bradshaw J., Darby D., Cumming T. Charting cognitive and volumetric trajectories after stroke: Protocol for the Cognition and Neocortical Volume after Stroke (CANVAS) study. Int. J. Stroke. 2014;9:824–828. doi: 10.1111/ijs.12301. [DOI] [PubMed] [Google Scholar]
- 66.Walker W.C., Carne W., Franke L.M., Nolen T., Dikmen S.D., Cifu D.X., Wilson K., Belanger H.G., Williams R. The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: Description of study and characteristics of early participants. Brain Inj. 2016;30:1469–1480. doi: 10.1080/02699052.2016.1219061. [DOI] [PubMed] [Google Scholar]
- 67.Zafonte R., Friedewald W.T., Lee S.M., Levin B., Diaz-Arrastia R., Ansel B., Eisenberg H., Timmons S.D., Temkin N., Novack T., et al. The citicoline brain injury treatment (COBRIT) trial: Design and methods. J. Neurotrauma. 2009;26:2207–2216. doi: 10.1089/neu.2009.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper D.B., Bowles A.O., Kennedy J.E., Curtiss G., French L.M., Tate D.F., Vanderploeg R.D. Cognitive Rehabilitation for Military Service Members with Mild Traumatic Brain Injury: A Randomized Clinical Trial. J. Head. Trauma. Rehabil. 2017;32:E1–E15. doi: 10.1097/HTR.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 69.Krch D., Frank L.E., Chiaravalloti N.D., Vakil E., DeLuca J. Cognitive Reserve Protects against Memory Decrements Associated with Neuropathology in Traumatic Brain Injury. J. Head. Trauma. Rehabil. 2019;34:E57–E65. doi: 10.1097/HTR.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 70.Eierud C., Nathan D.E., Bonavia G.H., Ollinger J., Riedy G. Cortical thinning in military blast compared to non-blast persistent mild traumatic brain injuries. Neuroimage Clin. 2019;22:101793. doi: 10.1016/j.nicl.2019.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kremen W.S., Franz C.E., Lyons M.J. Current Status of the Vietnam Era Twin Study of Aging (VETSA) Twin Res. Hum. Genet. 2019;22:783–787. doi: 10.1017/thg.2019.125. [DOI] [PubMed] [Google Scholar]
- 72.Vervoordt S.M., Arnett P., Engeland C., Rabinowitz A.R., Hillary F.G. Depression associated with APOE status and hippocampal volume but not cognitive decline in older adults aging with traumatic brain injury. Neuropsychology. 2021;35:863–875. doi: 10.1037/neu0000750. [DOI] [PubMed] [Google Scholar]
- 73.Thames A.D., Kuhn T.P., Mahmood Z., Bilder R.M., Williamson T.J., Singer E.J., Arentoft A. Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imaging Behav. 2018;12:96–108. doi: 10.1007/s11682-017-9676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grossner E.C., Bernier R.A., Brenner E.K., Chiou K.S., Hong J., Hillary F.G. Enhanced default mode connectivity predicts metacognitive accuracy in traumatic brain injury. Neuropsychology. 2019;33:922–933. doi: 10.1037/neu0000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oertel-Knochel V., Reinke B., Matura S., Prvulovic D., Linden D.E., van de Ven V. Functional connectivity pattern during rest within the episodic memory network in association with episodic memory performance in bipolar disorder. Psychiatry Res. 2015;231:141–150. doi: 10.1016/j.pscychresns.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Hwang W.J., Lee T.Y., Shin W.G., Kim M., Kim J., Lee J., Kwon J.S. Global and Specific Profiles of Executive Functioning in Prodromal and Early Psychosis. Front. Psychiatry. 2019;10:356. doi: 10.3389/fpsyt.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reyes A., Lalani S.J., Kaestner E., Hooper K., Chen A., Macari A.C., Paul B.M., Hermann B.P., McDonald C.R. The impact of cerebrovascular risk factors on postoperative memory decline in patients with left temporal lobe epilepsy. Epilepsy Behav. 2020;102:106558. doi: 10.1016/j.yebeh.2019.106558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuhn T., Sayegh P., Jones J.D., Smith J., Sarma M.K., Ragin A., Singer E.J., Albert Thomas M., Thames A.D., Castellon S.A., et al. Improvements in brain and behavior following eradication of hepatitis C. J. Neurovirol. 2017;23:593–602. doi: 10.1007/s13365-017-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marquardt C.A., Pokorny V.J., Disner S.G., Nelson N.W., McGuire K.A., Sponheim S.R. Inefficient Attentional Control Explains Verbal-Memory Deficits among Military Veterans with Posttraumatic Reexperiencing Symptoms. Clin. Psychol. Sci. 2022;10:499–513. doi: 10.1177/21677026211025018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bookheimer S.Y., Salat D.H., Terpstra M., Ances B.M., Barch D.M., Buckner R.L., Burgess G.C., Curtiss S.W., Diaz-Santos M., Elam J.S., et al. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage. 2019;185:335–348. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gollub R.L., Shoemaker J.M., King M.D., White T., Ehrlich S., Sponheim S.R., Clark V.P., Turner J.A., Mueller B.A., Magnotta V., et al. The MCIC collection: A shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics. 2013;11:367–388. doi: 10.1007/s12021-013-9184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien T., Vivash L. MCIS: Characterising Mild Cognitive Impairment Using Multimodal Biomarkers. [(accessed on 16 February 2023)]. Available online: https://research.monash.edu/en/projects/mcis-characterising-mild-cognitive-impairment-using-multimodal-bi.
- 83.Edlow B.L., Keene C.D., Perl D.P., Iacono D., Folkerth R.D., Stewart W., Mac Donald C.L., Augustinack J., Diaz-Arrastia R., Estrada C., et al. Multimodal Characterization of the Late Effects of Traumatic Brain Injury: A Methodological Overview of the Late Effects of Traumatic Brain Injury Project. J. Neurotrauma. 2018;35:1604–1619. doi: 10.1089/neu.2017.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broglio S.P., McAllister T., Katz B.P., LaPradd M., Zhou W., McCrea M.A., Investigators C.C. The Natural History of Sport-Related Concussion in Collegiate Athletes: Findings from the NCAA-DoD CARE Consortium. Sports Med. 2022;52:403–415. doi: 10.1007/s40279-021-01541-7. [DOI] [PubMed] [Google Scholar]
- 85.Kircher T., Wohr M., Nenadic I., Schwarting R., Schratt G., Alferink J., Culmsee C., Garn H., Hahn T., Muller-Myhsok B., et al. Neurobiology of the major psychoses: A translational perspective on brain structure and function-the FOR2107 consortium. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269:949–962. doi: 10.1007/s00406-018-0943-x. [DOI] [PubMed] [Google Scholar]
- 86.Wild K. Ph.D. Dissertation. Georgia State University; Atlanta, GA, USA: 2007. Neuroimmunoendocrine Pathology and Cognitive Function in Type 2 Diabetes. [Google Scholar]
- 87.Hoy K.E., McQueen S., Elliot D., Herring S.E., Maller J.J., Fitzgerald P.B. A Pilot Investigation of Repetitive Transcranial Magnetic Stimulation for Post-Traumatic Brain Injury Depression: Safety, Tolerability, and Efficacy. J. Neurotrauma. 2019;36:2092–2098. doi: 10.1089/neu.2018.6097. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez M., Knizkova K., Kerkova B., Sironova A., Sustova P., Jonas J., Spaniel F. The relationships between cognitive reserve, cognitive functioning and quality of life in first-episode schizophrenia spectrum disorders. Psychiatry Res. 2022;310:114479. doi: 10.1016/j.psychres.2022.114479. [DOI] [PubMed] [Google Scholar]
- 89.Adamson M.M. Repetitive Transcranial Magnetic Stimulation to Improve Cognitive Function in TBI. [(accessed on 16 February 2023)]; Available online: https://clinicaltrials.gov/study/NCT02152540.
- 90.Hillary F.G., Rajtmajer S.M., Roman C.A., Medaglia J.D., Slocomb-Dluzen J.E., Calhoun V.D., Good D.C., Wylie G.R. The rich get richer: Brain injury elicits hyperconnectivity in core subnetworks. PLoS ONE. 2014;9:e104021. doi: 10.1371/journal.pone.0104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petrovsky N., Quednow B.B., Ettinger U., Schmechtig A., Mossner R., Collier D.A., Kuhn K.U., Maier W., Wagner M., Kumari V. Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology. 2010;35:1429–1439. doi: 10.1038/npp.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bucker J., Popuri S., Muralidharan K., Kozicky J.M., Baitz H.A., Honer W.G., Torres I.J., Yatham L.N. Sex differences in cognitive functioning in patients with bipolar disorder who recently recovered from a first episode of mania: Data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) J. Affect. Disord. 2014;155:162–168. doi: 10.1016/j.jad.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 93.Mayeli A., Wilson J.D., Donati F.L., LaGoy A.D., Ferrarelli F. Sleep spindle alterations relate to working memory deficits in individuals at clinical high-risk for psychosis. Sleep. 2022;45:zsac193. doi: 10.1093/sleep/zsac193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt A., Crossley N.A., Harrisberger F., Smieskova R., Lenz C., Riecher-Rossler A., Lang U.E., McGuire P., Fusar-Poli P., Borgwardt S. Structural Network Disorganization in Subjects at Clinical High Risk for Psychosis. Schizophr. Bull. 2017;43:583–591. doi: 10.1093/schbul/sbw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spalletta G., Iorio M., Vecchio D., Piras F., Ciullo V., Banaj N., Sensi S.L., Gianni W., Assogna F., Caltagirone C., et al. Subclinical Cognitive and Neuropsychiatric Correlates and Hippocampal Volume Features of Brain White Matter Hyperintensity in Healthy People. J. Pers. Med. 2020;10:172. doi: 10.3390/jpm10040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yue J.K., Vassar M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Valadka A.B., Gordon W.A., Maas A.I., Mukherjee P., Yuh E.L., et al. Transforming research and clinical knowledge in traumatic brain injury pilot: Multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma. 2013;30:1831–1844. doi: 10.1089/neu.2013.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richard G., Petersen A., Ulrichsen K.M., Kolskar K.K., Alnaes D., Sanders A.M., Dorum E.S., Ihle-Hansen H., Nordvik J.E., Westlye L.T. TVA-based modeling of short-term memory capacity, speed of processing and perceptual threshold in chronic stroke patients undergoing cognitive training: Case-control differences, reliability, and associations with cognitive performance. PeerJ. 2020;8:e9948. doi: 10.7717/peerj.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nunes A., Schnack H.G., Ching C.R.K., Agartz I., Akudjedu T.N., Alda M., Alnaes D., Alonso-Lana S., Bauer J., Baune B.T., et al. Using structural MRI to identify bipolar disorders—13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Mol. Psychiatry. 2020;25:2130–2143. doi: 10.1038/s41380-018-0228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ayesa-Arriola R., Tordesillas-Gutierrez D., Setien-Suero E., Remon-Gallo D., Gonzalez M., Albacete A., Blanco-Campal A., Rodriguez-Sanchez J.M., Crespo-Facorro B. Verbal memory and voxel based morphometry in first episode non-affective psychosis: A process oriented approach. Neuropsychology. 2019;33:568–580. doi: 10.1037/neu0000540. [DOI] [PubMed] [Google Scholar]
- 100.Lundervold A.J., Halleland H.B., Brevik E.J., Haavik J., Sorensen L. Verbal Memory Function in Intellectually Well-Functioning Adults With ADHD: Relations to Working Memory and Response Inhibition. J. Atten. Disord. 2019;23:1188–1198. doi: 10.1177/1087054715580842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article and code used for analysis will be made available by the authors on reasonable request pending appropriate study approvals and data transfer agreements between participating institutions.